Abstract
The sensory organs of the vertebrate head originate from simple ectodermal structures known as cranial placodes. All cranial placodes derive from a common domain adjacent to the neural plate, the pre-placodal region, which is induced at the border of neural and non-neural ectoderm during gastrulation. Induction and specification of the pre-placodal region is regulated by the FGF, BMP, WNT and retinoic acid signaling pathways, and characterized by expression of the EYA and SIX family of transcriptional regulators. Once the pre-placodal region is specified, different combinations of local signaling molecules and placode-specific transcription factors, including competence factors, promote the induction of individual cranial placodes along the neural axis of the head region. In this review, we summarize the steps of cranial placode development and discuss the roles of the main signaling molecules and transcription factors which regulate these steps during placode induction, specification and development.
I. Introduction
Most sensory organs in the vertebrate head originate from simple ectodermal thickenings known as cranial placodes 1, 2. Together, these sensory organs coordinate with other components of the nervous system to contribute to the proper functioning of the organism in its environment by providing it with sensory information such as vision, hearing and balance, and olfaction. Cranial placodes are formed embryonically by a series of differentiation steps arising at the boundary between neural and non-neural ectoderm. Each step involves the cooperation of distinct signaling pathways and transcription factors which first divide neural and non-neural ectoderm, then promote formation of placodal progenitors and the neural crest, and finally act to induce each placode. In this review, we summarize the current understanding of cranial placode development and discuss the major signaling pathways and transcription factors that play important roles in the development of placodes. We also briefly discuss the role of factors which contribute towards developmental competence of placodal progenitors at different stages of differentiation.
1. Cranial placodes and their function
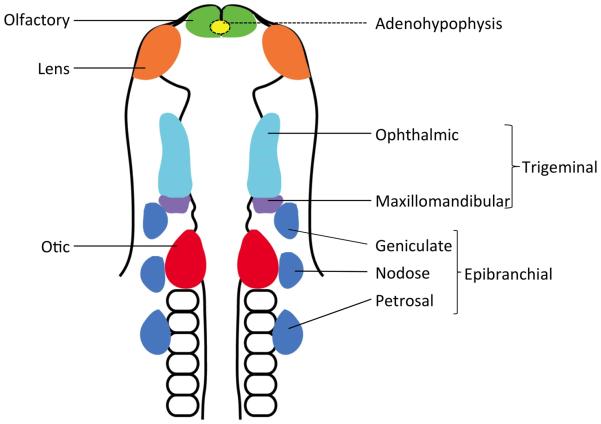
Cranial placodes can be divided into anterior, posterior and intermediate groups depending on their place of origin in the developing embryonic head (Figure 1). Anterior cranial placodes include the adenohypophyseal, olfactory and lens placodes 3. The adenohypophyseal placode invaginates from the roof of the mouth to form Rathke’s pouch which differentiates into the anterior pituitary and give rise to five types of endocrine hormone-secreting cells 4. The olfactory placode invaginates to form the olfactory sensory epithelium containing various types of secretory cells and olfactory sensory neurons, while the lens placode invaginates to give rise to the lens vesicle.
Figure 1. Location of cranial placodes in the embryonic vertebrate head.
Schematic representation of various types of cranial placodes in a 10-somite stage chick embryo (modified from Streit, 2004). Individual placodes develop in morphologically distinct domains along the neural tube in the head region. The adenohypophyseal placode develops ventral to the forebrain and is indicated here with a dotted line.
The posterior placodes comprise the otic, lateral line placodes and epibranchial placodes that give rise respectively to the inner ear, lateral line organs (in fish and amphibians) and sensory neurons of the geniculate, petrosal and nodose ganglia 5. The otic placode invaginates and pinches off from surface ectoderm to form the otic vesicle which then differentiates to generate the cochlear and vestibular systems of the inner ear, and the sensory neurons of its associated vestibulocochlear (VIIIth) ganglion. In fish and amphibians, lateral line placodes originate dorsolateral to otic placode and migrate extensively along the body before differentiating into neuromasts containing mechanoreceptors and, in some species, electroreceptors of the lateral line 6, 7.
Finally, the trigeminal placode develops between the anterior and posterior placodes, giving rise to the sensory neurons of the ophthalmic and maxilla-mandibular divisions of the trigeminal ganglion. With the exception of the adenohypophyseal and lens placodes, all other cranial placodes give rise to sensory neurons of their associated sensory structures 1, 2.
2. The emergence of placodal and neural crest progenitors at the neural plate border
Placode development is a multi-step process whose main features are conserved across all vertebrate groups. It begins at the border between neural and non-neural ectoderm that is induced during early gastrulation as a result of competing interactions between BMP, FGF and WNT signaling. BMP and WNT signaling have been shown to induce non-neural ectoderm while repressing neural differentiation 8-10, while FGF signaling, in combination with BMP and WNT antagonists, promotes neural induction 11. A number of different WNT molecules are expressed in lateral regions of the embryo where they block neural differentiation by inhibiting FGF signaling and instead induce epidermal fate in concert with BMP. WNT signaling is actively inhibited in medial regions of the embryo by secreted WNT inhibitors such as DKK and CERBERUS, which allow FGF signaling to repress BMP expression and activity and promotes neural fate in these cells 12. As neural induction proceeds, non-neural transcription genes such as Gata2 and 3, Foxi1 and 3, and Tfap2a/c are expressed more laterally, while neural genes including SoxB1, Geminin, Otx2, Gbx2, and Sox2/3 are enriched medially, but in an initially overlapping pattern with non-neural genes. This region gradually refines to become two mutually exclusive domains, the neural plate and future epidermis 13-15 (Figure 2).
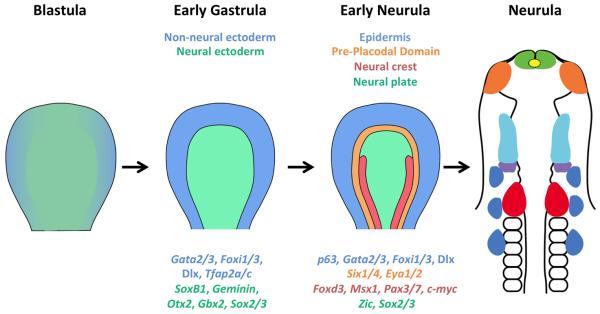
Figure 2. Overview of cranial placode development.
Placode development is a multi-step process which first starts with the induction of non-neural (blue) and neural (green) ectoderm from naïve ectoderm. The neural border (not shown) is formed in between neural and non-neural ectoderm, which gives rise to the pre-placodal region (orange) and neural crest (red). The pre-placodal region develops exclusively in the head region as a U-shaped domain surrounding the anterior neural plate and is absent from the trunk region. Neural crest is absent from the anterior-most part of the head and develops in the posterior part of head and throughout the trunk region. Some of the specific genes of each domain are also shown and color coded in accordance to their expression zone. Different cranial placodes are induced from the pre-placodal domain in response to local molecular signaling and specific transcription factors. The diagram represents a simplified and consensus view of a vertebrate embryo, but is closest to amniote embryos such as birds or humans that develop and gastrulate as a disc. Although the precise stages vary considerably in different vertebrate classes, the diagram shows an embryo prior to the onset of gastrulation (“blastula”), after initiation of gastrulation (“early gastrula”), after induction of the neural plate but before the formation of clear neural folds (“early neurula”) and after closure of the neural tube (“neurula”).
The emergence of distinct neural and non-neural ectodermal domains occurs through both positive regulatory feedback within each domain, and inhibitory negative interactions between the two domains. For example, the zebrafish non-neural genes tfap2, foxi1 and gata3 require BMP signals for their expression in non-neural ectoderm before gastrulation; however, after definitive neural tissue is induced, their expression become BMP independent due to positive autoregulation 16. In contrast, neural and non-neural genes mutually inhibit each other’s expression; for example, overexpression of dlx, gata, msx, foxi or tfap2 repress neural markers such as sox2, while inhibition of these genes results in the expansion of sox2 into the non-neural ectoderm 16, 17. As the boundary between neural and non-neural ectoderm becomes distinct, an intermediate border region emerges between these two populations. The neural plate border region is characterized by a distinct set of transcription factors such as members of the Msx, Dlx, Pax, Gbx and Zic families 18, 19.
After establishment of neural, non-neural and border domains in embryonic ectoderm, the border region is further subdivided into populations of neural crest and pre-placodal progenitors 9, 19-23. The combined action of FGF signals and inhibition of BMP and WNT signals induces the formation of the pre-placodal domain, while neural crest fate is induced in the presence of FGF, BMP and WNT activity 13, 14. It is notable that these two different progenitor populations form in close proximity, one (neural crest) in the presence of WNT and BMP signals, and the other (the pre-placodal domain) requiring inhibition of these signals. This raises the question of how distinct fates can be established in a rapidly changing environment of WNT and BMP signaling. Once again, positive autoregulation of transcription factors within neural crest or pre-placodal territories is used to stabilize their fates, while cross-repression between the two territories helps confine them to distinct regions 13, 14. Induction and development of neural crest cells has been studied in great detail over the past two decades and are well documented in recent reviews 18, 19, 24, 25. In the remainder of this review, we will focus on the induction of the pre-placodal region and the development of various cranial placodes from this region.
II. Induction and specification of the pre-placodal region
1. Similarities and differences between the pre-placodal region and neural crest
After non-neural and neural territories are established in the early embryo, the neural border region becomes subdivided into future neural crest and a domain of placodal progenitors termed the pre-placodal region 26. Several studies suggest that both the pre-placodal region and neural crest are derived from a common progenitor population originating in the neural border region 9, 21, 22. However, other studies have proposed an alternative ‘binary competence model’ which suggests that the pre-placodal region and neural crest derive from two distinct ectodermal regions of varying competence; with the pre-placodal region originating from non-neural ectoderm while neural crest arises from the neural plate 17, 27.
Cranial placodes are related to neural crest in several ways, for example, both originate from the neural border region, both are capable of producing migratory cells, and both give rise to glia, sensory and secretory components of the peripheral nervous system. However, the two progenitor populations also differ significantly regarding their origin and timing of induction during embryonic development, as well as their developmental potential. Cranial placodes develop exclusively in the head region from the U-shape pre-placodal domain surrounding the neural plate and are absent from the trunk region 28, while neural crest is absent from the anterior-most part of the head and develops in the posterior part of head and throughout the trunk region 29. These two domains are also induced and specified at different times during embryonic development; at least one study suggests that neural crest seems to be induced during late blastula or early gastrula, much earlier than pre-placodal region induction which happens around late gastrula 30. Indeed, a recent study in amphibians suggests that neural crest may even preserve some markers of pluripotency as they are specified from pluripotent progenitors in the very early embryo 31, although this finding has yet to be confirmed in other vertebrate groups. Finally, neural crest cells are developmentally more diverse than cranial placodes: they also form cartilage, bone, smooth muscle, mesenchymal and pigment cells in addition to forming sensory and secretory components of the peripheral nervous system 2, 25.
2. Induction of the pre-placodal region
The pre-placodal region is induced around the anterior border of the neural plate during gastrulation and forms various cranial placodes which give rise sensory organs of the head 2, 26, 32. Fate mapping and transplantation experiments in various vertebrates revealed that this domain is competent to give rise to all types of placodes at early developmental stages; however, the individual placodal fates become restricted as development proceeds.33. For example, the chicken trigeminal and otic placodes become committed within 8 hours of the neural folds first appearing1. This observation led to the concept of a common origin for all placodes, which is further strengthened by the expression in this domain of common transcription factors which play important roles in placode development such as members of the SIX and EYA transcription factor families 2, 26, 32. This concept is further supported by the studies showing that cells in any part of the pre-placodal domain are competent to induce an otic placode in response to FGF, while cells outside the pre-placodal domain fail to respond to FGF signaling in this manner 34. This demonstrates that the ectoderm must acquire pre-placodal properties to be able to respond to specific local inducing signals for individual placodes.
2.1 Signaling molecules involved in induction of the pre-placodal region
As discussed above, a BMP signaling gradient at early stages of gastrulation is proposed to regulate ectodermal patterning. In this model, high levels of BMP activity promote epidermis, intermediate levels and low levels induce neural crest and the pre-placodal region respectively, while still lower BMP activity is required for the formation of the neural plate 35-38. This is supported by studies which show that inhibition of BMP signaling by dominant-negative receptors or BMP antagonists induces some pre-placodal genes (Eya2 and Six4) and expand the pre-placodal region at the expense of non-neural ectoderm 22. Moreover, a number of BMP antagonists that are expressed near the non-neural and neural ectoderm and secreted from underlying mesoderm are important for pre-placodal development 39, 40.
BMP signaling has been proposed to regulate zebrafish pre-placodal induction in a two-step model 16, in which BMP is required only transiently during blastula/early gastrula stages to induce pre-placodal competence throughout non-neural ectoderm by promoting the expression of specific competence factors (Tfap2a/c, Gata3 and Foxi1). After this first induction step, expression of these factors becomes BMP-independent. In the late gastrula, BMP must be actively blocked by BMP antagonists for further induction and specification of the pre-placodal region within this zone of competence 16. Interestingly, different levels of BMP signaling regulate the fate choices between neural crest and pre-placodal cells by regulating different set of genes 16, 37, 38, 41. Gata3 and foxi1 are induced next to the epidermis in non-neural regions of high BMP activity; whereas tfap2a/c is induced by itself close to the neural plate by BMP activity, where it then induces other neural crest genes. Therefore gata3, foxi1 and tfap2a/c promote pre-placodal fates from non-neural ectoderm while tfap2a/c induces neural crest fates from neural ectoderm in a BMP-dependent manner 42, 43.
Several antagonists of both BMP and/or WNT are secreted from cranial mesoderm as well as from the anterior neural plate. Their combined action represses BMP and WNT signaling in the prospective pre-placodal region 39, 44, 45. In contrast, lateral and posterior mesoderm express high level of Wnt8c 22 while Wnt6 is expressed in trunk ectoderm 46. Interestingly, activation of WNT signaling in the pre-placodal region by overexpressing Wnt8c or active β-catenin attenuates expression of pre-placodal genes (Eya2, Six1 and Six4), whereas inhibition of WNT signaling by ectopic expression of WNT antagonists such as Crescent or Frizzled expands the expression domain of these genes 22. This indicates that inhibition of BMP and WNT together promotes the induction of pre-placodal genes, while they remain repressed outside the pre-placodal region by active WNT signaling. In this way, expression of placodal genes and therefore the placodes are restricted only to the border of the neural plate in the head. In contrast, high BMP and WNT activity promotes neural crest cell formation along the entire anterior-posterior extent of the body axis (Figure 3).
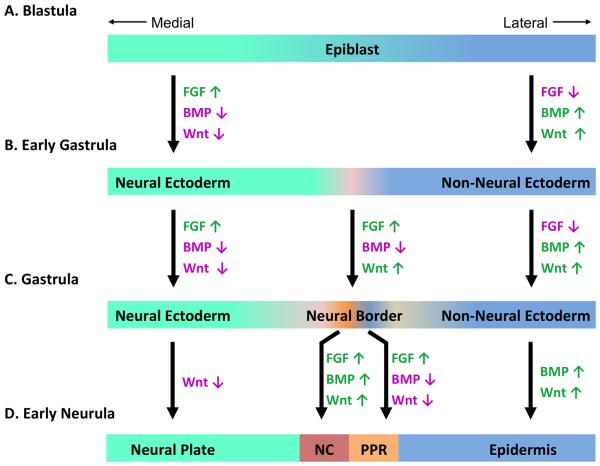
Figure 3. Induction of neural crest and the pre-placodal region.
(A) Prior to and during gastrulation, FGF, BMP and WNT signaling from the underlying hypoblast and mesoderm initiates differentiation in naïve ectoderm. (B) High BMP and WNT signaling along with FGF inhibition promotes non-neural ectoderm on the lateral side of the epiblast while high FGF signaling and reduced BMP and WNT signaling induce neural ectoderm formation in the medial side of epiblast in developing embryo. (C) The neural border is induced between non-neural and neural ectoderm. (D) Attenuation of BMP and WNT signaling in presence of FGF induces the pre-placodal region, while high BMP and WNT promote epidermis formation. On other hand, BMP and WNT along with FGF signaling promotes neural crest induction closer to the neural plate. Activation (green) and repression (magenta) of signaling pathways are color coded and the arrows indicate whether a signaling pathway is activated (↑) or inhibited (↓). BMP, Bone Morphogenetic Protein; FGF, Fibroblast Growth Factor; NC, Neural Crest; PPR, Pre-Placodal Region.
Unlike BMP and WNT which negatively regulate pre-placodal region induction, FGF signaling promotes the expression of pre-placodal genes 16, 22. FGF signals originate from tissues adjacent to the pre-placodal region during development; for example, Fgf4 and Fgf8 are expressed in mesoderm in chick, while fgf8 is expressed in the anterior neural plate in Xenopus 47, 48. Removing these tissues in chick and Xenopus blocks the induction of pre-placodal genes such as Six1 and Six4, whereas cranial mesodermal grafts activate them 27. Blocking FGF signaling by using dominant negative FGF receptors or fgf8 knockdown results in decreased six1 expression in Xenopus 27, 49. Simultaneous inhibition of FGF and PDGF signaling with the receptor antagonists SU5402 and AG1295 result in eya1 down-regulation, but not when either pathway is inhibited individually 16. Finally, in chick, the pre-placodal gene Eya2 can be ectopically induced in extra embryonic regions by activating FGF signaling in the presence of BMP and WNT inhibitors 22.
Retinoic acid (RA) also plays an important role in restricting the posterior boundary of the pre-placodal domain in a manner similar to WNT signaling. Raldh2, a RA generating enzyme, and Cyp26, a RA degrading enzyme, are expressed in a non-overlapping manner along the anteroposterior axis to create a RA gradient from low (anterior) to high (posterior) which plays an important role in regulating anteroposterior patterning in the developing embryo 50, 51. Inhibiting retinoic acid receptor activity causes posterolateral expansion of Fgf8 and Fgfr1/2 expression domains, indicating that RA and FGF may interact to restrict the posterior boundary of the pre-placodal region 52, 53.
In summary, inhibition of BMP and WNT signals in cranial ectoderm by antagonists in head mesoderm promotes a pre-placodal fate in the presence of FGF, whereas active BMP and WNT signaling induces the neural crest fate at the edge of the neural plate. FGF, WNT and RA signaling play important roles in restricting placode development to the head region, whereas neural crest induction occurs along the entire body axis. In the next section, we describe how combinations of transcription factors induced by these signals are important for placode development.
2.2 Transcription factors regulating specification of the pre-placodal region
The pre-placodal region is uniquely defined by two families of transcriptional regulators, the Six and Eya gene families. Six and Eya genes are expressed throughout the pre-placodal domain and later refine to individual placodes, where they play important roles in the development of each placode. Six and Eya genes were first identified in Drosophila as the genes sine oculis (so) and eyes absent (eya) that regulate compound eye development in combination with dachshund (dac) and eyeless/pax6 54. In vertebrates, there are 6 Six genes and 4 Eya genes based on their sequence homology and conserved domains 55. Six and Eya genes are expressed in the anterior neural plate border region that gives rise to all cranial placodes and they continue to be expressed in some of the individual placodes 56-59. All SIX proteins contain a C-terminal DNA binding SIX-type homeodomain (HD) and an N-terminal interaction domain known as the SIX Domain (SD) that interacts with other co-factors 54, 60. SIX proteins can act as both transcriptional activators and repressors depending on their interacting partners. SIX protein interaction with EYA proteins confers activator activity, whereas interactions with the Groucho co-repressor makes SIX proteins transcriptional repressors 49, 60.
SIX proteins play important roles in placodal development by positively regulating the expression of pre-placodal genes while also repressing epidermal and neural crest genes 49, 58. In Xenopus, overexpression of six1 at the neural plate border results in expansion of the pre-placodal region at the cost of epidermis and neural crest. Conversely, knockdown of six1 causes a reduction of the pre-placodal region and expansion of epidermal and neural crest domains as shown by up-regulation of keratin and FoxD3 respectively 49. In Xenopus and chick, expression of constitutively active SIX1 (VP16-SIX1) results in up-regulation of placode genes, whereas expression of a repressor form of SIX1 (EnR-SIX1) causes up-regulation of epidermal and neural crest genes. The activator and repressor activities of SIX1 are attributed to SIX1-EYA2 activator complexes and SIX1-Groucho repressor complexes respectively 49, 58. Mutation or removal of Six genes cause severe defects in different placodal derivatives, although they do not cause complete loss of particular placodal derivatives, which indicates a functional redundancy between different Six genes 61. For example, Six1/4 double knockouts show much more severe defects in olfactory placode development than Six1 knockouts alone 61.
EYA proteins are characterized by an N-terminal transactivation domain and a C-terminal interaction domain known as EYA domain (ED) which also harbors phosphatase activity 62. Unlike SIX proteins, EYA proteins cannot bind to DNA directly and require interactions with other proteins for their nuclear import and DNA binding 63. EYA proteins belong to a family of protein tyrosine phosphatases and act as a transcription co-activator of SIX proteins 62. Interactions between the SIX domain of SIX proteins and the EYA domain of EYA proteins facilitates the nuclear import of EYA and SIX complexes 62, 63. The phosphatase activity of EYA acts as a molecular switch to turn SIX proteins from repressors into activators by recruiting CREB binding protein (CBP) to SIX target sites 62. Eya1 knockout animals show severe defects in inner ear development, in addition to defects in other placodes and organs including kidney and thyroid 64, 65. Eya4 knockout mice also show inner ear defects leading to hearing loss 66. In humans, SIX1 and EYA1 mutations cause Branchio-Oto-Renal syndrome, where affected individuals suffer from branchial and kidney defects in addition to hearing loss 67-70.
As discussed in the previous section, several signaling molecules including FGF, WNT, BMP and RA as well as transcription factors including DLX, GATA and IROQUOIS (IRX) play important roles in regulating Six and Eya expression in the pre-placodal region. Six1 expression in the pre-placodal region is regulated by a placode-specific enhancer 59. The Six1 enhancer is bound directly by DLX5 and MSX1 proteins, which indicates roles of these transcription factors in Six1 regulation 59. Other transcription factors like Foxi1, Gata3, Tfap2a/c and Iroquois (Irx) also regulate six and eya expression during anamniote pre-placodal induction 16, 17, 71. By the neural plate stage, EYA and SIX protein expression is established in the pre-placodal region and they maintain their expression by positive autoregulation 58 and repress neural border genes such as dlx and gata family members which were initially required for the induction of the neural plate border 16, 17, 71, 72. Therefore, similar kinds of positive autoregulation and cross-inhibition which were initially used to establish the non-neural and neural territories of the embryo are employed again for establishing the pre-placodal domain in between the epidermis and neural crest.
III. Regionalization and specification of individual cranial placodes
At neural plate stages, the pre-placodal region and neural plate are divided into two domains along the rostro-caudal axis; an anterior domain expressing Otx2, and a posterior domain expressing Gbx2 73. These two transcription factors mutually repress each other to strengthen the boundaries between prospective placodal domains and establish individual placodal territories 73. During this process, Otx2 regulates the induction of olfactory, lens and trigeminal placodes, while Gbx2 regulates induction of the otic placode. The regionalization and specification of individual cranial placode is a multi-step process which is regulated by a number of signaling molecules and transcription factors (Figure 4). We will briefly discuss these steps in this section and direct readers to the following reviews for a more detailed discussion 2, 13, 74, 75.
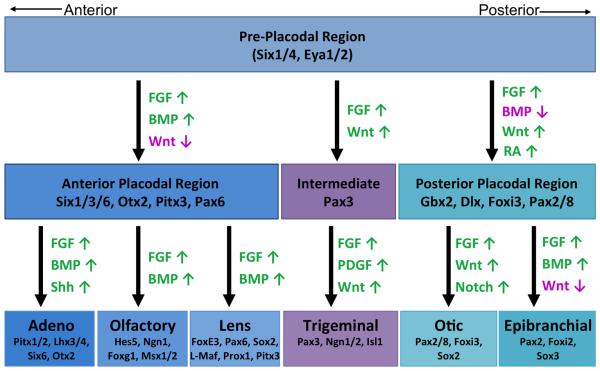
Figure 4. Regionalization and specification of individual cranial placodes from PPR.
Schematic depicting the major signaling pathways and some of the important genes involved in regionalization and specification of various cranial placodes from the pre-placodal region. Activation (green) and repression (magenta) of signaling pathways are color coded and the arrows indicate whether a signaling pathway is activated (↑) or inhibited (↓). BMP, Bone Morphogenetic Protein; FGF, Fibroblast Growth Factor; PDGF, Platelet Derived Growth Factor; RA, Retinoic Acid; SHH, Sonic hedgehog.
1. Induction of adenohypophyseal, olfactory and lens placodes
Induction of the anterior placodes is regulated by four of the major signaling pathways - FGF, BMP, SHH and WNTs. Pax6 is the earliest marker of the anterior placodes and is proposed to be induced by SIX1 in anterior domain 58. Experiments using cultured explants of chick pre-placodal ectoderm provide evidence for a ground state of competence to form Pax6-expressing lens placode tissue that extends throughout the entire pre-placodal region 76. This initial propensity to express Pax6 must be repressed by other placode inducing signals; for example, FGF signaling represses Pax6 expression during induction of the olfactory placode 76. Pax6 and Dlx5 are co-expressed in the prospective lens and olfactory placodes but as the separation of two placodal fates begins, Pax6 is transiently down-regulated by FGF8 in the prospective olfactory placode, while Dlx5 expression is lost from the prospective lens placode 76, 77. This results in a mutually exclusive expression pattern of Pax6 and Dlx5 expression in lens and olfactory placodes respectively, which is important for segregation of the two placodes. Next, BMP signaling regulates the fate choice between lens and olfactory placode, with a short exposure to BMP4 promoting olfactory fate while a more prolonged BMP4 exposure in combination with FGF signaling induces lens fate 78. Shh signaling regulates various steps of adenohypophyseal development including initial lineage commitment and differentiation while repressing lens and olfactory placodes 79.
2. Induction of otic and epibranchial placodes
FGF signaling (Fgf3/8 in zebrafish, FGF3/FGF19 in chick and FGF3/10 in mouse) has been proposed to be the main inducer of the posterior placodal domain which give rise to otic and epibranchial placodes 80. This domain is sometimes referred to as the otic-epibranchial placode domain or OEPD 5. FGF signaling induces Pax2 and Pax8 expression in the OEPD from which otic and epibranchial placodes develop 5, 81-84 , FGF signaling and WNT inhibition promotes epibranchial placode fate, while induction of the otic placode requires FGF signaling and activation of the WNT pathway 81, 85.
As the OEPD is induced, some markers of non-neural ectoderm, such as Dlx (dlx3b/4b in zebrafish, dlx3/5 in Xenopus, Dlx5/6 in chick) and Foxi (foxi1 in zebrafish, Foxi3 in chick and mouse) genes, become restricted to the pre-placodal region and then play roles in induction of the posterior placodes 5, 86. Loss of either dlx3b/4b or foxi1 results in smaller otic vesicle formation, while combined loss of both dlx3b and foxi1 causes complete ablation of the otic vesicle and failure of otic placode induction in zebrafish 87, 88. Recently, otic expression of chick Dlx3 was shown to be epigenetically regulated by Kdm4b which is also expressed during otic placode specification 89. Similarly, loss of Foxi3 results in complete failure of otic placode induction in chick 90 and mouse 91, 92. Foxi1 and dlx3b interact with FGF signaling to regulate pax8 and pax2 expression during OEPD induction 87, 88, 93. Once the OEPD is induced, foxi1 as well as FGF signaling must be downregulated towards medial regions of the OEPD to induce the otic placode in the presence of WNT signaling, which positively regulates otic fate and inhibit epibranchial fate 94. On the other hand, Foxi1 remains expressed in the lateral region of the OEPD where it induce pax8 and sox3 expression to promote epibranchial fate in presence of active FGF and BMP signaling 94, 95.
3. Induction of the trigeminal placode
The trigeminal placode develops immediately adjacent to the midbrain and is divided into ophthalmic and maxillomandibular divisions. Pax3 is the earliest marker of the trigeminal placode and is induced by signals from the brain 96, which include the combined activity of WNT and FGF signaling 97. In addition to WNT and FGF, PDGF and Notch signaling also contribute to the induction of ophthalmic placodes 98, 99. These signaling molecules also regulate the later steps of neuronal differentiation during trigeminal placode development 100, 101. The induction of Pax3 in the intermediate pre-placodal region causes Pax6 suppression, and the consequent mutually exclusive expression of Pax3 and Pax6 helps promote the proper regionalization and separation of anterior and intermediate placode territories 102.
IV. The role of transcription factors and pioneer factors in regulating placodal competence
Why do different regions within the pre-placodal domain respond to similar inducing signals, such as FGFs, but differentiate into different placodes? Similarly, why do cells within the pre-placodal region respond to these signals, whereas cells slightly more lateral to the pre-placodal domain remain refractory to induction and differentiate as epidermis 14? One explanation is the presence of transcription factors which provide the competence of ectoderm to respond to specific inducing signals. Such competence may be manifested by the induction of lineage-specific gene expression, or by the regulation of the cellular response towards extracellular signals by regulating surface receptors. For example, in zebrafish, Tfap2a/c, Gata3 and Foxi1 induce the expression of the pre-placodal genes dlx, eya and six which are important for initiation of placode induction and specification 16, 17, 71, by allowing this region to respond to FGF signals 76. In zebrafish, chick and mice, Foxi1/3 are necessary for otic and epibranchial placode development by regulating the cellular response towards FGF signaling. Foxi1/3 mutation or knockdown results in severe defects in otic and epibranchial placodes 90, 93, even though the FGF signaling pathway appears to be intact in cells lacking FOXI1/3 92.
In addition to the mechanisms of competence described above, the transcriptional competence of cells to respond to particular inducing signals is also regulated at the chromatin level by a specific class of transcription factors known as pioneer factors. Pioneer factors typically engage transcriptionally inactive regions of chromatin and allow them to attain a poised state which can be activated as soon as inductive signals are received 103, 104. These poised enhancers are characterized by H3K4me1 histone marks which later acquire H3K27ac as an additional histone mark upon gene activation 105, 106. Pioneer factors differ from conventional transcription factors in their ability to directly bind compact, DNA-methylated chromatin in the absence of other cofactors and chromatin remodelers. Often, engagement of a silent lineage-specific enhancer by a pioneer factor is accompanied by a progressive recruitment of DNA demethylases and histone-modifying enzymes that replace inactivating histone marks with activating marks during cellular reprogramming and differentiation 107-109.
Interestingly, members of the Forkhead family of transcription factors including FoxA1/2/3, FoxD3, FoxE1, and FoxO have been shown to act as pioneer factors in regulating gene expression 110, 111. The winged helix DNA binding domains 112 of many FOX proteins have structural similarities to linker H1 histones 113. This DNA binding domain, together with a C-terminal core histone binding domain and N-terminal transactivation domain allows FOXA proteins to bind directly to nucleosomes, open chromatin and promote accessibility and recruitment of other transcription factors to the DNA. Importantly, binding of FOXA to enhancers results in the recruitment of active histone marks such as H3K4me1/me2 and H3K9ac 114.
Fox proteins also play important roles in neural crest and pre-placodal specification; FoxD3 is an important regulator of neural crest specification 115 while zebrafish foxi1 (and its functional homologue in amniotes, Foxi3) is important for competence of non-neural ectoderm to respond to pre-placodal inducing signals 16. FOXD3 is already known to act as a pioneer factor in liver and ES cell differentiation 116 whereas FOXI1/3 shares several features of pioneer factors such as the ability to bind DNA during mitosis 117 and is a close homologue of another pioneer factor, FoxA 110. One important feature of pioneer factors is their stage-specific expression, where they begin to be expressed in a naïve, ground or undifferentiated state and keep their target genes in a poised configuration. Once differentiation signals are received, pioneer factors are often down-regulated and their target genes are induced 107. In the case of otic placode induction, foxi1/3 is initially expressed with other non-neural markers such as gata3, dlx3b, and tfap2a/c in non-neural ectoderm surrounding the future neural plate 16, 71, and is quickly down-regulated from the otic placode once it is induced 90, 92, 118, 119. Moreover, knockdown of Foxi3 in chick 90 or foxi1 in zebrafish 87, 88, causes a failure of otic placode induction. Foxi3 mutant mice lack an inner ear and are unable to induce even the earliest markers of the otic placode 91, 92.
We hypothesize that these competence factors including FOXI1/3 contribute to placode development in two ways - first, by regulating the expression of other co-expressed placodal genes 71, and second, by acting as a pioneer factor, where they remain bound to enhancers of specific placodal genes and keep them poised for activation until the appropriate induction signals are received. Recent evidence supports the idea that the gradual activation of otic-specific genes is accompanied by recruitment of enzymes which create activating histone marks, such as DNA demethylases 89.
V. Conclusion
Placode development is a multi-step process, with each of these steps being regulated by distinct signaling pathways and transcription factors. These signaling molecules create a local environment of differential expression of various transcription factors which first establish a non-neural and neural boundary followed by formation of the neural border which forms the neural crest and placodes. There are many examples of developmental defects in humans which are caused due to mutation in some of these genes regulating placode development 69, 70. Understanding the roles of these signaling pathways and various transcription factors during placode induction and development is important for understanding and designing treatment strategies for disease and defects associated with neural crest and placode derived sensory organs.
It remains unclear how the common pre-placodal region becomes competent to respond to similar inductive signaling and still be able to form different placode derivatives during embryonic development 26. We suggest that this lineage fate determination and differentiation towards a particular placodal fate may be regulated by pioneer factors 14. These pioneer factors may be expressed early during induction of the pre-placodal region, where they may be able to bind to the enhancers of lineage-specific genes keeping them poised for transcription by recruiting other co-factors. When lineage-specific inductive differentiation signals become available, they promote the differentiation of the cells towards their respective placodal fates. We hypothesize that FOXI3 may be acting as a pioneer factor in regulating otic placode development as supported by the absence of otic placode induction in Foxi3 knockout mice 91, 92. Pioneer factors are also known to regulate lineage specific gene expression during cellular reprogramming in stem cells 107-109. We suggest that similar kinds of pioneer factor-mediated gene regulation may be operational during regeneration and repair in various sensory neural crest and placode derived sensory organs in which adult stem cells are present 120, 121.
Footnotes
The authors declare they have no financial or other conflicts of interest
References
- 1.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 2.Schlosser G. Making Senses. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- 3.Toro S, Varga ZM. Equivalent progenitor cells in the zebrafish anterior preplacodal field give rise to adenohypophysis, lens, and olfactory placodes. Semin Cell Dev Biol. 2007;18:534–542. doi: 10.1016/j.semcdb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoti K. Genetic regulation of murine pituitary development. J Mol Endocrinol. 2015;54:R55–73. doi: 10.1530/JME-14-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladher RK, O'Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ED, Cruz IA, Hailey DW, Raible DW. There and back again: development and regeneration of the zebrafish lateral line system. Wiley Interdiscip Rev Dev Biol. 2015;4:1–16. doi: 10.1002/wdev.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker CV, Modrell MS, Gillis JA. The evolution and development of vertebrate lateral line electroreceptors. J Exp Biol. 2013;216:2515–2522. doi: 10.1242/jeb.082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 9.Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73–83. doi: 10.1242/dev.025890. [DOI] [PubMed] [Google Scholar]
- 10.Patthey C, Gunhaga L, Edlund T. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS One. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- 13.Grocott T, Tambalo M, Streit A. The peripheral sensory nervous system in the vertebrate head: a gene regulatory perspective. Dev Biol. 2012;370:3–23. doi: 10.1016/j.ydbio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Groves AK, LaBonne C. Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev Biol. 2014;389:2–12. doi: 10.1016/j.ydbio.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sargent TD. Transcriptional regulation at the neural plate border. Adv Exp Med Biol. 2006;589:32–44. doi: 10.1007/978-0-387-46954-6_3. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6:e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieper M, Ahrens K, Rink E, Peter A, Schlosser G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development. 2012;139:1175–1187. doi: 10.1242/dev.074468. [DOI] [PubMed] [Google Scholar]
- 18.Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. 2012;366:22–33. doi: 10.1016/j.ydbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- 21.Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 22.Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- 23.Patthey C, Gunhaga L. Specification and regionalisation of the neural plate border. Eur J Neurosci. 2011;34:1516–1528. doi: 10.1111/j.1460-9568.2011.07871.x. [DOI] [PubMed] [Google Scholar]
- 24.Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 26.Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- 27.Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. [DOI] [PubMed] [Google Scholar]
- 30.Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- 31.Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science. 2015;348:1332–1335. doi: 10.1126/science.aaa3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson AG. THE DETERMINATION AND POSITIONING OF THE NOSE, LENS AND EAR. III. EFFECTS OF REVERSING THE ANTERO-POSTERIOR AXIS OF EPIDERMIS, NEURAL PLATE AND NEURAL FOLD. J Exp Zool. 1963;154:293–303. doi: 10.1002/jez.1401540305. [DOI] [PubMed] [Google Scholar]
- 34.Martin K, Groves AK. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–887. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- 35.Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- 36.Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- 37.Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- 38.Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- 39.Ogita J, Isogai E, Sudo H, Sakiyama S, Nakagawara A, Koseki H. Expression of the Dan gene during chicken embryonic development. Mech Dev. 2001;109:363–365. doi: 10.1016/s0925-4773(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 40.Kwon HJ, Riley BB. Mesendodermal signals required for otic induction: Bmp-antagonists cooperate with Fgf and can facilitate formation of ectopic otic tissue. Dev Dyn. 2009;238:1582–1594. doi: 10.1002/dvdy.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12:452–458. doi: 10.1016/s0959-437x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman TL, Javier AL, Campeau SA, Knight RD, Schilling TF. Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zool B Mol Dev Evol. 2007;308:679–691. doi: 10.1002/jez.b.21189. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol. 2007;304:338–354. doi: 10.1016/j.ydbio.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley L, Sun B, Collins-Racie L, LaVallie E, McCoy J, Sive H. Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev Biol. 2000;227:118–132. doi: 10.1006/dbio.2000.9873. [DOI] [PubMed] [Google Scholar]
- 45.Carmona-Fontaine C, Acuna G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev Biol. 2007;309:208–221. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- 47.Shamim H, Mason I. Expression of Fgf4 during early development of the chick embryo. Mech Dev. 1999;85:189–192. doi: 10.1016/s0925-4773(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 48.Shim S, Bae N, Park SY, Kim WS, Han JK. Isolation of Xenopus FGF-8b and comparison with FGF-8a. Mol Cells. 2005;19:310–317. [PubMed] [Google Scholar]
- 49.Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Pollet N, Niehrs C, Pieler T. Increased XRALDH2 activity has a posteriorizing effect on the central nervous system of Xenopus embryos. Mech Dev. 2001;101:91–103. doi: 10.1016/s0925-4773(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Huang L, Solursh M. A concentration gradient of retinoids in the early Xenopus laevis embryo. Dev Biol. 1994;161:70–76. doi: 10.1006/dbio.1994.1008. [DOI] [PubMed] [Google Scholar]
- 52.Shiotsugu J, Katsuyama Y, Arima K, Baxter A, Koide T, Song J, Chandraratna RA, Blumberg B. Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. Development. 2004;131:2653–2667. doi: 10.1242/dev.01129. [DOI] [PubMed] [Google Scholar]
- 53.Janesick A, Shiotsugu J, Taketani M, Blumberg B. RIPPLY3 is a retinoic acid-inducible repressor required for setting the borders of the pre-placodal ectoderm. Development. 2012;139:1213–1224. doi: 10.1242/dev.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 55.Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes--structure and function as transcription factors and their roles in development. Bioessays. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- 57.Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christophorou NA, Bailey AP, Hanson S, Streit A. Activation of Six1 target genes is required for sensory placode formation. Dev Biol. 2009;336:327–336. doi: 10.1016/j.ydbio.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 59.Sato S, Ikeda K, Shioi G, Ochi H, Ogino H, Yajima H, Kawakami K. Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Dev Biol. 2010;344:158–171. doi: 10.1016/j.ydbio.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- 61.Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 63.Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 65.Zou D, Silvius D, Rodrigo-Blomqvist S, Enerback S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev Biol. 2006;298:430–441. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Depreux FF, Darrow K, Conner DA, Eavey RD, Liberman MC, Seidman CE, Seidman JG. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:651–658. doi: 10.1172/JCI32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Knosp BM, Maconochie M, Friedman RA, Smith RJ. A comparative study of Eya1 and Eya4 protein function and its implication in branchio-oto-renal syndrome and DFNA10. J Assoc Res Otolaryngol. 2004;5:295–304. doi: 10.1007/s10162-004-4044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Manaligod JM, Weeks DL. EYA1 mutations associated with the branchio-oto-renal syndrome result in defective otic development in Xenopus laevis. Biol Cell. 2010;102:277–292. doi: 10.1042/BC20090098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 70.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr., et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhat N, Kwon HJ, Riley BB. A gene network that coordinates preplacodal competence and neural crest specification in zebrafish. Dev Biol. 2013;373:107–117. doi: 10.1016/j.ydbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woda JM. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130:331–342. doi: 10.1242/dev.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steventon B, Mayor R, Streit A. Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning. Dev Biol. 2012;367:55–65. doi: 10.1016/j.ydbio.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jidigam VK, Gunhaga L. Development of cranial placodes: insights from studies in chick. Dev Growth Differ. 2013;55:79–95. doi: 10.1111/dgd.12027. [DOI] [PubMed] [Google Scholar]
- 75.Saint-Jeannet JP, Moody SA. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Dev Biol. 2014;389:13–27. doi: 10.1016/j.ydbio.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13:141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- 82.Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–3730. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- 83.McCarroll MN, Nechiporuk AV. Fgf3 and Fgf10a work in concert to promote maturation of the epibranchial placodes in zebrafish. PLoS One. 2013;8:e85087. doi: 10.1371/journal.pone.0085087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Culbertson MD, Lewis ZR, Nechiporuk AV. Chondrogenic and gliogenic subpopulations of neural crest play distinct roles during the assembly of epibranchial ganglia. PLoS One. 2011;6:e24443. doi: 10.1371/journal.pone.0024443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- 86.Chen J, Streit A. Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear Res. 2013;297:3–12. doi: 10.1016/j.heares.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 87.Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–433. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- 88.Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- 89.Uribe RA, Buzzi AL, Bronner ME, Strobl-Mazzulla PH. Histone demethylase KDM4B regulates otic vesicle invagination via epigenetic control of Dlx3 expression. J Cell Biol. 2015;211:815–827. doi: 10.1083/jcb.201503071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khatri SB, Edlund RK, Groves AK. Foxi3 is necessary for the induction of the chick otic placode in response to FGF signaling. Dev Biol. 2014;391:158–169. doi: 10.1016/j.ydbio.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edlund RK, Ohyama T, Kantarci H, Riley BB, Groves AK. Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers. Dev Biol. 2014;390:1–13. doi: 10.1016/j.ydbio.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Birol O, Ohyama T, Edlund RK, Drakou K, Georgiades P, Groves AK. The mouse Foxi3 transcription factor is necessary for the development of posterior placodes. Dev Biol. 2015 doi: 10.1016/j.ydbio.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Padanad MS, Riley BB. Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol. 2011;351:90–98. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–4295. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- 97.Canning CA, Lee L, Luo SX, Graham A, Jones CM. Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes. Neural Dev. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCabe KL, Bronner-Fraser M. Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development. 2008;135:1863–1874. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- 99.Lassiter RN, Ball MK, Adams JS, Wright BT, Stark MR. Sensory neuron differentiation is regulated by notch signaling in the trigeminal placode. Dev Biol. 2010;344:836–848. doi: 10.1016/j.ydbio.2010.05.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voelkel JE, Harvey JA, Adams JS, Lassiter RN, Stark MR. FGF and Notch signaling in sensory neuron formation: a multifactorial approach to understanding signaling pathway hierarchy. Mech Dev. 2014;134:55–66. doi: 10.1016/j.mod.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Lassiter RN, Stark MR, Zhao T, Zhou CJ. Signaling mechanisms controlling cranial placode neurogenesis and delamination. Dev Biol. 2014;389:39–49. doi: 10.1016/j.ydbio.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wakamatsu Y. Mutual repression between Pax3 and Pax6 is involved in the positioning of ophthalmic trigeminal placode in avian embryo. Dev Growth Differ. 2011;53:994–1003. doi: 10.1111/j.1440-169X.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- 103.Magnani L, Eeckhoute J, Lupien M. Pioneer factors: directing transcriptional regulators within the chromatin environment. Trends Genet. 2011;27:465–474. doi: 10.1016/j.tig.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smale ST. Pioneer factors in embryonic stem cells and differentiation. Curr Opin Genet Dev. 2010;20:519–526. doi: 10.1016/j.gde.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ram EV, Meshorer E. Transcriptional competence in pluripotency. Genes Dev. 2009;23:2793–2798. doi: 10.1101/gad.1881609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iwafuchi-Doi M, Zaret KS. Pioneer transcription factors in cell reprogramming. Genes Dev. 2014;28:2679–2692. doi: 10.1101/gad.253443.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol. 2008;73:119–126. doi: 10.1101/sqb.2008.73.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lalmansingh AS, Karmakar S, Jin Y, Nagaich AK. Multiple modes of chromatin remodeling by Forkhead box proteins. Biochim Biophys Acta. 2012;1819:707–715. doi: 10.1016/j.bbagrm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 112.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 113.Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 114.Serandour AA, Avner S, Percevault F, Demay F, Bizot M, Lucchetti-Miganeh C, Barloy-Hubler F, Brown M, Lupien M, Metivier R, et al. Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers. Genome Res. 2011;21:555–565. doi: 10.1101/gr.111534.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev Biol. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 116.Xu J, Watts JA, Pope SD, Gadue P, Kamps M, Plath K, Zaret KS, Smale ST. Transcriptional competence and the active marking of tissue-specific enhancers by defined transcription factors in embryonic and induced pluripotent stem cells. Genes Dev. 2009;23:2824–2838. doi: 10.1101/gad.1861209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol. 2006;26:155–168. doi: 10.1128/MCB.26.1.155-168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn. 2004;231:640–646. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- 119.Khatri SB, Groves AK. Expression of the Foxi2 and Foxi3 transcription factors during development of chicken sensory placodes and pharyngeal arches. Gene Expr Patterns. 2013;13:38–42. doi: 10.1016/j.gep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaltschmidt B, Kaltschmidt C, Widera D. Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev. 2012;8:658–671. doi: 10.1007/s12015-011-9340-9. [DOI] [PubMed] [Google Scholar]
- 121.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]