Abstract
Fanconi anemia (FA) is a rare inherited disorder caused by pathogenic variants in one of 19 FANC genes. FA patients display congenital abnormalities, and develop bone marrow failure, and cancer susceptibility. We identified homozygous mutations in four FA patients and, in each case, only one parent carried the obligate mutant allele. FANCA and FANCP/SLX4 genes, both located on chromosome 16, were the affected recessive FA genes in three and one family respectively. Genotyping with short tandem repeat markers and single nucleotide polymorphism (SNP) arrays revealed uniparental disomy (UPD) of the entire mutation-carrying chromosome 16 in all four patients. One FANCA patient had paternal UPD, whereas FA in the other three patients resulted from maternal UPD. These are the first reported cases of UPD as a cause of FA. UPD indicates a reduced risk of having another child with FA in the family and has implications in prenatal diagnosis.
Keywords: uniparental disomy, UPD16, Fanconi anemia, recurrence risk, FANCA, FANCP
Fanconi anemia (FA) is characterized by debilitating developmental abnormalities, progressive bone marrow failure, increased incidence of myelodysplastic syndrome, acute myeloid leukemia, and solid tumors, especially of the head and neck and genitourinary system (Kottemann and Smogorzewska, 2013). This rare genetic disorder, caused by mutations in any of at least 19 different genes, is both clinically and genetically heterogeneous. The genotype-phenotype relationship in FA remains largely unexplained. The Next-Gen sequencing (NGS) and arrayCGH technologies have been adopted to identify the gene and causal variants in FA families (Chandrasekharappa, et al., 2013; Flynn, et al., 2014). The inheritance of two mutant alleles resulting in FA can be from one of 17 autosomal recessive genes (FANCA, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ/BRIP1, FANCL, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ/ERCC4/XPF, FANCS/BRCA1, FANCT/UBE2T). Alternatively, acquiring only one X-linked mutant FANCB allele, or one autosomal dominant RAD51/FANCR mutant allele (Hira, et al., 2015; Rickman, et al., 2015; Wang, et al., 2015; Wang and Smogorzewska, 2015) can also cause FA. With the exception of FANCB and FANCR mutation carriers, affected individuals carry two mutations in the same FANC gene, generally compound heterozygous mutations. Though primarily a recessive disease, uniparental disomy (UPD) as a cause of disease has not been reported for FA patients. Search for parental inheritance of mutations is a critical component of a comprehensive molecular diagnosis in an FA family. Such an analysis, particularly, for FA patients with homozygous causal variants may reveal instances where only one of the parents is a heterozygous carrier of the obligatory mutation while the other is not, and thus may lead to discovery of uniparental disomy (UPD) as a cause of FA.
A search for biallelic pathogenic variants was performed in patients diagnosed with FA who were enrolled in the International Fanconi Anemia Registry (IFAR) (http://lab.rockefeller.edu/smogorzewska/ifar/). NGS and arrayCGH technologies were employed for mutation search in DNA from FA patients (see Supp. Methods). For those FA patients whose parental DNA were available, we also examined the parental inheritance of the observed pathogenic variants. Among the many families whose molecular diagnosis and the inheritance patterns of pathogenic variants were uncovered, there were four patients with unusual inheritance pattern. These include three patients with homozygous mutations in FANCA:FA1, FA2 and FA3, and one patient with a homozygous mutation in FANCP/SLX4: FA4 (Table 1). All four variants were independently confirmed by Sanger sequencing (Figure 1A). The c.2546delC variant in patient FA1, if expressed, is predicted to generate a non-functional truncation. It appears 22 times in the Fanconi Anemia Mutation Database (FAMutDB) (http://www.rockefeller.edu/fanconi/) and is confined to FA patients of Japanese and Korean ancestry (Park, et al., 2013; Yagasaki, et al., 2004). The c.3520_3522delTGG variant in patient FA2 appears 31 times in the FAMutDB, and its pathogenicity has been clearly demonstrated by complementation studies (Adachi, et al., 2002). The c.523_792del variant in patient FA3 is the result of a 9,290 bp genomic deletion (g;chr16:89867339–89876628), caused by non-allelic homologous recombination of AluY and AluSx1 repeats residing in IVS5 and IVS8, respectively (Supp. Figure S1), eliminating exons 6–8 (aa 175–264) of FANCA. Patient FA4 harbors a novel homozygous FANCP/SLX4 variant, c.1366G>A. Contrary to a predicted protein with a missense amino acid, this mutation, altering the last nucleotide of exon 6, results in aberrant splicing by retaining IVS6 in cDNA, creating an unstable message. A patient fibroblast cell line carrying this homozygous variant expressed no SLX4, and the defect of interstrand crosslinking and Camptothecin sensitivity was rescued by expression of the WT SLX4 cDNA (Supp. Figure S2). Thus, homozygous pathogenic variants caused FA in all four patients.
Table 1.
Clinical presentation of patients displaying UPD16
| Clinical Information | Patient | |||
|---|---|---|---|---|
| FA1 | FA2 | FA3 | FA4 | |
| Gender | F | M | M | F |
| Gene | FANCA | FANCA | FANCA | FANCP/SLX4 |
| Mutation (genomic)* | g.89833604delG | g.89811471–89811473delCCA | g.89867339–89876628del | g.3647798C>T |
| Mutation (cDNA) | c.2546delC | c.3520_3522delTGG | c.523_792del (Δexons 6–8) | c.1366G>A |
| Mutation (Protein**) | p.S849FfsX40 | p.W1174del | p.S175_Q264del | p.E456SfsX34, not expressed |
| Mutation (Zygosity) | Homozygous | Homozygous | Homozygous | Homozygous |
| Mother is carrier | No | Yes | Yes | Yes |
| Father is carrier | Yes | No | No | No |
| Ethnicity | Asian (Korean) | Caucasian | Caucasian | Caucasian |
| Consanguinity | No | No | No | No |
| Age at Diagnosis | 9 yrs. | 16 yrs. | 10 yrs. | 2 yrs. |
| BMF | 14 yrs. | 16 yrs. | 10 yrs. | Birth |
| BMT | No | 26 yrs. | 10 yrs. | 5 yrs. |
| Status | Deceased at 27yrs. | Deceased at 26 yrs. Complications of BMT | Alive and well at 22 years | Deceased at 5 yrs. Complications of BMT |
| Skin | Café-au-lait spots | Normal | Normal | Café-au-lait spots |
| Musculoskeletal | Hypoplastic thenar eminence | Abnormal thumb Hypoplastic thenar eminence | Mild thumb anomaly | Bilateral toe syndactyly, microcephaly |
| Scoliosis | ||||
| Mild pes planus | ||||
| Urogenital | Normal | Normal | Normal | Bilateral ectopic kidneys |
| Ears/Hearing | Small canals | Normal | Small canals | Normal |
| Gastrointestinal | Normal | Normal | Normal | Normal |
| Cardiopulmonary | Normal | Severe asthma | Normal | L. Superior vena cava, ASD |
| Eyes/Vision | Esotropia | Normal | Normal | Microophthalmia, Esotropia |
| Development | Normal | Normal | Normal | Severe, Global delay |
coordinates are in accord with UCSC genome build hg19
predicted
BMF = Bone Marrow Failure
BMT = Bone Marrow Transplantation
GenBank reference sequence for FANCA:NM_000135.2, and SLX4: NM_032444.2
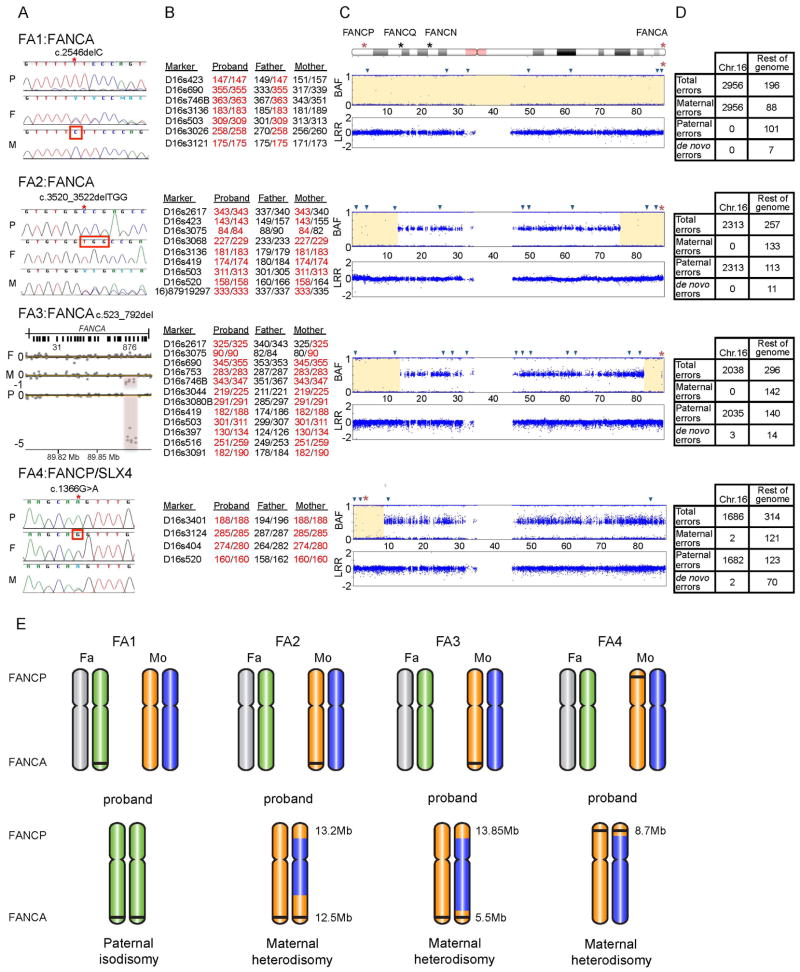
Figure 1. Homozygous mutations resulting from UPD of chromosome 16 in four FA patients.
A. Homozygous mutation in each proband and the heterozygous carrier status of each parent. For each family, data from the proband (P), father (F), and mother (M) are shown. Families FA1, FA2, and FA3 carry mutations in FANCA, and family FA4 carries a mutation in FANCP/SLX4. Sequence traces from Sanger sequencing are shown for families FA1, FA2, & FA4. The red star indicates the mutation site, homozygous in the proband and heterozygous in the carrier parent, and the red square indicates the presence of the WT sequence in the non-carrier parent. ArrayCGH intensity plot is shown for FA3 family. The red-shaded region indicates the site of deletion, and the length of the shaded region shows the state of zygosity: a heterozygous loss in the parental carrier and a homozygous loss in the proband.
B. Genotypes of proband/parents trio using chromosome 16 STRP markers. The markers and their two alleles in each member of the family are shown. The alleles of the proband are marked in red to emphasize the parent from whom they were inherited. Only the informative markers from STRP analysis are shown. The full list of tested markers is provided in Supp. Table S2. The positions of the informative markers on chromosome 16 are represented by blue triangles in Figure 1C.
C. Probands’ chromosome 16 SNP genotype data. An ideogram of chromosome 16 indicating the location of the four FA genes. The two affected genes in these four families, FANCA and FANCP/SLX4, are marked with a red asterisk. The top plot, for each proband, is the B Allele Frequency (BAF) showing the state of zygosity for each SNP. A BAF of 1 or 0 indicate a homozygous genotype, whereas a BAF of 0.5 indicates a heterozygous genotype. The bottom plot is the Log R Ratio (LRR) which shows the SNP intensity and represents the allelic copy number. A LRR of 0 indicates a copy number of two. The regions of isodisomy are highlighted in yellow.
D. Mendelian errors in the probands’ SNP genotypes. A mendelian error is identified as the presence of a genotype in the proband that cannot be explained by a classical inheritance pattern from both parents. The table shows the number of errors present in chromosome 16 in comparison to the number of errors present throughout the rest of the genome. Comparing the number of errors contributed from each parent elucidates whose chromosome 16 is absent from the proband.
E. Graphical representation of the likely mechanism leading to UPD in each family. The two chromosome 16 homologues of each parent and their presence in, or absence from, the proband are shown. The location of FANCA and FANCP/SLX4 are indicated, and the mutation is indicated with a black line. The sizes of the isodisomy regions in probands FA2-FA4 are presented in megabases. The complete paternal isodisomy in FA1 may have been generated by a mechanism involving monosomy rescue: maternal nondisjunction results in no chromosome 16 (nullisomy), leading to monosomy of paternal chromosome 16, which would be lethal and would be rescued by duplicating the paternal chromosome 16(Engel, 2006). The maternal heterodisomy in FA2, FA3, and FA4 may be explained by a mechanism involving trisomy rescue: where a supernumerary chromosome from a trisomic conceptus is lost, leaving behind two homologues from the same gamete (Engel, 2006). The homozygous regions distal to the crossover events during meiotic recombination appear as isodisomic regions in the pter of FA4 (a single crossover), and the pter and the qter of FA2 and FA3 (two crossover events). This reduction to homozygosity, of the regions harboring the FANCA (FA2 and FA3) and FANCP/SLX4 mutations (FA4), leads to FA.
Sequencing of parental DNA for each patient showed that the FANCA variant in FA1, c.2546delC, was present only in the paternal DNA, and not the patient’s mother. For patients FA2, FA3, and FA4, the mother was a heterozygous carrier (Figure 1A). ArrayCGH or multiplex ligation-dependent probe amplification (MLPA) was performed to confirm that the nature of the homozygous variants in FANCA (patients FA1, FA2) and FANCP/SLX4 (patient FA4) was not the result of an overlapping large heterozygous deletion (data not shown). This prompted the exploration of UPD as the cause of homozygous variants in the patients.
Each family was genotyped using STRP markers. Both parental alleles are accounted for in the proband (Supp. Table S1), except for the markers on chromosome 16 (Supp. Table S2) which showed inheritance from only one parent (Figure 1B). Similarly, SNP arrays demonstrate a contribution from both parental alleles for the entire genome of the probands (Supp. Figure S3), except for chromosome 16 (Figure 1C). Homozygosity was observed for the entire chromosome 16 in FA1, and large regions near the termini harboring the affected FA genes, FANCA (qter) in FA2 and FA3, or FANCP/SLX4 (pter) in FA4 (Figure 1C; Supp. Table S3). Mendelian error (ME) calculations revealed several chromosome 16 errors, ranging from 1682 to 2956 genotypes, originating exclusively from one parent. ME for the rest of the genome, ranging from 196–314 genotypes, were derived evenly from both parents (Figure 1D). The UPD16 extended throughout the entire chromosome 16 in all four families, with contribution from only one parent.
UPD describes the inheritance of a pair of chromosomes from only one parent, either both homologues (heterodisomy), or two copies of one homologue (isodisomy) (Shaffer, et al., 2001). In patient FA1, the paternal chromosome 16 bearing the FANCA variant c.2546delC was duplicated resulting in complete paternal isodisomy (Figure 1E). FANCA-deficient patients, FA2 and FA3, and FANCP/SLX4-deficient patient FA4 showed maternal heterodisomy with regions of isodisomy at the qter or pter harboring the maternal FANCA or FANCP/SLX4 mutant alleles. A mechanism involving monosomy rescue may explain the isodisomy in FA1, while trisomy rescue may have caused maternal heterodisomy in FA2, FA3, and FA4 (Figure 1E, see legend). We observe both paternal and maternal UPD16 in FA and, consequently, conclude no bias for parental origin of UPD.
UPD is often associated with defects in imprinting disorders resulting in lack of expression of a functional allele (Ishida and Moore, 2013). Potential consequences of imprinted genes in the UPD region may have additional clinical implications for patients (Engel, 2006). UPD of the entire chromosome 16, inherited paternally (FA1) and maternally (FA2-FA4), should expose phenotypes potentially associated with imprinting. However, typical clinical presentation of the four FA patients presented here (Table 1) suggests a lack of imprinted genes on chr16. Previous reports of UPD16 in five different recessive disorders involving genes MLYCD (Malonic aciduria), HBA1 (HB Bart hydrops fetalis), ABCA3 (Inherited surfactant deficiency), APRT (Adenine phosphoribosyltransferase deficiency) and GALNS (Morquio A syndrome) (Catarzi, et al., 2012) also showed no indication of imprinted genes causing a major phenotypic defect. The severe phenotype of patient FA4 could be due to the affected gene, FANCP/SLX4, or the 8.7 Mb pter homozygous region may harbor another unknown defective recessive gene.
UPD as a cause of FA is a novel mechanism that has not been previously reported. Four UPD cases in our mutation analysis of ~400 FA families suggests we may discover other FA cases with UPD by screening more patients using these methodologies. Exploring the inheritance pattern of a larger number of patients with homozygous pathogenic variants may define the precise frequency of UPD causing FA. Trisomy16 is the most common trisomy observed in human conceptions (Griffin, 1996), and thus as a consequence of trisomy rescue, children with UPD16 may be relatively more common. In addition to FANCA and FANCP/SLX4, FANCQ/ERCC4/XPF (chr16:g.14014014–14046205) and FANCN/PALB2 (chr16:g.23614483–23652678) also reside on chromosome 16, which suggests the possibility of unknown UPD16 in patients from other FA groups. The diagnosis of UPD as a cause of recessive disease drastically reduces the recurrence risk in a family from 25% to nearly zero, potentially making invasive prenatal diagnosis in subsequent pregnancies unwarranted. This has important implications for genetic counseling within affected families. Of all possibilities, UPD for only a few chromosomes results in abnormal phenotypes shown to be, or presumed to be, caused by imprinting (Shaffer, et al., 2001). It is thus important to rule out UPD as a cause of recessive disease in non-consanguineous homozygous patients as UPD cases identified following investigation of single gene often do not manifest additional anomalies beyond those expected for their disease.
Supplementary Material
Acknowledgments
Grant support: Grant R01 HL120922, Grant #UL1 TR000043 from NIH, and Intramural Research Program of the National Human Genome Research Institute, NIH.
National Human Genome Research Institute Intramural Research Program (FXD, DCK, AK, MPJ, UH, EAO, SCC), Grant R01 HL120922, Grant #UL1 TR000043, NIH; Support from Rita Allen Foundation (AS), Starr Cancer Consortium (AS), and Vilcek Foundation (ADA). We are most grateful to the individuals and families who participated in this study.
Footnotes
Authorship Contributions
Study design and manuscript preparation (SCC), patient care, enrollment, and sample collection (EMS, RT, JEW, MLM, EAO, ADA, AS), experiments and analysis (FXD, DCK, YK, FPL, UH, MPJ, AK, AS, SCC)
References
- Adachi D, Oda T, Yagasaki H, Nakasato K, Taniguchi T, D’Andrea AD, Asano S, Yamashita T. Heterogeneous activation of the Fanconi anemia pathway by patient-derived FANCA mutants. Human molecular genetics. 2002;11(25):3125–34. doi: 10.1093/hmg/11.25.3125. [DOI] [PubMed] [Google Scholar]
- Catarzi S, Giunti L, Papadia F, Gabrielli O, Guerrini R, Donati MA, Genuardi M, Morrone A. Morquio A syndrome due to maternal uniparental isodisomy of the telomeric end of chromosome 16. Mol Genet Metab. 2012;105(3):438–42. doi: 10.1016/j.ymgme.2011.11.196. [DOI] [PubMed] [Google Scholar]
- Chandrasekharappa SC, Lach FP, Kimble DC, Kamat A, Teer JK, Donovan FX, Flynn E, Sen SK, Thongthip S, Sanborn E, et al. Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia. Blood. 2013;121(22):e138–48. doi: 10.1182/blood-2012-12-474585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel E. A fascination with chromosome rescue in uniparental disomy: Mendelian recessive outlaws and imprinting copyrights infringements. Eur J Hum Genet. 2006;14(11):1158–69. doi: 10.1038/sj.ejhg.5201619. [DOI] [PubMed] [Google Scholar]
- Flynn EK, Kamat A, Lach FP, Donovan FX, Kimble DC, Narisu N, Sanborn E, Boulad F, Davies SM, Gillio AP, 3rd, et al. Comprehensive Analysis of Pathogenic Deletion Variants in Fanconi Anemia Genes. Human mutation. 2014 doi: 10.1002/humu.22680. [DOI] [PMC free article] [PubMed]
- Griffin DK. The incidence, origin, and etiology of aneuploidy. Int Rev Cytol. 1996;167:263–96. doi: 10.1016/s0074-7696(08)61349-2. [DOI] [PubMed] [Google Scholar]
- Hira A, Yoshida K, Sato K, Okuno Y, Shiraishi Y, Chiba K, Tanaka H, Miyano S, Shimamoto A, Tahara H, et al. Mutations in the Gene Encoding the E2 Conjugating Enzyme UBE2T Cause Fanconi Anemia. Am J Hum Genet. 2015;96(6):1001–7. doi: 10.1016/j.ajhg.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M, Moore GE. The role of imprinted genes in humans. Mol Aspects Med. 2013;34(4):826–40. doi: 10.1016/j.mam.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493(7432):356–63. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chung NG, Chae H, Kim M, Lee S, Kim Y, Lee JW, Cho B, Jeong DC, Park IY. FANCA and FANCG are the major Fanconi anemia genes in the Korean population. Clinical genetics. 2013;84(3):271–5. doi: 10.1111/cge.12042. [DOI] [PubMed] [Google Scholar]
- Rickman KA, Lach FP, Abhyankar A, Donovan FX, Sanborn EM, Kennedy JA, Sougnez C, Gabriel SB, Elemento O, Chandrasekharappa SC, et al. Deficiency of UBE2T, the E2 Ubiquitin Ligase Necessary for FANCD2 and FANCI Ubiquitination, Causes FA-T Subtype of Fanconi Anemia. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer LG, Agan N, Goldberg JD, Ledbetter DH, Longshore JW, Cassidy SB. American College of Medical Genetics statement of diagnostic testing for uniparental disomy. Genet Med. 2001;3(3):206–11. doi: 10.1097/00125817-200105000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Kim T, Wagner JE, Conti BA, Lach FP, Huang AL, Molina H, Sanborn EM, Zierhut H, Cornes BK, et al. A Dominant Mutation in Human RAD51 Reveals Its Function in DNA Interstrand Crosslink Repair Independent of Homologous Recombination. Mol Cell. 2015;59(3):478–90. doi: 10.1016/j.molcel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Smogorzewska A. SnapShot: Fanconi anemia and associated proteins. Cell. 2015;160(1–2):354–354. e1. doi: 10.1016/j.cell.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Yagasaki H, Hamanoue S, Oda T, Nakahata T, Asano S, Yamashita T. Identification and characterization of novel mutations of the major Fanconi anemia gene FANCA in the Japanese population. Human mutation. 2004;24(6):481–90. doi: 10.1002/humu.20099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.