Abstract
Urinary angiotensinogen excretion parallels albumin excretion, which is not the case for renin, while renin’s precursor, prorenin, is undetectable in urine. We hypothesized that renin and prorenin, given their smaller size, are filtered through the glomerulus in larger amounts than albumin and angiotensinogen, and that differences in excretion rate are due to a difference in reabsorption in the proximal tubule. To address this, we determined the glomerular sieving coefficient (GSC) of renin and prorenin, and measured urinary renin/prorenin 1) after inducing prorenin in Cyp1a1-Ren2 rats, and 2) in patients with Dent’s disease or Lowe syndrome, disorders characterized by defective proximal tubular reabsorption. GSCs followed molecular size (renin>prorenin>albumin). The induction of prorenin in rats resulted in a >300-fold increase in plasma prorenin and doubling of blood pressure, but did not lead to the appearance of prorenin in urine. It did cause parallel rises in urinary renin and albumin, which losartan but not hydralazine prevented. Defective proximal tubular reabsorption increased urinary renin and albumin 20-40-fold, and allowed prorenin detection in urine, at ≈50% of its levels in plasma. Taken together, these data indicate that circulating renin and prorenin are filtered into urine in larger amounts than albumin. All 3 proteins are subsequently reabsorbed in the proximal tubule. For prorenin such reabsorption is ≈100%. Minimal variation in tubular reabsorption (in the order of a few %) is sufficient to explain why urinary renin and albumin excretion do not correlate. Urinary renin does not reflect prorenin that is converted to renin in tubular fluid.
Keywords: renin, prorenin, angiotensinogen, glomerular filtration barrier, tubular reabsorption, Dent’s disease, Lowe syndrome
INTRODUCTION
Renal clearance of solutes occurs through a combination of glomerular filtration, tubular secretion and tubular reabsorption. The molecular weight (MW) cut-off for glomerular filtration is thought to be 30-50 kD.1 Urinary albumin (MW 67 kD) is widely accepted as a measure of damage to the glomerular filtration barrier (GFB). Recently, urinary levels of renin-angiotensin system (RAS) components have been suggested to reflect the activity of the intrarenal RAS, independently of GFB damage. This particularly concerns angiotensinogen and renin.2-4 Indeed, angiotensinogen synthesis has been demonstrated in the proximal tubule,5 suggesting that renal angiotensin (Ang ) II production might occur independently of circulating (hepatic) angiotensinogen. Yet, the urinary excretion pattern of angiotensinogen (MW 55-65 kD) is identical to that of albumin in a wide variety of patients.4,6-10 Moreover, two landmark studies, making use of a kidney-specific angiotensinogen knockout mouse, revealed that, both under normal and pathological conditions, renal Ang II production depends entirely on plasma-derived (i.e., hepatic) angiotensinogen.11,12 These studies suggested a new concept, namely that it is filtered, plasma-derived angiotensinogen that accumulates in the proximal tubule, rather than locally synthesized angiotensinogen, and that this filtered angiotensinogen contributes most to renal Ang II production.12 Indeed, filtered angiotensinogen largely, if not completely, is intact angiotensinogen (and not cleaved, des-Ang I-angiotensinogen), truly allowing a contribution to renal angiotensin generation.8 Pohl et al. have suggested that tubular angiotensinogen uptake occurs via endocytosis, in a megalin-dependent manner.13
Renal tubular renin expression is believed to occur more distally, in the collecting duct, in addition to its classical expression in the juxtaglomerular apparatus.14 Importantly, urinary renin excretion does not correlate with albumin excretion,4,15 except when the GFB is damaged and circulating renin levels are greatly elevated (e.g., during preeclampsia).8 Given its MW (48 kD), circulating renin is likely to pass through the glomerulus. To what degree this also applies to prorenin (MW 54 kD), the inactive precursor of renin, remains to be determined. Prorenin could be detected in urine of preeclamptic women,8 but not in urine of hypertensive patients or diabetic subjects, despite the fact that the latter have greatly elevated circulating prorenin levels.4,15 One possibility is that prorenin, once filtered, is converted to renin in tubular fluid. If so, this would explain why there is usually no prorenin in urine, and why urinary renin levels do not correlate with urinary albumin levels. Here, it should be kept in mind that renin, like angiotensinogen, is also endocytosed by the proximal tubule in a megalin-dependent manner.13
In the present study, we set out to delineate the origin of urinary renin. To do so, we used three approaches, based on the hypothesis that renin and prorenin, given their smaller size, are filtered in larger amounts than albumin, and that all three proteins are subsequently reabsorbed in the proximal tubule. First, the glomerular sieving coefficients (GSCs) for renin and prorenin versus albumin were determined, both under normal conditions and after doxorubicin-induced GFB damage. Second, urinary renin levels following the induction of prorenin production in the liver were studied, using Cyp1a1-Ren2 rats, i.e., transgenic rats which express the mouse Ren2 gene exclusively in the liver in an inducible manner.16 Renal Ren2 expression did not occur in these animals,16 and their elevated renal Ang II levels17 were therefore most likely due to sequestration of circulating Ang II.18,19 Third, blood and urine of patients with Dent’s disease or Lowe syndrome were collected. Such patients have a mutation that disturbs acidification of subapical endosomes in the proximal tubule, thereby disabling reabsorption by megalin.20 Results in such patients may mimic those in the megalin knockout mice that were used to demonstrate the contribution of megalin to the endocytosis of RAS components in the proximal tubule.13 Indeed, if so, their urinary renin, prorenin and angiotensinogen levels should be far above normal, supporting the concept that, normally, these levels are low or undetectable due to effective tubular reabsorption.
METHODS
Animal and human studies
Glomerular filtration of renin and prorenin were studied C57/Bl6 mice with or without glomerular filtration barrier damage due to doxorubicin administration. Urinary renin and prorenin excretion were quantified after hepatic prorenin induction with indole-3-carbinol (I3C) in Cyp1a1-Ren2 rats, treated with either placebo, the Ang II type 1 receptor antagonist losartan, or hydralazine. Plasma and urine for the determination of RAS components were collected from patients with Dent’s disease or Lowe syndrome visiting the outpatient clinic. For further details, see the Methods section in the online-only Data Supplement.
Statistical analysis
Data are expressed as mean±SEM when normally distributed, or as geometric mean and range. Differences between I3C and control animals, and I3C animals treated with losartan or hydralazine were assessed by one-way ANOVA with post-hoc Dunnet correction. Data with a non-normal distribution were logarithmically transformed prior to ANOVA. Differences between renin and total renin levels were assessed by Wilcoxon signed ranks tests. Correlations between urine/plasma concentration ratios (*100%) of parameters were assessed by Spearman’s correlation coefficient. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS (IBM SPSS Statistics 20, Chicago, IL, USA).
RESULTS
Glomerular filtration of renin and prorenin in normal and albuminuric mice
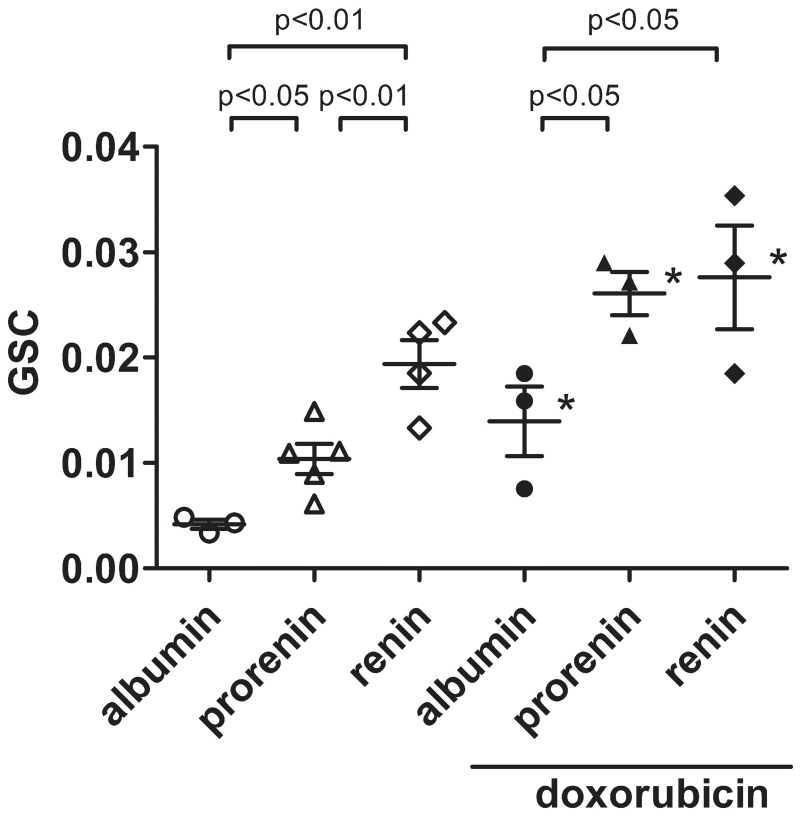
The average glomerular sieving coefficient (GSC) of mouse albumin in healthy C57/Bl6 mice was 0.00417±0.00043. The GSC of recombinant human prorenin was significantly greater than that of albumin (Figure 1). The GSC of recombinant human renin was significantly greater than that of both albumin and prorenin. In doxorubicin-treated mice, the GSC of each protein was greater than that in normal mice. Both prorenin and renin showed significantly greater GSCs than albumin, whereas the difference in GSC between prorenin and renin was diminished in doxorubicin-treated mice (Figure 1).
Figure 1.
Glomerular sieving coefficient (GSC) of albumin, prorenin, and renin in healthy C57/Bl6 mice (left) and C57/Bl6 mice treated with doxorubicin (right). N.S., not significant, *p<0.05 versus healthy mice. Data are mean±SEM.
Urinary renin and prorenin and renal megalin expression after hepatic prorenin induction in Cyp1a1-Ren2 rats
As reported previously,21 inducing hepatic prorenin synthesis with indole-3-carbinol (I3C) in Cyp1a1-Ren2 rats increased mean arterial blood pressure (MAP) from 111±4 to 197±6 mm Hg (P<0.01; n=9-11). Hydralazine partly (150±4 mm Hg; n=4) and losartan fully (121±7 mm Hg; n=7) prevented this rise. I3C exposure increased urinary volume from 10±1 to 57±13 mL/day (P<0.05), and the urinary creatinine concentration decreased accordingly from 5.9±0.3 to 0.6±0.2 mmol/L (P<0.05). Losartan and hydralazine normalized urinary volume to 6±1 and 9±1 mL/day, despite the fact that only losartan normalized MAP. Unfortunately, we did not monitor drinking behaviour, thus not allowing us to conclude whether the volume normalization during hydralazine was due to a reduction in water intake. The net creatinine excretion per day was identical in all groups (data not shown).
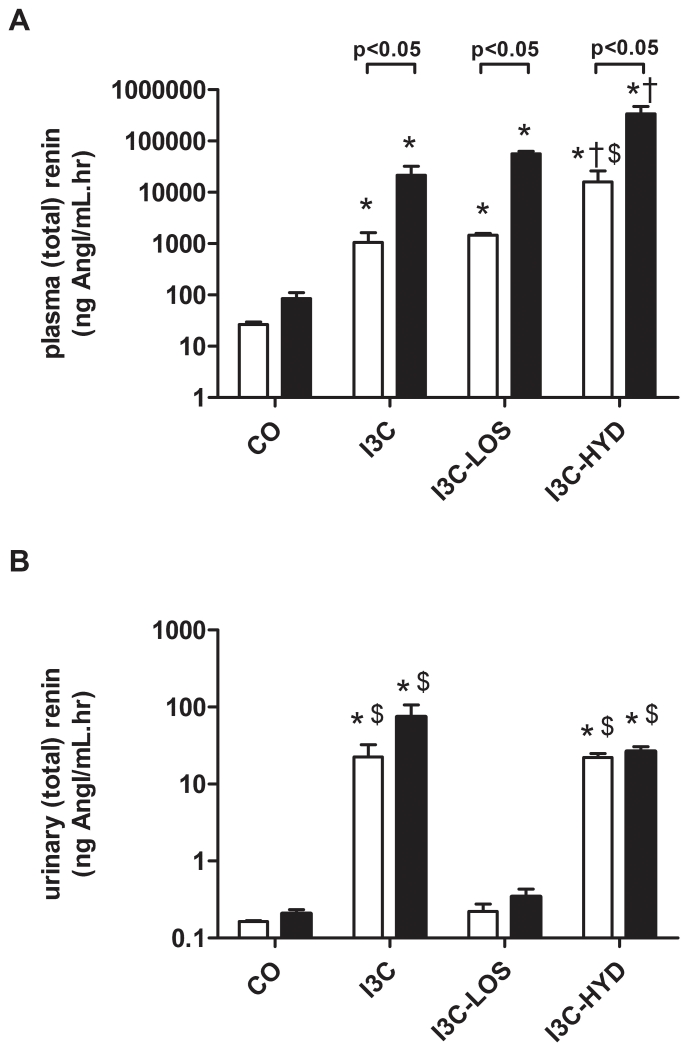
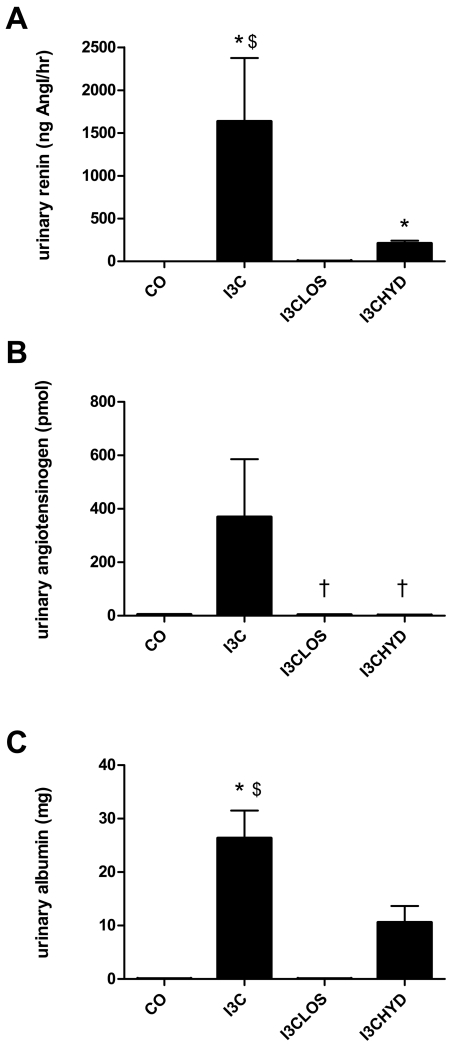
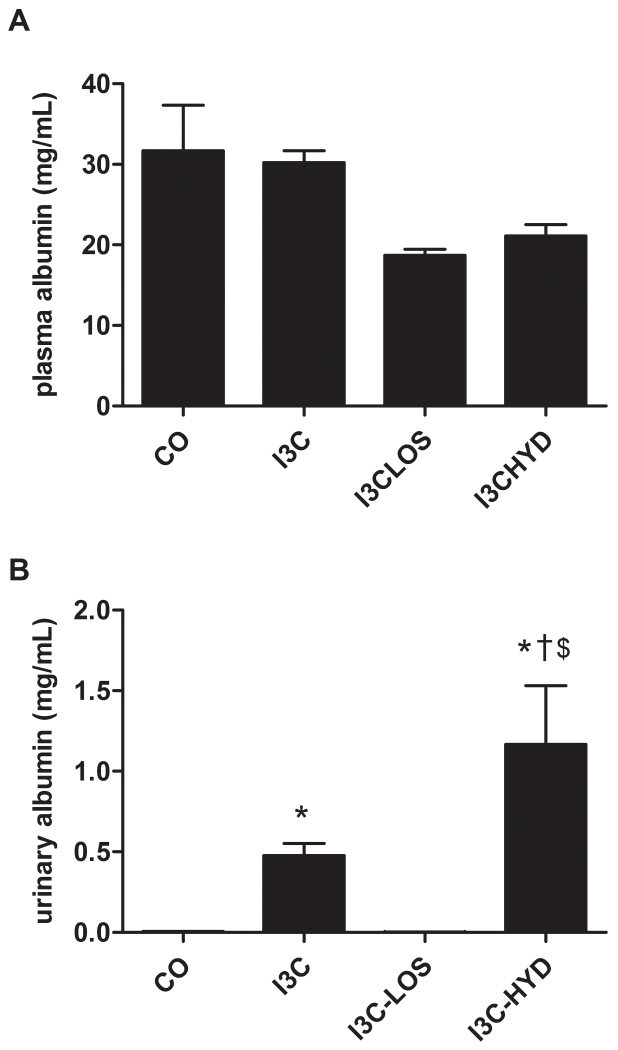
Compared to control (n=4), I3C exposure (n=5) increased plasma renin ≈20-fold, and plasma total renin ≈200-fold, confirming that its effect predominantly concerned prorenin (Figure 2A). On top of I3C, losartan (n=4) and hydralazine (n=4) additionally increased plasma renin and total renin ≈2- and ≈20-fold, although these changes were significant for hydralazine only (P<0.05 versus I3C). The I3C-induced rise in plasma renin and prorenin was accompanied by a ≈200-fold rise in urinary renin levels (P<0.05; Figure 2B), and a ≈1000-fold rise in urinary renin excretion (P<0.05; Figure 3A). Hydralazine partially prevented this rise (Figure 3A), without affecting the urinary renin levels, while losartan fully prevented it. Under all conditions, urinary total renin levels were not significantly different from urinary renin levels, suggesting that urine did not contain prorenin (Figure 2B).
Figure 2.
Plasma (A) and urinary (B) renin (white bars) and total renin (black bars) levels in transgenic Cyp1a1-Ren2 rats before (control, CO) and after treatment with indol-3-carbinol (I3C), with or without concomitant exposure to losartan (LOS) or hydralazine (HYD). *P<0.05 versus CO, †P<0.05 versus I3C, and $P<0.05 versus I3C-LOS. Data are mean±SEM of n=4-5.
Figure 3.
Urinary excretion (per 24 hours) of renin (A), angiotensinogen (B) or albumin (C) before (control, CO) and after treatment with indol-3-carbinol (I3C), with or without concomitant exposure to losartan (LOS) or hydralazine (HYD). *P<0.05 versus CO, †P<0.05 versus I3C, and $P<0.05 versus I3C-LOS. Data are mean±SEM of n=4-5.
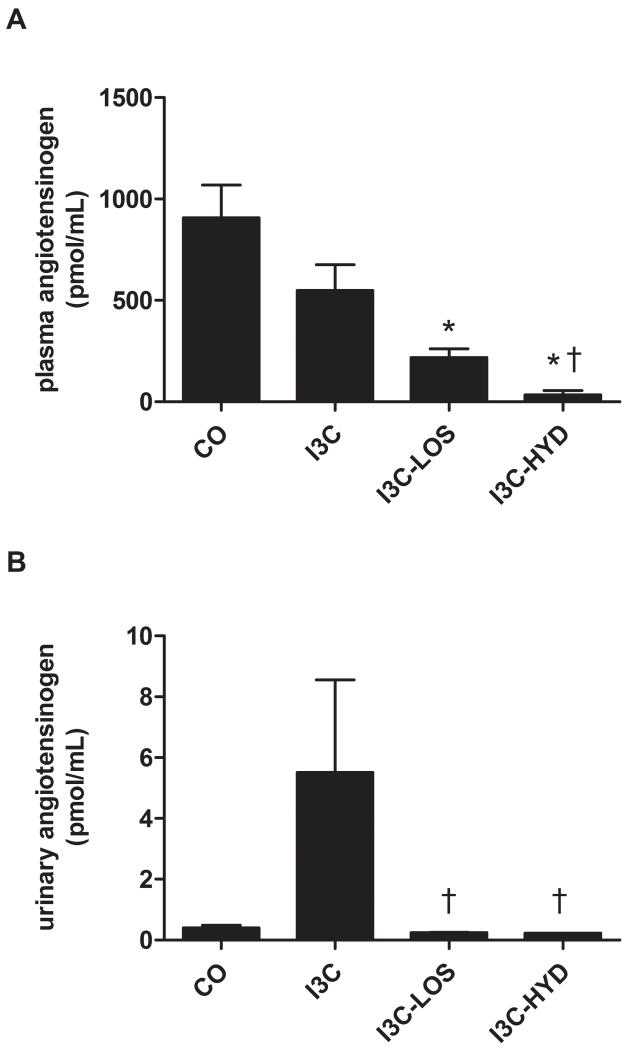
As expected based on the rise in renin levels, I3C lowered plasma angiotensinogen levels, particularly in combination with losartan and hydralazine (Figure 4A). In fact, after hydralazine, plasma angiotensinogen was close to zero. The urinary levels of angiotensinogen rose ≈10-fold after I3C, although this did not reach significance versus control (Figure 4B), and urinary angiotensinogen excretion increased ≈100-fold (Figure 3B). I3C in combination with losartan or hydralazine reduced urinary angiotensinogen excretion to that of control animals.
Figure 4.
Plasma (A) and urinary (B) angiotensinogen levels in transgenic Cyp1a1-Ren2 rats before (control, CO) and after treatment with indol-3-carbinol (I3C), with or without concomitant exposure to losartan (LOS) or hydralazine (HYD). *P<0.05 versus CO, and †P<0.05 versus I3C. Data are mean±SEM of n=4-5.
I3C with or without antihypertensive treatment did not affect plasma albumin levels (Figure 5A). Urinary albumin levels rose ≈100-fold after I3C (P<0.05), and urinary albumin excretion rose ≈500-fold (P<0.05; Figure 3C). Losartan fully, and hydralazine partially prevented this rise (Figures 3C and 5B).
Figure 5.
Plasma (A) and urinary (B) albumin levels in transgenic Cyp1a1-Ren2 rats before (control, CO) and after treatment with indol-3-carbinol (I3C), with or without concomitant exposure to losartan (LOS) or hydralazine (HYD). *P<0.05 versus CO, †P<0.05 versus I3C, and $P<0.05 versus I3C-LOS. Data are mean±SEM of n=4-5.
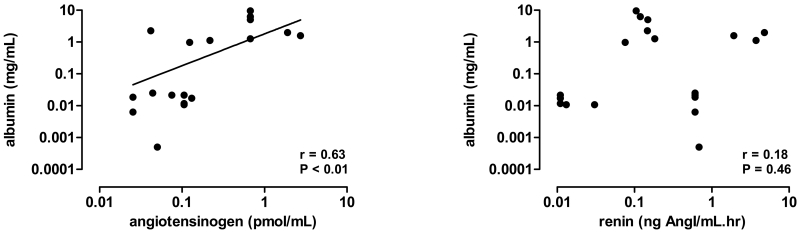
The urine/plasma concentration ratio of albumin correlated with that of angiotensinogen (r=0.63, P<0.05), but not with that of renin (r=0.18, P=0.46; Figure 6).
Figure 6.
Correlations between the urine/plasma concentration ratios of albumin and angiotensinogen (left) or renin (right) in transgenic Cyp1a1-Ren2 rats, at baseline or during treatment with indol-3-carbinol in the absence or presence of losartan or hydralazine.
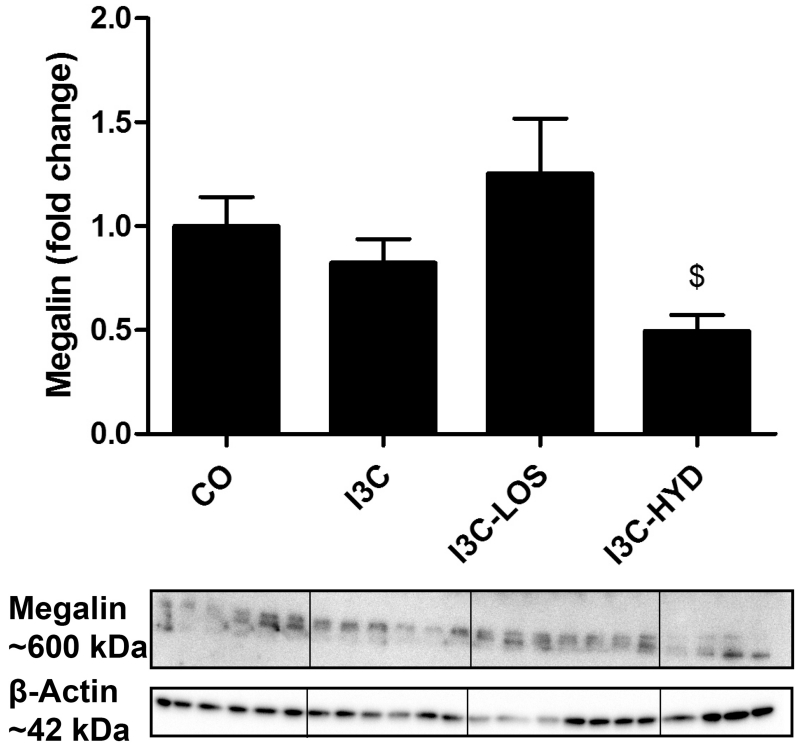
I3C treatment tended to reduce renal megalin expression (Figure 7), although this decrease became significant only (P<0.05) in the presence of hydralazine. Losartan prevented this phenomenon.
Figure 7.
Megalin protein expression in kidneys of transgenic Cyp1a1-Ren2 rats before (control, CO) and after treatment with indol-3-carbinol (I3C), with or without concomitant exposure to losartan (LOS) or hydralazine (HYD). $P<0.05 versus I3C-LOS. Data are mean±SEM of n=4-7.
Plasma and urinary RAS components in patients with Dent’s disease or Lowe syndrome
Plasma samples were obtained from 2 men with Dent’s disease (due to a CLC5 mutation) and 2 men with Lowe syndrome (due to a OCRL mutation, age 24-47 years), and in 3 of these men 1 or more urine samples could additionally be obtained (7 urine samples in total). All patients were normotensive, but one patient used an ACE inhibitor for proteinuria.
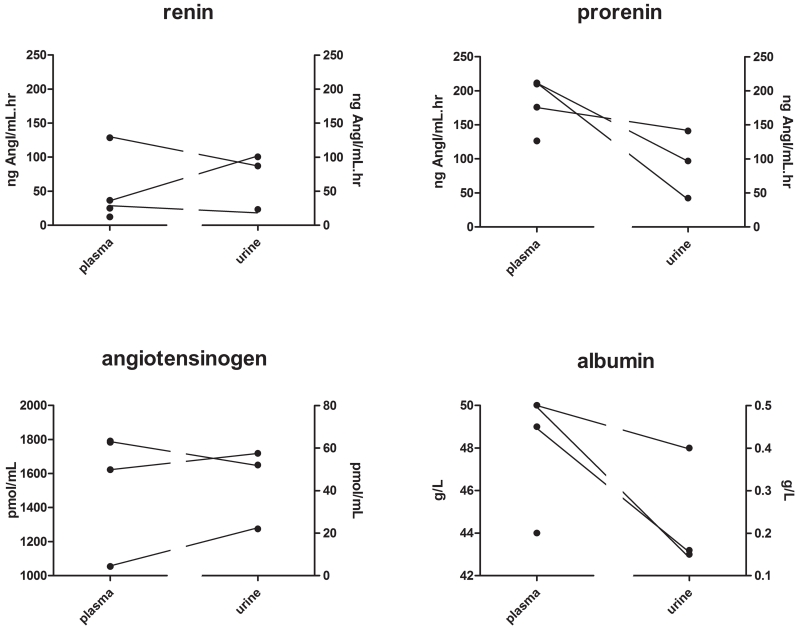
Urinary total renin levels were higher than urinary renin levels in all 7 urine samples, and thus the urine of these patients did contain prorenin. Figure 8 shows that urinary renin levels were comparable to plasma renin levels (range 68-275%), while urinary prorenin levels were ≈2-fold lower than plasma prorenin levels. Angiotensinogen and albumin levels in urine were 1-2% of the concomitantly measured plasma levels. Urinary aldosterone levels (6966 pg/mL) were higher than plasma aldosterone levels (485 pg/mL). Plasma creatinine levels averaged 284±72 μmol/L.
Figure 8.
Plasma and urinary levels of renin, prorenin, angiotensinogen, aldosterone, albumin and creatinine in 4 patients with Dent’s disease or Lowe syndrome. Urine was collected from 3 patients. Open symbols resemble the one patient that was treated with an ACE inhibitor.
DISCUSSION
This study shows that the GSCs of renin, prorenin and albumin correlate with their molecular size, albumin displaying the lowest GSC and renin the highest. The GSCs of all 3 proteins increased after inducing GFB damage with doxorubicin, and under this condition the GSC of prorenin was no longer different from that of renin. This is suggestive of a decrease in glomerular size-selectivity after exposure to doxorubicin. Severe prorenin-induced hypertension also resulted in rises in urinary renin and albumin. Since this was not accompanied by changes in nephrin expression or Wilms’ tumor staining (both suggestive for GFB damage),21 these rises were pressure-induced.22 Indeed, blood pressure lowering prevented or diminished these rises, complete normalization occurring only when blood pressure had been normalized completely (with losartan). Remarkably, even a >200-fold elevation of circulating prorenin, with or without hypertension, did not result in detectable prorenin levels in urine. Only when megalin-dependent tubular prorenin reabsorption was absent, did urinary prorenin become detectable. These data support the concept that urinary renin is plasma-derived, and does not represent activated prorenin of plasma or renal origin. Circulating prorenin apparently does filter into the proximal tubule, but is fully reabsorbed in a megalin-dependent manner.
Multiphoton imaging previously revealed that the GSC of angiotensinogen amounted to 25-50% of that of albumin,23 despite the fact that its MW is comparable to that of albumin. Since there are no reasons why the GSC of albumin would be up to 4-fold higher than that of angiotensinogen,23 the authors suggested that this difference is due to the presence of low-molecular weight fragments in their albumin preparation.24 Such fragments would have a GSC of 100%, and a fragmented fraction of labelled albumin of only 0.19% would have been enough to explain the difference. Thus, in reality, the GSCs of albumin and angiotensinogen are most likely comparable. Our earlier data in humans fully support this view: in hypertensive subjects the albumin and angiotensinogen levels were ≈0.05% of their concomitant plasma levels.4 The present study now reveals that the urinary albumin and angiotensinogen levels in patients with disabled tubular reabsorption are up to 1-2% of their plasma levels, i.e., 20-40-fold higher than normal. It is important to note that the plasma angiotensinogen levels in these patients were identical to those in hypertensive patients.4 These data therefore demonstrate that normally >95% of filtered angiotensinogen and albumin is reabsorbed. Given the strong correlation between the urinary/plasma concentration ratios of albumin and angiotensinogen in a wide variety of patients,4,6-9 as well as in healthy pregnant women (who display greatly elevated plasma angiotensinogen levels) and women with preeclampsia,8 it is highly likely that the process underlying tubular reabsorption (megalin-dependent endocytosis) is identical for both proteins.13 Our data in Cyp1a1-Ren2 rats stress the importance of normalizing the urinary angiotensinogen levels versus plasma angiotensinogen levels. Obviously, this is also true for urinary renin.25 Indeed, following I3C prorenin induction, particularly in combination with losartan or hydralazine, circulating renin levels became so high that circulating angiotensinogen started to get depleted. This resembles the situation in heart failure patients treated with RAS blockers, or the application of excessive RAS blocker doses.26,27 An additional reason for the high renin levels during hydralazine may be that this drug, through an interaction with CYP1A2, induces the generation of autoantibodies,28 thereby potentially worsening renal pathology. The virtual absence of circulating angiotensinogen during hydralazine explains why the urinary angiotensinogen levels in the hydralazine-treated rats were ‘normal’, while simultaneously urinary renin was excessively elevated.
Urinary renin levels in humans amount to ≈6-7% of the renin levels in plasma, normal values ranging from 0.05-5 ng Ang I/ml.hr (geometric mean 0.2 ng Ang I/ml.hr).4,29 The present study now reports urinary renin levels in patients with Dent’s disease or Lowe syndrome that are up to 68-275% of plasma renin. This is 20-40-fold higher than normal, identical to the rise observed for albumin and angiotensinogen in these patients. Blocking tubular reabsorption with lysine in mice even yielded a 100-fold rise in urinary renin, without affecting plasma renin.30 Combined with the larger GSC for renin versus albumin, these data indicate that circulating renin is readily filtered into urine, and subsequently reabsorbed for 90-98%. Small variations in reabsorption (in the order of a few %) may easily double or triple urinary renin. Such variations, if not exactly paralleled by the variation in albumin reabsorption, could explain why urinary renin excretion does not always correlate with urinary albumin excretion.
An alternative explanation for the different pattern of renin excretion versus albumin excretion is the release of renin into urine from the collecting duct. Yet, synthesis at this site largely concerns prorenin.31 Thus, such release, if occurring, would require the conversion of prorenin to renin. The same would apply to the possibility that urinary renin is in fact circulating prorenin that has been converted to renin within the tubular fluid. The present study allowed us to address these 2 scenarios. First, when elevating circulating prorenin several 100-fold in Cyp1a1-Ren2 rats (which is far above the rises observed in human physiology, which are <10-fold, and rather maximally 3-5-fold32), urinary renin excretion rose ≈1000-fold. A comparable (≈500-fold) rise was observed for albumin, while prorenin remained undetectable in urine. Had conversion of filtered, circulating prorenin contributed to urinary renin, the rise in urinary renin should have been many orders above that of albumin, given the much greater rise in circulating prorenin. Second, and most important, urine of the patients with Dent’s disease or Lowe syndrome did contain prorenin, albeit in amounts that, when corrected for its plasma concentration, were ≈2-3-fold lower than those of renin. This difference fits perfectly with the reduced GSC of prorenin versus renin under normal circumstances. Clearly, therefore, prorenin is filtered in the glomerulus, but its tubular reabsorption is virtually 100%, leaving no detectable prorenin in urine under normal circumstances. Early studies in mice, which observed prorenin in urine only when tubular reabsorption was blocked with lysine, complement this view.33 In summary, these findings argue against prorenin-renin conversion in tubular fluid, and also suggest that urinary renin is not activated prorenin from the collecting duct, at least under the conditions of the present study.
The circulating levels of renin and prorenin in patients with disabled proximal tubular reabsorption were 2-3-fold above normal,34 suggestive for RAS activation in these patients. This might relate to the polyuria in patients with Dent’s disease or Lowe syndrome.35 RAS activation also resulted in elevated plasma aldosterone.4 The urinary aldosterone levels in these patients were >1 order of magnitude above the plasma aldosterone levels. This is not different from previous studies,4,8 and reflects the well-known fact that aldosterone, as a small molecule, is readily filtered in the kidney, and subsequently concentrated in urine, without being reabsorbed. As a result, aldosterone concentrations in urine correlate with those in plasma, in full agreement with their common (adrenal) origin.4,8
Finally, the partial normalization of the elevated urinary excretion of renin and albumin by hydralazine in the I3C-treated Cyp1a1-Ren2 rats could be due to the fact that it did not fully normalize blood pressure. However, since Ang II suppresses the protein expression of megalin via its type 1 receptor,36,37 an alternative explanation is that RAS blockers not only lower urinary protein because they lower blood pressure, but also because they increase reabsorption of proteins via megalin. Conversely, intrarenal RAS activation will lower megalin expression, thereby increasing urinary renin levels. The elevated urinary renin levels in patients with diabetes,4 in whom such intrarenal RAS activation is well-known to occur,38,39 might be the consequence of this phenomenon. Our data on megalin expression are in complete agreement with this concept: expression decreased following RAS stimulation, particularly in the presence of hydralazine, and losartan prevented this.
PERSPECTIVE
Urinary renin originates in the circulation. Because of their smaller size, both plasma renin and prorenin are filtered into urine in larger amounts than plasma albumin. All 3 proteins are subsequently reabsorbed by >90% in the proximal tubule. Remarkably, for prorenin such reabsorption is virtually 100%, and there is no evidence for its conversion to urine in tubular fluid. Minor differences in tubular reabsorption (in the order of a few %) are sufficient to explain why urinary renin and albumin excretion, unlike urinary angiotensinogen and albumin excretion, are unrelated. Such differences may relate to the absolute molecular size and charge. An attractive concept would be that the presence of the prosegment, i.e., the 43 aminoacids that represent the difference between renin and prorenin, plays a decisive role. Such a role has also been demonstrated with regard to prorenin binding to the (pro)renin receptor.40,41 Glycosylation is less likely to contribute to this phenomenon, since Ren2 prorenin is non-glycosylated (unlike the recombinant human renin and prorenin preparations applied in the present study) and yet it behaved in exactly the same manner as prorenin in the human and mouse studies: it remained undetectable in urine, despite excessive rises and high blood pressure-induced reninuria and albuminuria.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Renin and prorenin are filtered through the glomerular filtration barrier to a greater extent than albumin, and differences in their tubular reabsorption rate, rather than release of (pro)renin from the collecting duct, are likely to explain the difference in excretion rate.
What is relevant?
Urinary renin is plasma-derived, and does not represent activated prorenin of plasma or renal origin.
SUMMARY
The glomerular sieving coefficients of renin, prorenin and albumin correlate with their molecular size, with albumin displaying the lowest GSC and renin the highest. All 3 proteins are subsequently reabsorbed by >90% in the proximal tubule. For prorenin such reabsorption is virtually 100%, and there is no evidence for its conversion to urine in tubular fluid. Minor differences in tubular reabsorption (in the order of a few %) are sufficient to explain why urinary renin and albumin excretion, unlike urinary angiotensinogen and albumin excretion, are unrelated.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by DK64324, DK100944, American Heart Association grant 15GRNT23040039, and American Diabetes Association grant #4-15-CKD-56 to J.P.P. EJH is supported by grants from the Netherlands Organisation for Scientific Research (NWO, Veni 916.12.140) and the Dutch Kidney Foundation (KSP-14OK19).
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Graham RC, Jr., Karnovsky MJ. Glomerular permeability. Ultrastructural cytochemical studies using peroxidases as protein tracers. J Exp Med. 1966;124:1123–1134. doi: 10.1084/jem.124.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Arthur JM, Investigators SA. Urinary angiotensinogen and risk of severe AKI. Clin J Am Soc Nephrol. 2013;8:184–193. doi: 10.2215/CJN.06280612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, Arthur JM. Association of elevated urinary concentration of renin-angiotensin system components and severe AKI. Clin J Am Soc Nephrol. 2013;8:2043–2052. doi: 10.2215/CJN.03510413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AHJ. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J Hypertens. 2011;29:2147–2155. doi: 10.1097/HJH.0b013e32834bbcbf. [DOI] [PubMed] [Google Scholar]
- 5.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Alper AB, Jr., Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills KT, Kobori H, Hamm LL, Alper AB, Khan IE, Rahman M, Navar LG, Liu Y, Browne GM, Batuman V, He J, Chen J. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012;27:3176–3181. doi: 10.1093/ndt/gfs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdonk K, Saleh L, Lankhorst S, Smilde JEI, van Ingen MM, Garrelds IM, Friesema ECH, Russcher H, van den Meiracker AH, Visser W, Danser AHJ. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65:1316–1323. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 10.Roksnoer LCW, Verdonk K, van den Meiracker AH, Hoorn EJ, Zietse R, Danser AHJ. Urinary markers of intrarenal renin-angiotensin system activity in vivo. Curr Hypertens Rep. 2013;15:81–88. doi: 10.1007/s11906-012-0326-z. [DOI] [PubMed] [Google Scholar]
- 11.Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85:1068–1077. doi: 10.1038/ki.2013.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson F, Lu X, Rossing P, Garrelds IM, Danser AHJ, Parving HH. Urinary renin and angiotensinogen in type 2 diabetes: added value beyond urinary albumin? J Hypertens. 2013;31:1646–1652. doi: 10.1097/HJH.0b013e328362217c. [DOI] [PubMed] [Google Scholar]
- 16.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, McGrath I, Kotelevtsev Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. J Renin Angiotensin Aldosterone Syst. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 18.van Kats JP, Schalekamp MADH, Verdouw PD, Duncker DJ, Danser AHJ. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 19.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–662. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 20.Claverie-Martin F, Ramos-Trujillo E, Garcia-Nieto V. Dent’s disease: clinical features and molecular basis. Pediatr Nephrol. 2011;26:693–704. doi: 10.1007/s00467-010-1657-0. [DOI] [PubMed] [Google Scholar]
- 21.Heijnen BFJ, Nelissen J, van Essen H, Fazzi GE, Tervaert JWC, Peutz-Kootstra CJ, Mullins JJ, Schalkwijk CG, Janssen BJA, Struijker-Boudier HAJ. Irreversible renal damage after transient renin-angiotensin system stimulation: involvement of an AT1-receptor mediated immune response. PLoS One. 2013;8:e57815. doi: 10.1371/journal.pone.0057815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maas RJ, Deegens JK, Wetzels JF. Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol Dial Transplant. 2014;29:2207–2216. doi: 10.1093/ndt/gfu355. [DOI] [PubMed] [Google Scholar]
- 23.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weyer K, Nielsen R, Christensen EI, Birn H. Generation of urinary albumin fragments does not require proximal tubular uptake. J Am Soc Nephrol. 2012;23:591–596. doi: 10.1681/ASN.2011101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roksnoer LCW, Verdonk K, Garrelds IM, van Gool JMG, Zietse R, Hoorn EJ, Danser AHJ. Methodologic issues in the measurement of urinary renin. Clin J Am Soc Nephrol. 2014;9:1163–1167. doi: 10.2215/CJN.12661213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AHJ. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J. 2009;30:805–812. doi: 10.1093/eurheartj/ehp012. [DOI] [PubMed] [Google Scholar]
- 27.Balcarek J, Sevá Pessôa B, Bryson C, Azizi M, Ménard J, Garrelds IM, McGeehan G, Reeves RA, Griffith SG, Danser AHJ, Gregg R. Multiple ascending dose study with the new renin inhibitor VTP-27999: nephrocentric consequences of too much renin inhibition. Hypertension. 2014;63:942–950. doi: 10.1161/HYPERTENSIONAHA.113.02893. [DOI] [PubMed] [Google Scholar]
- 28.Belloc C, Gauffre A, Andre C, Beaune PH. Epitope mapping of human CYP1A2 in dihydralazine-induced autoimmune hepatitis. Pharmacogenetics. 1997;7:181–186. doi: 10.1097/00008571-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lumbers ER, Skinner SL. Observations on the origin of renin in human urine. Circ Res. 1969;24:689–697. doi: 10.1161/01.res.24.5.689. [DOI] [PubMed] [Google Scholar]
- 30.Mazanti I, Hermann KL, Nielsen AH, Poulsen K. Ultrafiltration of renin in the mouse kidney studied by inhibition of tubular protein reabsorption with lysine. Clin Sci (Lond) 1988;75:331–336. doi: 10.1042/cs0750331. [DOI] [PubMed] [Google Scholar]
- 31.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batenburg WW, Danser AHJ. (Pro)renin and its receptors: pathophysiological implications. Clin Sci (Lond) 2012;123:121–133. doi: 10.1042/CS20120042. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen AH, Hermann KL, Mazanti I, Poulsen K. Evidence for a predominant renal secretion and clearance of inactive plasma renin, studied by in vivo inhibition of protein synthesis, and inhibition of renal tubular protein reabsorption. Clin Exp Hypertens A. 1988;10:1301–1303. doi: 10.1080/07300077.1988.11878925. [DOI] [PubMed] [Google Scholar]
- 34.Campbell DJ, Nussberger J, Stowasser M, Danser AHJ, Morganti A, Frandsen E, Ménard J. Activity assays and immunoassays for plasma renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem. 2009;55:867–877. doi: 10.1373/clinchem.2008.118000. [DOI] [PubMed] [Google Scholar]
- 35.Reinhart SC, Norden AG, Lapsley M, Thakker RV, Pang J, Moses AM, Frymoyer PA, Favus MJ, Hoepner JA, Scheinman SJ. Characterization of carrier females and affected males with X-linked recessive nephrolithiasis. J Am Soc Nephrol. 1995;5:1451–1461. doi: 10.1681/ASN.V571451. [DOI] [PubMed] [Google Scholar]
- 36.Hosojima M, Sato H, Yamamoto K, Kaseda R, Soma T, Kobayashi A, Suzuki A, Kabasawa H, Takeyama A, Ikuyama K, Iino N, Nishiyama A, Thekkumkara TJ, Takeda T, Suzuki Y, Gejyo F, Saito A. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology. 2009;150:871–878. doi: 10.1210/en.2008-0886. [DOI] [PubMed] [Google Scholar]
- 37.Tojo A, Onozato ML, Kurihara H, Sakai T, Goto A, Fujita T. Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens Res. 2003;26:413–419. doi: 10.1291/hypres.26.413. [DOI] [PubMed] [Google Scholar]
- 38.Hollenberg NK, Fisher ND, Nussberger J, Moukarbel GV, Barkoudah E, Danser AHJ. Renal responses to three types of renin-angiotensin system blockers in patients with diabetes mellitus on a high-salt diet: a need for higher doses in diabetic patients? J Hypertens. 2011;29:2454–2461. doi: 10.1097/HJH.0b013e32834c627a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price DA, Porter LE, Gordon M, Fisher NDL, De’Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 40.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AHJ. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 41.Morales R, Watier Y, Böcskei Z. Human prorenin structure sheds light on a novel mechanism of its autoinhibition and on its non-proteolytic activation by the (pro)renin receptor. J Mol Biol. 2012;421:100–111. doi: 10.1016/j.jmb.2012.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.