Abstract
Patients with generalized arterial calcification of infancy (GACI) develop vascular calcifications early in life. About half of them die within the first six months despite optimal medical care. A subset of those who survive eventually develop hypophosphatemic rickets. Since hypophosphatemia and hyperphosphaturia have been previously associated with increased survival in GACI patients, physicians often avoid phosphate repletion as treatment for rickets. As a consequence, GACI patients develop severe rachitic complications such as short stature and skeletal deformities. It appears that the recognition of hypophosphatemia later in life in some GACI patients is a consequence of having survived the first few months of life, and not the cause of their survival per se. Here, we report the long-term follow-up of a GACI patient who was phosphate-repleted for his rickets for more than seven years without worsening of vascular calcification.
Keywords: Generalized Arterial Calcification of Infancy, Hypophosphatemic Rickets, Hypophosphatemia, Hyperphosphaturia, ENPP1
INTRODUCTION
Generalized arterial calcification of infancy (GACI) is a disorder characterized by calcifications of large- to medium-sized vessels and/or fibrointimal hyperplasia resulting in cardiovascular morbidity either in utero or soon after birth [Ferreira et al., 2014]. Most cases are associated with biallelic loss-of-function mutations in the ENPP1 gene [Rutsch et al., 2003], although biallelic mutations in ABCC6 have been reported in a minority of patients [Nitschke et al., 2012]. A retrospective study of 55 patients with GACI found that hypophosphatemia and hyperphosphaturia were associated with increased survival [Rutsch et al., 2008]. However, the same study found that mortality was largely limited to the so called “critical period” encompassing the first 6 months of life, with only one death occurring later, at 7 months of age. Moreover, hypophosphatemia and hyperphosphaturia did not develop until later in life, so it is unlikely that these phosphate abnormalities themselves influenced survival. Later publications found that ENPP1 mutations are also associated with autosomal recessive hypophosphatemic rickets type 2 (ARHR2) [Levy-Litan et al., 2010; Lorenz-Depiereux et al, 2010], so it is not surprising that many patients with ENPP1-associated GACI who survive the critical period go on to develop rickets. Thus, the development of hypophosphatemia and hyperphosphaturia represent an acquired—rather than a congenital-—biochemical phenotype that does not appear until after the critical period of increased mortality in infancy. We are aware of several GACI patients who developed hypophosphatemic rickets that went untreated for years for fear that calcitriol and phosphorus supplementation would worsen the vascular calcifications, based on the previously reported association of hypophosphatemia with increased survival. These patients went on to develop profound skeletal deformities. In contrast, we now report a patient with GACI and subsequent hypophosphatemic rickets who was treated with calcitriol and phosphorus for more than 7 years, without developing new calcium deposits. This demonstrates that hypophosphatemic rickets in the setting of pre-existing GACI can be treated without detrimental effects; in fact, such therapy appears appropriate to prevent rachitic complications such as bone pain, deformities, and short stature.
CLINICAL REPORT
Our patient was born at 38 weeks of gestation by spontaneous vaginal delivery after an unremarkable prenatal course. Birth weight was 3.4 kg (25–50th centile) and birth length 48.9 cm (~25th centile); Apgar scores were 9 and 10 at 1 and 5 minutes, respectively. At day 29, he became cyanotic; in the emergency department, he was in severe respiratory distress, with mottled skin and metabolic acidosis. He was placed on a ventilator and on vasopressors, but was breathing room air on his own two days later, and was discharged home on an apnea monitor five days later. On day 48, the infant began crying inconsolably. In the emergency department, he was tachycardic with poor peripheral perfusion, hepatomegaly and splenomegaly. A chest radiograph revealed cardiomegaly and pulmonary edema, and an EKG showed left ventricular hypertrophy. An echocardiogram showed left ventricular enlargement, poor systolic function and moderate mitral regurgitation. A myocardial biopsy was unremarkable, with no findings of myocarditis. Cardiac catheterization showed severe attenuation of the left coronary artery with subtotal occlusion of the first obtuse marginal branch and occlusion proximal to the circumflex branch. The right coronary artery was occluded proximally. A CT scan revealed calcification of the descending aorta, and the renal, splenic, superior mesenteric, brachial and coronary arteries, consistent with the diagnosis of GACI. Biallelic mutations in ENPP1 (p.Arg481Gln and p.Tyr471Cys; NM_006208.2) confirmed the diagnosis. During that hospital admission, he was started on etidronate IV for 7 days and then orally at 20 mg/kg/d. During the hospitalization, the echocardiogram improved dramatically, with the left ventricular shortening fraction increasing from 13% on admission to 30% prior to discharge on day 71.
CT at 7 months of age showed reduced calcifications, and by 13 months the calcifications had regressed completely except for mild calcification of the aortic annulus. Etidronate was discontinued at 24 months of age.
At 13 years of age, the patient began complaining of significant, progressive pain in the ankles and knees, accompanied by stiffness of both joints, mainly in the morning. Radiographs revealed significant anterior bowing and thinning of the lower ends of both femora. A skeletal survey at 14 years 5 months showed resorption of the proximal medial metaphyses of both tibias and widening of the growth plates of the distal ulnae bilaterally and, to a lesser extent, the medial margins of the distal radius. Fusion of the posterior arches of C2, C3, C4 and C5 was also found.
At 14 years 5 months, his height was 154.4 cm (7th centile, −1.45 SD) for a mid-parental height of 180.3 cm ± 5 cm (75th centile). He was diagnosed with hypophosphatemic rickets, based on an elevated alkaline phosphatase of 631 U/L (reference: 166–571 U/L), bone specific alkaline phosphatase 241 µg/L (ref: 13–111 µg/L), serum phosphorus 2.5 mg/dL (ref: 3.5–5.3 mg/dL), tubular reabsorption of phosphorus (TRP) 88 % (low for his degree of hypophosphatemia), tubular maximum phosphorus reabsorption per glomerular filtration rate (TmP/GFR, or threshold above which phosphorus is no longer reabsorbed by the tubules) 2.3 mg/dL (ref: 2.8–5.2 mg/dL), intact PTH 39 pg/mL (ref: 15–65 pg/mL), 25-hydroxyvitamin D 35 ng/mL (ref: 30–50 ng/mL) and 1,25-dihydroxyvitamin D 36 pg/mL (ref: 24–86 pg/mL). Phosphorus supplementation was initiated with K-Phos Neutral at 250 mg every 6 hours (18 mg/kg/d), and calcitriol was begun at 0.5 µg twice a day (18 ng/kg/d) starting at 14 years 8 months of age.
Within a few weeks of starting therapy, the pain in his ankles and knees resolved completely. His deformities remained stable, with no progression or improvement. After his final height was achieved, he underwent two separate osteotomies at age 21 to correct the anterior femoral bowing with tibia vara.
Other pertinent medical findings included progressive hearing loss noted at age 7 years, for which he had PE tubes until the age of 20 years, followed by hearing aids. He also had enamel defects, requiring sealant application.
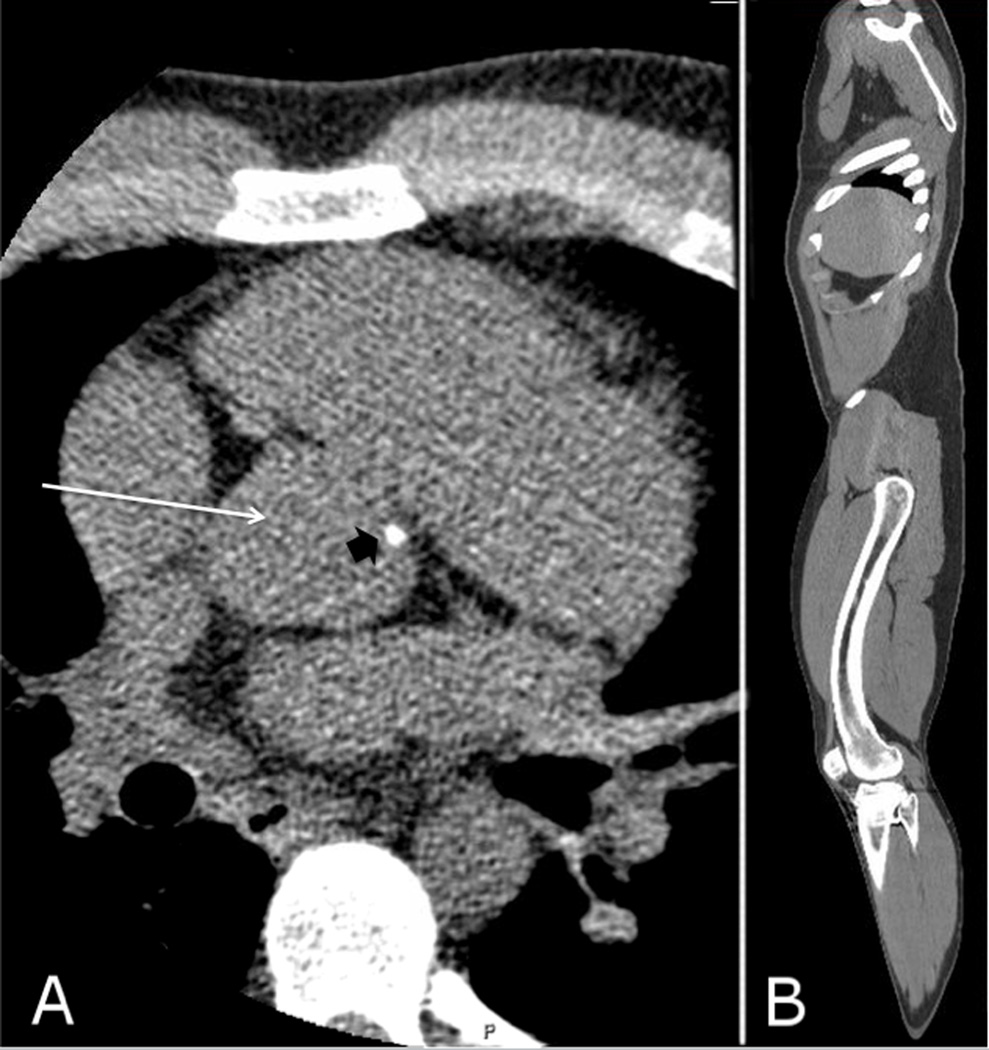
At the age of 21 years 11 months, the patient was taking calcitriol 0.75 µg every morning and 0.5 µg nightly (13.9 ng/kg/d) and phosphorus 500 mg twice a day (11.1 mg/kg/d). Dose adjustments were made based on blood and urine biochemical findings, although with some medication adherence issues. His ionized calcium was 1.23 mmol/L (ref: 1.1–1.35 mmol/L), serum phosphorus 2.1 mg/dL (ref: 2.5–4.5 mg/dL), alkaline phosphatase 191 U/L (ref: 45–115 U/L), and intact PTH 11 pg/mL (ref: 15–65 pg/mL). Multidetector helical CT from the neck to the legs revealed minimal calcification of the aortic root (figure 1A) and inferior portion of the heart, and minimal calcification of the left popliteal artery, with no calcifications elsewhere (including no nephrocalcinosis). Anterior femoral bowing could also be appreciated (figure 1B). A dedicated cardiac CT showed no coronary artery calcification, with an Agatston calcium score of zero.
Figure 1.
A) CT imaging showing minimal calcification (black arrowhead) along the aortic root (white arrow). B) Parasagittal CT reconstruction showing the anterior femoral bowing.
DISCUSSION
In 2008, Rutsch et al. reported the association of hypophosphatemia and hyperphosphaturia with increased survival in patients with GACI. Association, however, does not imply causality, and in fact this association was based upon the finding that GACI patients who survived beyond infancy were hypophosphatemic and hyperphosphaturic. The 2008 report did not address the fact that the GACI patients who died during their first year of life might also have been destined to develop renal phosphate wasting and hypophosphatemia later in life. Furthermore, there was no mortality in either patients with or without hypophosphatemia/hyperphosphaturia after 7.5 months of age, meaning that there is no evidence for a causal relationship between survival and hypophosphatemia after early infancy. Thus, the biochemical phenotype of hypophosphatemia and hyperphosphaturia will likely develop over time in many patients with GACI who survive the critical period, but it is not an explanation for survival. Rather, survival itself allows hypophosphatemia to be recognized; hypophosphatemia does not lead to survival.
There are various explanations why a “critical period” in the first 6 months of life might be followed by a more “refractory period” later. First, in most cases the calcifications regress either spontaneously [Sholler et al., 1984; Ciana et al., 2006] or after treatment with bisphosphonates [Meradji et al., 1978; van der Sluis et al., 2006]. Second, even though patients with GACI can have vessel narrowing in the presence or absence of calcifications, the authors are aware of several patients who received serial imaging showing that their vessels continued to grow in diameter with age. According to Poiseuille’s law, the flow through a vessel is directly proportional to the fourth power of the radius [Westerhof et al., 2010]. Thus, a linear increase in the caliber of a vessel will lead to an exponential increase in blood flow, so that small increases in the radius can lead to dramatic increases in flow.
Based upon the reported association of hypophosphatemia and hyperphosphaturia with increased survival, many physicians avoid treating the rickets that develop in children and young adults with GACI. Yet it is well known that these patients can have ENPP1-mediated hyperphosphaturia and hypophosphatemia and go on to develop florid rachitic deformities. Clearly, the decision to start therapy is difficult, since the effects of long-term calcitriol and phosphorus supplementation in GACI patients remain unknown. However, it should be noted that GACI is hypothesized to be caused by a cell-autonomous defect, leading to increased vascular calcification presumably due to lack of pyrophosphate synthesis in the local microenvironment of the vessel wall. Indeed, newborn patients with extensive vascular calcifications have normal phosphatemia and calcemia suggesting that vascular calcification does not appear to be related to any abnormalities in circulating levels of phosphate or calcium.
Moreover, there is one report of a patient with biallelic ENPP1 mutations who, after initiation of alfacalcidol, developed new-onset nephrocalcinosis, cardiac, hepatic and pancreatic calcifications as evaluated by ultrasound, and recurrence of previously regressed periarticular calcifications as assessed by radiograph [Freychet et al., 2014]. This, however, was an iatrogenic event, since the patient developed hypercalciuria during treatment, and once the calciuria was maintained at less than 4 mg/kg/d, most of these calcifications—with the exception of nephrocalcinosis—regressed. In another report, Rutsch et al. [2008] found one GACI patient treated with calcitriol and phosphorus supplementation whose arterial stenosis worsened. However, no clinical information was provided regarding the timing of institution of treatment in this patient, the degree of vascular calcification prior to treatment initiation, the dosage of the supplemented medications, or the possibility of iatrogenic adverse effects such as hypercalciuria.
In conclusion, we describe for the first time long-term treatment of hyphosphatemic rickets in the setting of GACI. We show that adequate treatment of rickets can be accomplished without worsening of vascular calcifications, as long as close monitoring is instituted so as to avoid iatrogenic complications.
Acknowledgments
We thank the patient and his family for their kind cooperation. This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute.
Footnotes
Conflict of interest: the authors report no conflict of interest.
REFERENCES
- Ciana G, Trappan A, Bembi B, Benettoni A, Maso G, Zennaro F, Ruf N, Schnabel D, Rutsch F. Generalized arterial calcification of infancy: two siblings with prolonged survival. Eur. J. Pediatr. 2006;165:258–263. doi: 10.1007/s00431-005-0035-6. [DOI] [PubMed] [Google Scholar]
- Ferreira C, Ziegler S, Gahl W. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Dolan CR, Fong C-T, Smith RJ, Stephens K. GeneReviews(®) Seattle (WA): University of Washington, Seattle; 2014. Generalized Arterial Calcification of Infancy. [PubMed] [Google Scholar]
- Freychet C, Gay C, Lavocat M-P, Teyssier G, Patural H, Bacchetta J, Cottalorda J, Meunier BB, Linglart A, Baujat G, Stephan J-L. [GACI syndrome: a case report with a neonatal beginning] Arch Pediatr. 2014;21:632–636. doi: 10.1016/j.arcped.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am. J. Hum. Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meradji M, de Villeneuve VH, Huber J, de Bruijn WC, Pearse RG. Idiopathic infantile arterial calcification in siblings: radiologic diagnosis and successful treatment. J. Pediatr. 1978;92:401–405. doi: 10.1016/s0022-3476(78)80427-2. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, Le Merrer M, Guest G, Lambot K, Tazarourte-Pinturier M-F, Chassaing N, Roche O, Feenstra I, Loechner K, Deshpande C, Garber SJ, Chikarmane R, Steinmann B, Shahinyan T, Martorell L, Davies J, Smith WE, Kahler SG, McCulloch M, Wraige E, Loidi L, Höhne W, Martin L, Hadj-Rabia S, Terkeltaub R, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P. Mutations in ENPP1 are associated with “idiopathic” infantile arterial calcification. Nat. Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- Rutsch F, Böyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1(2):133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholler GF, Yu JS, Bale PM, Hawker RE, Celermajer JM, Kozlowski K. Generalized arterial calcification of infancy: three case reports, including spontaneous regression with long-term survival. J. Pediatr. 1984;105:257–260. doi: 10.1016/s0022-3476(84)80123-7. [DOI] [PubMed] [Google Scholar]
- Van der Sluis IM, Boot AM, Vernooij M, Meradji M, Kroon AA. Idiopathic infantile arterial calcification: clinical presentation, therapy and long-term follow-up. Eur. J. Pediatr. 2006;165:590–593. doi: 10.1007/s00431-006-0146-8. [DOI] [PubMed] [Google Scholar]
- Westerhof N, Stergiopulos N, Noble MIM. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education. NY: Springer; 2010. Law of Poiseuille; pp. 9–14. [Google Scholar]