Abstract
Importance
Magnitude of association and quality of evidence for cutaneous squamous cell carcinoma (cSCC) and risk factors for outcomes have not been reviewed and analyzed systematically.
Objective
Review and systematically analyze all published data on risk factors for recurrence, metastasis, and disease-specific death (DSD) of cSCC.
Data Sources
Comprehensive medical literature search from each database’s inception to May 14, 2015.
Study Selection
Inclusion criteria were studies with ≥10 patients, comparative data for ≥1 cSCC risk factors, and an outcome of interest. Exclusion criteria were noncutaneous squamous cell carcinoma (SCC), anogenital SCC, inability to extract cSCC data from other malignancy data, SCC in situ, Marjolin tumor, and genetic disorders predisposing to cSCC.
Data Extraction and Synthesis
Two reviewers independently abstracted the data. Meta-analysis used the random effects model. Risk of bias was assessed through Newcastle-Ottawa Scale.
Main Outcomes and Measures
A priori outcomes were recurrence, metastasis, and DSD.
Results
Thirty-six studies (17,248 patients with 23,421 cSCCs) were included. Statistically significant risk factors for recurrence were Breslow >2 mm (risk ratio [RR] [95% CI], 9.64 [1.30–71.52]), invasion beyond subcutaneous fat (7.61 [4.17–13.88]), Breslow >6 mm (7.13 [3.04–16.72]), perineural invasion (PNI) (4.30 [2.80–6.60]), diameter >20 mm (3.22 [1.91–5.45]), location on temple (3.20 [1.12–9.15]), and poor differentiation (2.66 [1.72–4.14]). Statistically significant risk factors for metastasis were invasion beyond subcutaneous fat (RR [95% CI], 11.21 [3.59–34.97]); Breslow >2 mm (10.76 [2.55–45.31]); Breslow >6 mm (6.93 [4.02–11.94]); diameter >20 mm (6.15 [3.56– 10.65]); poor differentiation (4.98 [3.30–7.49]); PNI (2.95 [2.31–3.75]); location on temple (2.82 [1.72–4.63]), ear (2.33 [1.67–3.23), and lip (RR [1.54–3.37]); and immunosuppression (1.59 [1.07–2.37]). Factors for DSD were diameter >20 mm (RR [95% CI], 19.10 [5.80–62.95]), poor differentiation (5.65 [1.76–18.20]), location on ear (4.67 [1.28–17.12]) and lip (4.55 [1.41–14.69]), invasion beyond subcutaneous fat (4.49 [2.05–9.82]), and PNI (4.06 [3.10–5.32]). Evidence quality was considered low to moderate.
Conclusions and Relevance
Tumor depth is associated with highest RR of local recurrence and metastasis of cSCC; tumor diameter ≥20 mm is associated with highest risk of DSD. Unified, consistent collection and reporting of risk factors in a prospective, multicentered effort are needed to further understand the increasing cSCC.
Grade of Evidence
2A. Quality of Evidence: Level B.
Keywords: cutaneous squamous cell carcinoma, meta-analysis, outcomes
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common malignancy of the skin, with an estimated annual incidence of 700,000 in the United States.1,2 Most cases of cSCC portend an excellent prognosis following surgical removal.3 However, 3.7% to 5.2% of patients have nodal metastasis and 1.5% to 2.1% die of cSCC.4–8 Although these rates are relatively low compared with many other malignancies, the absolute number of cSCC patients who have nodal metastasis is estimated at 5,604 to 12,572 in the United States alone.1 Furthermore, the absolute number of cSCC-related deaths is estimated between 3,932 to 8,791 annually, the upper limit of which approaches annual melanoma-related deaths.1 The absence of a national tumor registry for cSCC complicates the analysis of prognostic factors related to outcomes on a broad scale. Thus, the current understanding of prognostic factors is based primarily on retrospective analyses of single-institution cohorts with heterogeneously reported data. The purpose of the present study was to perform a systematic review and meta-analysis of all published reports of cSCC risk factors and outcomes, to quantify the magnitude of each risk factor and the quality of the supporting data.
Methods
The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Literature Search
A comprehensive and systematic search of Ovid Medline In-Process and Other Non-Indexed Citations, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus was performed from each database’s earliest inception to May 14, 2015, by an experienced librarian (L.J.P.), with input from the study’s principle investigator (C.L.B.) and lead author (A.K.T.). Controlled vocabulary supplemented with keywords was used to search for studies of risk factors in cSCC and association with outcomes. The search strategy is outlined in eTable (online supplement). Bibliographies of selected review articles were reviewed for additional relevant studies. Only studies on human data, published or translated into English, were included.
Eligibility Criteria
Inclusion criteria were studies of ≥10 patients with cSCC that reported comparative data associating at least 1 defined risk factor (ie, depth of invasion; perineural invasion [PNI]; diameter; location on lip, ear, temple, or cheek; tumor differentiation; and immunosuppression) and an outcome of interest (ie, recurrence, nodal metastasis, and disease-specific death [DSD]).
Exclusion criteria for the studies included any of the following: squamous cell carcinoma (SCC) in situ, noncutaneous SCC, anogenital SCC, cSCC data that could not be extracted from data on other malignancies (eg, basal cell carcinoma, melanoma), cSCC in patients with genetic disorders that predispose to cSCC (eg, xeroderma pigmentosa), cSCC arising in scar tissue (Marjolin tumor), and an outcome of interest present at study initiation. No limitations were imposed on the basis of treatment modality.
Data Abstraction
Two reviewers (A.K.T. and B.F.K.) independently selected studies on the basis of inclusion and exclusion criteria. Disparities in selection were resolved through discussion and ultimately by a third reviewer (C.L.B.). Studies were initially reviewed on the basis of title and abstract; those deemed relevant were reviewed in full text to establish the final set of studies ultimately included. In cases of study duplicity, the more recent and complete studies were selected for inclusion. From these studies, data were abstracted in duplicate (A.K.T. and B.F.K.) to verify accuracy.
Risk of Bias Assessment
Risk of bias assessment was analyzed for each article by 2 investigators (A.K.T. and B.F.K.) using the Newcastle-Ottawa Scale.9 We considered that the most important factor in determining the risk of bias was the ability of a study to adjust for confounders (ie, other risk factors) using multivariate adjustment.
Statistical Analysis
From each study, we extracted or estimated the risk ratio (RR) (presented as hazard ratio [HR] or odds ratio [OR]) for each risk factor and outcome of interest, with 95% CI. Various association measures were assumed to be comparable statistically. Multivariate estimates were preferentially extracted when available. Meta-analysis was performed with the random effects model,10 reporting RR (95% CI). The I2 statistic11 also was calculated to measure inconsistency; a value >50% implied substantial heterogeneity (ie, difference in the estimates derived from each study). Forest plots were constructed for all associations of risk factor and outcome. Analysis was conducted through statistical software (Comprehensive Meta-Analysis, Version 3.0; Biostat).
Results
Literature Search
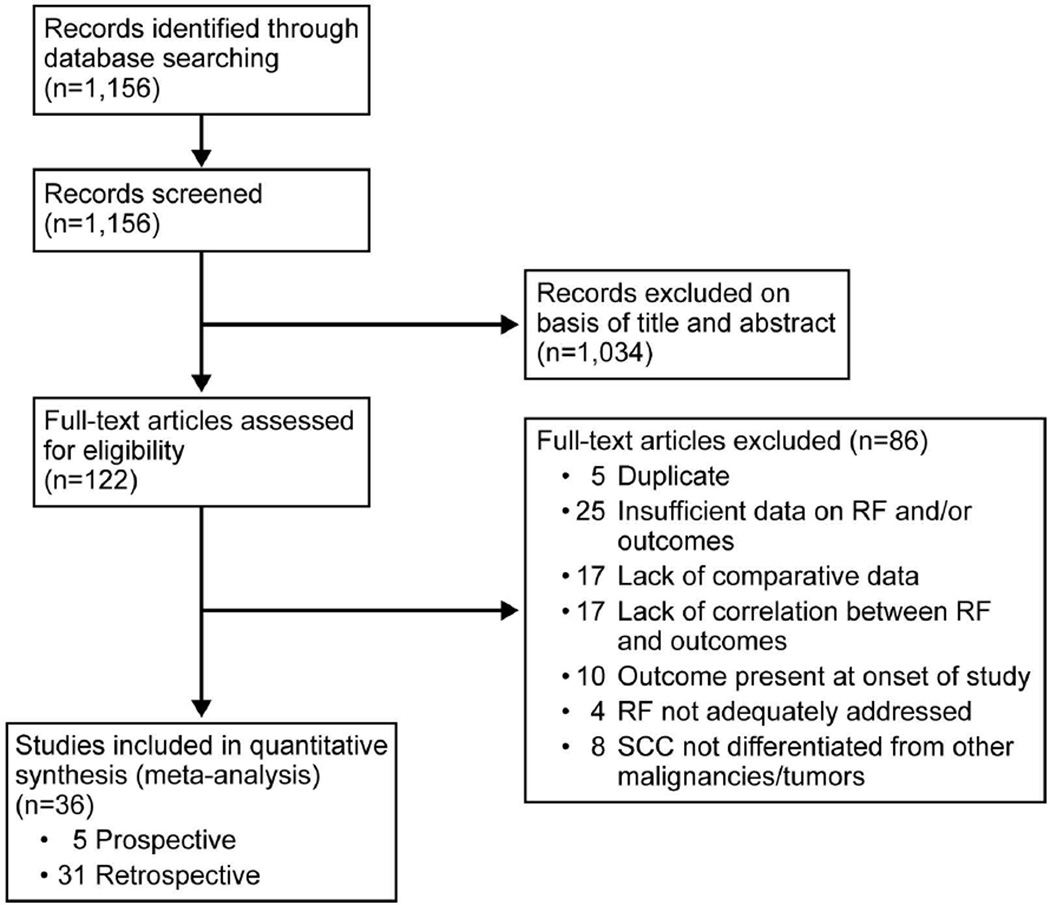
Database search identified 1,156 publications and meeting abstracts. After initial review of abstracts and titles, 1,034 records were excluded (Figure 1). The other 122 studies were reviewed in full text, and ultimately, 36 studies were included in our analysis.
Figure 1.
Flow Diagram of Preferred Reporting Items for Systematic Reviews and Meta-analyses for the Systematic Literature Search. RF indicates risk factor.
Study Characteristics and Meta-analysis
The 36 studies described 23,421 cSCCs in 17,248 patients; 5 were prospective cohort studies and 31 were retrospective studies. No randomized trials were identified. The number of patients in the included studies ranged from 41 to 6,164 (Table 1).
Table 1.
Study Characteristics
| First Author, Year |
Study Years |
Location | Study Type |
No. of Patients |
No. of cSCCs |
RF Addressed |
Outcomes | Inclusion Criteria |
Follow-up Data |
|---|---|---|---|---|---|---|---|---|---|
| Dinehart and Pollack, 198912 |
1979–1988 | North Carolina, single institution |
Retrospective cohort of consecutive cSCC |
365 | 365 | Location | Metastasis | Biopsy-proven cSCC; treated with Mohs |
Mean (range), 1.7 y (2 mo–7.8 y) |
| Dormand et al, 201013 |
1997–2004 | United Kingdom, single institution |
Retrospective cohort of limb cSCC |
243 | 517 | Differentiation | Metastasis | Biopsy-proven cSCC; extremities only |
Mean (range), 6.2 (4–22) y |
| Goepfert et al, 198414 |
1970–1979 | Texas, single institution |
Retrospective cohort of consecutive cSCC |
520 | 967 | PNI | Recurrence, metastasis, DSD |
Biopsy-proven cSCC | 98% of patients had minimum follow- up of 2 y |
| Quaedvlieg et al, 200615 |
1982–2002 | Netherlands, single institution |
Retrospective case-control of metastatic cSCC |
580 | 915 | Differentiation, PNI, location |
Metatasis | Using PALGA registry in Netherlands; contained all registered cSCC in University Hospital; biopsy proven |
At least 3 y of follow-up; mean (range), 5.7 (0.25–21) y |
| Brantsch et al, 20084 |
1990–2001 | Germany, single institution |
Prospective cohort of cSCC |
615 | 615 | Depth, diameter, location, differentiation, immunosuppression |
Recurrence, metastasis |
Biopsy-proven cSCC | Median (range), 43 (1–165) mo |
| Brinkman et al, 201416 |
2001–2008 | Netherlands, single institution |
Retrospective cohort of cSCC |
131 | 155 | Differentiation | Metastasis, DSD |
Biopsy-proven cSCC; treated with surgical excision |
Median (range), 81 (27–125) mo |
| Brougham et al, 20125 |
1997–2007 | New Zealand, regional NMSC database |
Retrospective cohort of cSCC |
6,164 | 8,997 | Location, PNI, differentiation |
Metastasis | Biopsy-proven cSCC | Minimum, 30-mo follow-up; mean (SD) 71 (25) mo; median (range), 70 (31–121) mo |
| Eroğlu et al, 199617 |
1980–1989 | Turkey, single institution |
Retrospective cohort of cSCC |
1,039 | 1,039 | Location, diameter, differentiation |
Recurrence | Biopsy-proven cSCC | Median (range), 28 (6–149) mo |
| Faustina et al, 200418 |
1952–2000 | Texas, single institution |
Retrospective cohort of periocular cSCC |
111 | 111 | PNI | Metastasis | Biopsy-proven cSCC; periocular only |
Median (range), 76.6 (6–484) mo |
| Gonzalez et al, 201419 |
1990–2012 | Argentina, single institution |
Retrospective cohort of head/neck cSCC |
434 | 434 | Location, diameter, differentiation |
Recurrence, metastasis |
Biopsy-proven cSCC; treated with Mohs |
Mean (range), 56.9 (10–251) mo |
| Griffiths et al, 200220 |
1990–1995 | United Kingdom, single institution |
Retrospective cohort of consecutive cSCC |
157 | 157 | Location | Metastasis, DSD |
Biopsy-proven cSCC | 93 patients had 5-y follow-up |
| Pugliano-Mauro et al, 201021 |
1996–2006 | Vermont, single institution |
Retrospective cohort of high- risk cSCC |
215 | 260 | Location, PNI | Metastasis | Biopsy-proven high- risk cSCC, including recurrent tumors; treated with Mohs |
Mean, 3.9 y |
| Immerman et al, 198322 |
1970–1980 | Illinois, single institution |
Retrospective cohort of consecutive cSCC |
86 | 86 | Differentiation | Recurrence | Biopsy-proven cSCC | Mean (range), 4.5 y (6 mo–10 y) |
| Karia et al, 20146 | 2000–2009 | Massachusetts, single institution |
Retrospective cohort of consecutive cSCC |
974 | 1,818 | Location, diameter, depth, differentiation, immunosuppression |
Recurrence, metastasis, DSD |
Biopsy-proven cSCC; excluded SCCIS, recurrent SCC, eyelid/anogenital SCC |
Median (range), 50 (2–142) mo |
| Mehrany et al, 200523 |
1988–1999 | Minnesota, single institution |
Retrospective case-control of cSCC in CLL patients |
142 | 171 | Differentiation, immunosuppression |
Recurrence | Biopsy-proven cSCC; treated with Mohs |
Mean, 3.3 y; median (range), 2.6 (0.04–13.7) y |
| Metchnikoff et al, 201224 |
2001–2010 | California, single institution |
Retrospective cohort of consecutive cSCC in heart/lung transplant recipients |
41 | 225 | Diameter, differentiation, PNI |
Recurrence | Biopsy-proven cSCC in heart/lung transplant recipients |
Median (range), 15.2 mo (6 d–9 y) |
| Moore et al, 200525 |
1996–2001 | Texas, single institution |
Prospective cohort of head and neck cSCC |
193 | 193 | Location, differentiation, depth, PNI |
Metastasis | Biopsy-proven head/neck cSCC |
Median (range), 20 (3.3–70) mo |
| Mourouzis et al, 20097 |
2000–2002 | United Kingdom, single institution |
Retrospective cohort of cSCC |
194 | 218 | Location, differentiation |
Metastasis | Biopsy-proven cSCC of the head and neck; treated with excision |
Monitored for 3 y; range, 30–60 mo |
| Mullen et al, 200626 |
1994–2004 | Texas, single institution |
Retrospective cohort of trunk/extremity cSCC |
136 | 149 | Diameter, differentiation |
Recurrence | Biopsy-proven cSCC | Median, 2.4 y |
| Peat et al, 201227 | 1996–2001 | New Zealand, single institution |
Retrospective case control of cSCC |
170 | 170 | Differentiation, PNI | Metastasis | Biopsy-proven cSCC | Follow-up, 5 y |
| Schmults et al, 20138 |
2000–2009 | Massachusetts, single institution |
Retrospective cohort of consecutive cSCC |
985 | 1,832 | Location, PNI | Recurrence, metastasis, DSD |
Biopsy-proven cSCC; excluded SCCIS, recurrent SCC |
Median (range), 50 (2–142) mo |
| Toll et al, 201228 | 2001–2010 | Spain, 6 tertiary care hospitals |
Retrospective case control of cSCC |
101 | 101 | Differentiation, PNI | Metastasis | Biopsy-proven cSCC; metastatic group vs nonmetastatic control group |
Mean, 24 mo |
| Baker et al, 200129 |
1990–1995 | United Kingdom, single institution |
Retrospective cohort of head/neck cSCC |
183 | 227 | Location | Regional metastasis |
Biopsy-proven cSCC | Minimum follow- up, 2 y |
| Cherpelis et al, 200230 |
1988–1998 | South Carolina, single institution |
Retrospective cohort of cSCC |
200 | 200 | Location, diameter, differentiation, PNI |
Metastasis | Biopsy-proven cSCC; treated with Mohs |
Range, 6 mo–10 y |
| Clayman et al, 200531 |
1996–2001 | Texas, single institution |
Prospective cohort of cSCC |
210 | 277 | Depth, PNI | DSD | Biopsy-proven cSCC | Median (range), 22 (2–72) mo |
| Kyrgidis et al, 201032 |
1996–2006 | Greece, single institution |
Prospective cohort of head/neck cSCC |
315 | 315 | Depth, differentiation, PNI |
DSD | Biopsy-proven cSCC | Mean (range), 46.7 (12–124) mo |
| Leibovitch et al, 200533 |
1993–2002 | Australia, multicenter |
Prospective cohort of cSCC with PNI |
1,177 | 1,177 | PNI | Recurrence | Biopsy-proven cSCC; treated with Mohs |
621 patients were lost to follow-up; 5-y follow-up |
| Roozeboom et al, 201334 |
2005–2007 | Netherlands, single institution |
Retrospective cohort of consecutive cSCC |
224 | 224 | Location, depth, differentiation, PNI |
Recurrence, metastasis |
Biopsy-proven cSCC | Median (range), 43 (0–73) mo |
| Pereira and Morgado, 199435 |
1983–1993 | Portugal, single institution |
Retrospective cohort of cSCC |
43 | 43 | Diameter | Recurrence | Biopsy-proven cSCC; treated with ED&C followed by cryotherapy |
Minimum follow- up, 4 y |
| Harwood et al, 200636 |
1995–1997 | United Kingdom, single institution |
Retrospective case control of cSCC in OTR |
65 | 100 | Immunosuppression | Recurrence, metastasis |
Biopsy-proven primary cSCC; immunocompromised group (OTR) with immunocompetent control group |
Follow-up,10 y |
| Friedman et al, 198537 |
1965–1975 | Virginia, single institution |
Retrospective cohort of cSCC |
63 | 71 | Depth, differentiation | Recurrence, DSD |
Biopsy-proven cSCC | Range, 8–18 y |
| Breuninger et al, 199038 |
Not specified |
Germany, single institution |
Retrospective cohort of cSCC |
571 | 673 | Location, diameter, differentiation, depth |
Metastasis | Biopsy-proven cSCC | Mean (range), 6 (1- 12) y |
| Krediet et al, 201539 |
2005–2009 | Germany, single institution |
Retrospective cohort of consecutive cSCC |
143 | 143 | Location, diameter, differentiation, immunosuppression |
Recurrence, metastasis |
Biopsy-proven cSCC treated with excision |
Minimum, 24 mo |
| Wermker et al, 201540 |
2005–2011 | Germany, single institution |
Retrospective cohort of consecutive cSCC of ear |
353 | 353 | Diameter, depth, differentiation, PNI, immunosuppression |
Metastasis | Biopsy-proven cSCC of external ear; treated surgically |
Minimum, 6 mo; mean (range), 43.3 (6–98) mo |
| Vasconcelos et al, 201441 |
1999–2003 | Brazil, single institution |
Retrospective cohort of head/neck cSCC |
61 | 79 | Differentiation, PNI | Recurrence | Biopsy-proven cSCC of head/neck; treated surgically |
Median (range), 5 (2–7) y |
| Stein and Tahan, 199442 |
1960–1991 | Massachusetts, single institution |
Retrospective cohort of lip cSCC |
44 | 44 | Differentiation, depth | Metastasis | Biopsy-proven cSCC of lip |
Median (range), 53 (5–212) mo |
Abbreviations: CLL, chronic lymphocytic leukemia; cSCC, cutaneous squamous cell carcinoma; DSD, disease-specific death; ED&C, electrodesiccation and curettage; Mohs, Mohs micrographic surgery; OTR, organ transplant recipient; PALGA, Pathological Anatomy National Automated Archive; PNI, perineural invasion; SCC, squamous cell carcinoma; SCCIS, squamous cell carcinoma in situ.
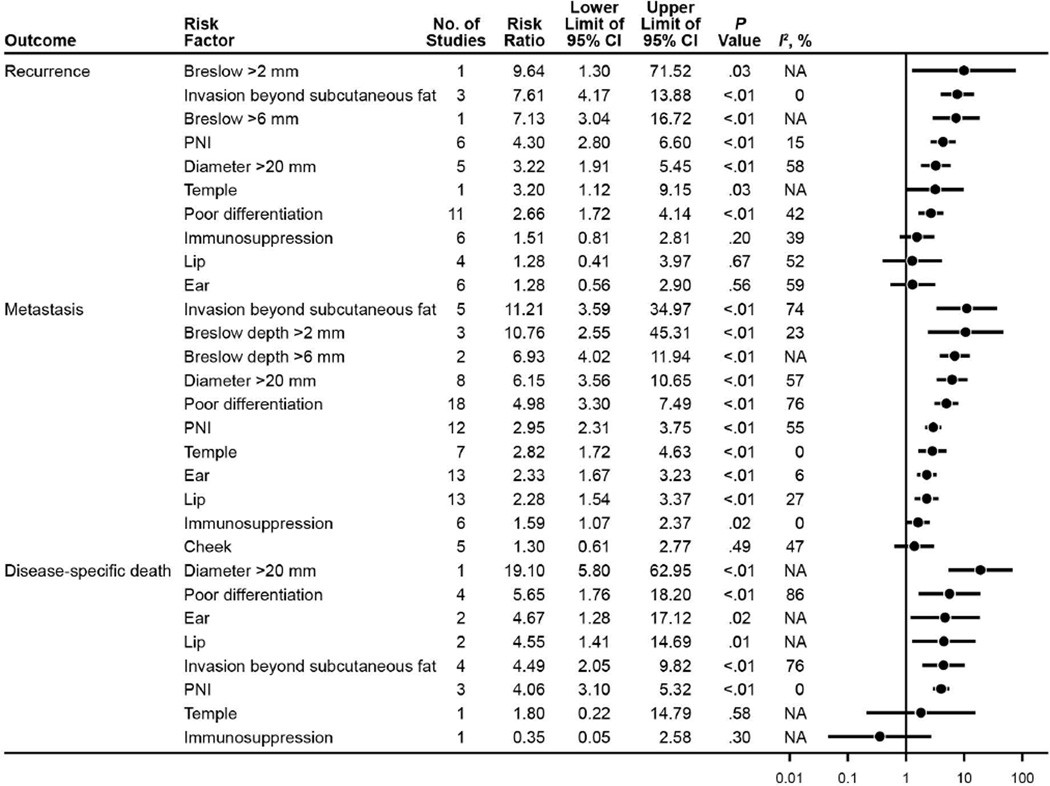
Analyzed risk factors and their respective associations with outcomes of recurrence, metastasis, and DSD are outlined in Figure 2, with corresponding RRs, 95% CIs, P values, I2 statistics, and forest plot. Risk factors associated with statistically significant increased risk of recurrence were Breslow >2 mm (RR [95% CI], 9.64 [1.30– 71.52]), invasion beyond subcutaneous fat (RR [95% CI], 7.61 [4.17–13.88]), Breslow >6 mm (RR [95% CI], 7.13 [3.04–16.72]), presence of PNI (RR [95% CI], 4.30 [2.80–6.60]), diameter >20 mm (RR [95% CI], 3.22 [1.91–5.45]), location on the temple (RR [95% CI], 3.20 [1.12–9.15]), and poor differentiation (RR [95% CI], 2.66 [1.72–4.14]).
Figure 2.
Summary of Risk Factors and Outcome Associations for cSCC. cSCC indicates cutaneous squamous cell carcinoma; PNI, perineural invasion.
Statistically significant risk factors for metastasis were invasion beyond subcutaneous fat (RR [95% CI], 11.21 [3.59–34.97]); Breslow >2 mm (RR [95% CI], 10.76 [2.55–45.31]); Breslow >6 mm (RR [95% CI], 6.93 [4.02–11.94]); diameter >20 mm (RR [95% CI], 6.15 [3.56–10.65]); poor differentiation (RR [95% CI], 4.98 [3.30– 7.49]); presence of PNI (RR [95% CI], 2.95 [2.31–3.75]); location on the temple (RR [95% CI], 2.82 [1.72–4.63]), ear (RR [95% CI], 2.33 [1.67–3.23]), and lip (RR [95% CI], 2.28 [1.54–3.37]); and immunosuppression (RR [95% CI], 1.59 [1.07–2.37]).
Several of the risk factors showed statistically significant association with DSD: diameter >20 mm (RR [95% CI], 19.10 [5.80–62.95]), poor differentiation (RR [95% CI], 5.65 [1.76–18.20]), location on the ear (RR [95% CI], 4.67 [1.28–17.12]) and lip (RR [95% CI], 4.55 [1.41–14.69]), invasion beyond subcutaneous fat (RR [95% CI], 4.49 [2.05–9.82]), and presence of PNI (RR [95% CI], 4.06 [3.10–5.32]).
Study Quality
Scores from the Newcastle-Ottawa Scale are provided in Table 2, with risk assessments for each study. Six studies (16.7%) were deemed to have low risk of bias due to presence of multivariate data; the other 30 studies were of high or unclear risk due to lack of adjustment for confounding variables. The number of studies for each risk factor ranged from 1 to 15. Risk factor and outcome associations are outlined in Figure 2, which visually depicts them in a forest plot.
Table 2.
Newcastle-Ottawa Scalea Scoring of Studies in the Meta-analysis
| Author, Year | Study Type | Selection | Comparability | Exposure/ Outcome |
Assessment of Risk |
|---|---|---|---|---|---|
| Dinehart and Pollack, 198912 | Cohort of consecutive SCC cases | *** | ** | ** | High/unclear |
| Dormand et al, 201013 | Cohort of limb SCC cases | *** | * | *** | High/unclear |
| Goepfert et al, 198414 | Cohort of consecutive SCC cases | **** | * | *** | High/unclear |
| Quaedvlieg et al, 200615 | Case control of metastatic SCC | **** | * | ** | High/unclear |
| Brantsch et al, 20084 | Cohort of SCC cases (prospective) | **** | ** | *** | High/unclear |
| Brinkman et al, 201416 | Cohort of SCC cases | **** | * | *** | Low |
| Brougham et al, 20125 | Cohort of SCC cases | **** | ** | ** | Low |
| Eroğlu et al, 199617 | Cohort of SCC cases | *** | ** | *** | High/unclear |
| Faustina et al, 200418 | Cohort of periocular SCC cases | *** | * | ** | High/unclear |
| Griffiths et al, 200220 | Cohort of consecutive SCC cases | **** | ** | *** | High/unclear |
| Pugliano-Mauro et al, 201021 | Cohort of high-risk SCC cases | *** | * | *** | High/unclear |
| Immerman et al, 198322 | Cohort of consecutive SCC cases | **** | ** | *** | High/unclear |
| Karia et al, 20146 | Cohort of consecutive SCC cases | **** | ** | *** | Low |
| Mehrany et al, 200523 | Case control | **** | ** | ** | High/unclear |
| Metchnikoff et al, 201224 | Cohort of consecutive heart/lung transplant SCC |
**** | ** | ** | High/unclear |
| Moore et al, 200525 | Cohort of SCC (prospective) | *** | * | ** | High/unclear |
| Mourouzis et al, 20097 | Cohort of excised SCC | **** | ** | ** | High/unclear |
| Mullen et al, 200626 | Cohort of trunk/extremity SCC | **** | ** | *** | High/unclear |
| Peat et al, 201227 | Case control | **** | ** | *** | High/unclear |
| Schmults et al, 20138 | Cohort of SCC cases | **** | ** | *** | Low |
| Toll et al, 201228 | Case control | **** | ** | *** | Low |
| Baker et al, 200129 | Cohort of SCC | **** | ** | ** | High/unclear |
| Cherpelis et al, 200230 | Cohort of SCC | **** | ** | ** | High/unclear |
| Clayman et al, 200531 | Cohort of SCC (prospective) | **** | ** | ** | High/unclear |
| Kyrgidis et al, 201032 | Cohort of consecutive SCC cases (prospective) |
**** | ** | *** | High/unclear |
| Leibovitch et al, 200533 | Cohort of PNI (prospective) | *** | * | ** | High/unclear |
| Roozeboom et al, 201334 | Cohort of consecutive SCC cases | **** | ** | ** | High/unclear |
| Pereira and Morgado, 199435 | Cohort of SCC | *** | * | * | High/unclear |
| Harwood et al, 200636 | Case control of cSCC in organ transplant recipients |
**** | * | ** | High/unclear |
| Friedman et al, 198537 | Cohort of SCC | **** | ** | *** | High/unclear |
| Breuninger et al, 199038 | Cohort of SCC | **** | ** | ** | High/unclear |
| Gonzalez et al, 201419 | Cohort of consecutive head/neck SCC cases |
*** | * | *** | Low |
| Krediet et al, 201539 | Cohort of consecutive cSCC | **** | ** | ** | High/unclear |
| Wermker et al, 201540 | Cohort of consecutive cSCC of ear | **** | * | *** | High/unclear |
| Vasconcelos et al, 201441 | Cohort of head/neck cSCC | **** | ** | *** | High/unclear |
| Stein and Tahan, 199442 | Cohort of cSCC of lip | **** | * | *** | High/unclear |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; PNI, perineural invasion; SCC, squamous cell carcinoma.
Possible scores are 0–4 asterisks for selection, 0–2 asterisks for comparability, and 0–3 asterisks for exposure/outcome, regarding risk of bias,
with indicating a low score
indicating the highest score.
Discussion
The meta-analysis of these data provides a quantitative value for each risk factor–outcome association. The pooled data for the majority of these risk factors showed a statistically significant association with the outcomes of interest; however, some previously reported risk factors for each outcome did not have a statistically significant association. Since the publication of the frequently cited report of cSCC outcomes by Rowe et al43 in 1992, several other studies have contributed to this body of knowledge. Most recently, staging systems have been proposed for the purpose of stratifying patients by outcomes. These include the American Joint Committee on Cancer (AJCC), Seventh Edition,44 Union for International Cancer Control (UICC),45 and Brigham and Women’s Hospital (BWH) staging systems,46 summarized in Table 3. Although the BWH system was developed on the basis of a single institution’s experience, the AJCC and UICC are based on expert consensus. The present meta-analysis summarizes the entire body of data on these previously reported risk factors. Therefore, our data provide, to our knowledge, the most comprehensive review of previously reported risk factors for cSCC related to outcomes of local recurrence, metastasis, and DSD.
Table 3.
Summary of the AJCC, UICC, and BWH Tumor (T) Staging Systems
| Tumor Staging System |
Definition |
|---|---|
| AJCC | |
| T1 | Tumor ≤2 cm in greatest dimension, with <2 high-risk factorsa |
| T2 | Tumor >2 cm in greatest dimension or with ≥2 high-risk factorsa |
| T3 | Tumor with invasion of orbit, maxilla, mandible, or temporal bones |
| T4 | Tumor with invasion of other bones or direct perineural invasion of skull base |
| UICC | |
| T1 | Tumor ≤2 cm in greatest dimension |
| T2 | Tumor >2 cm in greatest dimension |
| T3 | Tumor with invasion of deep structures (eg, muscle, cartilage, bone [excluding axial skeleton], orbit) |
| T4 | Tumor with invasion of axial skeleton or direct perineural invasion of skull base |
| BWH | |
| T1 | 0 high-risk factorsb |
| T2a | 1 high-risk factor |
| T2b | 2–3 high-risk factors |
| T3 | ≥4 high-risk factors or bone invasion |
Abbreviations: AJCC, American Joint Committee on Cancer; BWH, Brigham and Women’s Hospital; T, tumor stage from TNM staging system; UICC, International Union Against Cancer.
AJCC high-risk factors include >2 mm thickness, Clark level ≥IV, perineural invasion, primary site ear, primary site non-hair-bearing lip, or poorly differentiated histology.
BWH high-risk factors include tumor diameter ≥2 cm, poorly differentiated histologic findings, perineural invasion ≥0.1 mm, and tumor invasion beyond fat (excluding bone invasion, which automatically upgrades tumor to BWH T3).
Adapted from Karia et al.6 Used with permission.
Local Recurrence
Optimal management of cSCC is predicated on local tumor control because local recurrence is often the first indicator of aggressive biologic behavior.3,47 In our analysis, tumor depth, recorded as Breslow thickness (mm) or anatomic depth, was associated with the greatest RR of local recurrence. The highest-quality comparative data on Breslow depth was from Brantsch et al,4 one of the few prospective datasets in this analysis. The staging system reported in the AJCC Seventh Edition,44 for cSCC includes both Breslow depth >2 mm and Clark level ≥4 as high-risk characteristics. Meanwhile, the largest dataset describing anatomic depth was a retrospective analysis by Karia et al.6 In that study, depth was measured by anatomic depth rather than Breslow depth, with tumor depth beyond subcutaneous fat considered a high-risk characteristic. Although it is reassuring that the significance of tumor depth is verified through distinct methods of measure, the absence of a uniform measure and reporting together contribute to data heterogeneity and ambiguity for the clinician.
The theoretical advantage of Breslow depth measurement is the ability to perform refined analysis of data on a continuous numerical variable. From a practical perspective, however, measurement of Breslow depth is limited by time and the abundant frequency of transected shave biopsy specimens. Defining depth on the basis of the anatomic depth, such as an invasion beyond subcutaneous fat, simplifies the objective measure assessed in both horizontally and vertically sectioned tissue specimens. If adopted as a standard, the parameters for relation to anatomic structures, such as depth/invasion beyond subcutaneous fat, will require strict unambiguous definition.
PNI was associated with the greatest RR of local recurrence after tumor depth. As with tumor depth, the reporting of PNI was also heterogeneous. Several articles reported PNI as a binary variable, whereas others6,48 also analyzed on the basis of the diameter of the involved nerve. For the purpose of the present study, PNI was analyzed as a binary variable, including 6 articles, and was associated with an RR of local recurrence (RR [95% CI], 4.30 [2.80–6.60]; P <.01).8,14,24,33,34,41 A report by Karia et al6 also indicated that the RR increases with the size of the involved nerve, with RR (95% CI) of regional recurrence for nerves <0.1 mm in diameter of 5.6 (2.0–15.9) (P=.001) compared with nerves ≥0.1 mm in diameter (RR [95% CI], 10.4 [4.4–24.7]; P<.001). Interestingly, the AJCC Seventh Edition staging system for cSCC44 includes PNI as a binary variable for high risk, whereas the BWH staging system includes PNI as a risk factor when the involved nerve is ≥0.1 mm in diameter. As with tumor depth, the uniform definition and reporting of PNI are areas of critical need.
In descending order of RR, tumor diameter, location on the temple, and poor differentiation were associated with local recurrence. A cutoff of 20 mm was used for tumor diameter since it is uniformly included in the AJCC Seventh Edition staging system, the UICC staging system, and the BWH staging system. Although 4 studies in the analysis did show statistical significance,6,17,24,35 the prospective data from Brantsch et al4 did not find an association between diameter and recurrence on multivariate analysis; however, a statistically significant increase in risk was noted on univariate analysis (HR [95% CI], 3.47 [1.89–6.39]; P<.0001). Tumor location on the temple, although not part of the AJCC, UICC, or BWH staging system,44 was associated with a higher risk of recurrence (RR [95% CI], 3.20 [1.1–9.0]; P=.03) than tumor location on either lip or ear, albeit this finding was based on a single, but relatively large, retrospective analysis by Schmults et al.8 Poor differentiation also was found to have statistically significant association with recurrence (RR [95% CI], 2.66 [1.72–4.14]; P<.01), based on pooled data from 11 studies.4,6,17,19,22–24,34,37,39,41
Surprisingly, location on the lip or ear and immunosuppression were not associated with a statistically significant RR of local recurrence in the present meta-analysis. The lip43 and ear6,43 have been reported as risk factors in some studies; however, a sufficient body of data suggests otherwise.4,17,34 Interestingly, both the lip and the ear are included in the AJCC system but not in the UICC and BWH systems. Immunosuppression is a notable item within AJCC but is not included in the staging system, and it is not part of the BWH or UICC staging system.
For the purposes of the present analysis, immunosuppression was included as a general category without further stratification. Although immunosuppression was associated with recurrence in the prospective data by Brantsch et al4 and in certain subsets of well-defined patients, such as those with chronic lymphocytic leukemia,23 the results of these 2 studies were likely offset by other reports that did not stratify on the basis of the type of immunosuppression.6,34,39 Taken together, the data suggest that clarification of the nature of the immunosuppression is critical for understanding the factors that influence local recurrence. Notably, the I2 for location on the lip or the ear and for immunosuppression were increased at 52%, 59%, and 39%, respectively, indicating substantial data inconsistency, which also could contribute to a lack of statistical significance.
Metastasis
The correlation between cSCC risk factors and metastasis was the most well studied and well represented in our meta-analysis. The implications of metastasis are obvious since they generally are associated with poor outcomes.8,49,50 Thus, identifying risk factors associated with metastasis in cSCC is crucial for proper staging and for early identification of high-risk patients. As with local recurrence, tumor depth was associated with the highest RR of metastasis, our study showed, regardless of whether depth is measured as Breslow thickness or anatomic depth. These results are based on a total of 7 studies,4,6,25,34,38,40,42 one of which presents data on both Breslow depth and anatomic depth.38 Interestingly, the RR for metastasis in our analysis was greater for Breslow >2 mm than for Breslow >6 mm. This finding may reflect the absolute number of patients in the studies who had nodal metastases with primary tumor depths of 2 to 6 mm vs the smaller population of patients with the same outcome and a tumor depth >6 mm. Of importance, no patients in the prospective data from Brantsch et al4 had nodal metastasis with a Breslow depth <2 mm. The BWH group found that the anatomic depth associated with metastasis was the depth beyond subcutaneous fat (RR [95%CI], 7.0 [2.4–20.3]; P<.001).6 The practice gaps related to the reporting of depth hold true for association with metastasis and association with local recurrence.
Tumor diameter >20 mm and poor differentiation were associated with similar RRs of 6.15 (95% CI, 3.56–10.65) and 4.98 (95% CI, 3.30–7.49), respectively, for developing metastasis. A total of 8 included studies were based on tumor diameter and metastasis.4,6,19,26,30,38–40 Interestingly, the poorly differentiated cSCC and metastasis were the most well-studied association in this analysis, with a total of 18 publications that provided comparative data.4–7,13,15,16,19,25–28,30,34,38–40,42
The remaining risk factors associated with increased RR of metastasis in this analysis were PNI (RR [95% CI], 2.95 [2.31–3.75]), location on the temple (2.82 [1.72– 4.63]), location on the ear (2.33 [1.67–3.23]), location on the lip (2.28 [1.54–3.37]), and immunosuppression (1.59 [1.07–2.37]). The relative absence of heterogeneity for these risk factors is reflected in the I2 values of 0%, 6%, 27%, and 0% for location on the temple, ear, and lip and immunosuppression, respectively. It further underscores the association between these risk factors and metastasis. Tumor localization on the temple is not included as a risk factor in the AJCC, UICC, and BWH staging systems. Yet, these results suggest that it might be advisable to categorize this location with the other high-risk anatomic locations, such as ear and lip. Furthermore, a more stratified approach to the specific type of immunosuppression (eg, human immunodeficiency virus infection, solid-organ transplant, chronic lymphocytic leukemia) would afford greater precision in our risk analysis for metastasis in these patients.
Disease-Specific Death
Of all the outcomes, DSD had the least amount of comparative data available for analysis. The risk factor with the highest RR for DSD was a diameter >20 mm (RR [95% CI], 19.10 [5.80–62.95]). However, this conclusion is based solely on 1 study by Karia et al.6 Poor differentiation, location on the ear or the lip, invasion beyond subcutaneous fat, and PNI were associated with a statistically significant increase ranging from 4- to 6-fold, in the RR of DSD. Tumor depth, though significant, was not as highly associated with DSD as with local recurrence and metastasis—likely, a reflection of a paucity of data and the heterogeneity of available studies, reflected in the I2 of 76%. PNI as a risk factor for DSD had an I2 of 0%, reinforcing this association. The single report with the largest dataset related to DSD was from Karia et al.6 In their multivariate analysis, the risk factors with the greatest association to DSD were diameter >20 mm (HR [95% CI], 19.1 [5.8–63.0]; P<.001), invasion beyond subcutaneous fat (HR [95% CI], 11.1 [3.4–35.8]; P<.001), location on the ear (HR [95% CI], 10.1 [1.8–57.0]; P=.008), and poor differentiation (HR [95% CI], 10.0 [3.4–28.9]; P<.001). Only a single study that reported immunosuppression of 143 patients as a risk factor for DSD was included in our analysis, and the authors of that report acknowledged that their study was underpowered to assess prognostic significance.6,8 Given the increasing burden of cSCC and the estimated magnitude of DSD, the results of our analysis further emphasize the need for comprehensive reporting of these phenomena.
Limitations
This analysis was limited by several factors. First, it is possible that there were pertinent studies that were not identified because of inherent limitations in database literature searches. Second, the quality of evidence is limited by data largely derived from single-center experiences and retrospective analyses with heterogeneous data reporting and study design. Third, some included studies that had variable and at times limited follow-up data. However, no eligibility criteria were imposed on the basis of follow-up; rather, such limitations were reflected in the Newcastle-Ottawa scoring (Table 2). Fourth, many of the studies provided unadjusted estimates, and many of the risk factors can plausibly be codependent. Fifth, we included studies in which a small proportion of patients had SCC in the setting of scar, genetic disorder, or anogenital region.
Conclusions
This systematic review and meta-analysis study is, to our knowledge, the largest and most comprehensive study of risk factors related to outcomes for cSCC. These results verify the significance of many previously reported factors while providing a novel, robust quantitative risk for each risk factor and the associated outcomes of local recurrence, metastasis, and death. In the short term, these results may help guide clinicians in their risk assessment of patients, particularly patients with only 1 identified risk factor, while keeping in mind the inherent limitations of the data. In the long term, these results may be used to refine the evolving work on staging systems for cSCC while providing a renewed call to action for data collection. Not only are unambiguous definitions for each risk factor needed, but also a comprehensive, uniform reporting of risk factors and outcomes is needed, to provide optimal care for the increasing number of patients with cSCC in the United States and globally.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by Center for Clinical and Translational Science (CCaTS). This publication was supported in part by Clinical and Translational Science Award Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
| Funding/Sponsor was involved? | |
| Design and conduct of the study | Yes__ No_x_ |
| Collection, management, analysis and interpretation of data | Yes__ No_x_ |
| Preparation, review, or approval of the manuscript | Yes__ No_x_ |
| Decision to submit the manuscript for publication | Yes__ No_x_ |
Abbreviations
- AJCC
American Joint Commission of Cancer
- BWH
Brigham and Women’s Hospital
- cSCC
cutaneous squamous cell carcinoma
- DSD
disease-specific death
- HR
hazard ratio
- OR
odds ratio
- PNI
perineural invasion
- RR
risk ratio
- SCC
squamous cell carcinoma
- UICC
Union for International Cancer Control
Footnotes
A portion of this report’s results was presented at the annual meeting of the American College of Mohs Surgery, San Antonio, Texas, April 30, 2015.
Author Contributions: Drs Thompson and Baum had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Thompson, Murad, Baum. Analysis and interpretation of data: Thompson, Kelley, Murad, Baum. Drafting of the manuscript: Thompson, Baum. Critical revision of the manuscript for important intellectual content: Thompson, Kelley, Murad, Baum. Statistical analysis: Murad. Obtained funding: Murad. Administrative, technical, or material support: Prokop. Study supervision: Baum.
Financial Disclosure: None reported.
Publisher: To expedite proof approval, send proof via email to scipubs@mayo.edu.
References
- 1.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 4.Brantsch KD, Meisner C, Schonfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 5.Brougham ND, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106(7):811–815. doi: 10.1002/jso.23155. [DOI] [PubMed] [Google Scholar]
- 6.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327–334. doi: 10.1200/JCO.2012.48.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mourouzis C, Boynton A, Grant J, et al. Cutaneous head and neck SCCs and risk of nodal metastasis: UK experience. J Craniomaxillofac Surg. 2009;37(8):443–447. doi: 10.1016/j.jcms.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541–547. doi: 10.1001/jamadermatol.2013.2139. [DOI] [PubMed] [Google Scholar]
- 9.Deeks JJ, Dinnes J, D’Amico R, et al. International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinehart SM, Pollack SV. Metastases from squamous cell carcinoma of the skin and lip: an analysis of twenty-seven cases. J Am Acad Dermatol. 1989;21(2 Pt 1):241–248. doi: 10.1016/s0190-9622(89)70168-7. [DOI] [PubMed] [Google Scholar]
- 13.Dormand EL, Ridha H, Vesely MJ. Long-term outcome of squamous cell carcinoma of the upper and lower limbs. J Plast Reconstr Aesthet Surg. 2010;63(10):1705–1711. doi: 10.1016/j.bjps.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148(4):542–547. doi: 10.1016/0002-9610(84)90385-4. [DOI] [PubMed] [Google Scholar]
- 15.Quaedvlieg PJ, Creytens DH, Epping GG, et al. Histopathological characteristics of metastasizing squamous cell carcinoma of the skin and lips. Histopathology. 2006;49(3):256–264. doi: 10.1111/j.1365-2559.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkman JN, Hajder E, van der Holt B, Den Bakker MA, Hovius SE, Mureau MA. The effect of differentiation grade of cutaneous squamous cell carcinoma on excision margins, local recurrence, metastasis, and patient survival: a retrospective follow-up study. Ann Plast Surg. doi: 10.1097/SAP.0000000000000110. [published online January 7, 2014] [DOI] [PubMed] [Google Scholar]
- 17.Eroglu A, Berberoglu U, Berreroglu S. Risk factors related to locoregional recurrence in squamous cell carcinoma of the skin. J Surg Oncol. 1996;61(2):124–130. doi: 10.1002/(SICI)1096-9098(199602)61:2<124::AID-JSO6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Faustina M, Diba R, Ahmadi MA, Esmaeli B. Patterns of regional and distant metastasis in patients with eyelid and periocular squamous cell carcinoma. Ophthalmology. 2005;111(10):1930–1932. doi: 10.1016/j.ophtha.2004.02.009. Erratum in: Ophthalmology. 2005;112(3):446. Gutstein, Brett F [removed] [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez A, Etchichury D, Creydt MP, Rivero M, Arizmendi CS. Prognostic factors for local recurrence and lymph node metastasis in cutaneous squamous cell carcinoma of the head and neck treated with Mohs surgery. Br J Dermatol: abstracts of the XV World Congress on Cancers of the Skin. 2014;171(s4):59–60. [Google Scholar]
- 20.Griffiths RW, Feeley K, Suvarna SK. Audit of clinical and histological prognostic factors in primary invasive squamous cell carcinoma of the skin: assessment in a minimum 5 year follow-up study after conventional excisional surgery. Br J Plast Surg. 2002;55(4):287–292. doi: 10.1054/bjps.2002.3833. [DOI] [PubMed] [Google Scholar]
- 21.Pugliano-Mauro M, Goldman G. Mohs surgery is effective for high-risk cutaneous squamous cell carcinoma. Dermatol Surg. 2010;36(10):1544–1553. doi: 10.1111/j.1524-4725.2010.01576.x. [DOI] [PubMed] [Google Scholar]
- 22.Immerman SC, Scanlon EF, Christ M, Knox KL. Recurrent squamous cell carcinoma of the skin. Cancer. 1983;51(8):1537–1540. doi: 10.1002/1097-0142(19830415)51:8<1537::aid-cncr2820510830>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31(1):38–42. doi: 10.1111/j.1524-4725.2005.31006. [DOI] [PubMed] [Google Scholar]
- 24.Metchnikoff C, Mully T, Singer JP, Golden JA, Arron ST. The 7th edition AJCC staging system for cutaneous squamous cell carcinoma accurately predicts risk of recurrence for heart and lung transplant recipients. J Am Acad Dermatol. 2012;67(5):829–835. doi: 10.1016/j.jaad.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore BA, Weber RS, Prieto V, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(9):1561–1567. doi: 10.1097/01.mlg.0000173202.56739.9f. [DOI] [PubMed] [Google Scholar]
- 26.Mullen JT, Feng L, Xing Y, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol. 2006;13(7):902–909. doi: 10.1245/ASO.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Peat B, Insull P, Ayers R. Risk stratification for metastasis from cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg. 2012;82(4):230–233. doi: 10.1111/j.1445-2197.2011.05994.x. [DOI] [PubMed] [Google Scholar]
- 28.Toll A, Gimeno-Beltran J, Ferrandiz-Pulido C, et al. D2-40 immunohistochemical overexpression in cutaneous squamous cell carcinomas: a marker of metastatic risk. J Am Acad Dermatol. 2012;67(6):1310–1318. doi: 10.1016/j.jaad.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Baker NJ, Webb AA, Macpherson D. Surgical management of cutaneous squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2001;39(2):87–90. doi: 10.1054/bjom.2000.0584. [DOI] [PubMed] [Google Scholar]
- 30.Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28(3):268–273. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 31.Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 32.Kyrgidis A, Tzellos TG, Kechagias N, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563–1572. doi: 10.1016/j.ejca.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 33.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II: perineural invasion. J Am Acad Dermatol. 2005;53(2):261–266. doi: 10.1016/j.jaad.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Roozeboom MH, Lohman BG, Westers-Attema A, et al. Clinical and histological prognostic factors for local recurrence and metastasis of cutaneous squamous cell carcinoma: analysis of a defined population. Acta Derm Venereol. 2013;93(4):417–421. doi: 10.2340/00015555-1501. [DOI] [PubMed] [Google Scholar]
- 35.Pereira MF, Morgado MA. Cryosurgery of malignant cutaneous tumours: ten years experience. Skin Cancer. 1994;9(4):179–185. [Google Scholar]
- 36.Harwood CA, Proby CM, McGregor JM, Sheaff MT, Leigh IM, Cerio R. Clinicopathologic features of skin cancer in organ transplant recipients: a retrospective case-control series. J Am Acad Dermatol. 2006;54(2):290–300. doi: 10.1016/j.jaad.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Friedman HI, Cooper PH, Wanebo HJ. Prognostic and therapeutic use of microstaging of cutaneous squamous cell carcinoma of the trunk and extremities. Cancer. 1985;56(5):1099–1105. doi: 10.1002/1097-0142(19850901)56:5<1099::aid-cncr2820560524>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.Breuninger H, Black B, Rassner G. Microstaging of squamous cell carcinomas. Am J Clin Pathol. 1990;94(5):624–627. doi: 10.1093/ajcp/94.5.624. [DOI] [PubMed] [Google Scholar]
- 39.Krediet JT, Beyer M, Lenz K, et al. Sentinel lymph node biopsy and risk factors for predicting metastasis in cutaneous squamous cell carcinoma. Br J Dermatol. 2015;172(4):1029–1036. doi: 10.1111/bjd.13508. [DOI] [PubMed] [Google Scholar]
- 40.Wermker K, Kluwig J, Schipmann S, Klein M, Schulze HJ, Hallermann C. Prediction score for lymph node metastasis from cutaneous squamous cell carcinoma of the external ear. Eur J Surg Oncol. 2015;41(1):128–135. doi: 10.1016/j.ejso.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Vasconcelos L, Melo JC, Miot HA, Marques ME, Abbade LP. Invasive head and neck cutaneous squamous cell carcinoma: clinical and histopathological characteristics, frequency of local recurrence and metastasis. An Bras Dermatol. 2014;89(4):562–568. doi: 10.1590/abd1806-4841.20142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein AL, Tahan SR. Histologic correlates of metastasis in primary invasive squamous cell carcinoma of the lip. J Cutan Pathol. 1994;21(1):16–21. doi: 10.1111/j.1600-0560.1994.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 43.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–990. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 44.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 45.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Chichester, UK: Wiley-Blackwell; 2010. [Google Scholar]
- 46.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402–410. doi: 10.1001/jamadermatol.2013.2456. [DOI] [PubMed] [Google Scholar]
- 47.Dinehart SM, Peterson S. Evaluation of the American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma and proposal of a new staging system. Dermatol Surg. 2005;31(11 Pt 1):1379–1384. doi: 10.2310/6350.2005.31201. [DOI] [PubMed] [Google Scholar]
- 48.Carter JB, Johnson MM, Chua TL, Karia PS, Schmults CD. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149(1):35–41. doi: 10.1001/jamadermatol.2013.746. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt C, Martin JM, Khoo E, Plank A, Grigg R. Outcomes of nodal metastatic cutaneous squamous cell carcinoma of the head and neck treated in a regional center. Head Neck. doi: 10.1002/hed.23843. [published online July 4, 2014] [DOI] [PubMed] [Google Scholar]
- 50.Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33(8):885–899. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.