Abstract
Background
Osteosarcoma is the most common primary malignant bone tumor in many countries, with metastatic disease responsible for most patient deaths. This study compares the prevalence of metastatic osteosarcoma at diagnosis across countries to inform the critical question of whether diagnostic delay or tumor biology drives metastases development prior to diagnosis.
Procedure
A literature search of the PubMed database was conducted to compare the prevalence of metastatic disease at the time of OS diagnosis between countries. A pooled prevalence with 95% confidence intervals was calculated for each study meeting inclusion criteria. Studies were grouped for analysis based on human development index (HDI) scores.
Results
Our analysis found an 18% (95% CI: 15%, 20%) average global pooled proportion of metastasis at osteosarcoma diagnosis. The average prevalence of metastasis at diagnosis increased as HDI groupings decreased, with very high HDI, high HDI, and medium/ low HDI groups found to be 15% (95% CI: 13%, 17%), 20% (95% CI: 14%, 28%), and 31% (95% CI: 15%, 52%), respectively.
Conclusions
Our evidence suggests there is a biological baseline for metastatic OS at diagnosis, which is observed in countries with very high HDI. In countries with medium/ low HDI, where there are more barriers to accessing healthcare, the higher prevalence of metastasis may result from treatment delay or an artificial prevalence inflation due to patients with less severe symptoms not presenting to clinic. Additional research in countries with medium/ low HDI may reveal that earlier detection and treatment could improve patient outcomes in those countries.
Keywords: osteosarcoma, metastasis, bone cancer, epidemiology, human development index
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumor in many countries, [1–3] with a peak in adolescence and often a second smaller peak starting in the sixth decade of life. [3–6] Despite combined therapies, treatment failure is experienced within 5 years of diagnosis by over 40% of patients, generally due to metastatic disease developed before or after diagnosis. [7] Survival has not improved substantially over the past 30 years, [5] and metastatic OS is usually incurable and requires palliation. Older age, [6, 8, 9] axial tumor location, [6, 8–15] and tumor size [1, 9, 14] have been reported by a number of research groups to increase risk of metastatic disease and worsen survival outcomes. The incidence of OS is fairly constant worldwide, particularly among individuals ≤24 years, [4] but an international comparison of the prevalence of metastasis at diagnosis has not been compiled. The purpose of this study is to compare prevalence of metastatic OS at diagnosis across countries to inform the critical question of whether diagnostic delay or tumor biology drives metastases development prior to diagnosis.
Methods
International Literature Search
A literature search of the PubMed database was conducted to compare the prevalence of metastatic disease at the time of OS diagnosis between countries. All fields were searched for the terms osteosarcoma/ osteogenic sarcoma/ bone sarcoma AND metastases/ metastasis/ metastatic, yielding 9595 papers (last search conducted on May 19, 2015). Titles were screened and abstracts reviewed for single-institutional, multi-institutional, and population-based studies within a single continent that reported the prevalence of metastatic disease at diagnosis of high-grade, skeletal OS in a minimum of 20 patients. Papers were only included if information was available for all OS patients treated at a clinic(s) over the given period of data collection. Key phrases indicating a study fell into this search criteria were “all patients” and “consecutive patients.” Exceptions were made for publications reporting on patients of a specific age at diagnosis. Only those studies with a publication date of 1980 or later were considered. When studies with significantly overlapping datasets were encountered, to the best we could discern, the study covering the largest data collection period was included for analysis.
Effort was made to include papers written in any language. Seven papers were not available in English that were identified as possible candidates for our study. Three could not be readily translated and were excluded from analysis because data could not be extracted.
Several studies reported the prevalence of metastasis at OS diagnosis from single institutions in the United States. However, since Duong and Richardson provided a large, nationwide report using the Surveillance, Epidemiology, and End Results Program database in conjunction with the National Program of Cancer Registries’ central cancer registries,[16]this study was used to evaluate United States data.
Statistical Analysis
The summary statistic for each study is a prevalence proportion, calculated as the ratio of the number of individuals presenting with metastasis to the sample size of the study. A random-effects model with inverse-variance weighting was used to calculate a pooled prevalence and 95% confidence intervals (CI). [17] Statistical heterogeneity was evaluated with the Cochran’s Q statistic[18] and quantified using an I2 statistic.[19]The United Nations’ human development index (HDI) value for each country, which is based on the population’s average life expectancy, years of schooling, and gross national income, was used to group studies. Studies were categorized into groupings defined by the United Nations as very high HDI, high HDI, and medium/ low HDI. For multi-institutional studies that included countries from multiple HDI groups, the HDI group from which the majority of patients were seen was chosen.
Subgroup analysis was performed to account for heterogeneity. Subgroups were categorized from very high HDI studies into general age groupings: pediatric (upper age no greater than 18 years), adult (no pediatric cases), and mixed ages (all patients seen at a clinic that included pediatric and adult populations). A subgroup analysis was not performed from high HDI and medium/ low HDI studies because each had an insufficient number of studies restricted to either pediatric or adult populations for a pooled subgroup analysis. All meta-analysis was performed using R version 3.2.1. [20]
Results
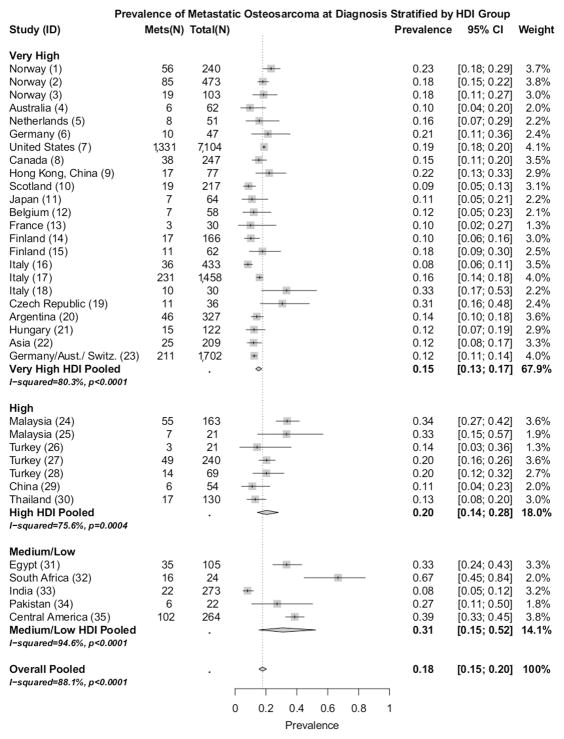
Thirty-five studies met the inclusion criteria (Table I): very high HDI (n=23), [1, 6, 8, 10, 13–16, 21–35] high HDI (n=7),[3, 11, 12, 36–39]and medium/ low HDI (n=5). [2, 40–43] Figure 1 depicts the prevalence of metastatic OS at diagnosis stratified by HDI group. The pooled proportion of patients presenting with metastatic OS at diagnosis in very high HDI, high HDI, and medium/ low HDI groups were found to be 15% (95% CI: 13%, 17%), 20% (95% CI: 14%, 28%), and 31% (95% CI: 15%, 52%), respectively. All 35 studies pooled together gave a global proportion of 18% (95% CI: 15%, 20%).
Table I.
Prevalence of Metastatic Osteosarcoma at Diagnosis Stratified by HDI Group
| ID | Country | HDI Value | HDI Group | Centers/Registries | Collection Period | Total Patients | Age Group |
|---|---|---|---|---|---|---|---|
| 1 | Norway | 0.944 | Very High | Norwegian Cancer Registry | 1953–1977 | 240 | Mixed Age |
| 2 | Norway | 0.944 | Very High | Norwegian Cancer Registry | 1975–2009 | 473 | Mixed Age |
| 3 | Norway | 0.944 | Very High | Norwegian Radium Hospital, Oslo | 1981–1995 | 103 | Mixed Age |
| 4 | Australia | 0.935 | Very High | Royal Prince Alfred Hospital, Camperdown | 1979–1994 | 62 | Adult |
| 5 | Netherlands | 0.922 | Very High | Nijmegen University Hospital, Nijmegen | 1974–1996 | 51 | Mixed Age |
| 6 | Germany | 0.916 | Very High | Hannover University Medical School, Hannover | 1980–1991 | 47 | Adult |
| 7 | United States | 0.915 | Very High | Surveillance, Epidemiology, and End Results Program (NCI); National Program of Cancer Registries (CDC) | 1999–2008 | 7104 | Mixed Age |
| 8 | Canada | 0.913 | Very High | Mount Sinai Hospital, Toronto | 1986–2003 | 247 | Mixed Age |
| 9 | Hong Kong, China | 0.910 | Very High | Chinese University of Hong Kong, Prince of Whales Hospital, Hong Kong | 1993–2008 | 77 | Pediatric |
| 10 | Scotland | 0.907 | Very High | University of Glosgow, Glosgow | 1933–2004 | 217 | Pediatric |
| 11 | Japan | 0.891 | Very High | Tohoku Musculoskeletal Tumor Society and the National Cancer Center, Tokyo | 1972–2002 | 64 | Adult |
| 12 | Belgium | 0.890 | Very High | University Hospital Leuven, Pellenberg | 1962–1987 | 58 | Pediatric |
| 13 | France | 0.888 | Very High | Hospital of Hautepierre, Strasbourg | 1983–1994 | 30 | Mixed Age |
| 14 | Finland | 0.883 | Very High | Finnish Cancer Registry | 1971–1990 | 166 | Mixed Age |
| 15 | Finland | 0.883 | Very High | Finnish Cancer Registry | 1991–2005 | 62 | Pediatric |
| 16 | Italy | 0.873 | Very High | Rizzoli Orthopedic Institute, Bologna | 1959–1979 | 433 | Mixed Age |
| 17 | Italy | 0.873 | Very High | Rizzoli Orthopedic Institute, Bologna | 1982–2002 | 1,458 | Mixed Age |
| 18 | Italy | 0.873 | Very High | Rizzoli Orthopedic Institute, Bologna | 1961–2006 | 30 | Adult |
| 19 | Czech Republic | 0.870 | Very High | Masaryk Memorial Cancer Institute, Brno | 1999–2010 | 36 | Adult |
| 20 | Argentina | 0.836 | Very high | Italian Hospital of Buenos Aires, Buenos Aires | 1980–2004 | 327 | Mixed Age |
| 21 | Hungary | 0.828 | Very High | Second Department of Pediatrics, Budapest | 1988–2006 | 122 | Pediatric |
| 22 | Asia | N/A | Very High | * | N/A-2001 | 209 | Adult |
| 23 | Germany, Austria, Switzerland | N/A | Very High | German-Austrian-Swiss Osteosarcoma Study Group | 1980–1998 | 1,702 | Mixed Age |
| 24 | Malaysia | 0.779 | High | Hospital Universiti Sains Malaysia, Kelantan | 2005–2010 | 163 | Mixed Age |
| 25 | Malaysia | 0.779 | High | Hospital of Kuala Lumpur, Kuala Lumpur | 1995–1999 | 21 | Mixed Age |
| 26 | Turkey | 0.761 | High | Ankara Numune Education and Research Hospital, Ankara | 2002–2012 | 21 | Adult |
| 27 | Turkey | 0.761 | High | ** | 1995–2011 | 240 | Mixed Age |
| 28 | Turkey | 0.761 | High | Hacettepe University, Ankara | 1985–2004 | 69 | Pediatric |
| 29 | China | 0.727 | High | Peking University People’s Hospital, Beijing | 1998–2011 | 54 | Adult |
| 30 | Thailand | 0.726 | High | Faculty of Medicine Ramathibodi Hospital Mahidol University, Bangkok | 1985–1988 | 130 | Mixed Age |
| 31 | Egypt | 0.690 | Medium/Low | University Hospital, Alexandria | 1979–1988 | 105 | Mixed Age |
| 32 | South Africa | 0.666 | Medium/Low | Greys Hospital, University of KwaZulu-Natal, Pietermaritzburg | 2009–2011 | 24 | Mixed Age |
| 33 | India | 0.609 | Medium/Low | Tata Memorial Hospital, Bombay | 1985–2988 | 273 | Mixed Age |
| 34 | Pakistan | 0.538 | Medium/Low | Aga Khan University Hospital, Kariachi | 2004–2008 | 22 | Adult |
| 35 | Central America | N/A | Medium/Low | *** | 2000–2009 | 264 | Pediatric |
N/A: Data not available or applicable.
Countries: S. Korea, Japan, Thailand, China, Philippines. Centers/ Registries: Catholic Center Hospital, Seoul; National Cancer Center Hospital, Tokyo; Seoul National University Hospital, Seoul; Siriraj Hospital, Bangkok; Korea Cancer Center Hospital, Seoul; Kosin University Gospel Hospital, Busan; Jishuitan Hospital, Beiging; Kanazawa University Hospital, Kanazawa; Tata Memorial Hospital, Mumbai; Philippine General Hospital, Manila.
Country: Turkey. Centers/ Registries: Ankara Oncology Training Center and Research Hospital, Ankara; Dicle University Hospital, Diyarbakir; Ankara Numune Training and Tresearch Hospital, Ankara; Erciyes University Hospital, Kayseri; 9 Eylul University Hospital, Izmir.
Countries: Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama. Centers/ Registries: National Children’s Hospital, San Jose; Benjamin Bloom National Children’s Hospital, San Salvedor, National Pediatric Oncology Unit, Guatemala City; Maternity and Children’s Hospital, Honduras; “La Mascota” Children’s Hospital, Managua; Children’s Hospital of Panama, Panama City; Pediatric Specialties Hospital, Panama.
Figure 1.
Prevalence (boxes), 95% confidence intervals (lines), and pooled prevalence (diamonds). ‘Overall Pooled is the pooled prevalence of all 35 studies. ‘ID’ refers to table I ID.
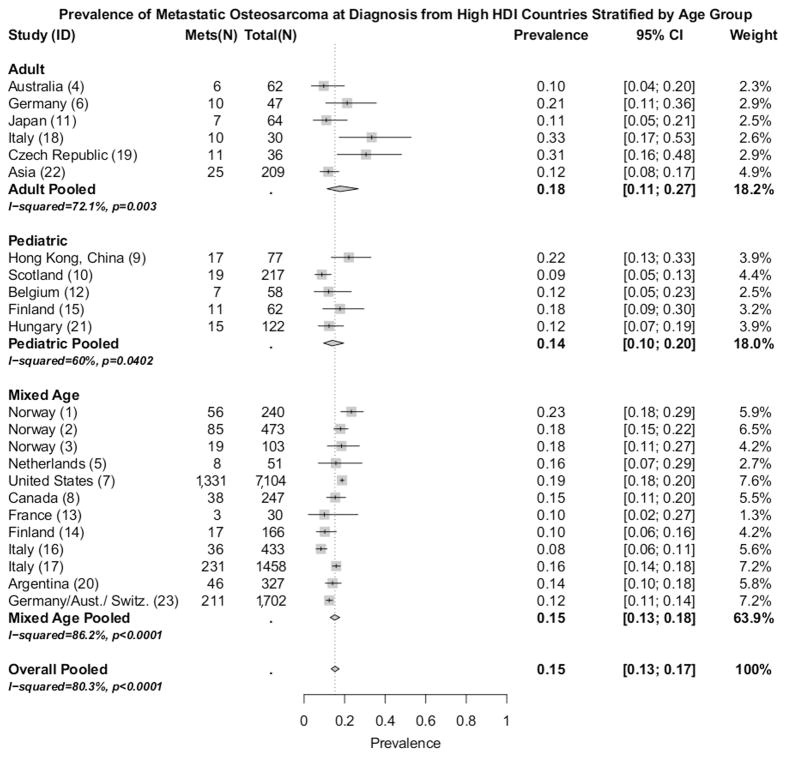
Figure 2 details information on the subgroup analysis of the 23 studies from very high HDI countries. The pooled prevalence for adult patients presenting with metastatic OS in very high HDI countries was found to be 18% (95% CI: 11%, 27%). Among pediatric patients presenting with metastatic OS in very high HDI countries, the pooled prevalence was 14% (95%CI: 10%, 20%). Studies that included a mixed age grouping from countries with a very high HDI had a pooled prevalence of metastatic OS at diagnosis of 15% (95%CI: 13%, 18%).
Figure 2.
Prevalence (boxes), 95% confidence intervals (lines), and pooled prevalence (diamonds). Study classifications: adult (no pediatric cases), pediatric (upper age no greater than 18 years), and mixed ages (all patients seen at a clinic that included pediatric and adult populations). ‘Overall Pooled’ is pooled prevalence of the 23 studies from very high HDI countries. ‘ID’ refers to table I ID.
Heterogeneity within HDI groups as measured by Cochran’s Q were all significant (p< 0.05), and remained statistically significant from the subgroup analysis of age groupings (pediatric, adult, and mixed age) (Figure 2). An apparent reduction in heterogeneity was noticed between studies restricted to either pediatric (I-squared = 60%) or adult cases (I-squared= 72.1%), but not between studies that included both pediatric and adult cases (I-squared = 86.2%). The lower heterogeneity from the pediatric and adult subgroups compared to the mixed age subgroup could reflect a difference in the prevalence of metastatic OS between pediatric and adult populations, and the heterogeneity between the studies from the same HDI group may be partially explained by differing age ranges.
Discussion
Prior to the introduction of high-dose chemotherapy to osteosarcoma (OS) treatment regiments in the United States, the 5- year survival rate of patients with localized disease was around 20% following amputation. [44] The improved survival with systemic chemotherapy likely results from the eradication of micrometastases not detected by current imaging techniques. The presence of detectable metastatic OS at diagnosis may be driven by two broad factors. If diagnosis is delayed, micrometastases may be allowed more time to leave dormancy and develop into macrometastases, increasing the observed prevalence of metastases at diagnosis. Alternatively, the biology of OS may drive the rate of metastasis, with a subset of OS having an intrinsically poor biology leading to macrometastases development.
Diagnostic delay may occur at the level of the patient (education, resources, socio-economic status), provider (referral centers, specialized oncology clinics, imaging facilities), and country (health care system organization, access to health care, social security). [2] If metastasis were attributable to diagnostic delay, one would expect longer duration of symptoms among these patients. One European group reported an association between increased time to diagnosis and metastasis at presentation. The German-Austrian-Swiss Osteosarcoma Study Group [8] observed an association of axial primary tumors (p<.001, X2), metastases at diagnosis (p<.007, X2), and increasing age (P<.001, t-test) with prolonged history of symptoms before diagnosis. However, given that axial tumors and older age are known to increase metastasis, [6, 9, 11–15] history of symptoms should be evaluated with multivariable analysis to determine if a correlation with metastases at presentation exists within their population.
Several research groups from single institutions in countries with high and very high HDI have also evaluated the effect of diagnosis delay on the prevalence of metastasis at OS diagnosis. No difference was observed in symptom duration to OS diagnosis between patients with or without metastasis at presentation by groups from Indianapolis, [45] Hong Kong, [46] and Taiwan. [47] Patients seen at the Italian Rizzoli Institute with extremity tumors had a shorter interval between onset of symptoms to time of diagnosis if metastases were found at presentation (2.17 months vs. 2.54 months; P<0.0002). [48] Although not statistically significant in all reports, there appears to be a trend that patients with metastases actually present earlier to clinic from symptom onset, likely due to the disease severity. These reports provide evidence that diagnosis delay does not increase the risk of developing detectable metastasis before OS diagnosis. Rather, they suggest that tumor biology is the driver of malignant tumor character.
Studies reporting the highest prevalence of metastasis at OS diagnosis were from countries with medium/ low HDI. [2, 41] Socio-economic status, educational levels, and healthcare systems and resources can negatively effect patient outcomes. [2] Within the United States’ healthcare system, counties with the lowest composite socioeconomic status scores had a higher proportion of patients with metastasis at diagnosis. [9] Socioeconomic status combines individual elements, social factors, and local infrastructure, and may identify communities with less access to medical care. Comparatively, Central American and African countries have a higher proportion of the population in underdeveloped communities with limited access to medical care. Barriers to accessing medical care may discourage individuals from seeking medical attention unless symptoms are severe. Similar to the observation of patients with metastatic disease presenting earlier to clinic in very high HDI countries, if individuals with severe symptoms are more likely present to clinic in medium/ low countries, the prevalence of metastatic disease at diagnosis will be artificially inflated in these countries.
Alternatively, the delay in diagnosis may be longer and driving a higher prevalence of metastasis at diagnosis in countries with medium/ low HDI as compared to countries with very high/ high HDI. Three of the four research groups evaluating the effect of diagnosis delay on the prevalence of metastasis at OS diagnosis had an upper range of 1–2 years from onset of symptoms to diagnosis. One group had a range of 10 years. [45] When comparing the lower and upper quartile of symptom length, a difference in diagnostic delay on metastatic development prior to diagnosis was still not observed. While this suggests that diagnosis delay does not have an effect up to a decade, the results cannot be extrapolated to countries with medium/ low HDI, where diagnostic delay may be even longer. The improved prognosis with addition of chemotherapy to OS treatments demonstrates that intervention is needed to prevent development of metastatic disease. It is unclear how well individuals in countries with medium/ low HDI are being diagnosed and treated compared to those in countries with very high/ high HDI. Research must be conducted in medium/ low HDI countries to determine if diagnosis delay is affecting patient outcomes.
The findings that the prevalence of metastasis is relatively constant and is not affected by diagnosis delay in countries with very high HDI values suggest there is a biological baseline for the presence of metastasis at diagnosis. Given that patients with metastases present earlier to clinic in countries with very high HDI, early detection may not be useful in improving survival rates. In countries with medium/ low HDI, where there are more barriers to accessing healthcare, two phenomenon may occur that give rise to the observed higher prevalence of metastasis at OS diagnosis. First, patients may delay seeking treatment significantly longer than patients in developed countries, allowing micrometastases time to develop into detectable metastasis above the 18% baseline observed in very high HDI countries. Second, patients with less severe symptoms may not present to clinic, artificially inflating the percentage of severe cases with detectable metastasis.
A limitation of this study is the lack of research that has been conducted in countries with medium/ low HDI. Research must be performed to address the question of whether delay in diagnosis increases the prevalence of detectable metastatic disease at the time of OS diagnosis in these countries. This knowledge will direct the treatment course if it can be determined whether earlier detection and treatment could improve patient outcomes in countries with medium/ low HDI.
Acknowledgments
This work was supported by the Zach Sobiech Osteosarcoma Fund of the Children’s Cancer Research Fund, Minneapolis, MN, and by NIH MSTP grant T32 GM008244 (T.M.)
Abbreviations
- OS
Osteosarcoma
- CI
Confidence Interval
- HDI
Human Development Index
Footnotes
Conflicts of Interest Statement:
No authors had a conflict of interest when generating this manuscript.
References
- 1.Serlo J, Tarkkanen M, Sampo M, Vettenranta K, Riikonen P, Helenius I. Incidence, treatment and survival of paediatric patients with bone sarcomas in Finland from 1991 to 2005. Acta Paediatr. 2015;104(7):738–45. doi: 10.1111/apa.12986. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich P, Ortiz R, Strait K, Fuentes S, Gamboa Y, Arambu I, Ah-Chu-Sanchez M, London W, Rodriguez-Galindo C, Antillon-Klussmann F, Baez F Central American Association of Pediatric Hematologists Oncologists AHOPCA. Pediatric sarcoma in Central America: outcomes, challenges, and plans for improvement. Cancer. 2013;119:871–9. doi: 10.1002/cncr.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan LL, Ahmad K, Kareem BA, Harwant S. Pattern of primary musculoskeletal sarcomas referred to Institute of Radiotherapy and Oncology, Hospital Kuala Lumpur, 1995–1999. Med J Malaysia. 2001;56(Suppl C):52–6. [PubMed] [Google Scholar]
- 4.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berner K, Johannesen TB, Berner A, Haugland HK, Bjerkehagen B, Bohler PJ, Bruland OS. Time-trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol. 2015;54:25–33. doi: 10.3109/0284186X.2014.923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 9.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am. 2013;95:e89. doi: 10.2106/JBJS.L.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada K, Hasegawa T, Nishida J, Ogose A, Tajino T, Osanai T, Yanagisawa M, Hatori M. Osteosarcomas after the age of 50: a clinicopathologic study of 64 cases—an experience in northern Japan. Annals of Surgical Oncology. 2004;11:998–1004. doi: 10.1245/ASO.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Tang S, Guo W, Yang RL, Tang XD, Li DS, Dong S. Surgical treatment and prognostic analysis of osteosarcoma in adults older than 40 years. Beijing Da Xue Xue Bao. 2015;47:165–9. [PubMed] [Google Scholar]
- 12.Seker MM, Seker A, Aksoy S, Ozdemir N, Uncu D, Zengin N. Clinicopathologic features and prognosis of osteosarcoma in Turkish adults. Asian Pac J Cancer Prev. 2014;15:3537–40. doi: 10.7314/apjcp.2014.15.8.3537. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss J, Szendroi M, Csoka M, Kovacs G. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57:415–22. doi: 10.1002/pbc.23172. [DOI] [PubMed] [Google Scholar]
- 14.Renard AJ, Veth RP, Schreuder HW, Pruszczynski M, Bokkerink JP, van Hoesel QG, van Der Staak FJ. Osteosarcoma: oncologic and functional results. A single institutional report covering 22 years. J Surg Oncol. 1999;72:124–9. doi: 10.1002/(sici)1096-9098(199911)72:3<124::aid-jso3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Saeter G, Bruland OS, Folleras G, Boysen M, Hoie J. Extremity and non-extremity high-grade osteosarcoma -- the Norwegian Radium Hospital experience during the modern chemotherapy era. Acta Oncol. 1996;35(Suppl 8):129–34. doi: 10.3109/02841869609098531. [DOI] [PubMed] [Google Scholar]
- 16.Duong LM, Richardson LC. Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999–2008. J Registry Manag. 2013;40:59–64. [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Cochran BG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Statistics in Medicine. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- 21.Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian National Data. Cancer. 1981;48:1719–23. doi: 10.1002/1097-0142(19811015)48:8<1719::aid-cncr2820480806>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Ellis PM, Tattersall MH, McCaughan B, Stalley P. Osteosarcoma and pulmonary metastases: 15-year experience from a single institution. Aust N Z J Surg. 1997;67:625–9. doi: 10.1111/j.1445-2197.1997.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 23.Bokemeyer C, Schmoll H, Harstrick A, Kohnewompner H, Poliwoda H. Treatment of adult osteosarcoma - single-center results in 47 patients. Int J Oncol. 1993;3:927–32. doi: 10.3892/ijo.3.5.927. [DOI] [PubMed] [Google Scholar]
- 24.Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol. 2009;20:1136–41. doi: 10.1093/annonc/mdn731. [DOI] [PubMed] [Google Scholar]
- 25.Rasalkar DD, Chu WC, Lee V, Paunipagar BK, Cheng FW, Li CK. Pulmonary metastases in children with osteosarcoma: characteristics and impact on patient survival. Pediatr Radiol. 2011;41:227–36. doi: 10.1007/s00247-010-1809-1. [DOI] [PubMed] [Google Scholar]
- 26.Foster L, Dall GF, Reid R, Wallace WH, Porter DE. Twentieth-century survival from osteosarcoma in childhood. Trends from 1933 to 2004. J Bone Joint Surg Br. 2007;89:1234–8. doi: 10.1302/0301-620X.89B9.19255. [DOI] [PubMed] [Google Scholar]
- 27.Meskens M, Burssens A, Hoogmartens M, Fabry G. Osteogenic sarcoma in children. A retrospective study of 58 cases. Acta Orthop Belg. 1993;59:64–8. [PubMed] [Google Scholar]
- 28.Babin SR, Simon P, Babin-Boilletot A, Bellocq JP, Marcellin L, Dosch JC. High grade osteosarcoma of the lower limb. Complications and results of the treatment of 20 patients. Rev Chir Orthop Reparatrice Appar Mot. 1996;82:14–21. [PubMed] [Google Scholar]
- 29.Sampo MM, Tarkkanen M, Kivioja AH, Taskinen MH, Sankila R, Bohling TO. Osteosarcoma in Finland from 1971 through 1990: a nationwide study of epidemiology and outcome. Acta Orthop. 2008;79:861–6. doi: 10.1080/17453670810016966. [DOI] [PubMed] [Google Scholar]
- 30.Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty year’s experience at the Istituto Ortopedico Rizzoli. Cancer. 1981;48:1569–81. doi: 10.1002/1097-0142(19811001)48:7<1569::aid-cncr2820480717>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Picci P, Mercuri M, Ferrari S, Alberghini M, Briccoli A, Ferrari C, Pignotti E, Bacci G. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol. 2010;21:1366–73. doi: 10.1093/annonc/mdp502. [DOI] [PubMed] [Google Scholar]
- 32.Longhi A, Errani C, Gonzales-Arabio D, Ferrari C, Mercuri M. Osteosarcoma in patients older than 65 years. J Clin Oncol. 2008;26:5368–73. doi: 10.1200/JCO.2007.14.9104. [DOI] [PubMed] [Google Scholar]
- 33.Adamkova Krakorova D, Vesely K, Zambo I, Tucek S, Tomasek J, Jureckova A, Janicek P, Cerny J, Pazourek L, Ondrusek S, Selingerova I. Analysis of prognostic factors in osteosarcoma adult patients, a single institution experience. Klin Onkol. 2012;25:346–58. [PubMed] [Google Scholar]
- 34.Ayerza MA, Farfalli GL, Aponte-Tinao L, Muscolo DL. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res. 2010;468:2854–9. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joo MW, Shin SH, Kang YK, Kawai A, Kim HS, Asavamongkolkul A, Jeon DG, Kim JD, Niu X, Tsuchiya H, Puri A, Wang EH, Chung SH, Chung YG. Osteosarcoma in Asian Populations Over the Age of 40 Years: A Multicenter Study. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4414-6. [DOI] [PubMed] [Google Scholar]
- 36.Faisham WI, Mat Saad AZ, Alsaigh LN, Nor Azman MZ, Kamarul Imran M, Biswal BM, Bhavaraju VM, Salzihan MS, Hasnan J, Ezane AM, Ariffin N, Norsarwany M, Ziyadi MG, Wan Azma WS, Halim AS, Zulmi W. Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia Pac J Clin Oncol. 2015 doi: 10.1111/ajco.12346. [DOI] [PubMed] [Google Scholar]
- 37.Durnali A, Alkis N, Cangur S, Yukruk FA, Inal A, Tokluoglu S, Seker MM, Bal O, Akman T, Inanc M, Isikdogan A, Demirci A, Helvaci K, Oksuzoglu B. Prognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patients. Med Oncol. 2013;30 doi: 10.1007/s12032-013-0624-6. 624,013–0624–6. [DOI] [PubMed] [Google Scholar]
- 38.Varan A, Yazici N, Aksoy C, Gedikoglu G, Yalcin B, Akyuz C, Kutluk T, Buyukpamukcu M. Treatment results of pediatric osteosarcoma: twenty-year experience. J Pediatr Orthop. 2007;27:241–6. [PubMed] [Google Scholar]
- 39.Pochanugool L, Sangthawan D, Subhadharaphandou T, Dangprasert S, Hathirat P, Sirikulchayanonta V, Ratanatharathorn V, Tannanonta C, Onsanit S, Yuktanonda P. Osteosarcoma: a study of 130 cases. J Med Assoc Thai. 1997;80:153–9. [PubMed] [Google Scholar]
- 40.Aouad AM, Badib AO, Aouad KM. Experience of treatment of osteogenic sarcoma at the University Hospital Center of Alexandria (Egypt): retrospective study of 105 cases. Bull Cancer. 1994;81:698–700. [PubMed] [Google Scholar]
- 41.Ferreira N, Marais LC. Osteosarcoma presentation stages at a tumour unit in South Africa. S Afr Med J. 2012;102:673–6. doi: 10.7196/samj.5835. [DOI] [PubMed] [Google Scholar]
- 42.Susnerwala SS, Pande SC, Dinshaw KA, Advani SH, Suraiya JN. Osteosarcoma: experience of the Tata Memorial Hospital, Bombay, India. Cancer Treat Res. 1993;62:365–9. doi: 10.1007/978-1-4615-3518-8_46. [DOI] [PubMed] [Google Scholar]
- 43.Ansari TZ, Masood N, Parekh A, Jafri RZ, Niamatullah SN, Zaidi AA, Umer M. Four year experience of sarcoma of soft tissues and bones in a tertiary care hospital and review of literature. World J Surg Oncol. 2011;9 doi: 10.1186/1477-7819-9-51. 51,7819–9–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rougraff BT, Davis K, Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin Orthop Relat Res. 2007;462:181–9. doi: 10.1097/BLO.0b013e3180f62608. [DOI] [PubMed] [Google Scholar]
- 46.Yang JY, Cheng FW, Wong KC, Lee V, Leung WK, Shing MM, Kumta SM, Li CK. Initial presentation and management of osteosarcoma, and its impact on disease outcome. Hong Kong Med J. 2009;15:434–9. [PubMed] [Google Scholar]
- 47.Hung GY, Yen HJ, Yen CC, Chen WM, Chen PC, Wu HT, Chiou HJ, Chang WH, Hsu HE. Experience of pediatric osteosarcoma of the extremity at a single institution in Taiwan: prognostic factors and impact on survival. Ann Surg Oncol. 2015;22:1080–7. doi: 10.1245/s10434-014-4154-z. [DOI] [PubMed] [Google Scholar]
- 48.Bacci G, Ferrari S, Longhi A, Forni C, Zavatta M, Versari M, Smith K. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. J Pediatr Hematol Oncol. 2002;24:27–30. doi: 10.1097/00043426-200201000-00008. [DOI] [PubMed] [Google Scholar]