Abstract
Background
Women with a cytological diagnosis of atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H) are usually immediately referred to colposcopy. However, triage may reduce the burden of diagnostic work-up and avoid over-treatment.
Methods
A meta-analysis was conducted to assess the accuracy of hrHPV testing, and testing for other molecular markers to detect CIN of grade II or III or worse (CIN2+ or CIN3+) in women with ASC-H. An additional question assessed was whether triage is useful given the relatively high pre-triage probability of underlying precancer.
Results
The pooled absolute sensitivity and specificity for CIN2+ of HC2 (derived from 19 studies) was 93% (95% CI: 89–95%) and 45% (95% CI: 41–50%), respectively. The p16INK4a staining (only 3 studies) has similar sensitivity (93%, 95% CI:75–100%) but superior specificity (specificity ratio: 1.69) to HC2 for CIN2+. Testing for PAX1 gene methylation (only 1 study) showed a superior specificity of 95% (specificity ratio: 2.08). The average pre-test risk was 34% for CIN2+ and 20% for CIN3+. A negative HC2 result decreased this to 8% and 5%, whereas a positive result upgraded the risk to 47% and 28%.
Conclusions
Due to the high probability of precancer in ASC-H, the utility of triage is limited. The usual recommendation to refer women with ASC-H to colposcopy is not altered by a positive triage test, whatever the test used. A negative hrHPV DNA or p16INK4a test may allow for repeat testing but this recommendation will depend on local decision thresholds for referral.
Keywords: cervical cancer, cervical intraepithelial neoplasia, triage, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion, ASC-H, HPV, meta-analysis, diagnostic test accuracy
INTRODUCTION
By repetitive high-quality cytological screening and treatment of cervical precancerous lesions, the incidence of and mortality from cervical cancer can be reduced substantially. However, still about halve a million of women are diagnosed every year with cervical cancer.1 The cytological diagnosis of ASC-H is a relatively new cytological classification and is a subset of atypical squamous cells (ASC) formally introduced in the 2001 Bethesda System (2001).2 ASC-H has cytological characteristics that are intermediate between atypical squamous cells of undetermined significance (ASC-US) and high-grade intraepithelial lesion (HSIL). As an uncommon cytological interpretation, ASC-H is reported infrequently and the prevalence of Pap smears interpreted as ASC-H varies significantly among laboratories with a mean reporting rate of 0.43% (5th–95th percentile: 0–2%) in the USA.3 The finding of ASC-H is often associated with a high HPV positive rate (10th –50th–90th percentile: 0%–54%–79%)4 and a relatively high risk of underlying intraepithelial neoplasia of grade II or III or worse (CIN2+, CIN3+), ranging from 13%5 to 66%6 and 11%7 to 35%,8 respectively. In comparison, ASC-US is a far more prevalent cytological category which has lower likelihood of underlying CIN2+ (pooled average of 12%) and CIN3+ (7%).9;10
Management of cervical cytological lesions depends on the severity of the lesion and its inherent underlying or future risk of high-grade cervical intraepithelial neoplasia or cancer.11;12 Consistent evidence is available supporting the recommendation to use high-risk HPV (hrHPV) testing to triage women ASC-US9;10 and general consensus exist regarding the recommendation to refer all women with HSIL directly to colposcopy.12;13 However, divergent recommendations are found in the international literature regarding the management with intermediately severe cytological abnormalities such as low-grade intraepithelial lesion (LSIL), atypical glandular cells (AGC) and ASC-H.
American and European guidelines recommend immediate colposcopy for women with ASC-H.11;12;14 The ASCCP (American Society of Colposcopy and Cervical Pathology) consensus guideline in 2006 was primarily based on data from the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesion (ASCUS/LSIL) Triage Study (ALTS), which indicated that ASC-H had a significantly greater hrHPV positivity (84%) and underlying risk of high-grade CIN compared with ASCUS.15 The 5-year cancer risk among 467 women of the Kaiser Permanente Northern California (KPNC) cohort with HPV-negative ASC-H was 2%, which is judged too high to justify observation16. Therefore, the ASCCP 2012 updated guidelines continues to recommend immediate colposcopy regardless of HPV result for women with ASC-H cytology11 even though the level of evidence of this recommendation was graded as moderate. However, a retrospective study carried out at University of Pittsburg Medical Center (UPMC) identified 885 HPV-negative ASC-H patients with available follow-up results. In an average follow-up period of 29 months, only 14 (1.6%) patients showed evidence of CIN2+ and no case of invasive cervical cancer was diagnosed.17 These data suggest that HPV triage in the management of women with ASC-H may be useful.
To reduce the burden of diagnostic work-up and to avoid over-treatment and adverse effects associated with excision of lesions,18;19 it is appropriate to identify markers which may increase safety and efficiency of management procedures for women with ASC-H. We therefore conducted a systematic review and meta-analysis to assess the accuracy not only of hrHPV testing but also of other molecular markers to predict presence or development of cervical precancer.
MATERIAL AND METHODS
Clinical question
This meta-analysis evaluates the test accuracy of HPV testing and other molecular markers to triage women with a cytological result of ASC-H to predict the presence of CIN2+ or CIN3+. The following clinical questions were addressed: A) what is the absolute accuracy (sensitivity and specificity) of hrHPV testing with the Hybrid Capture-2 assay (HC2)? and B) what is the absolute accuracy of other hrHPV assays and other molecular markers? and C) what is the relative accuracy of these other assays and markers compared to HC2?
Given the high underlying risk of cervical precancer associated with ASC-H, the review also assesses whether a negative triage test could downgrade the risk sufficiently to avoid immediate diagnostic work-up. The PICOS components of the clinical questions are explained in the Supplementary Material.
Inclusion criteria and search strategy
Studies were eligible if the following criteria were met: (1) women had cytological report of ASC-H (2) hrHPV testing was performed by HC2 and/or other assays and/or triage with other molecular markers (3) women were subsequently submitted to a reference test to verify presence or absence of CIN2+ or CIN3+ and (4) the separate accuracy data (number of false- and true-positive and negative results) were reported, computable or could be requested. Outcome assessment including colposcopy and directed biopsy, with or without endocervical curettage was considered as the reference standard. Only studies enrolling at least 20 women with ASC-H were selected. All retrospective or prospective studies that evaluated the accuracy of triage testing in women with ASC-H to predict the presence of CIN2+ or CIN3+ were eligible for this meta-analysis.
Twelve eligible studies had been identified through a previously performed literature search for a meta-analysis on hrHPV testing in triage of ASC-US and LSIL and for which details on the search strategy are described elsewhere.9;10 To update and extend retrieval of references, a new search string which focused on reports of ASC-H was performed in three databases, MEDLINE, EMBASE and CENTRAL. The search strategy used two terms only, “ASC-H” and “cannot exclude high grade intraepithelial lesion”, combined by the OR Boolean operator. No language restrictions were applied. Additionally, the references of included papers and citation lists of previous meta-analyses and other key studies were browsed using www.scopus.com. Eligibility of studies was evaluated by L.X. and F.V. In case of discordance, M.A. was consulted for final decision on in- and exclusion.
Evaluated tests
The main evaluated index test was HC2 test since it is the most widely studied hrHPV assay. HC2 targets DNA sequences of HPV types 16,18,31,33,35,38,39,45,51,52,56,58,59 and 68, using the standard cut off (signal strength, relative light units ≥ 1, expressing semi-quantitatively the viral load compared to a control sample with 1 pg of HPV DNA per millilitre). Other tests including other HPV assays identifying nucleic acid sequences of hrHPV types jointly or HPV assays detecting a limited number of HPV types were also included as well as other molecular markers (overexpression of proteins or methylation of certain viral or human genes) were also evaluated. The cut-off proposed by the manufacturer of each assay was accepted as the positivity criterion.
Reference standard
All women had to be submitted to verification with colposcopy, colposcopy-directed biopsies, possibly supplemented with endocervical curettage. The histological interpretation of biopsies was considered as the outcome, accepting negative colposcopy as sufficient ascertainment for the absence of disease, when no biopsies were taken in case of normal satisfactory colposcopic findings. Two levels of disease outcome were considered: CIN2+ and CIN3+.
Data extraction and statistical analysis
For all included studies, information on the design and characteristics of the study were abstracted by L.X. and F.V. and evaluated by M.A. The quality of each included study was evaluated using the second version of the check list for Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2).20 A score was given to four domains (participant selection, triage test, reference standard, and flow & timing), based on a set of signalling questions assigned to each domain.
When four or more studies were available, the absolute sensitivity and specificity of the tests were estimated jointly with a bivariate normal model for the logit transforms of sensitivity and specificity.21 Summary receiver-operating-characteristics (sROC) plots were drawn to show the joint overall and study-specific sensitivity and specificity.22 When less than four studies were available, sensitivity and specificity were pooled separately using random-effect models for pooling proportions and ratios of proportions.23;24
The relative sensitivity and specificity of other tests compared to HC2 were computed using metadas, a SAS macro for meta-analysis of diagnostic accuracy studies that allows the inclusion of test as a covariate making comparison of tests possible (See Supplementary Material Chapter 4).25 The influence of other study characteristics, such as the QUADAS-2 items, on test accuracy were also assessed with metadas.
Post-test probabilities of CIN2+ and CIN3+ were computed by applying the pooled sensitivity and specificity estimates on the observed average prevalence (=pre-test probabilities). The post-test risk if triage is positive corresponds with the positive predictive value (PPV), whereas the post-test risk in triage-negative women corresponds with the complement of the negative predictive value (cNPV=1-NPV). A positive triage was considered as efficient as the PPV exceeded 20% (for CIN2+) and 10% (for CIN3+) whereas a negative triage was considered as safe when cNPV was lower than 2% (for CIN2+) and 1% (for CIN3+).
RESULTS
Selection of studies
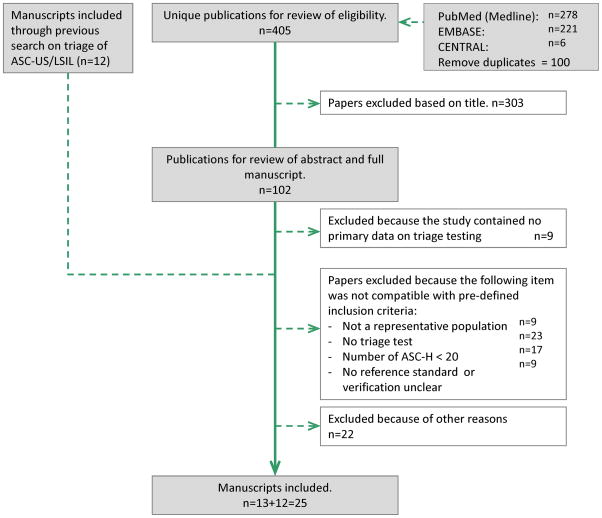
Twelve eligible studies had already been identified through a previously performed literature search for a meta-analysis on hrHPV testing in triage of ASC-US and LSIL.9;10 An additional systematic literature search in MEDLINE, EMBASE and CENTRAL, performed on March 31, 2015, resulted in 405 unique articles. After a primary eligibility check of the titles, 303 references were excluded. 88 manuscripts were excluded based on further evaluation of the abstracts and/or the full manuscripts. Among which two studies26;27 were excluded because no histologically confirmed CIN2+ were identified in the group with a negative triage test, resulting in zero true negatives or a specificity of 0%. From the KPNC cohort,28;29 only the report with complete cross-sectional accuracy data was chosen29. As a result, a total of 25 studies were selected. Two studies from an American laboratory were both included,30;31 since the proportion of overlapping participants was small. The process of study selection and the reason of exclusion of studies is shown in the PRISMA flow chart in Figure 1.
Figure 1.
PRISMA flow chart (Preferred Reporting Items for Systematic Reviews and Meta-analyses60) showing selection process of retrieved studies.
Study characteristics
An overview of study design, population and test characteristics of included studies is listed in Supplementary Table 1. Eight reports had a prospective design 5;7;8;31–35 and seventeen reports had a retrospective design.6;15;29;30;36–48 Eighteen studies included women with ASC-H identified through primary screening 6;7;29–32;35–39;42–48 and seven studies recruited subjects in colposcopy clinics where women were referred to because of prior cytological result of ASC-H. 5;8;15;33;34;40;41 Most included studies presented cross-sectional data. One study included women from a randomized controlled trial in which 110 ASC-H cases were retrospectively identified at cytology review by 4 pathologists.15
Nineteen studies reported accuracy data for the HC2 (Digene Corp., Gaithersburg, USA) assay.5–8;15;29–32;34–40;43;45;48 Four studies reported accuracy data of other hrHPV DNA testing, and among which, two with Linear Array® HPV Genotyping (Roche Molecular Diagnostics, Almede, USA) 33;49, one with the Cervista HPV HR test (Hologic, Marlborough, USA),46 and one with Multiplex PCR with genotype-specific primers (Eurofins MWG Operon, Huntsville, USA).41 For two studies accuracy data were obtained for HPV16/18 genotyping.8;33 For p16INK4a staining41;42;47 and p16INK4a/Ki-67 dualstaining,33;44;46 six studies were retrieved. In one study, accuracy of over-methylation of the paired boxed gene 1 (PAX1) gene was assessed.7
All the twenty-five included studies contributed accuracy data for CIN2+ but only seven for CIN3+. The evaluation of the quality of the included studies is summarized in Supplementary Table 2. Risks of selection bias were low to moderate, except for three studies 36;39;41 where women who had no biopsy data were not included. Concerns of bias regarding the reference standard were low to moderate. For more than half of the included studies (14/25), it was unclear whether the results of the reference test were masked towards the triage test or not. Complete verification with a valid reference standard was provided in 23/25 (92%) studies, and incorporation bias was avoided in 24/25 (96%) studies and was unclear in only 1 study.35 The delay between triage testing and verification outcome was not sufficiently documented in 8/25 (32%) studies. Withdraws were not well explained in 9/25 (36%) studies. Un-interpretable results for the triage test and reference test were not reported in 11/25 (44%) studies.
Absolute accuracy of HC2
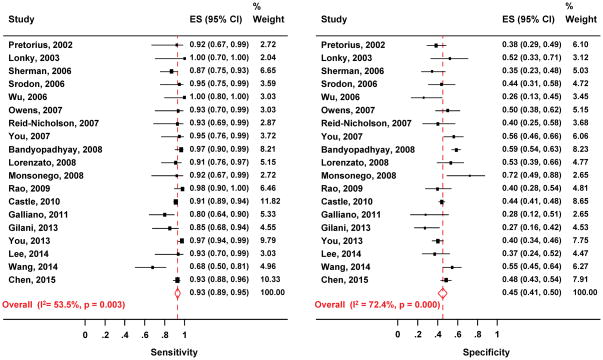
The pooled absolute sensitivity, specificity and the pooled disease rate and their 95% confidence intervals (CIs) are listed in Table 1. The pooled absolute sensitivity was 93% (95% CI: 89–95%) and 91% (95% CI, 81–96) to detect CIN2+ and CIN3+, respectively. The pooled absolute specificity for excluding CIN2+ and CIN3+ was 45% (95% CI: 41–50%) and 42% (95% CI: 34–51%), respectively. (Table 1, Figure 2). The corresponding SROC plots for outcome of CIN2+ and CIN3+ shown in Figure 3, display the variation of test accuracy in the individual studies as well as the overall pooled accuracy. The average hrHPV positivity rates was 67% (95% CI: 63–72%), ranging from 51% to 83%. The average prevalence of CIN2+ and CIN3+ among women triaged with HC2 was in 34% (95% CI: 28–40%, range:13–66%) and 20% (95% CI: 14–28%, range: 11–36%), respectively.
Table 1.
Pooled sensitivity and specificity of HC2 to detect CIN2+ and CIN3+, and pooled disease rate in women diagnosed with ASC-H on cytology.
| Outcome | Nb of studies | Sensitivity,% | Specificity,% | Disease rate, % | |||
|---|---|---|---|---|---|---|---|
| Pooled estimate (95% CI) | Range | Pooled estimate (95% CI) | Range | Pooled estimate (95% CI) | Range | ||
| CIN2+ | 19 | 93 (89–95) | 68–100 | 45 (41–50) | 26–72 | 34(28–40) | 13–66 |
| CIN3+ | 5 | 91(81–96) | 71–100 | 42 (34–51) | 32–65 | 20(14–28) | 11–36 |
Abbreviations: CIN2+, grade two cervical intraepithelial neoplasia or worse; CIN3+, grade three CIN or worse. HC2, Hybrid Capture-2 assay.
Figure 2.
Meta-analysis of the sensitivity (left) and specificity (right) of HC2 to detect CIN2+ in women with ASC-H. The pooled values are computed with a bivariate model.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; CIN2+, grade two cervical intraepithelial neoplasia or worse; CIN3+, grade three CIN or worse; HC2, Hybrid Capture-2 assay.
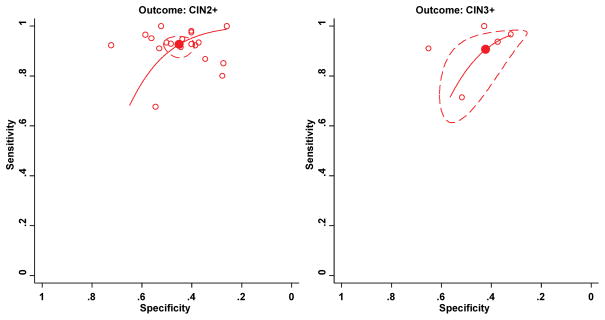
Figure 3.
Meta-analysis of the sensitivity and specificity of the HC2 assay to triage women with ASC-H to detect CIN2+ (left) and CIN3+ (right). Filled circles indicate the summary point; hollow circles, individual studies; solid line, summary receiver-operator curve; dashed line, 95% confidence ellipse.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; CIN2+, grade two cervical intraepithelial neoplasia or worse; CIN3+, grade three CIN or worse; HC2, Hybrid Capture-2 assay.
The variation of the accuracy of HC2 to detect CIN2+ according to study quality (assessed by the QUADAS items) and other covariates are shown in Supplementary Table 3 and Table 4. Study design (prospective or retrospective, psensi=0.90, pspeci=0.33), study setting (primary screening or referred population, psensi=0.88, pspeci=0.92) and study size (<100 vs ≥ 100 cases of ASC-H, psensi=0.81, pspeci=0.36) did not significantly affect the accuracy of HC2 in triage of women with ASC-H. A significantly higher sensitivity of HC2 was noted when the risk of inappropriate exclusion of patients was high compared to low risk studies. The sensitivity was significantly higher when the following reasons of concern regarding study quality were noted: a) the reference was not clearly described and b) avoidance of incorporation bias was unclear. When un-interpretable results were not reported for both triage and reference test, specificity was significantly lower.
Only three studies provided age-stratified accuracy data for HC2. 29;39;48 (See Supplementary Figure 1). In all studies, prevalence of CIN2/3+, test positivity rate and PPV for CIN2/3+ decreased by age. For the risk of CIN2+ and CIN3+ among test negative subjects, no relation with age was observed.
Accuracy of other triage tests
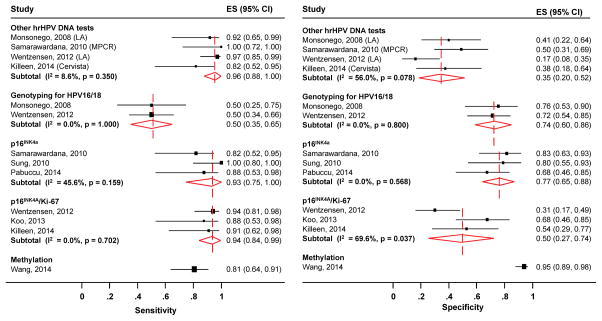
The pooled absolute accuracy, as well as the relative accuracy of other triage tests compared to the HC2 assay, for the detection of CIN2+, are shown in Table 2. The forest plot (Figure 4) shows the study-specific and pooled sensitivity and specificity for CIN2+ of the other triage tests. Given absence of significant heterogeneity in the sensitivity (I2=8.6% and p=0.35) and presence moderate but non-significant heterogeneity in the specificity (I2=56.0%% and p=0.08), pooled accuracy estimates could be computed for triage with hrHPV testing with other assays (Linear Arrary, Cervista and Multiplex PCR). The pooled absolute sensitivity and specificity for CIN2+ were 96% (95% CI: 88–100%) and 35% (95% CI: 20–52%), respectively. The relative sensitivity and specificity of other hrHPV tests versus HC2 for CIN2+ was 1.02 (95% CI: 0.95–1.10) and 0.76 (95% CI: 0.53–1.10), respectively. HPV16/18 genotyping identified on average 50% (95% CI: 35–65%) of CIN2+ and correctly excluded 74% (95% CI: 60–86%) of women without CIN2+. Genotyping for HPV16/18 was less sensitive (ratio of 0.50, 95% CI: 0.31–0.82) but more specific (ratio of 1.72. 95% CI: 1.41–2.10) than HC2.
Table 2.
Pooled absolute sensitivity and specificity of all triage tests and relative accuracy of other tests compared to HC2 to detect CIN2+ in women with ASC-H.
| Triage test | Nb of studies | Sensitivity, % | Sensitivity ratio (95% CI) | Specificity, % | Specificity ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | Range | Estimate (95% CI) | Range | ||||
| HC2 | 19 | 93 (89–95) £ | 57–100 | 1.00 | 45 (41–50)£ | 27–91 | 1.00 |
| Other hrHPV tests€ | 4 | 96 (88–100)£ | 82–100 | 1.02 (0.95–1.10) | 35 (20–52)£ | 17–50 | 0.76 (0.53–1.10) |
| HPV16/18 genotyping | 2 | 50 (35–65) † | 50–50 | 0.50 (0.31–0.82)* | 74 (60–86) † | 72–76 | 1.72 (1.41–2.10)* |
| p16INK4a | 3 | 93 (75–100) † | 82–100 | 0.99 (0.87–1.12) | 77 (65–88) † | 68–83 | 1.69 (1.39–2.06)* |
| p16INK4a/Ki-67 | 3 | 94 (84–99) † | 88–94 | 1.00 (0.92–1.10) | 50 (27–74) † | 31–68 | 1.12 (0.81–1.54) |
| Methylation | 1 | 81 (65–93) ‡ | -- | 1.01 (0.92–1.10) | 95 (90–99) ‡ | -- | 2.08 (1.85–2.32)* |
Pooled estimate of sensitivity & specificity computed jointly with the bivariate model,
Other hrHPV tests comprise Linear Array, Cervista and Multiplex PCR,
pooled estimate of sensitivity and specificity computed separately with a random effect model,
significant likelihood ratio test which assess whether the relative accuracy is statistically different from unity (p<0.05),
estimate from one study,
-- not applicable for only one study.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; CIN2+, grade two cervical intraepithelial neoplasia or worse; CIN3+, grade three CIN or worse; HC2, Hybrid Capture-2 assay.
Figure 4.
Subgroup meta-analysis of the sensitivity (left) and specificity (right) of other triage tests to detect CIN2+ in women with ASC-H. The pooled estimates of sensitivity and specificity are computed separately with a random effect model.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; CIN2+, grade two cervical intraepithelial neoplasia or worse; LA, Linear Array; MPCR, Multiplex PCR.
For p16INK4a staining, the pooled absolute sensitivity and specificity for CIN2+ were 93% (95% CI: 75–100%) and 77% (95% CI: 65–88%) respectively. The p16INK4a was as sensitive for detecting CIN2+ as HC2 is (ratio= 0.99, 95% CI: 0.87–1.12) but was substantially more specific (ratio =1.69; 95% CI: 1.39–2.06). For P16INK4a/Ki-67 dual staining, the pooled absolute sensitivity and specificity for CIN2+ were 94% (95% CI: 84–99%) and 50% (95% CI: 27–74%) respectively. Compared to HC2, P16INK4a/Ki-67 dual staining had similar sensitivity (ratio of 1.00, 95% CI: 0.92–1.10) and not significantly higher specificity (ratio of 1.12, 95% CI: 0.81–1.54).
With only one available study, the sensitivity of methylation of PAX1 to detect CIN2+ in women with ASC-H was 81% (95% CI: 65–93%), while its specificity was 95% (95%CI: 90–99%). Compared to HC2, the specificity of methylation to exclude CIN2+ is substantially higher with a ratio of 2.08 (95% CI: 1.85–2.32).
Post-test probabilities
The pre- and post-test probabilities of CIN2+ and CIN3+ after triage testing are presented in Table 3. Women with ASC-H have on average a pre-test risk of CIN2+ and CIN3+ of 34% (95% CI: 29–39%) and 20% (95% CI: 16–25%), respectively. Triage with the hrHPV testing of HC2 upgrades this risk (post-test risk), to 47% (for CIN2+) and 28% (for CIN3+) if HPV-positive and downgrades the risk to 8% (for CIN2+) and 5% (for CIN3+) if HPV-negative. After genotyping for HPV16/18, the risk of CIN2+ increases to 50% (for CIN2+) and 40%(for CIN3+) if HPV16/18-positive and decreases to 26% (for CIN2+) and 9% (for CIN3+) if HPV16/18-negative. Triage with p16INK4a staining stratifies the post-test risk of CIN2+ to 68% (if test+) and 5% (if test-). Triage of women with ASC-H using methylation of PAX1 lifts up the risks of CIN2+ to 89% and CIN3+ to 96% if test positive and brings them down to 9% (for CIN2+) and 2% (for CIN3+) if triage negative.
Table 3.
Sensitivity, specificity, likelihood ratios, pre- and post-test probabilities of CIN2+ and CIN3+ of triage with HC2 and other tests among women with ASC-H.
| Triage test | Outcome | # | Average pre-test risk, % (95% CI) | Pooled Sensitivity,% (95% CI) | Pooled specificity,% (95% CI) | PLR | NLR | Test+,% (95% CI) | Pooled post-test risk | |
|---|---|---|---|---|---|---|---|---|---|---|
| if test+ | if test− | |||||||||
| PPV,% (95% CI) | 1-NPV,% (95% CI) | |||||||||
| HC2 | CIN2+ | 19 | 34 (29–39) | 93 (89–95) | 45 (41–50) | 1.69 | 0.16 | 67 (63–72) | 47 (41–52) | 8 (6–9) |
| CIN3+ | 5 | 20 (16–25) | 91 (81–96) | 42 (34–51) | 1.57 | 0.22 | 67 (63–72) | 28 (23–34) | 5 (4–7) | |
| HPV16/18 genotyping | CIN2+ | 2 | 34 (29–39) | 50 (35–65) | 74 (60–86) | 1.92 | 0.68 | 38 (28–48) | 50 (44–55) | 26 (22–30) |
| CIN3+ | 2 | 20 (16–25) | 73 (53–91) | 73 (62–83) | 2.70 | 0.37 | 38 (28–48) | 40 (34–48) | 9 (7–11) | |
| p16INK4a | CIN2+ | 3 | 34 (29–39) | 93 (75–100) | 77 (65–88) | 4.04 | 0.09 | 48 (36–61) | 68 (62.4–72.2) | 5 (4–6) |
| Methylation | CIN2+ | 1 | 34 (29–39) | 81 (65–93) | 95 (90–99) | 16.20 | 0.20 | 23 (17–31) | 89 (87–91) | 9 (8–11) |
| CIN3+ | 1 | 20 (16–25) | 93 (69–99) | 99 (95–100) | 93.00 | 0.07 | 23 (17–31) | 96 (95–97) | 2 (1–3) | |
Abbreviations: #, number of studies; CIN2+, grade two cervical intraepithelial neoplasia or worse; CIN3+, grade three CIN or worse; PLR: positive likelihood ratio, NLR: negative likelihood ratio, test+: triage test positivity rate. HC2, Hybrid Capture-2 assay.
As shown in Supplementary Table 5, in women younger than 50, the average pre-test risk of CIN2+ and CIN3+ is 33% (95% CI: 17–48%) and 25% (95% CI: 22–28%), respectively. Women 50 or older have on average a pre-test risk of 14% (95% CI: 3–26%) and 13% (95% CI: 10–16%), respectively. The post-test probabilities of CIN2+ after triage with HC2 are: 47% (if test+ and <50 years), 27% (if test+ and ≥ 50 years), 6% (if test- and <50 years) and 4% (if test- and ≥50 years). Triage with hrHPV testing of HC2 upgrades the post-test risk of CIN3+, to 51% (women <50 years) and 53% (women ≥50 years) if HPV-positive and downgrades the risk to 6% (women <50 years) and 3% (women ≥50 years) if HPV-negative.
In Supplementary Table 6, we estimated the number of useful referrals (TP), missed cases (FN), unnecessary referrals (FP), and correctly reassured cases or colposcopies avoided (TN) when a given triage method is applied on 1,000 women with ASC-H. These numbers are computed from the meta-analytically pooled sensitivity and specificity assuming an average, low or high background risk of CIN2+ and CIN3+ observed from the range of studies included in the systematic review. Triage with HC2 of an average ASC-H population (pre-test probability of CIN2+ of 34%) would identify 316 women needing treatment, would miss 24 cases of CIN2, would generate 363 unnecessary referrals and would avoid 297 colposcopies.
DISCUSSION
Across all 25 included studies, over 4,000 women with a diagnosis of ASC-H were identified. Among those women, CIN2+ and CIN3+ was detected in 34% (95%CI: 29–39%, range: 13– 66%) and 20% (95% CI: 16–25%, range: 11–36%) of the cases. Although disease rates vary among different studies, the pooled values are in line with those of a previous systematic review.3
In this meta-analysis, testing for hrHPV infection by HC2 demonstrated good sensitivity of 93% but a rather low specificity of 45%. In comparison, in triage of ASC-US a similar average sensitivity (90%, 95% CI: 88–92) but higher specificity (58%, 95% CI: 54–63) have been observed for HC2.9;10 The underlying pre-triage risk of CIN2+ is considerably higher in women with ASC-H (on average 34%) compared to with those with ASC-US (on average 12%)9;10 Whereas it is accepted that a women with ASC-US and a negative hrHPV test may be released to routine screening, this recommendation seems not permitted for hrHPV negative women with ASC-H.12;50 Indeed, in women who were hrHPV-negative the risk is 8% for CIN2+ and 5% for CIN3+, which is much lower than that in hrHPV-positive women with ASC-H, but still is too high to withdraw them from follow-up. The post-test risk of CIN3+ among hrHPV-negative women aged 50 or older was lower (3%) than among younger women (5%), but the plausible recommendation of repeat testing would not be different (risk of CIN3+ in both age categories>1%51).
The large range of hrHPV positivity (51–90%) and underlying range of prevalence of CIN2+ (13–66%) and CIN3+(11–35%), observed in our meta-analysis, illustrates the subjectivity of the cytological diagnosis of ASC-H. In practice, the variation of hrHPV positivity is even larger (10th–90th percentile range of 0–79% in a recent survey in the USA)4. We postulate that the low rates of hrHPV positivity in ASC-H may reveal cytological overcalling in certain cytological laboratories. In the ALTS study, where the cytological diagnosis of ASC-H was based on expert quality control review, the hrHPV positivity was 84%. In Supplementary Figure 2, we have pooled the hrHPV positivity and underlying prevalence of CIN2+ and assessed the correlation between both in a scatter plot (See Supplementary Figure3). The clear positive trend between both parameters can be observed (Slope=0.53, 95% CI: 0.26–0.80, p=0.0001, R2= 0.46). It suggests that the hrHPV positivity rate in ASC-H as well as in other cytology categories may be used as an indicator for the quality of cervical cytology interpretation.52 Monitoring of the hrHPV positivity rate in women with ASC-H and correlating this to the subsequent risk of cervical precancer according to HPV status (assessed routinely or for reasons of surveillance) may be a good procedure to improve the quality of cytological interpretation as well as to challenge local guidelines for triage.
We included studies with diagnostic follow-up of less than 2 years, so these risks are cross-sectional rather than longitudinal in nature. In European guidelines, a PPV for CIN2+ ≥20% or PPV for CIN3+ ≥ 10%, and cNPV for CIN2+ ≤ 2% or for CIN3+ ≤1% are often accepted as decision thresholds to define management.51;53;54 Since the PPV of hrHPV testing in women with ASC-H clearly exceeds the positive triage criterion, the recommendation to refer immediately to colposcopy is clear, whatever the used hrHPV assay. However, the hrHPV negative women with ASC-H show a risk which is within the 1%–10% and 2–20% benchmarks for CIN3+ and CIN2+ respectively, therefore, a recommendation for repeat testing 6–12 months later would be acceptable in an European context.
In Supplementary Table 5, we provide a framework for clinicians and decision makers to judge whether triage of women with ASC-H is useful or acceptable in a given setting (low, average or high pre-test probability of CIN2+ and CIN3+). In a European context (where referral is acceptable if risk of CIN3+ exceeds 10%), repeat testing, would be appropriate when the pre-test risk is low or average. In the USA, however, where referral thresholds may be lower55, only in a low background risk situation (for instance in the UPMC study 39), retesting after 6–12 months would be acceptable.
Several other authors also suggest that hrHPV testing for the triage of women with ASC-H would be useful for selecting patients with ASC-H who should undergo immediate colposcopic examination.31;36;37;56;57 However, the use of HPV DNA triage testing in the primary work-up of ASC-H is not recommended in the ASCCP guidelines.11 This consensus guideline in 201211 is based on the data from KPNC cohort study, in which 44 (10.6%) of 414 patients with HPV-negative ASC-H results were diagnosed of CIN2+ and who were followed-up for at least 5 years.55 However, a large cohort study carried out in UPMC had a larger group of 885 HPV-negative ASC-H women, of whom only 14 (1.6%) of 885 patients developed high-grade CIN and no case of invasive cancer were diagnosed during a mean follow-up period of 29 months.17 The authors considered the risk of CIN2+ among HPV-negative women is sufficiently low to recommend women from this group to repeat Pap and HPV testing after 1 year. However, given the rather low hrHPV rate (51%, 95% CI: 47–55%) in this ASC-H study population, we suspect a certain degree of overcall in cytological interpretation. A prior meta-analysis of the proportion of HC2 positivity in ASC-US showed an average estimate of 39% (95% CI: 39–46%, range 26–74%).58 So we assume that in this UPMC study, a substantial proportion of ASC-H might be classified as ASC-US in other laboratories.
Genotyping for the most important carcinogenic HPV types (HPV16 and 18) results in tremendous gain of specificity (74%) but also large loss of sensitivity (relative sensitivity: 0.50) compared to HC2 in excluding patients with CIN2+, suggesting that HPV16/18 genotyping has limited utility in the management of women with ASC-H. The p16INK4a staining has superior specificity (55%) but similar sensitivity (95%) to HC2 and seems therefore more useful in management of patients with ASC-H. PAX1 gene methylation7 showed excellent specificity (95%) but its sensitivity was significantly lower than HC2. As shown in Supplementary Table 5, risk of CIN2+ and CIN3+ after a negative triage result with p16INK4a and methylation markers is sufficiently downgraded to accept repeat testing instead of referral but the level of evidence is low given the small number of studies.
For a patient with an ASC-H and a normal or an unsatisfactory colposcopy, hrHPV DNA testing or p16INK4a cyto-immunochemistry could play a role in the follow-up decisions. However, the improved risk stratification for a small group of screen-positive women needs to be weighed against the increased complexity of triage and management algorithms.
The included studies were of moderate to good methodological quality according to QUADAS-2 criteria, providing us reasonable confidence in the reliability of the meta-analysis. We found rather precise estimates of the accuracy to detect CIN2+ (rather narrow confidence intervals) but substantial heterogeneity in the accuracy estimates of HC2. However, for the outcome of CIN3+, the 95% confidence ellipse in SROC plots was rather wide, due to the small number of studies, which downgrades the quality of evidence. For the other triage tests, even smaller number of studies could be retrieved resulting in a low level evidence for the use of these molecular markers in clinical practice59
Another limitation of this meta-analysis is the lack of long-term longitudinal outcomes for women with ASC-H. The predictive values of triage tests in this meta-analysis are based on short-term colposcopic and histologic examination while the cumulative risk of high grade CIN and cancer increases over time16. Limitations are also connected with the infrequent occurrence of ASC-H in screening, often resulting in retrospective study designs that are generally limited by the difficulty to obtain complete clinical and virology data for participants with sufficiently similar characteristics.
CONCLUSION
Our meta-analysis shows that a cytological result of ASC-H is associated with a high risk of cervical precancer, which justifies immediate referral for colposcopy. However, our results support a certain utility of hrHPV DNA testing and in particular of p16INK4a cyto-immunochemistry. A positive triage result does not alter the decision to refer, but those testing negative could be recalled for a repeat test 6–12 months later in countries with a conservative follow-up policy. Nonetheless, in countries with a low decision threshold for colposcopy referral, triage of ASC-H would be considered as not useful to orient diagnostic work-up.
Supplementary Material
Footnotes
Funding and financial disclosure:
M. Arbyn, L. Xu and F. Verdoodt were supported by the seventh framework program of DG Research of the European Commission, through the COHEAHR Network (grant No 603019). The work of M. Arbyn was supported by the Joint Action CANCON which has received funding from the European Union in the framework of the Health Programme (2008–13). Other funding was received from the German Guideline Program in Oncology (German Cancer Aid project # 110163).
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 3.Davey DD, Greenspan DL, Kurtycz DF, Husain M, Austin RM. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion: review of ancillary testing modalities and implications for follow-up. J Low Genit Tract Dis. 2010;14:206–14. doi: 10.1097/LGT.0b013e3181ca66a6. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Moriarty AT, Ghofrani M, et al. Human papillomavirus testing and reporting rates in 2012: results of a College of American Pathologists national survey. Arch Pathol Lab Med. 2015;139:757–61. doi: 10.5858/arpa.2014-0393-CP. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius RG, Peterson P, Novak S, et al. Comparison of two signal-amplification DNA tests for high-risk HPV as an aid to colposcopy. J Reprod Med. 2002;47:290–6. [PubMed] [Google Scholar]
- 6.Galliano GE, Moatamed NA, Lee S, Salami N, Apple SK. Reflex high risk HPV testing in atypical squamous cells, cannot exclude high grade intraepithelial lesion: a large institution’s experience with the significance of this often ordered test. Acta Cytol. 2011;55:167–72. doi: 10.1159/000323319. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZM. PAX1 methylation analysis by MS-HRM is useful in triage of high-grade squamous intraepithelial lesions. Asian Pac J Cancer Prev. 2014;15:891–4. doi: 10.7314/apjcp.2014.15.2.891. [DOI] [PubMed] [Google Scholar]
- 8.Monsonego J, Pollini G, Evrard MJ, et al. Detection of Human Papillomavirus Genotypes Among High-Risk Women: A Comparison of Hybrid Capture and Linear Array Tests. Sex Transm Dis. 2008;35:521–7. doi: 10.1097/OLQ.0b013e318164e567. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding HPV testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 10.Arbyn M, Roelens J, Simoens C, et al. Human papillomavirus testing versus repeat cytology for triage of minor cytological cervical lesions. Cochrane Database Syst Rev. 2013;3:1–201. doi: 10.1002/14651858.CD008054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Anttila A, Jordan J, et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition - Summary Document. Ann Oncol. 2010;21:448–58. doi: 10.1093/annonc/mdp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 14.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Sherman ME, Castle PE, Solomon D. Cervical cytology of atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H): characteristics and histologic outcomes. Cancer. 2006:298–305. doi: 10.1002/cncr.21844. [DOI] [PubMed] [Google Scholar]
- 16.Katki HA, Schiffman M, Castle PE, et al. Five-Year Risks of CIN 3+ and Cervical Cancer Among WomenWho Test Pap-Negative But Are HPV-Positive. J Low Genit Tract Dis. 2013;17:S56–S63. doi: 10.1097/LGT.0b013e318285437b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen D, Austin RM, Gilbert C, Freij R, Zhao C. Follow-up Outcomes in a Large Cohort of Patients With Human Papillomavirus–Negative ASC-H Cervical Screening Test Results. Am J Clin Pathol. 2012;138:517–23. doi: 10.1309/AJCPYK60BZRNNAHQ. [DOI] [PubMed] [Google Scholar]
- 18.Arbyn M, Kyrgiou M, Simoens C, et al. Peri-natal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: a meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyrgiou M, Arbyn M, Martin-Hirsch P, Paraskevaidis E. Preterm birth after treatment for cervical precancer: an issue yet to be resolved. BMJ. 2012;345:e5847. doi: 10.1136/bmj.e5847. [DOI] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 21.Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Harbord RM, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. The Stata Journal. 2009;9:211–29. [Google Scholar]
- 23.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris R, Bradburm M, Deeks J, et al. metan: fixed- and random-effects meta-analysis. The Stata Journal. 2008;8:3–26. [Google Scholar]
- 25.Takwoingi Y, Deeks J. METADAS: A SAS macro for meta-analysis of diagnostic accuracy studies. Quick reference and worked example. 2010 http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/MetaDAS%20Quick%20Reference%20v1.3%20May%202012.pdf, -Version 1.3.
- 26.Passamonti B, Gustinuci D, Rechia P, et al. Expression of p16 in abnormal pap-tests as an indicator of CIN2+ lesions: a possible role in the low grade ASC/US and L/Sil (lg) cytologic lesions for screening prevention of uterine cervical tumours. Pathologica. 2010;102:6–11. [PubMed] [Google Scholar]
- 27.Alaghehbandan R, Fontaine D, Bentley J, et al. Performance of ProEx C and PreTect HPV-proofer E6/E7 mRNA tests in comparison with the hybrid capture 2 HPV DNA test for triaging ASCUS and LSIL cytology. Diagn Cytopathol. 2013;41:767–75. doi: 10.1002/dc.22944. [DOI] [PubMed] [Google Scholar]
- 28.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. J Low Genit Tract Dis. 2013;17:S50–S55. doi: 10.1097/LGT.0b013e3182854282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castle PE, Fetterman B, Thomas CJ, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010;116:76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 30.Owens CL, Moats DR, Burroughs FH, Gustafson KS. “Low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion” is a distinct cytologic category: histologic outcomes and HPV prevalence. Am J Clin Pathol. 2007;128:398–403. doi: 10.1309/QRDNMWWAKJQTVJGF. [DOI] [PubMed] [Google Scholar]
- 31.Srodon M, Parry DH, Ronnett BM. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion. Cancer. 2006;108:32–8. doi: 10.1002/cncr.21388. [DOI] [PubMed] [Google Scholar]
- 32.Lonky NM, Felix JC, Naidu YM, Wolde Tsadik G. Triage of atypical squamous cells of undetermined significance with Hybrid Capture II: colposcopy and histologic human papillomavirus correlation. Obstet Gynecol. 2003;101:481–9. doi: 10.1016/s0029-7844(02)02715-1. [DOI] [PubMed] [Google Scholar]
- 33.Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012;18:4154–62. doi: 10.1158/1078-0432.CCR-12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzato M, Caudroy S, Nou JM, et al. Contribution of DNA ploidy image cytometry to the management of ASC cervical lesions. Cancer. 2008;114:263–9. doi: 10.1002/cncr.23638. [DOI] [PubMed] [Google Scholar]
- 35.You K, Geng L, Guo Y, Qiao J. The use of HPV test in patients with cervical cytology interpreted as ASC-H. Acta Cytol. 2013;57:85. [Google Scholar]
- 36.Wu HH, Allen SL, Kirkpatrick JL, Elsheikh TM. Reflex high-risk human papilloma virus DNA test is useful in the triage of women with atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion. Diagn Cytopathol. 2006;34:707–10. doi: 10.1002/dc.20497. [DOI] [PubMed] [Google Scholar]
- 37.Reid-Nicholson M, Gatscha RM, Riedel ER, Lin O. Atypical squamous cells, cannot exclude high grade intraepithelial lesion (ASC-H): Does HPV matter? Diagn Cytopathol. 2007;35:1–5. doi: 10.1002/dc.20576. [DOI] [PubMed] [Google Scholar]
- 38.You K, Liang X, Qin F, Guo Y, Geng L. High-risk human papillomavirus DNA testing and high-grade cervical intraepithelial lesions. Aust N Z J Obstet Gynaecol. 2007;47:141–4. doi: 10.1111/j.1479-828X.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 39.Bandyopadhyay S, Austin RM, Dabbs D, Zhao C. Adjunctive human papillomavirus DNA testing is a useful option in some clinical settings for disease risk assessment and triage of females with ASC-H Papanicolaou test results. Arch Pathol Lab Med. 2008;132:1874–81. doi: 10.5858/132.12.1874. [DOI] [PubMed] [Google Scholar]
- 40.Rao A, Pather S, Dalrymple C, et al. The role of HPV testing in patients with possible high-grade cervical cytology. J Obstet Gynaecol Res. 2009;35:503–6. doi: 10.1111/j.1447-0756.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 41.Samarawardana P, Dehn DL, Singh M, et al. p16(INK4a) is superior to high-risk human papillomavirus testing in cervical cytology for the prediction of underlying high-grade dysplasia. Cancer Cytopathol. 2010;118:146–56. doi: 10.1002/cncy.20078. [DOI] [PubMed] [Google Scholar]
- 42.Sung CO, Kim SR, Oh YL, Song SY. The use of p16(INK4A) immunocytochemistry in “Atypical squamous cells which cannot exclude HSIL” compared with “Atypical squamous cells of undetermined significance” in liquid-based cervical smears. Diagn Cytopathol. 2010;38:168–71. doi: 10.1002/dc.21164. [DOI] [PubMed] [Google Scholar]
- 43.Gilani SM, Tashjian R, Fathallah L. Cervical cytology with a diagnosis of atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H): a follow-up study with corresponding histology and significance of predicting dysplasia by human papillomavirus (HPV) DNA testing. Arch Gynecol Obstet. 2014;289:645–8. doi: 10.1007/s00404-013-3015-5. [DOI] [PubMed] [Google Scholar]
- 44.Koo YJ, Hahn HS, Lee IH, et al. Dual immunostaining of cervical cytology specimens with atypical squamous cells for p16/Ki-67 does not exclude the existence of a high-grade squamous intraepithelial lesion. Virchows Arch. 2013;463:689–96. doi: 10.1007/s00428-013-1483-4. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Kim JW, Hong JH, et al. Clinical significance of HPV DNA cotesting in Korean women with ASCUS or ASC-H. Diagn Cytopathol. 2014 doi: 10.1002/dc.23173. [DOI] [PubMed] [Google Scholar]
- 46.Killeen JL, Dye T, Grace C, Hiraoka M. Improved Abnormal Pap Smear Triage Using Cervical Cancer Biomarkers. J Low Genit Tract Dis. 2014;18:1–7. doi: 10.1097/LGT.0b013e31828aeb39. [DOI] [PubMed] [Google Scholar]
- 47.Pabuccu EG, Taskin S, Ustun H, et al. Diagnostic performance of p16 staining in atypical squamous cells ‘cannot exclude high-grade squamous epithelial lesion’ in predicting high-grade cervical pathology. J Obstet Gynaecol. 2014:1–5. doi: 10.3109/01443615.2014.930107. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Baker S, De PG, Yang B. HPV testing results and histologic follow-up in women with ASC-H cytology in different age groups. J Am Soc Cytopathology. 2015;4:225–31. doi: 10.1016/j.jasc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Monsonego J, Pollini G, Evrard MJ, et al. Linear array genotyping and hybrid capture II assay in detecting human papillomavirus genotypes in women referred for colposcopy due to abnormal Papanicolaou smear. Int J STD AIDS. 2008;19:385–92. doi: 10.1258/ijsa.2007.007259. [DOI] [PubMed] [Google Scholar]
- 50.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 52.Arbyn M, Roelens J, Martin-Hirsch P, Leeson S, Wentzensen N. Use of HC2 to triage women with borderline and mild dyskaryosis in the UK. Br J Cancer. 2011;105:877–80. doi: 10.1038/bjc.2011.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol. 2007;197:356. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbyn M, Martin-Hirsch P, Wentzensen N. Human papillomavirus-based triage of women showing a cervical cytology result of borderline or mild dyskaryosis. BJOG. 2010;117:641–4. doi: 10.1111/j.1471-0528.2010.02521.x. [DOI] [PubMed] [Google Scholar]
- 55.Katki HA, Schiffman M, Castle PE, et al. Five-Year Risks of CIN 3+ and Cervical Cancer Among Women With HPV Testing of ASC-US Pap Results. J Low Genit Tract Dis. 2013;17:S36–S42. doi: 10.1097/LGT.0b013e3182854253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S, Sodhani P, Chachra KL, Singh V, Sehgal A. Outcome of “Atypical squamous cells” in a cervical cytology screening program: implications for follow up in resource limited settings. Diagn Cytopathol. 2007;35:677–80. doi: 10.1002/dc.20719. [DOI] [PubMed] [Google Scholar]
- 57.Liman AK, Giampoli EJ, Bonfiglio TA. Should women with atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion, receive reflex human papillomavirus-DNA testing? Cancer. 2005;105:457–60. doi: 10.1002/cncr.21387. [DOI] [PubMed] [Google Scholar]
- 58.Arbyn M, Martin-Hirsch P, Buntinx F, et al. Triage of women with equivocal or low-grade cervical cytology results. A meta-analysis of the HPV test positivity rate. J Cell Mol Med. 2009;13:648–59. doi: 10.1111/j.1582-4934.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.