Abstract
Diffusion kurtosis imaging (DKI) can offer a useful complementary tool to routine diffusion MRI for improved stratification of heterogeneous tissue damage in acute ischemic stroke. However its relatively long imaging time has hampered its clinical applications in the emergency setting. Recently proposed fast DKI approach substantially shortens the imaging time, which may help overcome the scan time limitation. However, to date, the sensitivity of fast DKI protocol for imaging acute stroke has not yet been fully described. In this study, we performed routine and fast DKI scans in a rodent model of acute stroke, and compared sensitivity of diffusivity and kurtosis indices (i.e. axial, radial and mean) in depicting acute ischemic lesion. In addition, we analyzed the contrast-to-noise ratio (CNR) between the ipsilateral ischemic and contralateral normal regions using both conventional and fast DKI methods. We found that mean kurtosis shows a relative change of 47.1±7.3% between the ischemic and contralateral normal regions, being the most sensitive parameter in revealing acute ischemic injury. The two DKI methods yielded highly correlated diffusivity and kurtosis measures and lesion volumes (R2≥0.90, P<0.01). Importantly, the fast DKI method exhibited significantly higher CNR of mean kurtosis (1.6±0.2) compared to the routine tensor protocol (1.3±0.2, P<0.05), with its CNR per unit time (CNR efficiency) approximately doubled when the scan time is taken into account. In conclusion, the fast DKI method provides excellent sensitivity and efficiency to image acute ischemic tissue damage, which are essential for imaging-guided and individualized stroke treatment.
Keywords: acute stroke, contrast-to-noise ratio, diffusion kurtosis imaging, mean diffusion, mean kurtosis
INTRODUCTION
Diffusion kurtosis imaging (DKI) is a relatively new MRI technique that measures the degree of peakedness of a random walk distribution, providing unique information on non-Gaussian diffusion in complex biological tissues (1-14). Since previous studies have demonstrated that mean kurtosis (MK) (i.e. the average kurtosis along all uniformly distributed directions) is sensitive to subtle structural alterations following acute ischemia (15,16), DKI has gained tremendous interest as a complementary MRI technique to the widely-used diffusion-weighted imaging (DWI) in acute stroke (17). In animal stroke models, initially decreased mean diffusivity (MD) renormalizes typically in 1-2 days after ischemia and then becomes elevated afterwards, while MK elevation persists up to one week (15). The relative magnitude change of MK is generally twice larger than that of MD (16,18). Furthermore, ischemic tissue with decreased diffusivity without changes in kurtosis shows a propensity of restoration following early reperfusion whereas areas with concurrent abnormalities in diffusion and kurtosis show poor recovery (19). These findings strongly suggest that DKI is a promising diagnostic method that can unravel complex ischemic tissue injury and provide improved tissue stratification, which are essential for imaging-guided and individualized stroke management (20,21).
The commonly-used DKI protocol requires DWI images at multiple b-values along fifteen or more directions in order to resolve diffusion and kurtosis tensors; thereby significantly lengthens its scan time (1,22). As millions of neurons are lost each minute in acute stroke patients, it is important to minimize the scan time before any new imaging methods can be adopted to the emergency setting (23). Recently, Hansen et al. introduced a fast DKI protocol for mapping mean diffusivity and kurtosis (24,25). With acquisition of thirteen DWI images, followed by linear combination of log diffusion signals, they found that the obtained diffusion and kurtosis maps agree well with those estimated from conventional DKI. This approach has been further validated in applications such as tumor grading in glioma patients (26) and acute stroke imaging in rodent models (27). Our previous work revealed that MD/MK measurements from the conventional DTI tensor-model (6 directions) and the fast DKI method were in good agreement (27). However, it is not clear whether mean diffusion and kurtosis measurements are sufficient when compared to conventional DKI approach during acute stroke imaging. Herein we evaluated the sensitivity of tensor indices to detect acute ischemic injury, and the commonly used tensor-model DKI (conventional) and fast DKI methods were compared by means of the contrast-to-noise ratio (CNR) and CNR per unit time (efficiency) using an acute ischemic stroke model to elucidate its diagnostic value in the acute stroke setting.
METHODS
Animals
All experiments were approved by the institutional animal care and use committee. Adult male Wistar rats (n=12) were anesthetized with inhalation of 1.5-2.0% Isoflurane-air mixture throughout all procedures. Heart rate and oxygen content of the blood (SpO2) were monitored continuously (Nonin Pulse Oximeter 8600, Plymouth, MN), and body temperature was maintained by a circulating warm water jacket positioned around the torso. Unilateral stroke was induced following a standard intraluminal middle cerebral artery occlusion (MCAO) procedure. Rats underwent MR experiments 60 minutes after the procedure. One rat was excluded from the analysis due to failed MCAO surgery, which displayed a very small ischemic lesion in the hypothalamus region.
MRI
MRI scans were performed at a 4.7-Tesla small-bore scanner (Bruker Biospec, Billerica, MA). Animals were immobilized with a tightly fitted MRI-compatible cradle with bite and ear bars and anesthetized throughout the study. Five slices, with slice thickness/gap = 1.8/0.2 mm, were acquired with a single-shot echo-planar imaging (EPI) sequence. Imaging parameters applied to both conventional and fast DKI protocols include: field of view = 20x20 mm2, acquisition matrix = 48x48, the gradient pulse duration/diffusion time (δ/Δ) = 6/20 ms, repetition time (TR)/echo time (TE) = 2,500/36.6 ms, and number of signal average (NSA) = 4. For the conventional DKI protocol, two b-values of 1,000 and 2,500 s/mm2 were applied in fifteen diffusion directions in addition to a single reference image of b = 0. The scan time was 5 min and 10 s. The fast DKI protocol consisted one reference image of b = 0 , three images of b = 1,000 s/mm2 along gradient directions of (1,0,0), (0,1,0) and (0,0,1), and nine images of b = 2,500 s/mm2 along nine diffusion directions of , and , which were defined as and , and similarly for i =2 and 3. Note that the superscript i in labels the position of the “1” while in and it labels the position of the “0” (24). The fast DKI scan time was 2 min and 10 s.
Image Analysis
The data acquired with conventional DKI scheme was analyzed using the Diffusion Kurtosis Estimator (DKE; http://academicdepartments.musc.edu/cbi/dki/dke.html). We chose linearly constrained algorithm for the tensor estimation in DKI model fitting, and fractional anisotropy (FA), axial (D∥) and radial (D⊥) diffusivity, axial (K∥) and radial (K⊥) kurtosis, and tensor-based MD and MK (denoted as MDtensor and MKtensor, respectively) were derived (28). Due to sufficient anesthesia and restricted motion using a rat cradle, we observed no head motion and hence, postprocessing motion correction was not necessary. For the fast DKI, images were analyzed in MATLAB (MathWorks, Natick, MA). Mean diffusion (denoted as MDfast) was calculated as the mean of MDx,y,z following the formula of Jensen et al. (29):
| (1) |
where , and b3 = 2,500 s/mm2. Furthermore, directional average kurtosis (denoted as MKfast) was obtained using the method described by Hansen et al. (24):
| (2) |
Note that MKtensor is the mean kurtosis averaged over all directions, whereas MKfast represents average of the kurtosis tensor (24,25). The conventional DKI acquisition scheme was denoted as the tensor approach because of its capability in measuring axial and radial diffusivity/kurtosis.
Image Segmentation
Baseline mean MD and its standard deviation (SD) were measured from the contralateral normal brain. The background and ventricles were selected and removed with empirical thresholding of DWI signal(with the b-value of 1000 s/mm2)>1200 (a.u.) and MDtensor<1.2 μm2/ms (30). The contralateral normal region was determined from the manually drawn brain midline for each animal. The ischemic lesion was defined using an MDtensor threshold criterion that was two SDs below the mean MD of the contralateral normal region (27,31). The reference region of interest (ROI) was designated by mirroring of the semiautomatically segmented lesion into the contralateral brain. The MKtensor, MDfast and MKfast lesions were similarly defined by thresholding at two SDs outside their respective means. The semiautomatic segmentation results were reviewed by a board-certified neuroradiologist, whose visual assessments were in good agreement with computational method.
Data Analysis
The contrast-to-noise ratio (CNR) was calculated as:
| (3) |
where S and σ represent the signal of the diffusion or kurtosis and their SDs from the ischemic and contralateral regions, respectively. Note that the CNR index calculated here includes not only image noise but also tissue heterogeneity. Additionally CNR per unit time (CNR efficiency), defined as , was calculated for each protocol (32).
Two-tailed Student’s t-test was performed between paired measurements in ipsilateral ischemic and contralateral normal regions. Statistical significance of measurement differences across the ROIs was tested using one-way analysis of variance (ANOVA) with Bonferroni correction (P < 0.05).
Results
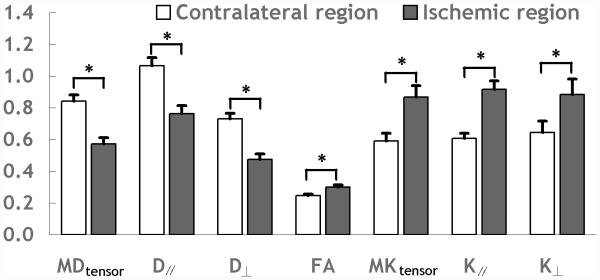
Fig. 1 shows representative multi-parametric maps of diffusion indices (MDtensor, D∥ and D⊥), FA and kurtosis indices (MKtensor, and K∥ and K⊥) from the conventional DKI scan on an acute stroke rat. Ischemic lesion showed diffusion decrease and FA and kurtosis increase when compared to the contralateral normal region, consistent with prior studies (15,33). Fig. 2 compares diffusion and kurtosis measurements of the contralateral normal and ipsilateral ischemic regions from the conventional DKI method. The ipsilateral ischemic region was defined by thresholding MDtensor at two SDs below the mean value in the contralateral normal region, so that the paired measurements of the two DKI methods could be compared. There were significant decreases in diffusion indices (MDtensor: 32.1±2.1%, D∥: 28.4±2.1%, and D⊥: 34.9±2.3%), and increase of FA (22.5±4.5%) and kurtosis indices (MKtensor: 47.1±7.3%, K∥: 50.6±6.2%, and K⊥: 37.4±7.6%) in the ischemic regions compared to those in the contralateral regions (P<0.05). Notably, the magnitude of percentage changes were significantly higher in MKtensor and K∥ than other indices (P<0.05). In addition, the difference between the percentage changes of MKtensor and K∥ was not statistically significant (P>0.05). The results indicate that MKtensor and/or K∥ provide the most sensitive measurements to detect acute ischemia-induced structural changes. Notably, the percentage change of MKfast was 50.9±4.7% (not shown), slightly higher than that of the MKtensor (P = 0.05), suggesting elevated sensitivity of MKfast for revealing acute ischemic tissue damage than that of conventional approach.
Fig. 1.
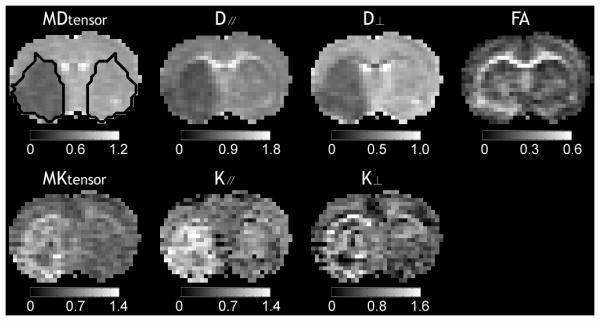
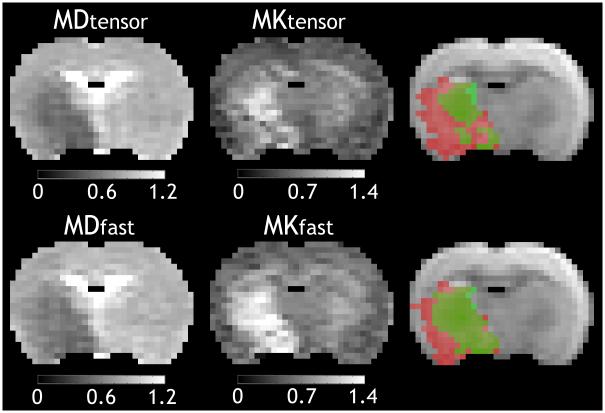
Multi-parametric maps of mean diffusivity (MDtensor), axial diffusivity (D∥), radial diffusivity (D⊥), fractional anisotropy (FA), mean kurtosis (MKtensor), axial kurtosis (K∥), and radial kurtosis (K⊥) obtained using the conventional DKI method from a representative acute ischemic stroke rat. Ipsilateral ischemic lesion was defined using a threshold-based algorithm and the lesion was mirrored into the contralateral normal region as the reference region of interest (ROI). The unit of diffusivity is μm2/ms.
Fig. 2.
Comparison of change of multiple indices between ischemic and contralateral regions obtained using the conventional DKI method. The unit of diffusivity is μm2/ms.
Fig. 3 compares MD and MK maps between the conventional and fast DKI methods from a representative acute stroke animal. MD and MK lesions determined from the aforementioned threshold-based algorithm were overlaid on a diffusion-weighted image and were colored in red and green, respectively. Note that regions with concurrent mean diffusivity and mean kurtosis abnormalities were shown in deep green, and there were only a few pixels in the medial caudate-putamen showing kurtosis abnormality without diffusion deficit (light green). Importantly, there was noticeable mismatch between diffusion and kurtosis lesions. In comparison of relative CNR over the MK maps between the two methods, fast DKI method yielded significantly higher CNR between the reference and ischemic regions (1.6±0.2), compared to that from the conventional DKI method (1.3±0.2, P<0.05). The acquisition time of the fast DKI method was about 50% shorter than that of the conventional DKI protocol, leading to 1.9 times higher gain of CNR efficiency. For the representative rat in Fig. 3, its lateral striatum showed slightly higher MKtensor in the ipsilateral ischemic lesion than that of the contralateral region, but was not detected using the threshold-based algorithm. This is likely due to the relatively low sensitivity of the routine DKI method, demonstrating the benefits of the fast DKI approach in capturing kurtosis abnormality. Despite that CNR of MDfast (3.0±0.4) is slightly lower than that of MDtensor (3.4±0.4, P<0.05), the CNR efficiency from fast DKI method was 1.4 times of that of conventional DKI, owing to its shortened scan time. These observations suggest that the fast DKI protocol is advantageous in circumstances where MK and MD need to be quickly mapped, which would be particularly useful in the acute stroke setting.
Fig. 3.
Comparison of mean diffusivity and mean kurtosis maps obtained using the conventional and the fast DKI methods. Lesions of mean diffusivity and mean kurtosis were overlaid on a diffusion-weighted image and were colored in red and green, respectively. Note that regions with concurrent diffusivity and mean kurtosis abnormalities were shown in deep green, and there were only a few pixels in the medial caudate-putamen showing kurtosis abnormality without diffusion deficit (light green). The unit of diffusivity is μm2/ms.
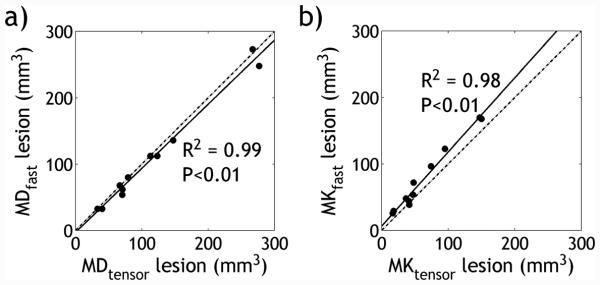
Fig. 4 shows that the lesion sizes between the two DKI methods are highly correlated (Pearson Correlation, P<0.01). Specifically, average volumes of the MD abnormality from the conventional and fast methods measured at 117.0±79.9 mm3 and 110.0±77.8 mm3, respectively (R2 = 0.99). The volumes of the MK abnormality from the two methods were 64.9±44.8 mm3 and 78.9±50.6 mm3, respectively (R2 = 0.98). In addition, values of MD and MK were in good agreement between the two DKI methods (Pearson Correlation, P<0.01). Explicitly, the baseline MD from the conventional and fast methods were 0.84±0.04 and 0.87±0.04 μm2/ms (R2 = 0.93) in the contralateral region, and 0.57±0.04 and 0.60±0.04 μm2/ms (R2 = 0.94) in the ipsilateral ischemic region, respectively. In comparison, the baseline MK was 0.59±0.05 and 0.66±0.05 (R2 = 0.83) in the contralateral region, and 0.87±0.07 and 1.00±0.09 (R2 = 0.90) in the ischemic region, from the conventional and fast DKI protocols, respectively.
Fig. 4.
Correlation of lesion volume size of (a) mean diffusion and (b) mean kurtosis determined from the conventional and fast DKI methods. The linear regression is shown as a solid line, and the identity line shown as a dash-dotted line.
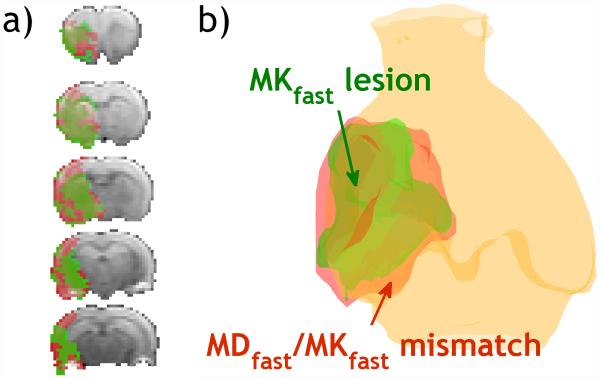
Fig. 5a illustrates representative multi-slice diffusion (MDfast) and kurtosis (MKfast) lesion mismatch from an acute stroke rat, and the corresponding 3D volume rendering of MDfast and MKfast lesions overlaid on a structural scan (Fig. 5b). Notably, there was lesion mismatch with MKfast lesion significantly smaller than MDfast lesion, with their normalized lesion area ratio being 0.75±0.11 (P<0.05). Fig. 6 compares MDfast and MKfast values in the contralateral normal area, ipsilateral MDfast lesion and MKfast lesion (defined as two SDs outside their respective means from the contralateral normal region) as well as their mismatch. Although MDfast values were significantly reduced in ischemic lesions (MDfast lesion, MKfast lesion, and their mismatch area) from the contralateral normal area (P<0.05), differences of MDfast values across the three lesions were statistically insignificant. In comparison, MKfast across the three lesion areas was significantly elevated from the normal area (P<0.05), and more importantly, MKfast shows significant difference across the three lesion areas (P<0.05). This shows that the fast DKI method can indeed resolve the heterogeneity within the commonly used DWI approach.
Fig. 5.
Demonstration of fast DKI in an acute stroke rat. (a) Multi-slice diffusion and kurtosis lesion mismatch from fast DKI. (b) 3D illustration of mean diffusion/mean kurtosis lesions and their mismatch in a representative acute stroke rat using the fast DKI method.
Fig. 6.
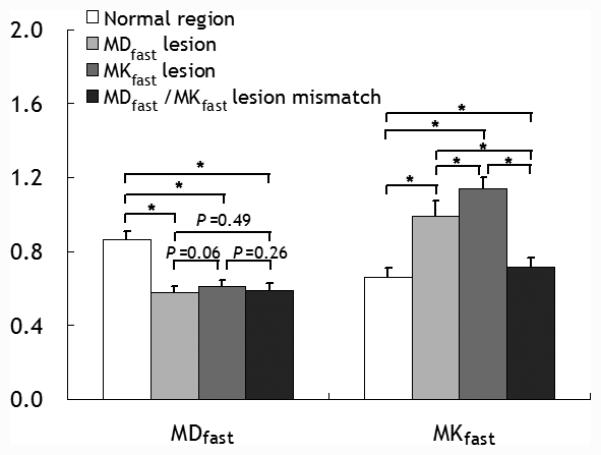
Comparison of mean diffusion and kurtosis values in the contralateral normal area, ipsilateral ischemic diffusion lesion (MDfast lesion), kurtosis lesion (MKfast lesion), and MDfast/MKfast lesion mismatch obtained using the fast DKI method. The unit of diffusivity is μm2/ms.
Discussion
DKI is an emerging method complementary to the conventional diffusion MRI that enables refined characterization of ischemic tissue injury (15,18,34-37). Previous in vivo DKI studies mostly used the diffusion kurtosis tensor approach that models anisotropic properties of the brain structure. However, its scan time is relatively long, limiting its adoption in the acute stroke setting. MK, a composite parameter of non-Gaussian water diffusion free from tensor estimation, provides sensitive and fast characterization of acute ischemic tissue injury (29). Our study demonstrated concordant results with previously published data that indicated MK renders the highest sensitivity to detect early structural alterations in acute stroke (16,18). Moreover, we found significant difference in MKfast values across three distinctive ischemic regions (e.g., MDfast lesion, MKfast lesion and their mismatch) but no differences in their MDfast. Notably, MK difference between the ischemic and contralateral normal regions (Sischemia - Scontralateral) was 0.28±0.04 and 0.34±0.04 for the routine and fast DKI MRI, respectively (P<0.05), while their noise level was similar (0.22±0.02 vs. 0.21±0.02, P = 0.33). The higher CNR in MK measurement observed in the fast DKI method is largely due to increased signal contrast between normal and ischemic regions, as shown in Fig. 3, which nearly doubles the CNR efficiency when the scan time is taken into account. This upholds our postulation that the fast DKI method is suitable for acute stroke imaging.
It is important to point out that MD lesions and part of MK lesions showed gradual transition from the normal brain tissue to the ischemic area. The threshold-based lesion segmentation algorithm performed reasonably well with less bias in the situation where manual determination of transitional regions would be otherwise extremely difficult. Although higher spatial resolution may better resolve ischemic lesions, our study chose to balance between SNR and scan time without significant penalty in lesion definition. It has been shown that MKfast and MKtensor convey similar diagnostic information on the underlying ischemic tissue damage (27). Indeed, MK can be accurately determined based on measures along nine directions without need of computationally expensive nonlinear regression fitting of diffusion signals (24,38,39). Notably, for in vivo applications, the fast DKI method tends to slightly overestimate both MD and MK indices compared to the conventional method. Apart from the differences in direction and number of diffusion measurements, this discordance may also be attributed partly to the different mathematical models assumed in equations for diffusion and kurtosis indices. Specifically, the fast DKI method approximates kurtosis equation using the Taylor expansion of the logarithm of the diffusion-weighted signal, assuming negligible high order diffusion (e.g., O(b3)) and Rician noise terms (24), whereas such terms are treated as the residual fitting error in the conventional DKI approach (27). Nevertheless, pairwise statistical test of MD and MK measurements showed significant correlations between the two methods. MDfast lesion volume was found to be slightly smaller than MDtensor lesion volume with marginal significance (P=0.04), while MKfast lesion was significantly larger than that of MKtensor. This may be because that the sensitivity advantage in the fast DKI method allows more accurate lesion segmentation (Fig. 3). Besides, considering the increased risk of intracranial hemorrhage upon late recanalization, a slightly more conservative estimation of diffusion/kurtosis mismatch may be beneficial to minimize risk of unnecessary interventional rescue therapy (40-42). One pitfall of the fast DKI method is its inability to resolve the full diffusion and kurtosis tensors, which limits its general applicability (43-49). However, for the case of acute stroke imaging, MD and MK maps render superior sensitivity compared to the anisotropy maps as the anisotropic diffusion measurement may not consistently ascertain ischemic insult (50-52). Therefore, fast DKI could serve as a time-efficient imaging approach to quickly assess tissue salvageability, advancing imaging-guided triage for recanalization therapy.
CONCLUSION
In this study, we recapitulated that mean kurtosis is one of the most sensitive parameters to detect acute stroke injury, and demonstrated the enhanced sensitivity of the fast DKI method for stratification of graded ischemic tissue injury during acute stroke. The fast DKI method may provide critically important information for resolving heterogeneous DWI lesion, particularly translatable in the acute stroke setting.
Acknowledgments
Sources of Support: National Basic Research Program of China (2015CB755500), NSFC (81571668 and 81471721), Shenzhen Science and Technology Program (JCYJ20140610151856743), NIH/NINDS (1R21NS085574 and 1R01NS083654).
Abbreviations
- CNR
contrast-to-noise ratio
- DKI
diffusion kurtosis imaging
- MD
mean diffusivity
- MK
mean kurtosis
REFERENCES
- 1.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 2.Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19(2):236–247. doi: 10.1002/nbm.1020. [DOI] [PubMed] [Google Scholar]
- 3.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29(3):514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A, Ferris SH, Helpern JA. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 2008;28(6):1345–1350. doi: 10.1002/jmri.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. NeuroImage. 2009;45(2):386–392. doi: 10.1016/j.neuroimage.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23(7):836–848. doi: 10.1002/nbm.1506. [DOI] [PubMed] [Google Scholar]
- 7.Van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, De Keyzer F, Van Gool SW, Van Calenbergh F, De Vleeschouwer S, Van Hecke W, Sunaert S. Gliomas: Diffusion Kurtosis MR Imaging in Grading. Radiology. 2012;263(2):492–501. doi: 10.1148/radiol.12110927. [DOI] [PubMed] [Google Scholar]
- 8.Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. NeuroImage. 2012;59(1):467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Zhang Y, Wong CS, Wu PM, Zhang Z, Gao J, Qiu D, Huang B. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR Biomed. 2012;25(12):1369–1377. doi: 10.1002/nbm.2809. [DOI] [PubMed] [Google Scholar]
- 10.Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, Silver JM, Grossman RI, Inglese M. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma. 2012;29(13):2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhoutte G, Pereson S, Delgado YPR, Guns PJ, Asselbergh B, Veraart J, Sijbers J, Verhoye M, Van Broeckhoven C, Van der Linden A. Diffusion kurtosis imaging to detect amyloidosis in an APP/PS1 mouse model for Alzheimer's disease. Magn Reson Med. 2013;69(4):1115–1121. doi: 10.1002/mrm.24680. [DOI] [PubMed] [Google Scholar]
- 12.Falangola MF, Jensen JH, Tabesh A, Hu C, Deardorff RL, Babb JS, Ferris S, Helpern JA. Non-Gaussian diffusion MRI assessment of brain microstructure in mild cognitive impairment and Alzheimer's disease. Magn Reson Imaging. 2013;31(6):840–846. doi: 10.1016/j.mri.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong NJ, Wong CS, Chan CC, Leung LM, Chu YC. Correlations between microstructural alterations and severity of cognitive deficiency in Alzheimer's disease and mild cognitive impairment: a diffusional kurtosis imaging study. Magn Reson Imaging. 2013;31(5):688–694. doi: 10.1016/j.mri.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Gooijers J, Leemans A, Van Cauter S, Sunaert S, Swinnen SP, Caeyenberghs K. White matter organization in relation to upper limb motor control in healthy subjects: exploring the added value of diffusion kurtosis imaging. Brain Struct Funct. 2014;219(5):1627–1638. doi: 10.1007/s00429-013-0590-y. [DOI] [PubMed] [Google Scholar]
- 15.Hui ES, Du F, Huang S, Shen Q, Duong TQ. Spatiotemporal dynamics of diffusional kurtosis, mean diffusivity and perfusion changes in experimental stroke. Brain Res. 2012;1451(0):100–109. doi: 10.1016/j.brainres.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinberg F, Ciobanu L, Farrher E, Shah NJ. Diffusion kurtosis imaging and log-normal distribution function imaging enhance the visualisation of lesions in animal stroke models. NMR Biomed. 2012;25(11):1295–1304. doi: 10.1002/nbm.2802. [DOI] [PubMed] [Google Scholar]
- 17.Jensen JH, Falangola MF, Hu C, Tabesh A, Rapalino O, Lo C, Helpern JA. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2011;24(5):452–457. doi: 10.1002/nbm.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui ES, Fieremans E, Jensen JH, Tabesh A, Feng W, Bonilha L, Spampinato MV, Adams R, Helpern JA. Stroke Assessment With Diffusional Kurtosis Imaging. Stroke. 2012;43(11):2968–2973. doi: 10.1161/STROKEAHA.112.657742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung JS, Wang E, Lo EH, Sun PZ. Stratification of heterogeneous diffusion MRI ischemic lesion with kurtosis imaging – Evaluation of mean diffusion and kurtosis MRI mismatch in an animal model of transient focal ischemia. Stroke. 2012;43(8):2252–2254. doi: 10.1161/STROKEAHA.112.661926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber RA, Hui ES, Jensen JH, Nie X, Falangola MF, Helpern JA, Adkins DL. Diffusional kurtosis and diffusion tensor imaging reveal different time-sensitive stroke-induced microstructural changes. Stroke. 2015;46(2):545–550. doi: 10.1161/STROKEAHA.114.006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tax CM, Otte WM, Viergever MA, Dijkhuizen RM, Leemans A. REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn Reson Med. 2015;73(2):794–808. doi: 10.1002/mrm.25165. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga I, Hori M, Masutani Y, Hamasaki N, Sato S, Suzuki Y, Kumagai F, Kosuge M, Hoshito H, Kamagata K, Shimoji K, Nakanishi A, Aoki S, Senoo A. Effects of diffusional kurtosis imaging parameters on diffusion quantification. Radiol Phys Technol. 2013;6(2):343–348. doi: 10.1007/s12194-013-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saver JL. Time Is Brain—Quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 24.Hansen B, Lund TE, Sangill R, Jespersen SN. Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med. 2013;69(6):1754–1760. doi: 10.1002/mrm.24743. [DOI] [PubMed] [Google Scholar]
- 25.Hansen B, Lund TE, Sangill R, Jespersen SN. Erratum: Hansen, Lund, Sangill, and Jespersen. Experimentally and Computationally Fast Method for Estimation of a Mean Kurtosis. Magnetic Resonance inn Medicine. 2013;69:1754–1760. doi: 10.1002/mrm.24743. Magn Reson Med 2014;71:2250. [DOI] [PubMed] [Google Scholar]
- 26.Tietze A, Hansen MB, Ostergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean Diffusional Kurtosis in Patients with Glioma: Initial Results with a Fast Imaging Method in a Clinical Setting. AJNR Am J Neuroradiol. 2015;36(8):1472–1478. doi: 10.3174/ajnr.A4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun PZ, Wang Y, Mandeville E, Chan S-T, Lo EH, Ji X. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR in Biomed. 2014;27(11):1413–1418. doi: 10.1002/nbm.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65(3):823–836. doi: 10.1002/mrm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. J Magn Reson Imaging. 2008;27(1):20–26. doi: 10.1002/jmri.21231. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K, Song SK. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma. 2010;27(3):587–598. doi: 10.1089/neu.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun PZ, Lu J, Wu Y, Xiao G, Wu R. Evaluation of the dependence of CEST-EPI measurement on repetition time, RF irradiation duty cycle and imaging flip angle for enhanced pH sensitivity. Phys Med Biol. 2013;58:N229–N240. doi: 10.1088/0031-9155/58/17/N229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nael K, Trouard TP, Lafleur SR, Krupinski EA, Salamon N, Kidwell CS. White matter ischemic changes in hyperacute ischemic stroke: voxel-based analysis using diffusion tensor imaging and MR perfusion. Stroke. 2015;46(2):413–418. doi: 10.1161/STROKEAHA.114.007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umesh Rudrapatna S, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, Leemans A, van der Toorn A, Dijkhuizen RM. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage 2014;97:363-373. doi: 10.1016/j.neuroimage.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35:2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 36.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27(6):1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 37.Sun PZ, Wang E, Cheung JS. Imaging acute ischemic tissue acidosis with pH-sensitive endogenous amide proton transfer (APT) MRI – Correction of tissue relaxation and concomitant RF irradiation effects toward mapping quantitative cerebral tissue pH. NeuroImage. 2012;60(1):1–6. doi: 10.1016/j.neuroimage.2011.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen JH, Hui ES, Helpern JA. Double-pulsed diffusional kurtosis imaging. NMR Biomed. 2014;27(4):363–370. doi: 10.1002/nbm.3030. [DOI] [PubMed] [Google Scholar]
- 39.Glenn GR, Helpern JA, Tabesh A, Jensen JH. Quantitative assessment of diffusional kurtosis anisotropy. NMR Biomed. 2015;28(4):448–459. doi: 10.1002/nbm.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–1025. [PubMed] [Google Scholar]
- 41.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35(11 Suppl 1):2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 42.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7:CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui ES, Cheung MM, Qi L, Wu EX. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. NeuroImage. 2008;42(1):122–134. doi: 10.1016/j.neuroimage.2008.04.237. [DOI] [PubMed] [Google Scholar]
- 44.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254(3):876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 45.Wang JJ, Lin WY, Lu CS, Weng YH, Ng SH, Wang CH, Liu HL, Hsieh RH, Wan YL, Wai YY. Parkinson Disease: Diagnostic Utility of Diffusion Kurtosis Imaging. Radiology. 2011;261(1):210–217. doi: 10.1148/radiol.11102277. [DOI] [PubMed] [Google Scholar]
- 46.Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J, Silver JM, Grossman RI, Inglese M. Thalamus and Cognitive Impairment in Mild Traumatic Brain Injury: A Diffusional Kurtosis Imaging Study. J Neurotrauma. 2011;29(13):2318–2327. doi: 10.1089/neu.2011.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinberg F, Farrher E, Kaffanke J, Oros-Peusquens A-M, Shah NJ. Non-Gaussian diffusion in human brain tissue at high b-factors as examined by a combined diffusion kurtosis and biexponential diffusion tensor analysis. NeuroImage. 2011;57(3):1087–1102. doi: 10.1016/j.neuroimage.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 48.Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. NeuroImage. 2011;58(1):177–188. doi: 10.1016/j.neuroimage.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenkrantz AB, Sigmund EE, Winnick A, Niver BE, Spieler B, Morgan GR, Hajdu CH. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012;30(10):1534–1540. doi: 10.1016/j.mri.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Shen Q, Ren H, Fisher M, Duong TQ. Statistical prediction of tissue fate in acute ischemic brain injury. J Cereb Blood Flow Metab. 2005;25(10):1336–1345. doi: 10.1038/sj.jcbfm.9600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu O, Sumii T, Asahi M, Sasamata M, Ostergaard L, Rosen BR, Lo EH, Dijkhuizen RM. Infarct prediction and treatment assessment with MRI-based algorithms in experimental stroke models. J Cereb Blood Flow Metab. 2007;27(1):196–204. doi: 10.1038/sj.jcbfm.9600328. [DOI] [PubMed] [Google Scholar]
- 52.Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol. 2014;202(1):W26–33. doi: 10.2214/AJR.13.11365. [DOI] [PubMed] [Google Scholar]