SUMMARY
Although 5%–10% weight loss is routinely recommended for people with obesity, the precise effects of 5% and further weight loss on metabolic health are unclear. We conducted a randomized controlled trial that evaluated the effects of 5.1±0.9% (n=19), 10.8±1.3% (n=9) and 16.4±2.1% (n=9) weight loss, and weight maintenance (n=14) on metabolic outcomes. Five percent weight loss improved adipose tissue, liver and muscle insulin sensitivity, and β-cell function, without a concomitant change in systemic or subcutaneous adipose tissue markers of inflammation. Additional weight loss further improved β-cell function and insulin sensitivity in muscle, and caused stepwise changes in adipose tissue mass, intrahepatic triglyceride content, and adipose tissue expression of genes involved in cholesterol flux, lipid synthesis, extracellular matrix remodeling and oxidative stress. These results demonstrate that moderate 5% weight loss improves metabolic function in multiple organs simultaneously, and progressive weight loss causes dose-dependent alterations in key adipose tissue biological pathways.
eTOC
Magkos et al. demonstrate the profound therapeutic effects of weight loss on metabolic function in subjects with obesity. Even a moderate 5% weight loss has considerable health benefits, including decreased intra-abdominal and intra-hepatic fat, and increased multi-organ insulin sensitivity and β-cell function. Additional weight loss further improves many cardiometabolic outcomes.
INTRODUCTION
Obesity is associated with a constellation of cardiometabolic abnormalities including insulin resistance, β-cell dysfunction, nonalcoholic fatty liver disease, dyslipidemia, and hypertension, which are important risk factors for the development of serious medical complications such as type 2 diabetes and coronary heart disease (Klein et al., 2002; Kopelman, 2000). Most treatment guidelines, including those recently proposed by several major medical and scientific societies, recommend moderate weight loss of 5%–10% to achieve improvements in metabolic function and health outcomes (Jensen et al., 2014). However, it is much easier to achieve a 5% weight loss than it is to achieve a 10% weight loss, so it is important to understand the cardiometabolic benefits that occur with a 5% weight loss and what additional benefits, if any, can be expected with more weight loss in people with obesity. Several large randomized controlled weight loss trials retrospectively evaluated the effects of different amounts of weight loss on clinical outcomes (Wing et al., 1987; Wing et al., 2011). However, the weight loss stratification used in these studies combined the results from subjects who lost 5% through 10% of their body weight into one group; we are not aware of any trials that separated the weight loss outcomes in those who achieved 5% from those who achieved 10% weight loss, either prospectively or retrospectively.
The mechanism(s) responsible for the beneficial effects of weight loss on cardiometabolic outcomes is not known, but presumably involves a reversal of the mechanism(s) responsible for the adverse effects of weight gain. It has been proposed that a pathological expansion of adipose tissue mass causes an increase in adipose tissue inflammation, manifested by alterations in adipose tissue immune cell populations and increased gene expression of pro-inflammatory cytokines and chemokines, which cause systemic inflammation and insulin resistance (Berg and Scherer, 2005; Ferrante, 2007; Hotamisligil, 2006; Sun et al., 2013). However, the importance of decreasing adipose tissue and systemic inflammation in the beneficial metabolic effects of weight loss is unclear because of conflicting results from different studies, reporting decreases, increases and no changes in markers of inflammation after diet-induced weight loss (Capel et al., 2009; Clement et al., 2004; Dahlman et al., 2005; Johansson et al., 2012; Malisova et al., 2014; Sola et al., 2009). Therefore, a simultaneous assessment of the effects of moderate weight loss on metabolic function and adipose tissue inflammation in people with obesity could help elucidate the potential physiological significance of inflammation on metabolic dysfunction.
The purpose of the present study was to conduct a randomized controlled trial, in persons who are obese and have evidence of insulin-resistant glucose metabolism, to determine: 1) the therapeutic effects of 5% weight loss on cardiometabolic outcomes, including body composition (total body fat mass, intra-abdominal fat volume, and intrahepatic triglyceride content), 24-hour ambulatory blood pressure and heart rate, plasma lipid profile, β-cell function, and multi-organ (adipose tissue, liver, and muscle) insulin sensitivity; 2) whether 5% weight loss-induced cardiometabolic benefits are associated with a reduction in systemic or subcutaneous adipose tissue markers of inflammation; and 3) the effects of progressive 5%, 10% and 15% weight loss on cardiometabolic outcomes and global adipose tissue gene expression profile.
RESULTS AND DISCUSSION
Weight loss targets were effectively achieved
Forty subjects were randomized to either weight maintenance (n=20; 14 completed [five withdrew after being informed of their randomization and one subsequently dropped out], 46 ± 13 years old, 11 women and 3 men) or diet-induced weight loss (n=20; 19 completed [one dropped out], 43 ± 11 years old; 16 women and 3 men) (Supplemental Figure S1). Nineteen subjects in the weight loss group achieved the initial targeted 5% weight loss (5.1 ± 0.9% actual weight loss); 9 of these subjects (44 ± 12 years old; 8 women and 1 man) successfully achieved the subsequent weight loss targets of ~10% and ~15% (the actual mean weight losses achieved were 10.8 ± 1.3% and 16.4 ± 2.1%). Subjects were studied when they were weight stable (<2% weight change for at least 3 weeks; Supplemental Figure S2) before and after a median (quartiles) of 6.1 (5.9, 6.7) months in the weight maintenance group, and after 5% weight loss at 3.5 (2.9, 4.6) months, 11% weight loss at 6.8 (6.0, 8.6) months, and 16% weight loss at 10.4 (9.6, 11.4) months in the weight loss group.
Five percent weight loss improves body composition and multiple risk factors for cardiometabolic disease, and progressive weight loss causes further benefits
Five percent weight loss resulted in a 2 ± 2 % decrease in fat-free mass (FFM), an 8 ± 3 % decrease in body fat mass, a 7 ± 12 % decrease in intra-abdominal adipose tissue (IAAT) volume, and a 40 ± 21 % decrease in intrahepatic triglyceride (IHTG) content (Table 1). Five percent weight loss significantly decreased the plasma concentrations of some risk factors for cardiometabolic disease (glucose, insulin, triglyceride, alanine transaminase, and leptin), but did not affect others (free fatty acids, low- and high-density lipoprotein [LDL and HDL, respectively] cholesterol, and adiponectin) (Table 1). Five percent weight loss decreased 24-h ambulatory heart rate and 24-h ambulatory systolic, but not diastolic, blood pressure (Table 1). The reductions in FFM, fat mass, IAAT volume, IHTG content, fasting plasma insulin, leptin, and triglyceride concentrations continued with progressive weight loss up to 16% of initial body weight in a predominantly linear fashion, whereas plasma free fatty acid and CRP concentrations decreased and plasma adiponectin concentration increased significantly only after 16% weight loss (Table 2).
Table 1.
Effect of 5% weight loss on body composition and cardiometabolic risk factors
| Weight maintenance (n = 14) | Weight loss (n = 19) | Interaction P-value |
|||
|---|---|---|---|---|---|
| Baseline | Weight maintenance | Baseline | 5% weight loss | ||
| Weight (kg) | 106.6 ± 15.0 | 106.7 ± 14.7 | 106.2 ± 16.8 | 100.8 ± 16.2* | <0.001 |
| Body mass index (kg/m2) | 37.9 ± 4.4 | 38.0 ± 4.4 | 37.8 ± 4.4 | 35.9 ± 4.3* | <0.001 |
| Body fat (%) | 45.4 ± 6.3 | 45.6 ± 6.5 | 47.9 ± 4.9 | 46.3 ± 5.2* | <0.001 |
| Fat mass (kg) | 48.7 ± 11.6 | 49.0 ± 11.8 | 51.0 ± 10.1 | 46.7 ± 9.6* | <0.001 |
| Fat free mass (kg) | 57.1 (53.3, 63.4) | 56.9 (53.2, 62.4) | 53.0 (46.7, 56.8) | 51.5 (47.0, 55.5)* | 0.032 |
| Intra-abdominal adipose tissue (cm3) | 1456 ± 593 | 1585 ± 733* | 1409 ± 508 | 1294 ± 431* | 0.004 |
| Intrahepatic triglyceride (%) | 7.5 (4.1, 16.2) | 6.0 (3.4, 16.8) | 6.7 (3.3, 11.2) | 3.8 (1.5, 7.8)* | 0.023 |
| Free fatty acids, basal (mmol/L) | 0.48 (0.40, 0.52) | 0.49 (0.44, 0.59) | 0.55 (0.47, 0.58) | 0.54 (0.45, 0.66) | 0.341 |
| Glucose, basal (mg/dL) | 98 (91, 101) | 98 (93, 104) | 95 (92, 103) | 91 (88, 96)*† | 0.040 |
| Insulin, basal (mU/L) | 20.6 (15.7, 29.0) | 21.3 (19.1, 26.7) | 16.7 (13.3, 22.6) | 15.0 (9.6, 18.9)*† | 0.028 |
| SBP, 24-hour (mmHg) | 117 ± 12 | 121 ± 13 | 122 ± 11 | 118 ± 11* | 0.028 |
| DBP, 24-hour (mmHg) | 67 (58, 72) | 67 (65, 72) | 72 (66, 77) | 71 (66, 74) | 0.176 |
| Heart rate, 24-hour (bpm) | 78 ± 9 | 79 ± 6 | 78 ± 9 | 74 ± 9* | 0.034 |
| Triglyceride (mg/dL) | 107 (86, 141) | 114 (63, 158) | 153 (106, 201) | 105 (69, 162)* | 0.023 |
| HDL-cholesterol (mg/dL) | 47 ± 17 | 46 ± 15 | 41 ± 8 | 40 ± 7 | 0.724 |
| LDL-cholesterol (mg/dL) | 119 (88, 136) | 105 (91, 114) | 100 (90, 126) | 98 (86, 121) | 0.888 |
| Alanine transaminase (U/L) | 17.0 (12.5, 30.5) | 17.0 (14.8, 25.5) | 18.0 (13.5, 23.5) | 15.0 (12.0, 17.0)*† | 0.048 |
| Leptin (ng/mL) | 45.5 (25.9, 48.8) | 40.4 (27.9, 55.9) | 47.3 (25.2, 57.8) | 38.2 (26.7, 46.2)* | 0.006 |
| Adiponectin (μg/mL) | 4.36 (2.38, 8.31) | 4.89 (2.88, 8.95) | 6.06 (4.58, 6.85) | 6.06 (4.72, 7.44) | 0.408 |
| C-reactive protein (mg/L) | 3.59 (1.21, 4.92) | 4.36 (8.21, 6.13) | 3.70 (2.34, 5.90) | 4.68 (2.38, 7.30) | 0.538 |
| Interleukin-6 (ng/mL) | 2.5 ± 1.0 | 2.7 ± 1.5 | 2.0 ± 0.5 | 2.2 ± 0.7 | 0.909 |
| MCP-1 (pg/mL) | 150 (125, 194) | 174 (149, 190)* | 136 (115, 168) | 144 (123, 166)* | 0.260 |
| WBC count (103/mL) | 6.2 ± 1.4 | 6.1 ± 1.7 | 6.8 ± 1.8 | 7.0 ± 1.7 | 0.466 |
Data are means ± SD for normally distributed variables or medians (quartile 1, quartile 3) for not normally distributed variables. The effect of time (before vs. after) and differences between groups (weight maintenance vs. weight loss) were evaluated with repeated measures analysis of variance for normally distributed variables or Friedman’s test for not normally distributed variables. Significant time-by-group interactions were followed by appropriate within- and between-group post-hoc tests.
P<0.05 vs. baseline and
P<0.05 vs. weight maintenance group after the intervention. There were no significant differences between groups at baseline.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; WBC, white blood cell.
Table 2.
Effect of progressive weight loss on body composition and cardiometabolic risk factors
| Progressive weight loss (n = 9) | Effect of time P-value |
||||
|---|---|---|---|---|---|
| Baseline | 5% weight loss | 11% weight loss | 16% weight loss | ||
| Weight (kg)† | 103.8 ± 16.4 | 97.9 ± 15.7* | 92.5 ± 14.4* | 87.0 ± 14.9* | <0.001 |
| Body mass index (kg/m2)† | 37.7 ± 4.5 | 35.5 ± 4.3* | 33.6 ± 3.9* | 31.6 ± 4.1* | <0.001 |
| Body fat (%)† | 48.3 ± 4.3 | 46.3 ± 4.8* | 44.2 ± 4.5* | 42.2 ± 5.0* | <0.001 |
| Fat mass (kg)† | 50.3 ± 10.2 | 45.4 ± 9.6* | 41.1 ± 8.6* | 36.9 ± 8.8* | <0.001 |
| Fat free mass (kg)† | 53.0 (46.3, 55.6) | 51.5 (45.7, 54.1)* | 50.2 (45.2, 53.2)* | 49.0 (44.1, 51.4)* | <0.001 |
| Intra-abdominal adipose tissue (cm3)† | 1656 ± 559 | 1501 ± 469 | 1277 ± 474* | 1154 ± 457* | <0.001 |
| Intrahepatic triglyceride (%)†‡ | 8.5 (3.9, 25.9) | 7.4 (3.0, 12.5)* | 4.1 (1.1, 10.2)* | 3.0 (1.1, 5.2)* | <0.001 |
| Free fatty acids, basal (mmol/L)† | 0.56 (0.55, 0.65) | 0.56 (0.48, 0.64) | 0.49 (0.46, 0.58) | 0.47 (0.44, 0.55)* | 0.050 |
| Glucose, basal (mg/dL) | 92.7 ± 8.9 | 89.4 ± 4.9 | 89.3 ± 5.7 | 88.6 ± 2.7 | 0.288 |
| Insulin, basal (mU/L)† | 18.3 ± 7.7 | 15.5 ± 6.1 | 12.6 ± 5.5* | 9.5 ± 2.8* | <0.001 |
| Triglyceride (mg/dL)† | 153 ± 56 | 130 ± 71 | 110 ± 59* | 97 ± 39* | 0.003 |
| HDL-cholesterol (mg/dL) | 43 ± 10 | 41 ± 7 | 42 ± 7 | 44 ± 7 | 0.261 |
| LDL-cholesterol (mg/dL) | 115 (95, 135) | 98 (91, 127) | 101 (90, 136) | 91 (85, 125) | 0.162 |
| Alanine transaminase (U/L)† | 18.0 (11.5, 25.0) | 15.0 (12.0, 16.5) | 11.0 (10.0, 16.0) | 11.0 (9.5, 14.5)* | 0.015 |
| Leptin (ng/mL)† | 43.0 ± 13.5 | 32.8 ± 13.6* | 27.1 ± 9.9* | 18.6 ± 6.5* | <0.001 |
| Adiponectin (μg/mL)†‡ | 6.23 ± 2.73 | 6.34 ± 2.35 | 6.87 ± 2.63 | 8.30 ± 3.51* | <0.001 |
| C-reactive protein (mg/L)†‡ | 4.69 (3.44, 7.84) | 4.74 (2.40, 11.24) | 5.47 (2.27, 8.30) | 3.14 (1.01, 4.43)* | 0.019 |
| Interleukin-6 (ng/mL) | 2.0 ± 0.6 | 2.1 ± 0.8 | 2.1 ± 0.7 | 2.3 ± 1.0 | 0.826 |
| MCP-1 (pg/mL) | 136 (124, 159) | 144 (127, 162) | 140 (122, 153) | 138 (129, 173) | 0.790 |
| WBC count (103/mL) | 6.8 ± 1.4 | 7.5 ± 1.5 | 7.4 ± 1.8 | 6.8 ± 1.3 | 0.056 |
Data are means ± SD for normally distributed variables or medians (quartile 1, quartile 3) for not normally distributed variables. The main effect of time was evaluated with repeated measures analysis of variance for normally distributed variables or Friedman’s test for not normally distributed variables. Significant effects of time were followed by simple contrasts to assess differences from baseline, and trend analysis to assess the linear, quadratic and cubic components of the overall time-related change.
P<0.05 vs. baseline;
P<0.05 for linear component and
P<0.05 for quadratic component.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; WBC, white blood cell.
Five percent weight loss improves multi-organ insulin sensitivity, and progressive weight loss has organ-specific and dose-dependent effects
Five percent weight loss did not affect standard measures of glycemic control evaluated by using the oral glucose tolerance test (OGTT), including the 2-h plasma glucose concentration and total glucose area-under-the-curve (AUC) (Table 3). In contrast, more sensitive measures of organ-specific insulin action, assessed by using a two-stage hyperinsulinemic-euglycemic clamp procedure in conjunction with infusion of stable isotopically labeled tracers, demonstrated an improvement in adipose tissue insulin sensitivity (insulin-mediated suppression of palmitate rate of appearance [Ra] in plasma), liver insulin sensitivity (insulin-mediated suppression of glucose Ra in plasma) and skeletal muscle insulin sensitivity (insulin-mediated stimulation of glucose rate of disappearance [Rd] from plasma) (Table 3). The improvements in insulin-mediated suppression of palmitate Ra and glucose Ra plateaued after 5% weight loss, whereas insulin-mediated stimulation of glucose Rd increased further with 11%–16% weight loss (Table 4).
Table 3.
Effect of 5% weight loss on multi-organ insulin sensitivity and β-cell function
| Weight maintenance (n = 14) | Weight loss (n = 19) | Interaction P-value |
|||
|---|---|---|---|---|---|
| Baseline | Weight maintenance | Baseline | 5% weight loss | ||
| Glucose, 2-hour (mg/dL) | 157 ± 29 | 151 ± 36 | 138 ± 22 | 141 ± 24 | 0.295 |
| Glucose AUC (mg/dL·min) | 18115 (15844, 19883) | 17876 (15237, 20352) | 16991 (15182, 19244) | 16692 (15612, 18084) | 0.889 |
| Insulin AUC (mU/L·min) | 12379 (10591, 17468) | 13912 (11919, 17370) | 12365 (8515, 18250) | 12554 (6952, 18063) | 0.346 |
| Insulin secretion rate AUC (pmol/L) | 34606 ± 5470 | 34468 ± 5658 | 31820 ± 9706 | 32700 ± 12059 | 0.717 |
| Φ-static (109/min) | 63.6 (45.4, 85.9) | 67.5 (49.1, 84.9) | 66.9 (48.8, 104.3) | 72.0 (56.1, 134.4) | 0.556 |
| Φ-dynamic (109) | 939 (700, 1975) | 1201 (803, 1599) | 1263 (733, 1934) | 1340 (822, 2214) | 0.750 |
| Φ-total (109/min) | 35.2 (32.2, 40.9) | 35.3 (33.5, 42.1) | 32.8 (27.2, 42.7) | 36.6 (27.9, 43.4) | 0.952 |
| Insulin clearance rate (pools/min) | 0.39 ± 0.10 | 0.36 ± 0.07 | 0.37 ± 0.08 | 0.41 ± 0.11* | 0.023 |
| β-cell functiona | 6555 ± 3768 | 6195 ± 3692 | 7102 ± 4321 | 9165 ± 3609*† | 0.026 |
| Basal palmitate Ra (μmol/kg FFM·min) | 2.0 (1.7, 2.2) | 2.1 (1.6, 2.8) | 2.1 (1.7, 2.7) | 1.9 (1.4, 2.3)* | 0.047 |
| Palmitate Ra suppression (%) | 53 ± 14 | 49 ± 13 | 52 ± 12 | 58 ± 14*† | 0.009 |
| Basal glucose Ra (μmol/kg FFM·min) | 15.7 ± 1.3 | 16.4 ± 2.3 | 16.4 ± 2.5 | 15.9 ± 2.0 | 0.088 |
| Glucose Ra suppression (%) | 70 (51, 76) | 64 (46, 74) | 71 (66, 76) | 75 (64, 83)*† | 0.026 |
| Basal glucose Rd (μmol/kg FFM·min) | 16.2 ± 1.3 | 16.9 ± 2.3 | 16.9 ± 2.6 | 16.4 ± 2.0 | 0.084 |
| Glucose Rd stimulation (%) | 161 (103, 236) | 139 (98, 216) | 185 (109, 248) | 235 (179, 421)*† | 0.002 |
Data are means ± SD for normally distributed variables or medians (quartile 1, quartile 3) for not normally distributed variables. The effect of time (before vs after) and differences between groups (weight maintenance vs weight loss) were evaluated with repeated measures analysis of variance for normally distributed variables or Friedman’s test for not normally distributed variables. Significant time-by-group interactions were followed by appropriate within- and between-group post-hoc tests.
P<0.05 vs. baseline and
P<0.05 vs. weight maintenance group after the intervention. There were no significant differences between groups at baseline.
An index of β-cell function was calculated as the product of Φ-total assessed during the oral glucose tolerance test and the relative increase in glucose Rd during high-dose insulin infusion (stage 2) of the clamp procedure.
Abbreviations: AUC, area under the concentration vs. time curve; Ra, rate of appearance; FFM, fat-free mass; Rd rate of disappearance.
Table 4.
Effect of progressive weight loss on multi-organ insulin sensitivity and β-cell
| Progressive weight loss (n = 9) | Effect of time P-value |
||||
|---|---|---|---|---|---|
| Baseline | 5% weight loss | 11% weight loss | 16% weight loss | ||
| Glucose, 2-hour (mg/dL) | 132.6 ± 24.6 | 136.8 ± 29.1 | 140.2 ± 24.0 | 143.8 ± 37.3 | 0.659 |
| Glucose AUC (mg/dL·min) | 16558 (15031, 18463) | 15790 (15190, 18785) | 17300 (15772, 19977) | 16665 (16211, 18812) | 0.182 |
| Insulin AUC (mU/L·min) | 12365 (9025, 21012) | 12950 (7352, 17370) | 11137 (7965, 17654) | 9534 (6548, 14417)* | 0.024 |
| Insulin secretion rate AUC (pmol/L) | 33269 ± 9105 | 33550 ± 9246 | 33952 ± 10172 | 31648 ± 8859 | 0.727 |
| Φ-static (109/min) | 67.1 (51.5, 113.4) | 59.7 (53.8, 118.7) | 70.5 (62.0, 74.8) | 69.4 (57.1, 84.4) | 0.865 |
| Φ-dynamic (109) | 1264 (735, 1861) | 1206 (769, 1985) | 1125 (981, 1605) | 1243 (769, 1390) | 0.435 |
| Φ-total (109/min) | 36.1 ± 9.3 | 35.7 ± 7.6 | 38.3 ± 9.1 | 36.7 ± 10.1 | 0.839 |
| Insulin clearance rate (pools/min)† | 0.36 ± 0.09 | 0.40 ± 0.12 | 0.41 ± 0.11 | 0.48 ± 0.14* | 0.016 |
| β-cell functiona | 6860 ± 4808 | 8130 ± 3565 | 10607 ± 2508* | 11107 ± 2666* | 0.003 |
| Basal palmitate Ra (μmol/kg FFM·min)† | 2.7 ± 1.1 | 2.4 ± 1.0 | 2.0 ± 0.5* | 1.9 ± 0.4* | 0.022 |
| Palmitate Ra suppression (%)†‡ | 53 ± 12 | 63 ± 14* | 67 ± 11* | 62 ± 15* | 0.006 |
| Basal glucose Ra (μmol/kg FFM·min) | 16.8 ± 2.3 | 16.3 ± 2.3 | 16.4 ± 2.8 | 16.1 ± 1.3 | 0.573 |
| Glucose Ra suppression (%)† | 71 ± 13 | 77 ± 10* | 76 ± 11* | 80 ± 6* | 0.028 |
| Basal glucose Rd (μmol/kg FFM·min) | 17.2 ± 2.3 | 16.7 ± 2.3 | 16.8 ± 2.8 | 16.5 ± 1.3 | 0.530 |
| Glucose Rd stimulation (%)† | 168 (94, 297) | 207 (149, 306)* | 326 (233, 379)* | 311 (248, 388)* | 0.009 |
Data are means ± SD for normally distributed variables or medians (quartile 1, quartile 3) for not normally distributed variables. The main effect of time was evaluated with repeated measures analysis of variance for normally distributed variables or Friedman’s test for not normally distributed variables. Significant effects of time were followed by simple contrasts to assess differences from baseline, and trend analysis to assess the linear, quadratic and cubic components of the overall time-related change.
P<0.05 vs. baseline;
P<0.05 for linear component and
P<0.05 for quadratic component.
An index of β-cell function was calculated as the product of Φ-total assessed during the oral glucose tolerance test and the relative increase in glucose Rd during high-dose insulin infusion (stage 2) of the clamp procedure.
Abbreviations: AUC, area under the concentration vs. time curve; Ra, rate of appearance; FFM, fat-free mass; Rd rate of disappearance.
These data show that the relationship between weight loss and improvement in insulin sensitivity is organ-specific; maximal benefits in insulin-mediated suppression of hepatic glucose production and adipose tissue lipolytic activity occur after 5% weight loss, whereas insulin-stimulated muscle glucose uptake continues to increase with greater amounts of weight loss. In addition, these results help clarify the relationship between weight loss and skeletal muscle insulin sensitivity. The minimum amount of weight loss needed to increase skeletal muscle insulin sensitivity has been unclear because of conflicting data from different studies reporting either no change or increased insulin-stimulated glucose uptake after 6%–8% weight loss (Kirk et al., 2009; Petersen et al., 2005; Petersen et al., 2012). However, those studies were conducted in small numbers of subjects, which might have limited their ability to detect statistically significant effects. Our results, obtained from a much larger group, demonstrate that 5% weight loss increases insulin-stimulated glucose uptake by ~25% in people who are obese and have some degree of insulin resistance but not diabetes. Furthermore, skeletal muscle insulin sensitivity continued to increase with greater weight loss. Although a maximal two-fold increase in insulin-stimulated glucose uptake was observed after 11%–16% weight loss in our subjects, we cannot exclude the possibility that greater weight loss would result in even greater improvement. However, we previously found that 20% weight loss induced by bariatric surgery also caused a two-fold increase in insulin-stimulated glucose uptake (Bradley et al., 2012), suggesting that most of the beneficial effect of weight loss on muscle insulin action in people occurs after only an 11%–16% decline in body weight.
Weight loss increases insulin clearance and improves β-cell function
Five percent weight loss significantly increased insulin clearance rate but did not affect indices of insulin secretion, determined by modeling the data from the OGTT, including insulin concentration AUC, insulin secretion rate AUC, and β-cell responsivity (Φ-dynamic, which is a measure of insulin secretion in response to the rate of change in glucose concentration; Φ-static, which is a measure of insulin secretion in response to a given glucose concentration; and Φ-total, which is a measure of the total insulin secretory response) (Table 3). However, 5% weight loss improved overall β-cell function, determined by an assessment of insulin secretion in response to glucose ingestion in relationship to insulin sensitivity (product of Φ-total during the OGTT and the relative increase in insulin-stimulated glucose disposal during the hyperinsulinemic euglycemic clamp procedure) (Table 3). Progressive weight loss decreased insulin AUC during the OGTT after 16% weight loss, but did not affect the insulin secretory response to plasma glucose (Φ-static, Φ-dynamic, and Φ-total indices of β-cell responsivity and total insulin secretion rate AUC) (Table 4). These results suggest the decrease in plasma insulin was primarily due to an increase in insulin clearance, not a decrease in insulin secretion. The overall index of β-cell function increased with progressive weight loss in concert with the improvement in muscle insulin sensitivity (Table 4).
The assessment of overall β-cell function is more informative than simply measuring the insulin secretory response alone, because the insulin secretion rate and plasma insulin concentration needed to maintain normal glucose homeostasis depends on a person’s sensitivity to insulin (Bergman et al., 2002). Accordingly, a lower plasma insulin concentration is needed to maintain normal glucose homeostasis in insulin-sensitive than in insulin-resistant people, and a compensatory increase in insulin secretion can maintain normal glucose homeostasis in those who are insulin-resistant. The improvement in overall β-cell function observed in our subjects was primarily due to an increase in insulin sensitivity without a significant change in the insulin secretory response to plasma glucose. However, plasma insulin concentrations in response to glucose ingestion decreased with progressive weight loss because of a progressive increase in insulin clearance. Weight loss-induced changes in β-cell function have important clinical implications in preventing and treating type 2 diabetes: impaired β-cell function is an important risk factor for future development of type 2 diabetes (Lorenzo et al., 2010), and an improvement in β-cell function after weight loss induced by Roux-en-Y gastric bypass surgery is an important determinant of which patients will achieve diabetes remission (Khanna et al., 2015; Lund et al., 2015).
Five percent weight loss does not affect systemic or subcutaneous adipose tissue markers of inflammation, but progressive weight loss causes progressive changes in subcutaneous adipose tissue metabolic pathways involved in regulating lipid metabolism, extracellular matrix remodeling, and oxidative stress
Markers of inflammation in the systemic circulation (plasma concentrations of interleukin-6 [IL-6], C-reactive protein [CRP] and white blood cell [WBC] count) and in subcutaneous adipose tissue (gene expression of IL-6, monocyte chemoattractant protein-1 [MCP1] and CD68) were increased in subjects with obesity compared with a group of lean adults that we studied previously (Yoshino et al., 2012) (Supplemental Table S1 and Supplemental Figure S3). However, 5% weight loss did not decrease the plasma concentrations of several circulating inflammatory markers, such as IL-6, MCP1, CRP or WBC count (Table 1), and did not significantly alter, or tended to increase, subcutaneous adipose tissue gene expression of the pro-inflammatory cytokines IL-6 and tumor necrosis factor (TNF), major chemokines (MCP1 and regulated on activation normal T cell expressed and secreted [RANTES]) and macrophage markers (CD68 and EMR1) (Figure 1).
Figure 1. Effect of 5% weight loss on subcutaneous adipose tissue gene expression of inflammatory markers.
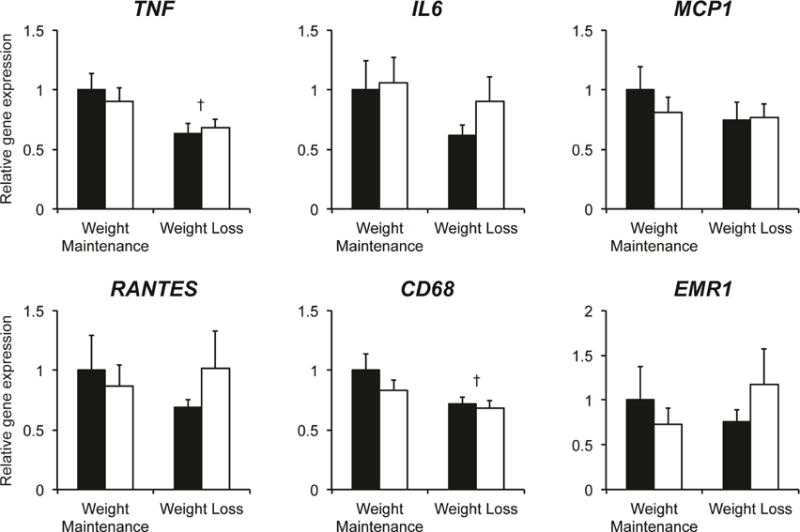
Subcutaneous abdominal adipose tissue expression of genes involved in inflammation was determined by real-time PCR before (black bars) and after (white bars) 5% weight loss (n = 19) or weight maintenance (n = 12). The effect of time (before vs. after) and differences between groups (weight maintenance vs. weight loss) were evaluated by using repeated measures analysis of variance. Significant time-by-group interactions were followed by appropriate within-and between-group post-hoc tests. Non-normally distributed variables were log transformed for analysis and back transformed for presentation. Data are means ± SEM. No effects of weight loss were detected. †P<0.05 vs. weight maintenance group before and after the intervention. Abbreviations: TNF, tumor necrosis factor; IL6, interleukin 6; MCP1, monocyte chemoattractant protein 1; RANTES, regulated on activation normal T cell expressed and secreted; CD68, cluster of differentiation 68; EMR1, EGF-like module-containing mucin-like hormone receptor-like 1.
Parametric analysis of gene-set enrichment (PAGE) was performed on microarray data to determine global transcriptional changes in abdominal subcutaneous adipose tissue induced by progressive weight loss (Figure 2A and Supplemental Table S2). Biological pathways related to lipid flux (e.g. REACTOME_HDL_MEDIATED_LIPID_TRANSPORT and REACTOME_LIPOPROTEIN_METABOLISM) were significantly up-regulated by weight loss, whereas numerous pathways related to lipid synthesis (e.g. KEGG_BIOSYNTHESIS_OF_UNSATURATED_FATTY_ACIDS and REACTOME_TRIGLYCERIDE_BIOSYNTHESIS), extracellular matrix (ECM) remodeling (e.g. NABA_MATRISOME, REACTOME_CELL_EXTRACELLULAR_MATRIX_INTERACTIONS and KEGG_FOCAL_ADHESION), and oxidative stress (e.g. OXIDOREDUCTASE_ACTIVITY and RESPONSE_TO_OXIDATIVE_STRESS) were markedly down-regulated by weight loss (Figure 2B). Consistent with these alterations in biological pathways, progressive weight loss caused a progressive increase in subcutaneous adipose tissue expression of genes involved in cholesterol flux (ABCG1, ABCA1, APOE, and CETP) and a progressive decrease in adipose tissue expression of genes involved in lipid synthesis (SCD, FADS1, FADS2, and ELOVL6), ECM remodeling (SPARC, MAFP5, LOX, LOXL2, ANGPT1, and ADAM12), and oxidative stress (NQO1, DHCR24 and UCHL1) (Figure 2C). In addition, progressive weight loss moderately decreased (COL3A1) or did not affect (COL1A1 and COL6A1) gene expression of ECM structural markers and decreased gene expression of selected markers of adipogenesis (PPARG and CEBPA) in subcutaneous adipose tissue (Supplemental Figures S4A and S4B, respectively). There was also a trend towards an increase in subcutaneous adipose tissue biological pathways involved in immune function and inflammation (e.g. REACTOME_ADAPTIVE_IMMUNE_SYSTEM, REACTOME_IMMUNE_SYSTEM, IMMUNE_SYSTEM_PROCESS) after 5% weight loss, followed by a subsequent decline in these pathways with progressive weight loss and a significant decrease after 16% weight loss (Supplemental Figure S4C). Progressive weight loss tended to down-regulate subcutaneous adipose tissue expression of genes involved in inflammation after 11%–16% weight loss (Supplemental Figure S4D).
Figure 2. Effect of progressive weight loss on subcutaneous adipose tissue gene expression profile.
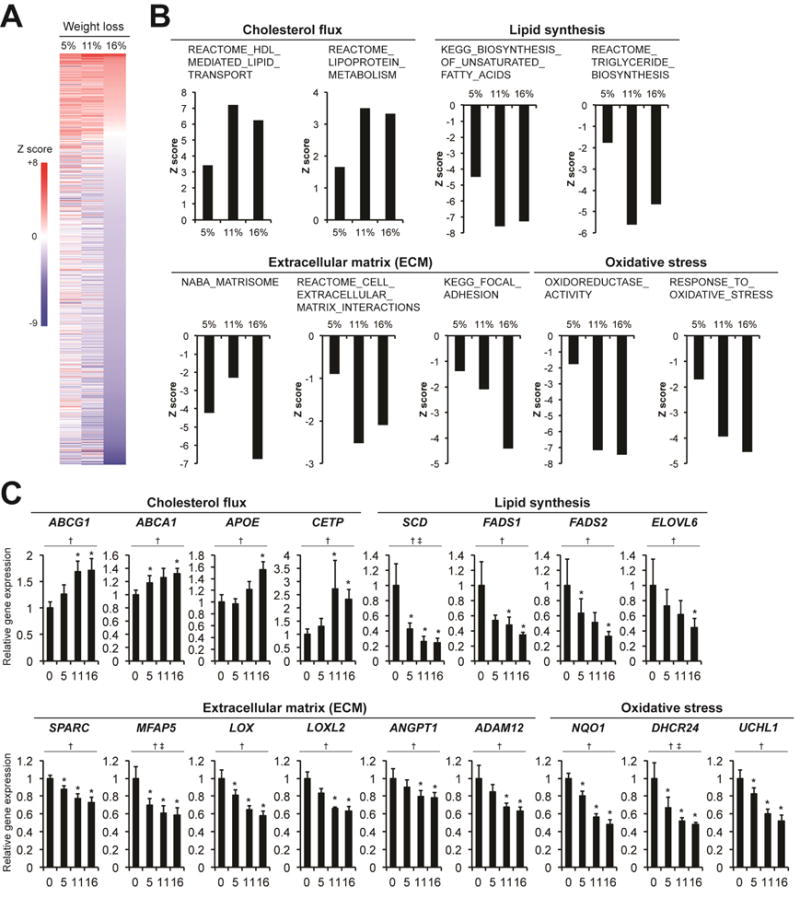
Parametric analysis of gene-set enrichment (PAGE) was performed on microarray data to identify biological pathways in subcutaneous abdominal adipose tissue that increased (red) or decreased (blue) with progressive weight loss in subjects with obesity (n = 9). Biological pathways that were significantly affected by 5%, 11%, or 16% weight loss, based on the Z score between baseline (before weight loss) and 16% weight loss (A). Biological pathways involved in regulating cholesterol flux were significantly up-regulated, and pathways involved in lipid synthesis, regulating extracellular matrix (ECM) remodeling, and oxidative stress were significantly down-regulated by progressive weight loss (B). Subcutaneous abdominal adipose tissue expression of genes involved in regulating cholesterol flux, synthesis, ECM remodeling, and oxidative stress was determined by real-time PCR before (0) and after progressive 5% (5), 11% (10), and 16% (15) weight loss (C). The main effect of time was evaluated with repeated measures analysis of variance, which revealed significant linear changes for all genes. Non-normally distributed variables were log transformed for analysis and back transformed for presentation. Data are means ± SEM. *P<0.05 vs. baseline; †P<0.05 for linear component and ‡P<0.05 for quadratic component.
Abbreviations: ABCG1, ATP-binding cassette sub-family G member 1; ABCA1, ATP-binding cassette transporter ABCA1; APOE, apolipoprotein E; CETP, cholesteryl ester transfer protein; SCD, stearoyl-CoA desaturase; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; ELOVL6, elongation of very long chain fatty acids protein 6; SPARC, secreted protein acidic and rich in cysteine; MFAP5, microfibrillar-associated protein 5; LOX, lysyl oxidase; LOXL2, lysyl oxidase homolog 2; ANGPT1, angiopoietin 1; ADAM12, disintegrin and metalloproteinase domain-containing protein 12; NQO1, NAD(P)H dehydrogenase, quinone 1; DHCR24, 24-dehydrocholesterol reductase; UCHL1, ubiquitin carboxyl-terminal esterase L1.
Our findings demonstrate that adipose tissue is a dynamic organ that is extraordinarily responsive to diet-induced weight loss. Adipose tissue biological pathways and genes involved in cholesterol flux progressively increased, whereas those involved in lipid synthesis, ECM remodeling and oxidative stress progressively decreased, with continued 5% through 16% weight loss. Data from studies conducted in rodent models demonstrate that adipocyte-specific genetic manipulation of many of the same metabolic pathways that were affected by diet-induced weight loss in our subjects, namely the cholesterol transporter ABCA1 (de Haan et al., 2014), regulators of cellular oxidative stress (Chutkow et al., 2010; Xue et al., 2013), and production of ECM components (Halberg et al., 2009; Sun et al., 2013), can influence insulin sensitivity and whole-body glucose and lipid metabolism. Moreover, it was recently shown that increased adipose tissue markers of oxidative stress are associated with high-calorie diet-induced insulin resistance in people (Boden et al., 2015). Although our study cannot determine whether the weight loss-induced changes in adipose tissue biological pathways contributed to the improvement observed in multi-organ insulin sensitivity, our data support the mechanistic links between whole-body metabolic function and adipose tissue cholesterol transport, ECM formation and oxidative stress identified in animal models.
People with obesity and metabolic abnormalities often have concomitant systemic and adipose tissue “inflammation,” manifested by increased circulating inflammatory proteins and white blood cells, and increased subcutaneous adipose tissue macrophages, pro-inflammatory CD4+ T-lymphocytes, and increased gene expression of pro-inflammatory cytokines and chemokines (Fabbrini et al., 2013; Weisberg et al., 2003). Although a mechanistic relationship between inflammation and metabolic dysfunction has been demonstrated in rodent models (Berg and Scherer, 2005; Brestoff and Artis, 2015; Ferrante, 2007; Hotamisligil, 2006; Hotamisligil et al., 1995; Sun et al., 2013), the importance of low-grade inflammation in the pathogenesis of obesity-related insulin resistance in people is not clear, and moderate 5–6% weight gain decreases insulin sensitivity in people without increasing systemic or subcutaneous adipose tissue markers of inflammation (Boden et al., 2015; Fabbrini et al., 2015). Therefore, we sought to evaluate the relationship between moderate 5% weight loss and progressively greater amounts of weight loss on the relationship between metabolic function and both systemic and adipose tissue markers of inflammation. Our data demonstrate that the improvement in multi-organ insulin sensitivity after 5% weight loss was not accompanied by an improvement in either systemic or subcutaneous adipose tissue markers of inflammation. However, 11%–16% weight loss was associated with a reduction in both systemic and subcutaneous adipose tissue inflammation. This biphasic adipose tissue immune response to weight loss observed in our subjects is consistent with the early increase and subsequent decrease in adipose tissue macrophage content observed after calorie restriction and weight loss in obese mice (Kosteli et al., 2010). In fact, it is possible that a moderate increase in adipose tissue inflammation during early weight loss provides a beneficial adaptive response to energy restriction, because adipose tissue inflammation could be required for appropriate adipose tissue remodeling (Rutkowski et al., 2015; Wernstedt Asterholm et al., 2014). Together, these findings suggest that the beneficial effect of 5% weight loss on insulin action is not mediated by a reduction in subcutaneous adipose tissue inflammation. However, we did not evaluate potential alterations in inflammation in other adipose tissue depots, such as intra-abdominal fat, or evaluate the potential paracrine effects of adipose tissue cytokines that would not be detected by our study methods. Moreover, we cannot exclude the possibility that decreased adipose tissue inflammation contributes to the improvement in insulin sensitivity observed with greater weight loss, e.g. after bariatric surgery-induced 20% weight loss that has been associated with decreased gene expression of pro-inflammatory cytokines and macrophage number in adipose tissue of people with obesity (Bradley et al., 2012; Cancello et al., 2005; Moschen et al., 2010).
PERSPECTIVE
Although 5% to 10% weight loss is a commonly recommended therapeutic target for people with obesity (Jensen et al., 2014), the differences between 5% and 10% weight loss and the effects of additional diet-induced weight loss on body composition, adipose tissue biology, and cardiometabolic health outcomes are not clear. Therefore, we conducted a randomized controlled trial to determine: 1) the effects of 5% weight loss on metabolic function and both systemic and subcutaneous adipose tissue markers of inflammation; and 2) the effects of subsequent progressive weight loss on body composition, metabolic function, and global adipose tissue gene expression profile. The major findings from our study demonstrate that 5% weight loss improves multi-organ (adipose tissue, liver and skeletal muscle) insulin sensitivity, β-cell function, and multiple risk factors for cardiometabolic disease. These therapeutic effects occurred without a concomitant change in systemic or subcutaneous adipose tissue markers of inflammation, demonstrating that improvement of these selected markers of inflammation is not necessary for weight loss-induced improvements in metabolic function. Progressive 11% and 16% weight loss caused stepwise reductions in body fat mass, IAAT volume and IHTG content, progressive changes in adipose tissue biology (i.e. up-regulation of metabolic pathways and genes involved in cholesterol flux and down-regulation of metabolic pathways and genes involved in lipid synthesis, ECM remodeling and oxidative stress), further improvement in skeletal muscle, but not liver or adipose tissue, insulin sensitivity, and continued improvement in β-cell function.
The results from the present study demonstrate the profound therapeutic effects of weight loss on metabolic function and other risk factors for cardiometabolic disease in people with obesity. Even a moderate 5% weight loss has considerable health benefits, including decreased IAAT volume, IHTG content, systolic blood pressure and plasma triglyceride concentration and increased multi-organ insulin sensitivity and β-cell function. Additional weight loss further improves many cardiometabolic outcomes and has a progressive effect on adipose tissue expression of genes involved in cholesterol flux, lipid synthesis, ECM remodeling and oxidative stress. Future studies are needed to determine whether the weight loss-induced changes in adipose tissue biology contribute to the observed beneficial effects on cardiometabolic outcomes.
EXPERIMENTAL PROCEDURES
Study Subjects
Forty sedentary (<2 hours of exercise/week) men and women (44 ± 12 years old) who were obese (body mass index = 37.9 ± 4.3 kg/m2) participated in this study (ClinicalTrials.gov, NCT01299519). All subjects completed a screening history and physical examination, a resting electrocardiogram, and standard blood tests. Subjects with obesity had evidence of multi-organ insulin resistance [based on a homeostasis model assessment of insulin resistance score >2.0 (Levy et al., 1998) and results of a hyperinsulinemic-euglycemic clamp procedure conducted in conjunction with infusion of stable isotopically labeled tracers] and an increase in both systemic and adipose tissue markers of inflammation (compared with a group of lean adults that we studied previously) (Yoshino et al., 2012) (Supplemental Table S1 and Supplemental Figure S3). No subject had evidence of serious illness or organ dysfunction (e.g., diabetes), were taking medications that could interfere with insulin action, consumed excessive alcohol (>14 drinks/week for women and >21 drinks/week for men) or smoked tobacco products. This study was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis, MO, and written informed consent was obtained from all subjects before their participation.
Study Design
Subjects were randomly assigned to weight loss (n = 20) or weight maintenance (n = 20) therapy. Subjects in both groups participated in a lifestyle intervention program that included weekly individual behavior education sessions and dietary counseling. Initial dietary recommendations were based on an estimate of each subject’s total daily energy expenditure (1.5 × measured resting energy expenditure, assessed by using an automated metabolic measurement system [TrueOne 2400, ParvoMedics, Salt Lake City, UT]) to help ensure subjects in the weight maintenance group maintained the same body weight for 6 months, and that subjects in the weight loss group achieved their weight loss targets. All subjects were able to achieve a 5% weight loss by consuming a low-calorie diet of self-prepared foods. Solid and liquid meal replacements were provided to participants, as needed, to achieve the 10% (11% actual weight loss) and 15% (16% actual weight loss) weight loss targets. The precise intervention was individualized based on the judgment of the study dietitian and behavioral psychologist after discussion with the participant. More details about the lifestyle intervention program are provided in the Supplement. All 20 subjects in the weight loss group were required to lose 5% of their weight; half (n=10) were assigned to continue to lose ~10% and then ~15% of their initial body weight. After subjects achieved each weight loss target, a weight maintenance diet was prescribed to maintain a stable body weight (<2% change) for at least 3 weeks before repeat testing was performed. Subjects were studied at baseline and after 6 months in the weight maintenance group and after targeted weight loss in the weight loss group.
Experimental Procedures
Body composition
Body fat mass and FFM were determined by dual energy x-ray absorptiometry, IAAT volume by magnetic resonance imaging, and IHTG content by magnetic resonance spectroscopy (Fabbrini et al., 2015).
24-hour ambulatory blood pressure and heart rate
Subjects were fitted with a portable blood pressure recording device (Ultralite 90217 monitor, Spacelabs Healthcare, Snoqualmie, WA) to monitor 24-h ambulatory blood pressure and heart rate (every 20 min from 0600 h to 2400 h, and every hour from 2400 h to 0600 h).
Oral glucose tolerance test
After subjects fasted for 12 hr overnight, they were admitted to the Clinical Research Unit at 0700 h. An intravenous catheter was placed into a hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized venous blood samples. After three blood samples were obtained at 5 min intervals, subjects ingested a 75 g glucose drink, and additional blood samples were collected at 10, 20, 30, 60, 90 and 120 min after glucose ingestion to determine plasma glucose, insulin, and C-peptide concentrations.
Hyperinsulinemic-euglycemic clamp procedure and adipose tissue biopsies
Subjects were admitted to the Clinical Research Unit in the afternoon and consumed a standard evening meal. After subjects fasted for 12 hr overnight, a 10.5-hr, two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled tracer infusions and subcutaneous abdominal adipose tissue biopsies from the periumbilical area, were performed as previously described (Fabbrini et al., 2015).
Sample Analyses and Calculations
Real-time PCR
Total RNA was isolated from frozen subcutaneous adipose tissue samples by using QIAzol and RNeasy mini kit (Qiagen, Valencia, CA). Gene expression was determined by using an ABI 7500 real-time PCR system (Invitrogen, Carlsbad, CA) and SYBR Green Master Mix (Invitrogen) as previously described (Fabbrini et al., 2015; Yoshino et al., 2014). The expression of each gene was determined by normalizing the cycle threshold value of each sample to the housekeeping control gene, ribosomal protein (36B4). Primer details are listed in Supplemental Table S3.
Microarray
Microarray analyses were performed with the GeneChip Human Gene 1.0 ST array (Affymetrix, Santa Clara, CA, USA). To identify biological pathways that were significantly altered by weight loss, normalized data were subjected to parametric analysis of gene set enrichment (PAGE) as previously described (Fabbrini et al., 2015; Pearson et al., 2008; Yoshino et al., 2011). Canonical pathway and GO gene sets used in PAGE were obtained from http://www.broad.mit.edu/gsea/msigdb/msigdb_index.html (C2: curated gene sets collection and C5: GO gene sets collection). Z scores and P-values were calculated for each gene set. Other sample analyses and calculations used to evaluate metabolic function are available in the Supplement.
Statistical Analyses
Multi-organ insulin sensitivity and intrahepatic triglyceride content were the primary outcomes of our study; other components of body composition and other metabolic variables were secondary outcomes; and adipose tissue gene expression was an exploratory outcome. The effect of weight loss and differences between groups were evaluated by using repeated measures analysis of variance (RANOVA) for normally distributed variables or Friedman’s test for not normally distributed variables. Significant time-by-group interactions in the statistical analysis of the effects of 5% weight loss, and significant main effects of time in the statistical analysis of the effects of progressive weight loss, were followed by appropriate between- and within-group post-hoc tests to adjust for multiple comparisons. Results are shown as means ± SD for normally distributed variables or medians (quartile 1, quartile 3) for not normally distributed variables, unless otherwise indicated. Based on the inter-individual variability of insulin sensitivity we have observed previously (Magkos et al., 2011), we estimated that 8 subjects per group would be needed to detect between-group differences of ≥19% in hepatic, ≥25% in adipose tissue, and ≥29% in skeletal muscle insulin sensitivity, with a power of 0.8 and an α value of 0.05. Therefore, we estimated that 15–20 subjects would need to be recruited in each group to ensure that an adequate number of subjects completed the study. Fewer subjects would be needed to detect differences of similar magnitude within the same group (Magkos et al., 2011).
Supplementary Material
Highlights.
Moderate 5% weight loss improves multi-organ insulin sensitivity and β-cell function
Additional weight loss of 11%–16% further increases insulin sensitivity in muscle
Progressive weight loss causes stepwise changes in adipose tissue biology
Acknowledgments
This study was supported by National Institutes of Health grants DK 37948, DK 104995, DK 56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center) and RR024992 (Clinical and Translational Science Award), a KL2 Career Development Award (TR 000450), and grants from the Pershing Square Foundation and the Longer Life Foundation.
The authors thank Freida Custodio and Jennifer Shew for technical assistance, the research coordinators of the Center for Human Nutrition and the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: ClinicalTrials.gov, NCT01299519
AUTHOR CONTRIBUTIONS
Conceptualization, S.K.; Methodology, S.K.; Validation, S.C.K., A.L.O., and B.W.P.; Investigation, F.M., G.F., C.L., K.K., and S.H.; Formal Analysis, F.M. and J.Y.; Writing – Original Draft, F.M.; Writing – Review & Editing, G.F., J.Y, C.L., S.C.K., K.K., L.d.l.F., S.H., A.L.O., B.W.P, and S.K.; Supervision, F.M., J.Y. and S.K.; Resources, S.K.; Funding Acquisition, S.K.
ACCESSION NUMBERS
All microarray data used in this study have been deposited into the NCBI GEO database under accession number GSE70529.
SUPPLEMENTAL INFORMATION
The online Supplement includes four figures, three tables, and additional experimental procedures.
CONFLICT OF INTEREST STATEMENT
S. Klein is a shareholder of Aspire Bariatrics and has served on scientific advisory boards for Takeda Pharmaceuticals and NovoNordisk. None of the other authors have any conflicts of interest relevant to this manuscript.
References
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation research. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, Fecchio C, Koller S, Merali S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Science translational medicine. 2015;7:304re307. doi: 10.1126/scitranslmed.aac4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122:4667–4674. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Capel F, Klimcakova E, Viguerie N, Roussel B, Vitkova M, Kovacikova M, Polak J, Kovacova Z, Galitzky J, Maoret JJ, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58:1558–1567. doi: 10.2337/db09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Birkenfeld AL, Brown JD, Lee HY, Frederick DW, Yoshioka J, Patwari P, Kursawe R, Cushman SW, Plutzky J, et al. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- Dahlman I, Linder K, Arvidsson Nordstrom E, Andersson I, Liden J, Verdich C, Sorensen TI, Arner P. Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am J Clin Nutr. 2005;81:1275–1285. doi: 10.1093/ajcn/81.6.1275. [DOI] [PubMed] [Google Scholar]
- de Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. Journal of lipid research. 2014;55:516–523. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–374. e361–363. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, Fraterrigo G, Okunade AL, Patterson BW, Klein S. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. Journal of internal medicine. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Johansson LE, Danielsson AP, Parikh H, Klintenberg M, Norstrom F, Groop L, Ridderstrale M. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. Am J Clin Nutr. 2012;96:196–207. doi: 10.3945/ajcn.111.020578. [DOI] [PubMed] [Google Scholar]
- Khanna V, Malin SK, Bena J, Abood B, Pothier CE, Bhatt DL, Nissen S, Watanabe R, Brethauer SA, Schauer PR, et al. Adults with long-duration type 2 diabetes have blunted glycemic and beta-cell function improvements after bariatric surgery. Obesity (Silver Spring) 2015;23:523–526. doi: 10.1002/oby.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MT, Hansen M, Skaaby S, Dalby S, Stockel M, Floyd AK, Bech K, Helge JW, Holst JJ, Dela F. Preoperative beta-cell function in patients with type 2 diabetes is important for the outcome of Roux-en-Y gastric bypass surgery. The Journal of physiology. 2015;593:3123–3133. doi: 10.1113/JP270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes (Lond) 2011;35:1233–1240. doi: 10.1038/ijo.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisova L, Rossmeislova L, Kovacova Z, Kracmerova J, Tencerova M, Langin D, Siklova-Vitkova M, Stich V. Expression of inflammation-related genes in gluteal and abdominal subcutaneous adipose tissue during weight-reducing dietary intervention in obese women. Physiol Res. 2014;63:73–82. doi: 10.33549/physiolres.932537. [DOI] [PubMed] [Google Scholar]
- Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Morino K, Yoo PS, Cline GW, Shulman GI. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci U S A. 2012;109:8236–8240. doi: 10.1073/pnas.1205675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. The Journal of cell biology. 2015;208:501–512. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola E, Jover A, Lopez-Ruiz A, Jarabo M, Vaya A, Morillas C, Gomez-Balaguer M, Hernandez-Mijares A. Parameters of inflammation in morbid obesity: lack of effect of moderate weight loss. Obes Surg. 2009;19:571–576. doi: 10.1007/s11695-008-9772-8. [DOI] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749–1753. [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue P, Hou Y, Chen Y, Yang B, Fu J, Zheng H, Yarborough K, Woods CG, Liu D, Yamamoto M, et al. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes. 2013;62:845–854. doi: 10.2337/db12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Almeda-Valdes P, Patterson BW, Okunade AL, Imai S, Mittendorfer B, Klein S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab. 2014;99:E1666–1670. doi: 10.1210/jc.2014-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


