Abstract
Phorbol, the flagship member of the tigliane diterpene family, has been known for over 80 years and has attracted attention from scores of chemists and biologists due to its intriguing chemical structure and the medicinal potential of phorbol esters.1 Access to useful quantities of phorbol and related analogs has relied upon isolation from natural sources and semisynthesis. Despite relentless efforts spanning 40 years, chemical synthesis has been unable to compete with these strategies due to its sheer complexity and unusual oxidation pattern. In fact, purely synthetic enantiopure phorbol has remained elusive and efforts on the synthetic biology side have not led to even the simplest members of this terpene family. Recently the chemical syntheses of eudesmanes,2 germacrenes,3 taxanes,4,5 and ingenanes6-8 have all benefited from a strategy inspired by the logic of two-phase terpene biosynthesis where powerful C–C bond constructions and C–H bond oxidations go hand in hand. In this manuscript, we show how a two-phase terpene synthesis strategy can be enlisted to achieve the first enantiospecific total synthesis of (+)-phorbol in only 19 steps from the abundant monoterpene (+)-3-carene. The purpose of this route is not to displace isolation/semisynthesis as a means to generate the natural product per se, but rather to enable access to analogs containing unique oxidation patterns that are otherwise inaccessible.
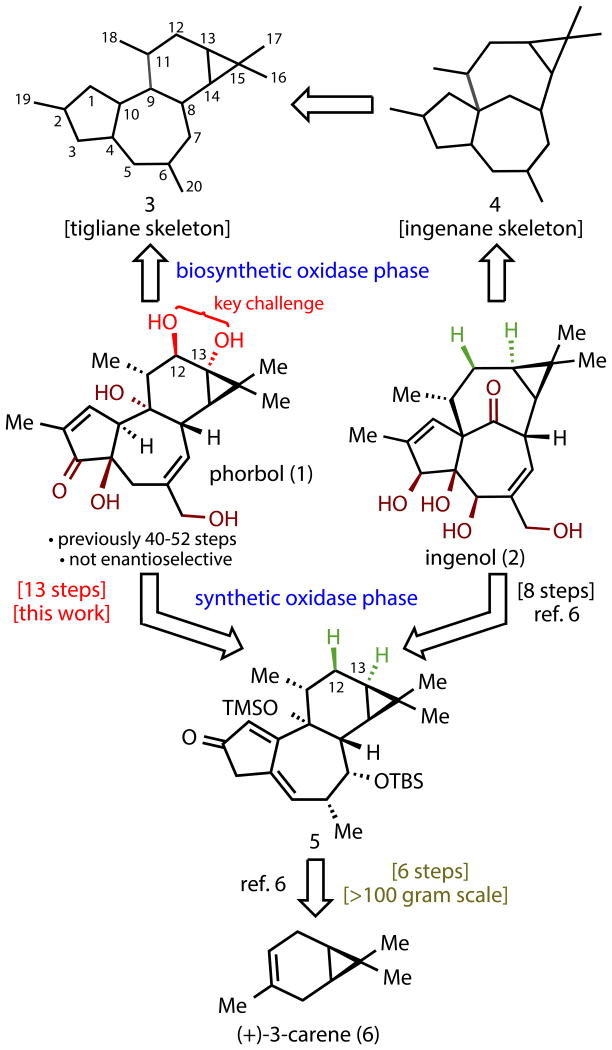
Tigliane diterpenes have been viewed as promising leads in a myriad of different medicinal applications ranging from immunomodulatory9 to anti-viral10 to anti-cancer.11 The most progressed compound in this class of natural products is phorbol 12-myristate 13-acetate (PMA), which is currently in phase II clinical trials for treatment of acute myeloid leukemia (AML).11 Remarkably, subtle perturbations of their structures can have a dramatic effect on their biological profiles, perhaps as a result of differing protein kinase C (PKC) subtype selectivity.9,10 As such, phorbol esters and related terpenes have been a hotly pursued area of natural products research.1 Our interest in this family stemmed from the anti-cancer effects of certain phorbol analogs whose access was limited. In 2009, our lab described a fundamentally new strategy for the chemical synthesis of complex terpenes following the underlying logic of biosynthesis and demonstrated its utility by concisely accessing numerous eudesmane family members.2 This strategy has been successfully applied to germacrenes,3 complex taxanes,4,5 and most recently ingenanes.6-8 In particular, the two-phase synthesis of (–)-ingenol (2) is only 14 steps from (+)-3-carene (6) and has enabled access to a variety of analogs that were not accessible by any other means.6-8 Since ingenanes (4) and tiglianes (3) are intimately related in nature it was hypothesized that intermediate 5, available in >100 gram quantities from LEO Pharma, might serve as an ideal starting point for a short route to 1. The execution of this plan hinged on the invention of a simple solution to the challenge of incorporating the C-12 and C-13 oxygen atoms of 1. Indeed, the difficulty in installing this functionality is notorious in the field. To date, only two total syntheses from the Wender group12-14 and two formal syntheses (one from the Wender15 and one from the Cha group16) of 1 have been reported in 40-52 steps (see Supplementary Information for summary).12-16 In these landmark studies, an α-oxygenated enone was prepared from a ketone and subsequently cyclopropanated to build in the C-13 oxidation via a six-step sequence. In addition, many elegant efforts towards 1 have been reported by the Shibasaki,17,18 Wilson, Rigby, Harwood, Little, Page, Dauben, McMills, Paquette, Singh, Ovaska, West, Evans, and Li groups (for a listing of the 36 papers in this area, see Supplementary Information). The Shibasaki route is particularly instructive and illustrates the inherent challenge of using (+)-3-carene (6) as a starting material to (+)-phorbol (1) since 38 steps were required to reach a phorbol analog lacking the C-11 methyl and C-13 oxygen from 6.17,18 For the purposes of contextualizing the current studies, we define a reaction step as one in which a substrate is converted to a product in a single reaction flask (irrespective of the number of transformations) without intermediate workup or purification. Outlined below are studies that culminated in a two-phase, 19-step synthesis of 1 that solves these problems in an unusually concise and unique way.
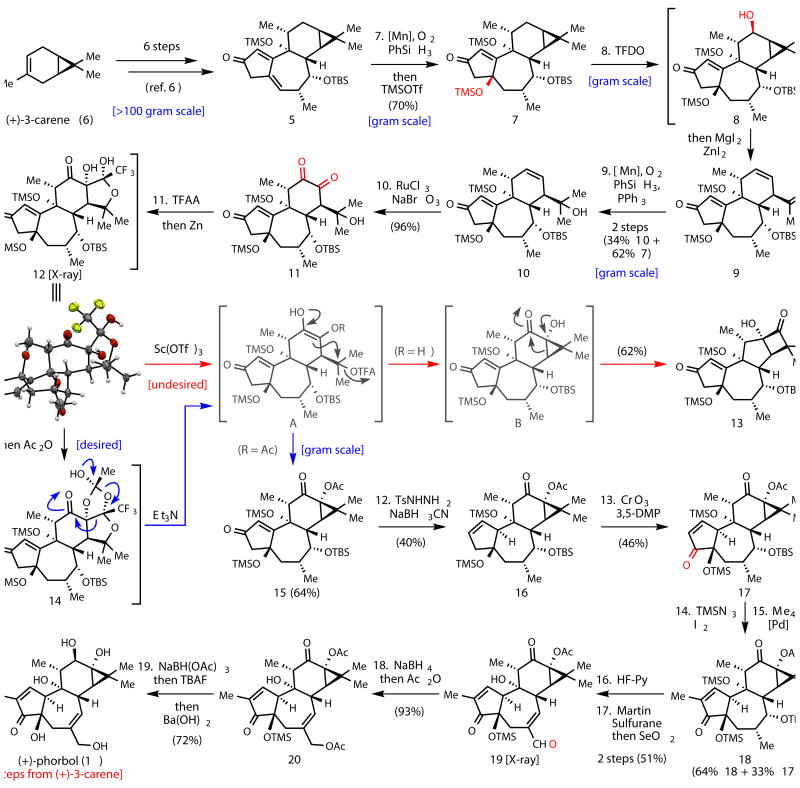
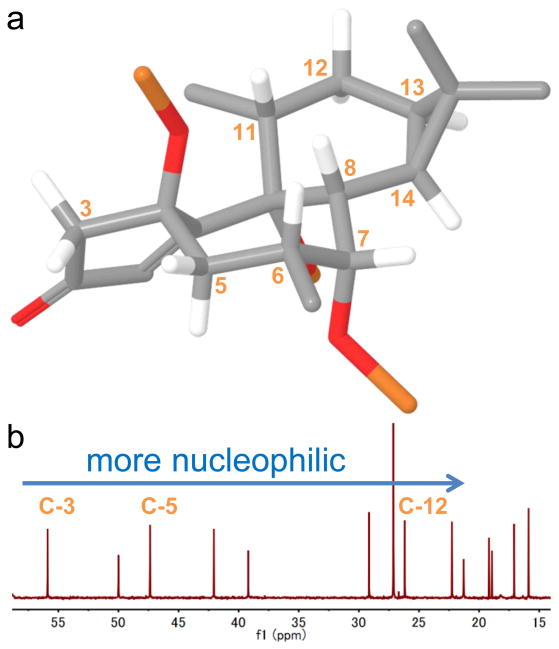
The synthesis commenced with intermediate 5 (Figure 2), provided by LEO Pharma in large quantities using our previously described route.6 Installation of the C-4 oxygen was accomplished using a Mukaiyama hydration and in situ silyl group installation to furnish 7 in 70% yield (gram scale). At this juncture, installation of the C-12 oxygen atom was pursued, requiring the selective oxidation of a methylene position in the presence of an enone, six tertiary C–H bonds, and two other competing methylene sites. In order to choose the proper oxidant for this bold transformation the structure was analyzed computationally (Figure 3a) and by inference of innate reactivity using NMR (Figure 3b). Thus, the pseudo-equatorial C–H bond at C-12 was predicted to be the most reactive based on the following considerations:19 (1) steric shielding of the C-6, C-7, C-8, and C-11 positions would decrease their rate of oxidation; (2) the higher s-character of the tertiary cyclopropane C–H bonds (C-13/14) makes them notoriously difficult to oxidize; (3) of the remaining carbon centers, 13C NMR indicated that C-12 is the most “nucleophilic”; (4) hyperconjugation from the π-like cyclopropane system should facilitate oxidation of the pseudo-equatorial C–H bond on C-12, and finally (5) strain-release may, to a small extent, accelerate such an oxidation.20 The small, reactive, electrophilic oxidant methyl(trifluoromethyl)dioxirane (TFDO) was therefore chosen due to its straightforward preparation and success in other challenging methylene oxidations.21 As predicted, TFDO cleanly achieved C–H oxidation at C-12 to deliver the intermediate cyclopropyl carbinol intermediate 8 (single diastereomer) which could be isolated and fully characterized on a gram scale. In practice, however, it was directly treated in the same flask with MgI2/ZnI2 to elicit a dehydrative ring opening22 to furnish diene 9 along with unreacted enone 7. The anticipated tertiary iodide product was not observed presumably due to rapid elimination to form a diene system. The mixture of 7 and 9 was directly subjected to another Mukaiyama hydration to furnish the tertiary carbinol 10 in 34% isolated yield from 7 along with 62% recovered 7. The addition of PPh3 to this type of reaction has not been reported before and was found to be essential to prevent the reactive intermediate peroxide adduct from generating an unwanted byproduct (see Supplementary Information for details).
Figure 2.
19-step total synthesis of 1. Reagents and conditions: 7. Mn(acac)2, PhSiH3, O2, EtOH, then TMSOTf, Et3N, CH2Cl2, 0 °C, 70%. 8. TFDO, CH2Cl2, 0 °C, then ZnI2, MgI2, Et2O. 9. Mn(acac)2, PhSiH3, O2, PPh3, EtOH, 2 steps (34% 10 + 62% 7). 10. RuCl3, NaBrO3, NaHCO3, EtOAc, CH3CN, H2O, 96%. 11. TFAA, DMAP, CH2Cl2, 0 °C, then Zn, AcOH, CH2Cl2, then Ac2O, DMAP, CH2Cl2, 0 °C, then Et3N, DMF, 60 °C, 64%. 12. TsNHNH2, MeOH, reflux, then NaBH3CN, AcOH, reflux, 40%. 13. CrO3, 3,5-DMP, CH2Cl2, 0 °C to rt, 46%. 14. TMSN3, DCE, 70 °C, then I2, DCE, pyridine, 70 °C. 15. Me4Sn, PdCl2(PhCN)2, AsPh3, CuI, NMP, 80 °C, 2 steps (64% 18 + 33% 17). 16. HF·Pyridine, THF, 0 °C. 17. Martin Sulfurane, DCE, 60 °C, then SeO2, benzene, 80 °C, 2 steps 51%. 18. NaBH4, MeOH, -40 °C, then Ac2O, DMAP, CH2Cl2, 93%. 19. NaBH(OAc)3, benzene, reflux, then TBAF, THF, 0 °C, then Ba(OH)2, MeOH, 72%.
Figure 3.
(a) Structural analysis of intermediate 7 calculated using MacroModel 10.8 (Schrödinger LLC) (b) 13C NMR can be used to predict the most nucleophilic methylene C–H bond after taking into account the role of other factors (see text)
With the cyclopropane ruptured, the stage was now set to install the C-12/13 oxygenation pattern along with reclosure of the cyclopropane. Overall, this was a maneuver without precedent that needed to be accomplished in a scalable and short fashion. Towards this end, chemoselective oxidation of the C-12/13 olefin to diketone 11 took place in near quantitative yield using Ru/NaBrO3. In order to reform the gem-dimethylcyclopropane moiety and access the correct oxidation state, a two-electron reduction of the diketone to an enediol would need to take place followed by intramolecular cyclization. Whereas attempts at acetylation of the hindered tertiary alcohol were fruitless and mesylation led to rapid elimination (as with the putative iodide intermediate in 8 → 9), treatment with trifluoroacetic anhydride smoothly led to an intermediate trifluoroacetate. This ester could be treated in the same flask with Zn to deliver an intermediate (12) that was difficult to assign without the aid of X-ray crystallography. Although the desired reduction took place, it was accompanied by the formation of an additional, unwanted, and bizarre C–C bond between C-13 and the carbonyl group of the trifluoroester. Although aldol additions of enediols to aldehydes23 and ketones24 are known, there is no precedent for such reactivity with esters. It was reasoned that this product originated from a highly reactive enediol intermediate (such as A; R = H) that might be regenerated under equilibrating conditions. In support of this hypothesis was the empirical observation that conversion of 11 to 12 initially resulted in the formation of a ca. 1:1 mixture of 12 along with an isomeric species tentatively assigned as the diastereomeric aldol adduct. Over 24 hours this mixture equilibrated to 12 exclusively perhaps due to stabilizing intramolecular hydrogen bonding interactions. Conditions were thus evaluated to coax the enediol back into existence (retro aldol) with the hope that concomitant irreversible cyclopropane formation (via attack at C-15) would take place. However, treatment of 12 with catalytic Sc(OTf)3 in DMF led instead to the cyclobutanone 13. Although clearly undesired, this was encouraging since 13 must have been derived from the desired hydroxy-cyclopropane B via a 1,2-shift which in turn was produced via the enediol intermediate A (R = H). Indeed, the formation of cyclobutanones such as 13 from hydroxycyclopropanes is precedented.25 In order to circumvent this undesired pathway, intermediate 12 was converted to hemiorthoester 14 by simple treatment with Ac2O followed by exposure to Et3N and gentle heating to 60 °C to deliver 15. The remarkable single flask conversion of 11 to 15 can be conducted on a gram scale and presumably succeeds due to the in situ formation of intermediate A (R = Ac). The conversion of A to the desired cyclopropane is believed to be concerted since no elimination products were observed (vide supra). This reaction cascade consists of thirteen discreet events occuring in the same flask, forming two new C–C bonds, three new ring systems, and three C–O bonds along with the cleavage of one C–C bond, two ring systems, and two C–O bonds. It unravels a complex polycyclic network to a kinetic endpoint using simple reagents thereby solving the most daunting challenge posed by 1.
The final eight steps of the synthesis served to install two additional oxygen atoms, one methyl group, and the proper C-12 and C-10 stereochemistry. Attempts to install the key C-10 stereocenter by way of conjugate reduction were met with failure despite extensive experimentation (see Supplementary Information for details). Thus, allylic transposition of 15, the Achilles heel of the synthesis from an efficiency standpoint, employed TsNHNH2 under reductive conditions leading to 16 in 40%. Allylic oxidation with CrO3,26 delivered enone 17 in 46% yield. A two-step methyl group introduction was accomplished by formation of an α-iodoenone (TMSN3, I2)27 followed by a Stille coupling28 affording 18 in 64% overall yield along with 33% recovered 17. Selective removal of the C-7 and C-9 silyl groups with HF·pyridine complex led to a diol that could be selectively dehydrated using Martin Sulfurane reagent to an unstable olefin that was directly oxidized using SeO2 to aldehyde 19 in 51% overall yield. The selective cleavage of the C-7 and C-9 silyl groups may stem from the inductive effect of the C-3 carbonyl group, which could make C-4 silyl group less reactive to acids. X-ray crystallographic analysis of 19 confirmed the structural assignments made thus far. To complete the synthesis, 19 was reduced to the allylic alcohol and shielded as the acetate ester. Attempts to directly reduce the C-20 aldehyde and the C-12 ketone in 19 were thwarted by the rapid rate of the C-3 enone carbonyl reduction in the presence of the C-20 free primary alcohol (the C-20 hydroxy group is actually proximate to C-3 as judged by molecular models). Stereoselective ketone reduction using NaBH(OAc)3 followed by successive treatment with fluoride and hydroxide sources (TBAF and Ba(OH)2 respectively) led to optically pure (+)-1 (synthetic, [α]26 D 105.37 (c 0.23, CH3OH); natural, [α]27 D 104.25 (c 0.20, CH3OH)) in 72% isolated yield (15 mg prepared by this route).
The concise route to 1 is enabled by a fundamentally different retrosynthetic approach to terpene synthesis in the laboratory rather than focusing on the invention of a new synthetic method. Indeed, the newest method employed in this synthesis is a Stille coupling, invented in the 1980's.28 Although the development of new synthetic methodologies are of paramount importance to push the field of organic chemistry forward, this work emphasizes the equally important role of strategy design in complex natural product total synthesis.29 By holistically mimicking the biosynthesis of ingenol (2) in the laboratory,6,7 the interrelationship between ingenanes and tiglianes could be leveraged using the same building block for both synthetic routes. Since two-phase terpene synthesis builds the carbon skeleton with a strategically planned redox state, the majority of steps can focus on installing key oxidations rather than repositioning functional groups and C–C bonds. Indeed, access to phorbol (1) only requires five additional steps compared to ingenol (2) due to the presence of two additional oxygen atoms (C-12/13) placed at particularly challenging locations on the carbon skeleton. Successful installation of these oxygenations and two others directly or indirectly relied on the application of C–H functionalization logic to pinpoint both the location and choreography of reactions.19,30 This artificial oxidase phase will enable the synthesis of analogs containing deep-seated modifications in the same fashion that has recently been reported for ingenol (2).8 Although the route, in its current concise form, cannot compete with isolation for the procurement of large quantities of phorbol (1), it can be argued that it could be adapted to become truly scalable if the need arose. Rather, the purpose of this synthesis is to enable rapid access to new tigliane family members with exciting bioactivity that are either difficult or impossible to access through isolation or semisynthesis.
Supplementary Material
Figure 1. A two-phase approach to ingenanes and tiglianes enables a concise approach to the legendary phorbol structure.
Acknowledgments
This work was supported by LEO Pharma, the Uehara Memorial Foundation (postdoctoral fellowship to S.K.) and the National Institute of General Medical Sciences Grant GM-097444. We are especially grateful to Swaminathan Natarajan of KemXtree and his team for providing ample quantities of compound 5. We thank Dr. D.-H. Huang and Dr. L. Pasternack for assistance with NMR spectroscopy; and Dr. A. L. Rheingold and Dr. C. E. Moore for X-ray crystallographic analysis.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions S.K. and P.S.B. conceived this work; J.F. provided compound 5; S.K., H.C., and P.S.B. designed experiments and analyzed data; S.K. and H.C. conducted experiments; S.K. performed molecular mechanics calculations; and S.K. and P.S.B. wrote the manuscript.
Author Information Metrical parameters for the structure of 12 and 19 are available free of charge from the Cambridge Crystallographic Data Center under reference number CCDC-1434376 and 1434377. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Wang HB, Wang XY, Liu LP, Qin GW, Kang TG. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem Rev. 2015;115:2975–3011. doi: 10.1021/cr200397n. [DOI] [PubMed] [Google Scholar]
- 2.Chen K, Baran PS. Total synthesis of eudesmane terpenes by site-selective C-H oxidations. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 3.Foo K, et al. Scalable, Enantioselective Synthesis of Germacrenes and Related Sesquiterpenes Inspired by Terpene Cyclase Phase Logic. Angew Chem Int Ed. 2012;51:11491–11495. doi: 10.1002/anie.201206904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza A, Ishihara Y, Baran PS. Scalable enantioselective total synthesis of taxanes. Nat Chem. 2012;4:21–25. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilde NC, Isomura M, Mendoza A, Baran PS. Two-Phase Synthesis of (−)-Taxuyunnanine D. J Am Chem Soc. 2014;136:4909–4912. doi: 10.1021/ja501782r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jørgensen L, et al. 14-Step Synthesis of (–)-Ingenol from (+)-3-Carene. Science. 2013;341:878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]
- 7.McKerrall SJ, Jørgensen L, Kuttruff CA, Ungeheuer F, Baran PS. Development of a Concise Synthesis of (–)-Ingenol. J Am Chem Soc. 2014;136:5799–5810. doi: 10.1021/ja501881p. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, et al. C-H Oxidation of Ingenanes Enables Potent and Selective Protein Kinase C Isoform Activation. Angew Chem Int Ed. 2015;54:14044–14048. doi: 10.1002/anie.201507977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakov N, Altman A. Regulation of immune system cell functions by protein kinase C. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKernan LN, Momjian D, Kulkosky J. Protein kinase C: one pathway towards the eradication of latent HIV-1 reservoirs. Advances in Virology. 2012;2012 doi: 10.1155/2012/805347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 12.Wender PA, et al. Studies on tumor promoters 8. The synthesis of phorbol. J Am Chem Soc. 1989;111:8957–8958. [Google Scholar]
- 13.Wender PA, Lee HY, Wilhelm RS, Williams PD. Studies on tumor promoters 7. The synthesis of a potentially general precursor of the tiglianes, daphnanes, and ingenanes. J Am Chem Soc. 1989;111:8954–8957. [Google Scholar]
- 14.Wender PA, Rice KD, Schnute ME. The First Formal Asymmetric Synthesis of Phorbol. J Am Chem Soc. 1997;119:7897–7898. [Google Scholar]
- 15.Wender PA, McDonald FE. Studies on tumor promoters 9. A second-generation synthesis of phorbol. J Am Chem Soc. 1990;112:4956–4958. [Google Scholar]
- 16.Lee K, Cha JK. Formal Synthesis of (+)-Phorbol. J Am Chem Soc. 2001;123:5590–5591. doi: 10.1021/ja010643u. [DOI] [PubMed] [Google Scholar]
- 17.Sugita K, Shigeno K, Neville CF, Sasai H, Shibasaki M. Synthetic Studies towards Phorbols: Synthesis of B or C Ring Substituted Phorbol Skeletons in the Naturally Occurring Form. Synlett. 1994;1994:325–329. [Google Scholar]
- 18.Sugita K, Neville CF, Sodeoka M, Sasai H, Shibasaki M. Stereocontrolled syntheses of phorbol analogs and evaluation of their binding affinity to PKC. Tetrahedron Lett. 1995;36:1067–1070. [Google Scholar]
- 19.Newhouse T, Baran PS. If C-H Bonds Could Talk: Selective C-H Bond Oxidation. Angew Chem Int Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou L, et al. Enhanced Reactivity in Dioxirane C–H Oxidations via Strain Release: A Computational and Experimental Study. J Org Chem. 2013;78:4037–4048. doi: 10.1021/jo400350v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaudel Q, et al. Improving Physical Properties via C-H Oxidation: Chemical and Enzymatic Approaches. Angew Chem Int Ed. 2014;53:12091–12096. doi: 10.1002/anie.201407016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick JP, Barton DL. Synthetic applications of metal halides. Conversion of cyclopropylmethanols into homoallylic halides. J Org Chem. 1980;45:2566–2570. [Google Scholar]
- 23.Miyoshi N, Takeuchi S, Ohgo Y. A Facile Synthesis of 2,3-Dihydroxyketones from 1,2-Diketones and Aldehydes Using Samarium Diiodide. Chem Lett. 1993;22:959–962. [Google Scholar]
- 24.Krohn K, Frese P, Flörke U. Biomimetic Synthesis of the Racemic Angucyclinones of the Aquayamycin and WP 3688-2 Types. Chemistry – A European Journal. 2000;6:3887–3896. doi: 10.1002/1521-3765(20001103)6:21<3887::aid-chem3887>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch H, Hecker E. Zur Chemie des Phorbols, XIII1) Über eine Acyloin-Umlagerung des 12-Desoxy-12-oxo-phorbol-13.20-diacetats. Justus Liebigs Ann Chem. 1969;725:142–153. [Google Scholar]
- 26.Salmond WG, Barta MA, Havens JL. Allylic oxidation with 3,5-dimethylpyrazole. Chromium trioxide complex steroidal .DELTA.5-7-ketones. J Org Chem. 1978;43:2057–2059. [Google Scholar]
- 27.Sha CK, Huang SJ. Synthesis of β-substituted α-iodocycloalkenones. Tetrahedron Lett. 1995;36:6927–6928. [Google Scholar]
- 28.Stille JK. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles [New Synthetic Methods (58)] Angew Chem, Int Ed. 1986;25:508–524. [Google Scholar]
- 29.Nicolaou KC, Sorensen EJ. Classics in Total Synthesis: Targets, Strategies, Methods 821. Wiley; 1996. [Google Scholar]
- 30.Gutekunst WR, Baran PS. C-H functionalization logic in total synthesis. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.