Abstract
A better understanding of factors associated with early death and survival among children, adolescents and young adults with acute myeloid leukaemia (AML) may guide health policy aimed at improving outcomes in these patients. We examined trends in early death and survival among 3935 patients aged 0 to 39 years with de novo AML in California during 1988–2011 and investigated the associations between sociodemographic and selected clinical factors and outcomes. Early death declined from 9.7% in 1988–1995 to 7.1% in 2004–2011 (P = 0.062), and survival improved substantially over time. However, 5-year survival was still only 50% (95% confidence interval 47%–53%) even in the most recent treatment period (2004–2011). Overall, the main factors associated with poor outcomes were older age at diagnosis, treatment at hospitals not affiliated with National Cancer Institute-designated cancer centres, and black race/ethnicity. For patients diagnosed during 1996–2011, survival was lower among those who lacked health insurance compared to those with public or private insurance. We conclude that mortality after AML remained strikingly high in California and increased with age. Possible strategies to improve outcomes include wider insurance coverage and treatment at specialized cancer centres.
Keywords: acute myeloid leukaemia, survival, early death, population-based
INTRODUCTION
Acute myeloid leukaemia (AML) is a complex and highly heterogeneous disease. Without treatment, most patients die within weeks or months of diagnosis (Appelbaum, et al 2006). Survival among patients with AML has increased over the last 3 decades, mostly among patients younger than 60 years of age, but progress has now reached a plateau (Pritchard-Jones, et al 2013; Ribeiro 2014) and acute leukaemias, including AML, remain the leading cause of cancer deaths among patients aged 39 years or younger (Deschler and Lubbert 2006; Wingo, et al 2003). Although complete remission can be achieved in approximately 75% to 90% of patients younger than 60 years of age, approximately 35% to 50% of these patients experience relapse within the following 2 years (Burnett 2005; Hann, et al 2004). Disturbingly, children, adolescents and young adults who survive AML may suffer long-term debilitating complications of treatment, such as secondary malignancies, cardiovascular and neurocognitive dysfunctions, as well as severe psychosocial effects (Byrne, et al 2011; Dores, et al 2012; Mulrooney, et al 2008; Schultz, et al 2014; Sekeres, et al 2004; Sullivan, et al 2013).
Given the lack of population-based studies focusing on young patients with AML (Pulte, et al 2009), we aimed to evaluate trends in survival and early death (i.e., death occurring within 30 days of diagnosis) among patients aged 0 to 39 years with AML in California, and investigate sociodemographic and selected clinical factors associated with poor outcomes.
PATIENTS AND METHODS
Patients
Our data were obtained from the California Cancer Registry (CCR), which participates in the Survival Epidemiology and End Results (SEER) Programme of the National Cancer Institute (NCI). Reporting of all malignant neoplasms is compulsory in California, and the standard for completeness of ascertainment is at least 98% (Hayat, et al 2007). In addition to relevant variables available in the SEER datasets, the CCR provides information on hospital designation (i.e., whether the initial reporting hospital is affiliated with a NCI-designated cancer centre), whether the patient has undergone chemotherapy or haematopoietic stem cell transplantation (HSCT) and neighbourhood socioeconomic status (SES).
Ethics approval for human subject research was obtained from the Cancer Prevention Institute of California Institutional Review Board. As the analysis was based on state-mandated cancer registry data, the study was conducted in accordance with the waivers of individual informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization.
We identified all patients aged 0 to 39 years who were diagnosed with de novo AML between 1 January 1988 and 31 December 2011, and excluded those with acute promyelocytic leukaemia, which has a much more favourable prognosis than the other subtypes of AML and was the focus of a separate study (Abrahão, et al 2015a). Information on patients with AML associated with Down syndrome (who also have a better prognosis) was only available in the CCR from 2010 onwards; prior to that, these cases were classified as ‘AML not otherwise specified’. Therefore, it was not possible to study these patients separately.
To identify cases of AML diagnosed during 1988–2011, we used the following morphology codes from the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) (World Health Organization 2000): 9840, 9861, 9867, 9870–9874, 9891, 9895–9898, 9910, 9920, and 9931. We excluded patients diagnosed by autopsy or death certificate only (n = 12), patients of non-Hispanic American Indian (n = 20) or unknown (n = 18) race/ethnicity and patients with a missing month of diagnosis (n = 22). Patients who died on the day of diagnosis (n = 28) were included. Of the 4007 patients reviewed, 3935 (98.2%) were included in the analyses. All the patients were followed from the date of diagnosis until death, loss to follow-up or the end of the study (31 December 2012), whichever occurred first.
Demographic and clinical variables
We examined early death and survival with a comprehensive set of variables in order to identify the main factors associated with poorer prognosis among young patients (≤ 39 years of age). Age is independently associated with survival after AML, and a progressive survival decline is observed from 10 years of age (Gatta, et al 2014, Horibe, et al 2001, Ofran and Rowe 2014, Razzouk, et al 2006, Walter, et al 2011). Based on these observations, we categorized age in 4 groups (0–9, 10–19, 20–29, and 30–39 years). To evaluate trends in outcomes, we used 3 calendar periods of diagnosis (1988–1995, 1996–2003, and 2004–2011). Race/ethnicity was classified in 4 groups [non–Hispanic white (white), non-Hispanic black (black), Hispanic, and non-Hispanic Asian/Pacific Islander (Asian)]. Neighbourhood SES was divided into quintiles by using a previous developed index (Yost, et al 2001), which is based on block-level census data, and is considered an adequate surrogate to SES at the individual level (Glaser, et al 2014; Tao, et al 2014). Patients’ health insurance status was routinely reported by the CCR from 1996 onwards and was categorized in 4 groups [uninsured, publicly insured, privately insured or unknown/not otherwise specified (NOS)]. Binary variables were sex (male/female) and initial care at hospitals affiliated with NCI-designated cancer centres (Y/N).
We provided descriptive information on chemotherapy and HSCT, that, like all treatment data collected by the CCR, is limited to the first course of treatment, with no details on treatment regimens or intensity. Information on HSCT was routinely reported from 2003 onwards; however, it was also abstracted for patients diagnosed during 1996–2002, when available.
Statistical analysis
Our analyses investigated how the following variables representing sociodemographic and clinical characteristics were associated with early death and overall survival: age at diagnosis, treatment period, sex, race/ethnicity, neighbourhood SES, health insurance status, and treatment facility. All of the variables considered had a priori hypothesized or previously observed (Bradley, et al 2011, Patel, et al 2015a, Percival, et al 2015, Pulte, et al 2013, Walter, et al 2011, Wolfson, et al 2012) associations with early death or survival. We also hypothesized that sociodemographic factors would have a greater impact on survival in older versus younger patients and investigated this hypothesis by analysing the hazard of death by age group.
Early death
Chi-squared tests were used for testing whether early death differed among groups for each covariate. The Kruskal-Wallis test was also used for ordinal covariates (age group, neighbourhood SES and calendar period). We used multivariate logistic regression to obtain the odds ratios (ORs) for early death (death within 30 days of diagnosis) and the corresponding 95% confidence intervals (95% CIs) associated with sociodemographic and clinical characteristics. We used the likelihood ratio test as an overall significance test for the association of each independent variable with early death.
Survival
We estimated the overall (all causes) survival at 1, 5, and 10 years by using the Kaplan-Meier method and tested differences in survival across strata of each variable with the log-rank test (the log-rank test for trend was also estimated for ordinal variables). Twenty-eight patients who died on the day of diagnosis were considered to have a survival time of 1 day.
The 5-year survival in the 3 calendar periods examined and the 10-year survival in 1988–1995 and 1996–2003 were estimated using the traditional cohort-based approach, because most patients had been followed for at least 5 or 10 years, respectively, during these time periods. For patients who had all been followed up for at least 10 years, the classical cohort approach provided survival estimates using all the observed follow-up data. For patients with less than 5 (or 10) years of follow-up, we used the period approach (Brenner, et al 2004) to obtain a short-term prediction of their survival up to 5 (or 10) years after diagnosis on the assumption that their partial probabilities of survival will be the same as those observed during the most recent years for which follow-up data were available.
We used multivariate Cox regression to obtain the hazard ratios (HRs) and corresponding 95% CIs for each variable, and the likelihood ratio test as an overall significance test for the association of each independent variable with survival. The proportional hazard assumption, assessed by looking at Schoenfeld residuals, was met for all variables in the multivariate model. To investigate whether the association of survival with sociodemographic and clinical factors varied with age, we fitted separate Cox models by age group (0–9, 10–19, 20–29 and 30–39 years) and tested for interactions between age group and each variable using the likelihood ratio test. Statistical analyses were performed using Stata 13 software (StataCorp, College Station, TX), and a 2-sided P value of less than 0.05 was considered statistically significant.
RESULTS
Sociodemographic and clinical characteristics
Among 3935 patients, the median age at diagnosis was 23 years (range, 0–39 years), with a slight predominance of males (53.5%) (Table I). Most patients were white (41%) or Hispanic (39%) and were treated at hospitals that were not affiliated with NCI-designated cancer centres (74%). For patients diagnosed during 1996–2011, 85% had health insurance (46% had private insurance and 39% had public insurance), 4% were uninsured and 11% had unknown or not otherwise specified health insurance status.
Table I.
Patient characteristics, early death and overall survival in patients aged 0 to 39 years with acute myeloid leukaemia in California, 1988–2011.
| Characteristics |
Total
N (%) |
Early death
N (%) |
Pa |
1-year OS
(95% CI) |
5-year OS
(95% CI) |
10-year OS*
(95% CI) |
Pb |
|---|---|---|---|---|---|---|---|
| Total | 3935 (100) | 332 (8.4) | 66.8 (65.3–68.3) | 42.8 (41.2–44.4) | 39.6 (38.0–41.3) | ||
| Calendar period | |||||||
| 1988–1995 | 1303 (33.1) | 126 (9.7) | 59.3 (56.6–62.0) | 32.9 (30.3–35.5) | 30.7 (28.3–33.3) | ||
| 1996–2003 | 1299 (33.0) | 111 (8.6) | 68.1 (65.4–70.5) | 45.8 (43.0–48.5) | 42.4 (39.6–45.1) | ||
| 2004–2011 | 1333 (33.9) | 95 (7.1) | 0.0620/0.0626 | 72.8 (70.3–75.1) | 50.0 (47.0–52.9) | 45.2 (42.5–47.9) | <0.0001/<0.0001 |
| Age at diagnosis, years | |||||||
| 0–9 | 964 (24.5) | 55 (5.7) | 73.2 (70.3–75.9) | 52.4 (49.1–55.6) | 50.0 (46.1–52.9) | ||
| 10–19 | 733 (18.6) | 52 (7.1) | 69.8 (66.3–73.0) | 44.7 (40.9–48.4) | 41.4 (37.6–45.2) | ||
| 20–29 | 951 (24.2) | 94 (9.9) | 64.8 (61.6–67.7) | 40.4 (37.2–43.7) | 37.9 (34.6–41.1) | ||
| 30–39 | 1287 (32.7) | 131 (10.2) | <0.0001/0.0003 | 61.7 (58.9–64.3) | 36.2 (33.5–38.9) | 32.6 (29.9–35.4) | <0.0001/<0.0001 |
| Median | 23 | 27 | |||||
| Race/ethnicity | |||||||
| Non–Hispanic white | 1607 (40.8) | 131 (8.2) | 65.4 (63.0–67.7) | 44.3 (41.8–46.7) | 40.8 (38.2–43.3) | ||
| Non–Hispanic black | 276 (7.0) | 27 (9.8) | 60.7 (54.6–66.1) | 33.1 (27.4–38.8) | 31.5 (25.8–37.2) | ||
| Hispanic | 1545 (39.3) | 147 (9.5) | 68.2 (65.8–70.5) | 42.8 (40.2–45.4) | 39.6 (36.9–42.3) | ||
| Asian/Pacific Islander | 507 (12.9) | 27 (5.3) | 0.0230 | 70.2 (65.9–74.0) | 42.8 (38.3–47.3) | 40.3 (35.7–44.8) | 0.0087 |
| Sex | |||||||
| Male | 2106 (53.5) | 188 (8.9) | 66.8 (64.7–68.8) | 41.8 (39.6–44.0) | 39.0 (36.8–41.2) | ||
| Female | 1829 (46.5) | 144 (7.9) | 0.2360 | 66.7 (64.5–68.9) | 43.9 (41.6–46.3) | 40.4 (38.0–42.8) | 0.3151 |
| Initial care at hospitals affiliated with NCI-designated cancer centres | |||||||
| Yes | 1039 (26.4) | 53 (5.1) | 72.3 (69.5–75.0) | 49.4 (46.2–52.5) | 46.8 (43.5–50.0) | ||
| No | 2896 (73.6) | 279 (9.6) | < 0.0001 | 64.8 (63.0–66.5) | 40.4 (38.6–42.3) | 37.1 (35.2–39.0) | < 0.0001 |
| Neighbourhood socioeconomic status (quintiles) | |||||||
| 1. Lowest 20% | 986 (25.1) | 108 (11.0) | 65.1 (62.0–68.4) | 42.1 (38.9–45.4) | 38.8 (35.4–42.1) | ||
| 2. | 826 (21.0) | 61 (7.9) | 68.3 (65.0–71.4) | 41.0 (37.5–44.5) | 37.7 (34.2–41.2) | ||
| 3. Middle 20% | 783 (19.9) | 64 (8.2) | 64.8 (61.3–68.0) | 40.3 (36.7–43.8) | 37.1 (33.5–40.6) | ||
| 4. | 714 (18.1) | 57 (8.0) | 68.0 (64.4–71.3) | 46.2 (42.4–50.0) | 42.9 (39.0–46.7) | ||
| 5. Highest 20% | 626 (15.9) | 42 (6.7) | 0.0180/0.0178 | 68.4 (64.6–71.9) | 45.5 (41.4–49.4) | 43.1 (39.0–47.1) | 0.1446/0.0338 |
| Health insurance status (limited to patients diagnosed in 1996–2011, N = 2632) | |||||||
| None | 99 (3.8) | 21 (21.2) | 56.3 (45.7–65.7) | 37.9 (27.7–48.0) | 37.9 (27.7–48.0) | ||
| Public | 1038 (39.4) | 78 (7.5) | 71.9 (69.0–74.5) | 47.6 (44.4–50.9) | 43.8 (40.3–47.2) | ||
| Private | 1207 (45.9) | 86 (7.1) | 71.0 (68.3–73.5) | 49.9 (47.0–52.8) | 46.5 (43.5–49.5) | ||
| Unknown/NOS | 288 (10.9) | 21 (7.3) | < 0.0001 | 67.9 (62.1–73.0) | 42.6 (36.6–48.4) | 37.1 (31.1–43.2) | 0.0045 |
Abbreviations: OS, overall survival; CI, confidence interval; NOS, not otherwise specified; NCI, National Cancer Institute.
The chi-squared was used to test whether early death differs among groups for each variable. For ordinal variables, the Kruskal-Wallis test also is reported (value on the right).
The log-rank was used to test differences in survival across strata for each variable. The log-rank test for trend also is reported for ordinal variables (value on the right)
Ten-year survival during 2004–2011 was estimated using the period approach.
Chemotherapy was administered to 93% of patients; it was recommended, but not given, to 2% of patients, and refused by 0.2% of patients (or their families). A total of 690 patients (26%) received HSCT; 324 (27%) of those diagnosed during 1996–2003 and 366 (30%) of those diagnosed during 2004–2011. Leukaemia was the cause of death in 88% of patients; a small percentage died of other (9%) or unknown (3%) causes. Of the deaths resulting from other causes, 3% were caused by infections (data not shown).
Early death
In total, 332 patients (8.4%) died within 30 days of diagnosis. There was a trend towards a reduction in early death over time, from 9.7% in 1988–1995 to 8.6% in 1996–2003 to 7.1% in 2004–2011 (P = 0.062) (Table I). Overall, in unadjusted analyses, early death was strongly associated with age, hospital designation, neighbourhood SES, and health insurance status (Table I). In multivariate analyses in which all variates were mutually adjusted (Table II). the odds of early death increased progressively with age: the OR for older patients (aged 30 to 39 years) was increased by 70% relative to that for younger patients (aged 0 to 9 years) (OR = 1.70, 95% CI 1.22–2.38). Patients treated at hospitals not affiliated with NCI-designated cancer centres had a higher risk of early death compared with those treated at hospitals affiliated with such centres (OR = 1.75, 95% CI 1.28–2.39). Uninsured patients diagnosed during 1996–2011 had an approximately 3 times greater risk of early death than privately insured patients (OR = 2.91, 95% CI 1.65–5.12); there was no evidence of such a difference between publicly and privately insured patients (P = 0.849). Patients living in the lowest SES neighbourhoods had a significantly greater risk of early death than patients living in the highest SES neighbourhoods (OR = 1.57, 95% CI 1.05–2.34).
Table II.
Relationship of sociodemographic and clinical factors to early death in patients aged 0 to 39 years with acute myeloid leukaemia in California, 1988–2011
| Characteristics |
Adjusted OR1
(95% CI) 1988–2011 |
P-value* |
Adjusted OR2
(95% CI) 1996–2011 |
P-value* |
Adjusted OR3
(95% CI) 1996–2011 |
P-value* |
|---|---|---|---|---|---|---|
| Calendar period | ||||||
| 1988–1995 | 1.38 (1.04–1.83) | N/A | N/A | |||
| 1996–2003 | 1.22 (0.92–1.63) | 1.23 (0.92–1.64) | 1.20 (0.90–1.61) | |||
| 2004–2011 | 1 (reference) | 0.0799 | 1 (reference) | 0.1552 | 1 (reference) | 0.2208 |
| Sex | ||||||
| Male | 1.11 (0.88–1.40) | 1.21 (0.91–1.62) | 1.20 (0.90–1.61) | |||
| Female | 1 (reference) | 0.3656 | 1 (reference) | 0.1908 | 1 (reference) | 0.2153 |
| Age at diagnosis, years | ||||||
| 0–9 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 10–19 | 1.21 (0.82–1.40) | 1.16 (0.90–2.76) | 1.13 (0.70–1.81) | |||
| 20–29 | 1.64 (1.16–2.34) | 1.58 (1.03–2.42) | 1.44 (0.93–2.21) | |||
| 30–39 | 1.70 (1.22–2.38) | 0.0049 | 1.36 (0.89–2.06) | 0.1743 | 1.27 (0.84–1.94) | 0.3915 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Non-Hispanic black | 1.15 (0.74–1.79) | 1.07 (0.58–1.97) | 1.06 (0.58–1.96) | |||
| Hispanic | 1.14 (0.86–1.49) | 1.22 (0.86–1.73) | 1.12 (0.78–1.61) | |||
| Asian/Pacific Islander | 0.65 (0.42–0.99) | 0.0599 | 0.66 (0.38–1.15) | 0.1533 | 0.66 (0.38–1.14) | 0.2791 |
| Neighbourhood socioeconomic status (quintiles) | ||||||
| 1. Lowest 20% | 1.57 (1.05–2.34) | 1.58 (0.90–2.76) | 1.54 (0.87–2.72) | |||
| 2. | 1.04 (0.68–1.57) | 1.29 (0.73–2.27) | 1.28 (0.72–2.26) | |||
| 3. Middle 20% | 1.18 (0.78–1.77) | 1.51 (0.86–1.73) | 1.53 (0.87–2.69) | |||
| 4. | 1.19 (0.78–1.81) | 1.54 (0.87–2.70) | 1.58 (0.90–2.80) | |||
| 5. Highest 20% | 1 (reference) | 0.0934 | 1 (reference) | 0.4512 | 1 (reference) | 0.4411 |
| Initial care at hospitals affiliated with NCI-designated cancer centres | ||||||
| Yes | 1 (reference) | 1 (reference) | 1 (reference) | |||
| No | 1.75 (1.28–2.39) | 0.0002 | 1.96 (1.32–2.92) | 0.0004 | 1.99 (1.33–2.97) | 0.0004 |
| Health insurance status (limited to patients diagnosed in 1996–2011, N=2632) | ||||||
| Uninsured | N/A | N/A | 2.91 (1.65–5.12) | |||
| Public | N/A | N/A | 1.03 (0.73–1.46) | |||
| Private | N/A | N/A | 1 (reference) | |||
| Unknown/NOS | N/A | N/A | N/A | N/A | 1.04 (0.01–0.43) | 0.0046 |
Abbreviations: OR, odds ratio; CI, confidence interval; NOS, not otherwise specified; NCI, National Cancer Institute. OR1: adjusted model without insurance (1988–2011); OR2: adjusted model without insurance (1996–2011); OR3: adjusted model with insurance (1996–2011).
Likelihood ratio test.
Survival
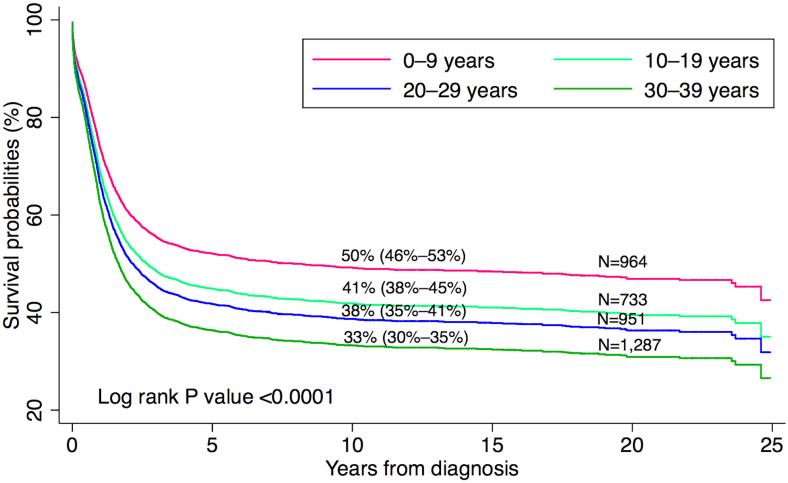
Of 3935 patients included in the analysis, 2272 (58%) died over the course of follow-up. Approximately 93% of patients had confirmation of vital status within 18 months of the study end date. The median time to death for deceased patients was 0.9 years, the median follow-up time for surviving patients was 8.8 years, and the overall median follow-up time using reverse censoring (Schemper and Smith 1996) was 10.0 years. Overall survival improved substantially over time for all ages and racial/ethnic groups. Five-year survival increased from 32.9% (95% CI 30.3–35.5) in 1988–1995 to 50% (95% CI 47.0–52.9) in 2004–2011 (Table I). Based on the log-rank test, there was evidence of an association between worse survival and older age at diagnosis (Figure 1), black race/ethnicity, receipt of initial care in hospitals not affiliated with NCI-designated cancer centres, and, for patients diagnosed during 1996–2011, lack of health insurance. In a multivariate Cox regression analysis in which all variables were mutually adjusted (Table III), we found an increased hazard of death for older patients compared with younger patients (30 to 39 vs. 0 to 9 years of age) (HR = 1.55, 95% CI 1.38–1.74), for black patients compared with white patients (HR = 1.27, 95% CI 1.08–1.49), and for patients who received initial care at hospitals not affiliated with NCI-designated cancer centres compared with those initially treated at such facilities (HR = 1.18, 95% CI 1.07–1.31). For patients diagnosed during 1996–2011, the hazard of death was higher among uninsured patients than among privately insured patients (HR = 1.34, 95% CI 1.01–1.78), with no evidence of a difference in hazard between privately and publicly insured patients (P = 0.429).
Figure 1.
Overall survival after acute myeloid leukaemia by age group at diagnosis, in California, 1988–2011 (percentages in the graph correspond to 10-year survival)
Table III.
Relationship of sociodemographic and clinical factors to the hazard of death after acute myeloid leukaemia in patients aged 0 to 39 years in California, 1988–2011
| Characteristics |
Adjusted HR1
(95% CI) 1988–2011 |
P-value* |
Adjusted HR2
(95% CI) 1996–2011 |
P-value* |
Adjusted HR3
(95% CI) 1996–2011 |
P-value* |
|---|---|---|---|---|---|---|
| Calendar period | ||||||
| 1988–1995 | 1.58 (1.43–1.76) | N/A | N/A | |||
| 1996–2003 | 1.14 (1.03–1.27) | 1.14 (1.02–1.27) | 1.12 (1.00–1.25) | |||
| 2004–2011 | 1.0 (reference) | <0.0001 | 1.0 (reference | 0.0211 | 1.0 (reference) | 0.0460 |
| Age at diagnosis, years | ||||||
| 0–9 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| 10–19 | 1.23 (1.07–1.40) | 1.28 (1.08–1.52) | 1.28 (1.07–1.51) | |||
| 20–29 | 1.34 (1.18–1.52) | 1.39 (1.18–1.64) | 1.38 (1.17–1.62) | |||
| 30–39 | 1.55 (1.38–1.74) | <0.0001 | 1.49 (1.28–1.74) | <0.0001 | 1.49 (1.28–1.74) | <0.0001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| Non-Hispanic black | 1.27 (1.08–1.49) | 1.33 (1.08–1.65) | 1.34 (1.08–1.65) | |||
| Hispanic | 1.05 (0.95–1.16) | 1.10 (0.96–1.25) | 1.08 (0.94–1.24) | |||
| Asian/Pacific Islander | 0.98 (0.86–1.13) | 0.0318 | 1.00 (0.83–1.18) | 0.0505 | 1.00 (0.84–1.19) | 0.0629 |
| Sex | ||||||
| Male | 1.03 (0.95–1.12) | 0.99 (0.89–1.10) | 0.99 (0.89–1.10) | |||
| Female | 1.0 (reference) | 0.4806 | 1.0 (reference | 0.8900 | 1.0 (reference) | 0.8349 |
| Neighbourhood socioeconomic status (quintiles) | ||||||
| 1. Lowest 20% | 1.14 (0.99–1.31) | 1.23 (1.01–1.49) | 1.22 (1.00–1.48) | |||
| 2. | 1.10 (0.95–1.27) | 1.20 (1.00–1.46) | 1.20 (0.99–1.45) | |||
| 3. Middle 20% | 1.13 (0.98–1.30) | 1.30 (1.08–1.58) | 1.31 (1.08–1.59) | |||
| 4. | 1.01 (0.87–1.15) | 1.07 (0.88–1.30) | 1.07 (0.88–1.31) | |||
| 5. Highest 20% | 1.0 (reference) | 0.1868 | 1.0 (reference | 0.0490 | 1.0 (reference) | 0.0453 |
| Initial care at hospitals affiliated with NCI-designated cancer centres | ||||||
| Yes | 1.0 (reference) | 1.0 (reference | 1.0 (reference) | |||
| No | 1.18 (1.07–1.31) | 0.0009 | 1.26 (1.11–1.43) | 0.0004 | 1.27 (1.11–1.45) | 0.0002 |
| Health insurance status (limited to patients diagnosed in 1996–2011, N=2632) | ||||||
| None | N/A | N/A | 1.34 (1.01–1.78) | |||
| Public | N/A | N/A | 1.05 (0.93–1.19) | |||
| Private | N/A | N/A | 1.0 (reference) | |||
| Unknown/NOS | N/A | N/A | N/A | N/A | 1.27 (1.07–1.51) | 0.0204 |
Abbreviations: HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; NCI, National Cancer Institute. HR1: adjusted model without insurance, 1988–2011; HR2: adjusted model without insurance, 1996–2011; HR3: adjusted model with insurance, 1996–2011.
Likelihood ratio test.
When we fitted separate Cox models by age at diagnosis (Tables IV and V), we observed that the association between the hazard of death and sociodemographic and clinical factors varied by age group. Table IV presents Cox models for the factors available during 1988–2011 (all variables except health insurance status) by age group at diagnosis. Table V additionally includes health insurance status, but is limited to patients diagnosed during 1996–2011. For patients aged 0 to 9 years, we found no association between the risk of death and sociodemographic or clinical factors, whereas associations were found with advancing age (Table IV). Markedly, for patients aged 30 to 39 years, the hazard of death was substantially higher among those who received initial care at hospitals not affiliated with NCI-designated cancer centres (HR = 1.31, 95% CI 1.08–1.58) (Table IV) and, during 1996–2011, among uninsured patients (HR = 1.78, 95% CI 1.14–2.76) (Table V). We also observed an increased risk of death among black patients, particularly those aged 20 to 29 years (HR = 1.70, 95% CI 1.21–2.39) (Table IV). However, despite observed differences in associations between the explanatory variables and survival by age group, none of these were found to be statistically significant when tested for interactions between age group and each variable, and the results should therefore be interpreted with caution.
Table IV.
Relation of sociodemographic and clinical factors to the hazard of death after acute myeloid leukaemia by age group at diagnosis, California, 1988–2011
| Characteristics (Total = 3935) |
HR1 (95% CI) 0–9 years N = 964 |
P-value* | HR2 (95% CI) 10–19 years N = 733 |
P-value* | HR3 (95% CI) 20–29 years N = 951 |
P-value* | HR4 (95% CI) 30–39 years N = 1287 |
P-value* |
|---|---|---|---|---|---|---|---|---|
| Calendar period | ||||||||
| 1988–1995 | 1.84 (1.45–2.34) | 1.52 (1.19–1.93) | 1.29 (1.05–1.59) | 1.71 (1.44–2.04) | ||||
| 1996–2003 | 1.36 (1.07–1.73) | 1.27 (0.99–1.63) | 0.95 (0.76–1.18) | 1.14 (0.95–1.36) | ||||
| 2004–2011 | 1.0 (reference) | <0.0001 | 1.0 (reference) | 0.0034 | 1.0 (reference) | 0.0049 | 1.0 (reference) | <0.0001 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | ||||
| Non-Hispanic black | 1.22 (0.86–1.74) | 1.19 (0.81–1.74) | 1.70 (1.21–2.39) | 1.19 (0.92–1.54) | ||||
| Hispanic | 1.02 (0.82–1.28) | 1.06 (0.83–1.35) | 1.05 (0.86–1.30) | 1.10 (0.93–1.30) | ||||
| Asian/Pacific Islander | 0.79 (0.57–1.09) | 0.2468 | 1.16 (0.84–1.60) | 0.7294 | 1.28 (0.99–1.64) | 0.0122 | 0.84 (0.67–1.05) | 0.0821 |
| Sex | ||||||||
| Male | 0.93 (0.77–1.12) | 0.89 (0.73–1.08) | 1.17 (0.99–1.38) | 1.06 (0.92–1.21) | ||||
| Female | 1.0 (reference) | 0.4455 | 1.0 (reference) | 0.2287 | 1.0 (reference) | 0.0734 | 1.0 (reference) | 0.4152 |
| Neighbourhood socioeconomic status (quintiles) | ||||||||
| 1. Lowest 20% | 0.88 (0.63–1.22) | 1.11 (0.80–1.53) | 1.26 (0.94–1.68) | 1.19 (0.94–1.51) | ||||
| 2. | 1.07 (0.77–1.47) | 0.96 (0.69–1.32) | 1.03 (0.77–1.38) | 1.21 (0.96–1.53) | ||||
| 3. Middle 20% | 0.86 (0.63–1.20) | 0.93 (0.66–1.30) | 1.14 (0.86–1.52) | 1.31 (1.05–1.53) | ||||
| 4. | 0.83 (0.59–1.17) | 0.82 (0.58–1.16) | 0.84 (0.62–1.14) | 1.31 (1.04–1.64) | ||||
| 5. Highest 20% | 1.0 (reference) | 0.4063 | 1.0 (reference) | 0.4579 | 1.0 (reference) | 0.0583 | 1.0 (reference) | 0.1260 |
| Initial care at hospitals affiliated with NCI-designated cancer centres | ||||||||
| Yes | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | ||||
| No | 1.10 (0.91–1.32) | 0.3314 | 1.29 (1.03–1.61) | 0.0220 | 1.11 (0.90–1.37) | 0.3310 | 1.31 (1.08–1.58) | 0.0042 |
Abbreviations: HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; NCI, National Cancer Institute.
Likelihood ratio test.
Table V.
Relationship of sociodemographic and clinical factors to the hazard of death after acute myeloid leukaemia by age group at diagnosis, including health insurance status, California, 1996–2011
| Characteristics (Total = 2632) |
HR1 (95% CI) 0–9 years N = 671 |
P-value* | HR2 (95% CI) 10–19 years N = 510 |
P-value* | HR3 (95% CI) 20–29 years N = 619 |
P-value* | HR4 (95% CI) 30–39 years N = 832 |
P-value* |
|---|---|---|---|---|---|---|---|---|
| Calendar period | ||||||||
| 1996–2003 | 1.31 (1.02–1.68) | 1.28 (0.99–1.64) | 0.92 (0.74–1.15) | 1.13 (0.94–1.36) | ||||
| 2004–2011 | 1.0 (reference) | 0.0308 | 1.0 (reference) | 0.0580 | 1.0 (reference) | 0.4640 | 1.0 (reference) | 0.2000 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | ||||
| Non-Hispanic black | 1.63 (1.04–2.57) | 1.23 (0.74–2.05) | 1.95 (1.17–3.25) | 1.11 (0.78–1.56) | ||||
| Hispanic | 1.27 (0.93–1.72) | 1.05 (0.76–1.44) | 1.17 (0.88–1.56) | 0.99 (0.79–1.24) | ||||
| Asian/Pacific Islander | 0.87 (0.55–1.36) | 0.0821 | 1.01 (0.66–1.55) | 0.8872 | 1.40 (1.01–1.92) | 0.0392 | 0.83 (0.62–1.11) | 0.4981 |
| Sex | ||||||||
| Male | 0.89 (0.70–1.12) | 0.84 (0.65–1.08) | 1.08 (0.86–1.35) | 1.06 (0.88–1.27) | ||||
| Female | 1.0 (reference) | 0.3220 | 1.0 (reference) | 0.1688 | 1.0 (reference) | 0.5054 | 1.0 (reference) | 0.5343 |
| Neighbourhood socioeconomic status (quintiles) | ||||||||
| 1. Lowest 20% | 0.92 (0.59–1.43) | 1.12 (0.71–0.78) | 1.37 (0.92–2.04) | 1.34 (0.95–1.88) | ||||
| 2. | 1.16 (0.76–1.77) | 0.92 (0.59–1.44) | 1.03 (0.69–1.53) | 1.56 (1.14–2.15) | ||||
| 3. Middle 20% | 1.02 (0.67–1.56) | 0.99 (0.64–1.53) | 1.21 (0.82–1.78) | 1.76 (1.28–2.42) | ||||
| 4. | 0.92 (0.59–1.45) | 0.87 (0.54–1.40) | 0.77 (0.51–1.16) | 1.60 (1.17–2.20) | ||||
| 5. Highest 20% | 1.0 (reference) | 0.6758 | 1.0 (reference) | 0.7838 | 1.0 (reference) | 0.0281 | 1.0 (reference) | 0.0035 |
| Initial care at hospitals affiliated with NCI-designated cancer centres | ||||||||
| Yes | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | ||||
| No | 1.12 (0.88–1.43) | 0.3512 | 1.44 (1.09–1.90) | 0.0078 | 1.24 (0.93–1.66) | 0.1414 | 1.39 (1.08–1.80) | 0.0095 |
| Health insurance status | ||||||||
| None | 1.60 (0.63–4.02) | 1.78 (0.85–3.75) | 0.94 (0.57–1.55) | 1.78 (1.14–2.76) | ||||
| Public | 0.93 (0.69–1.25) | 1.21 (0.90–1.64) | 0.99 (0.77–1.27) | 1.10 (0.90–1.36) | ||||
| Private | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | ||||
| Unknown/NOS | 1.21 (0.83–1.75) | 0.4384 | 1.35 (0.92–1.99) | 0.2399 | 1.45 (1.02–2.07) | 0.1965 | 1.17 (0.86–1.59) | 0.0986 |
Abbreviations: HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; NCI, National Cancer Institute.
Likelihood ratio test.
DISCUSSION
Our study found evidence of a reduction in early death and an improvement in survival after AML over a 25-year period for patients of all age and racial/ethnic groups in California. Overall, early death and survival were associated with several sociodemographic and clinical factors, including age at diagnosis, race/ethnicity, neighbourhood SES, hospital designation, and health insurance status. Despite substantial improvements, approximately half of the patients died in the most recent treatment period (2004–2011).
We found worse survival among black patients than white patients, consistent with previous studies of AML and acute lymphoblastic leukaemia (ALL) (Abrahão, et al 2015b; Aplenc, et al 2006; Byrne, et al 2011; Patel, et al 2015b; Pulte, et al 2012; Pulte, et al 2013; Rubnitz, et al 2007; Sekeres, et al 2004). Results from several clinical trials at a single institution in the US showed survival in black children with AML to be similar to that in white children (Rubnitz, et al 2007). However, a recent trial at the same institution showed a trend towards worse outcomes in black children compared to those in white and Hispanic children (Rubnitz, et al 2007). It is not yet clear what factors accounted for the disparities in survival among black patients with AML that were observed in our and other studies. Black race/ethnicity has been associated with both favourable and unfavourable cytogenetic subtypes (Rubnitz, et al 2007; Sekeres, et al 2004). It is possible that pharmacogenetic differences between black and white patients contribute to different responses to chemotherapy (Pui, et al 2004; Rubnitz, et al 2007). Another possibility is that black patients have had less access to chemotherapy and/or HSCT. A recent study using CCR data linked to hospital discharge data showed that the odds of receipt of HSCT and chemotherapy were lower among black than non-black patients (Patel, et al 2015a).
Interestingly, we found no evidence of differences in survival between Hispanic and white patients in any age group. This differs from the results of 2 consecutive clinical trials by the Children’s Oncology Group (patients aged 0 to 21 years) (Aplenc, et al 2006), but is consistent with the population-based study mentioned above (Patel, et al 2015a) that found survival among Hispanics to be similar to that among white patients after adjustment for age (all ages included), and with paediatric clinical trials that showed favourable outcomes among Hispanic patients with AML (Rubnitz, et al 2007). These observations contrast with the worse survival observed among Hispanic children and adolescents with ALL in the US (Abrahão, et al 2015b; Goggins and Lo 2012; Lim, et al 2014; Pulte, et al 2013), and suggest that unfavourable biological characteristics are associated with survival after ALL, (Lim, et al 2014) but may not contribute, to the same extent, to the worse outcomes after AML. In fact, clinical trials have shown favourable cytogenetic characteristics among Hispanic children with AML (Rubnitz, et al 2007).
Clinical (Aplenc, et al 2006) and population-based studies (Patel, et al 2015a) that looked at the association of race/ethnicity with survival lacked information on SES. Our information on neighbourhood SES found a significant association between lower SES and higher early death, but there was no evidence of an association between neighbourhood SES and survival. This suggests that some patients with lower neighbourhood SES lacked access to optimal treatment during the critical initial days after AML diagnosis.
Our findings showed that survival was better among patients aged 0 to 9 years and there was no evidence of increased hazard of death associated with sociodemographic and clinical characteristics in this age group. However, among older patients, particularly those aged 30 to 39 years, we observed an association between increased risk of death and several sociodemographic and clinical factors, including treatment at hospitals not affiliated with NCI-designated cancer centres, lack of health insurance and black race/ethnicity. The diagnosis of AML in older patients may carry a worse prognosis and probably requires more intensive chemotherapy and, in some cases, HSCT. Consequently, these patients possibly have a higher probability of treatment-related complications (mainly haemorrhage and infection) requiring more aggressive treatment and long-term supportive care.
Recent studies have shown that the biology of paediatric AML differs from that of adult AML and that structural and numerical chromosome alterations have prognostic implications (Grimwade, et al 1998; Harrison, et al 2010; Tarlock and Meshinchi 2015). For instance, core-binding factor AML [CBF AML: t(8;21) and inv(16)/t(16;16)], which has a favourable prognosis, is more frequent in children and adolescents than in adults. In contrast, abnormalities of chromosomes 5 and 7 are more common in adults and are associated with a dismal prognosis (Tarlock and Meshinchi 2015). Additionally, somatic mutations in selected genes, such as FLT3, NPM1 and CEBPA, are known to have prognostic clinical significance in paediatric and adult AML. Whereas double CEBPA and isolated NPM1 mutations are associated with a reduced risk of relapse and better survival (Ho, et al 2009; Yoon, et al 2015), patients with internal tandem mutations of FLT3 (FLT3-ITD mutations) have a higher risk of relapse and worse survival and may benefit from receipt of HSCT (Schlenk, et al 2008). Adult AML has a higher prevalence of FLT3-ITD mutations compared to paediatric AML (27% vs. 12%) (Tarlock and Meshinchi 2015). These cytogenetic and genomic differences may partly account for the inferior outcomes we observed among older patients and explain the association between worse survival and sociodemographic and clinical factors. Hence, interventions to improve timely access to high-quality complex therapy and optimal supportive care for all individuals with AML have the potential to reduce mortality and morbidity, particularly among higher-risk and minority patients.
Other factors that may contribute to the worse outcomes among older patients with AML include the lower participation of adolescents and young adults in clinical trials or treatment at hospitals that are not affiliated with NCI-designated cancer centres compared with that of paediatric patients (Bleyer and Barr 2009). We had no information on patients’ clinical trial enrolment, but our observations support the results from a previous study (Wolfson, et al 2012) showing that adolescents and young adults with cancer who were treated at hospitals affiliated with NCI-designated cancer centres had better outcomes than those treated at hospitals not affiliated with such centres.
Moreover, we found evidence of increased early death and worse survival among uninsured patients compared to privately or publicly insured patients. These results agree with recent studies that showed health insurance status to be independently associated with the risk of death (Bradley, et al 2011; Robbins, et al 2014; Rosenberg, et al 2014), and highlight the importance of health systems that provide timely access to adequate treatment (chemotherapy and, when recommended, HSCT) and optimal supportive care, including prophylaxis and control of invasive fungal infection.
Intensive chemotherapy regimens, improvements in supportive care, development of risk-adapted treatment strategies (through cytogenetic studies and early response to treatment as measured by minimal residual disease) and provision of HSCT to a greater number of high-risk patients are considered the primary causes of better outcomes in AML, rather than novel therapeutic agents (Ferrara and Schiffer 2013). Although improvements in HSCT have led to a significant decrease in transplant-related morbidity and mortality in patients with AML (Ferrara and Schiffer 2013), the role of HSCT remains controversial. With the progress in the use of chemotherapy and the improvement in risk assessment over the last 25 years, HSCT in first remission is not recommended for AML patients that have a favourable prognosis (CBF AML) (Carpenter, et al 2012), and the use of HSCT may be limited to intermediate-risk patients who experience relapse after undergoing initial therapy (Burnett, et al 2013).
Because AML is a complex disease characterized by morphological and cytogenetic heterogeneity, we believe that multiple factors may have contributed to the lower survival we observed among older patients and those of black race/ethnicity. Further improvements in disease outcomes will also require the development of more effective and less toxic agents for each subtype of the disease (precision medicine) (Rubnitz and Inaba 2012). Conventional genetic and, more recently, genomic studies have played a key role in advancing the cure for ALL over a period of almost 30 years (Evans, et al 2013), and the same benefit is expected for AML. In the new era of basket trials [clinical trial design based on the hypothesis that the presence of a molecular marker predicts response to a targeted therapy regardless of tumour histology (Redig and Janne 2015)] and big data infrastructure (including access to electronic medical records and linkage of cancer registry data with insurance claims information) (Meyer and Basch 2015), national and international collaborations are fundamental to help to answer questions regarding treatment efficacy, toxicity and long-term survival.
Our study has several limitations. Hospital designation was limited to the location of care at the first reporting facility, so it is possible that some patients who were initially treated at one type of facility were subsequently treated at another. Nevertheless, the majority of our patients (90%) received at least part of their treatment at the reporting hospital. The CCR, like the majority of population-based cancer registries, does not collect information on patients’ performance status, baseline cytogenetic risk assessment or relapse. Without these additional data, it was not possible to clearly investigate whether there was an association between the receipt of HSCT and survival. Although supplementary clinical information would have contributed additional important findings and explained some of the variability of our results, our study provided relevant information on survival and early death over a 25-year period in the most populous and racial/ethnically diverse state of the United States, using high-quality data. We have also provided important information on factors that may have influenced AML outcomes. To our knowledge, this is the first population-based study to consider the association between neighbourhood SES and outcomes (survival and early death) and to identify associations of several sociodemographic and clinical factors with survival, both overall and stratified by age group among children, adolescents and young adults with AML. Whereas clinical trials are essential to develop guidelines for the best therapeutic regimen (better efficacy with less toxicity), they provide data in less than 3% of the cancer population (Meyer and Basch 2015), although this proportion is usually higher among paediatric patients. In addition, clinical trials commonly report relatively short outcomes (i.e., event-free survival and 1 to 5 years overall survival). Our study included up to 10 years of survival estimates on virtually all patients in California, important information to evaluate long-term outcomes and excess mortality after treatment.
In conclusion, survival after AML increased over time among children, adolescents and young adults, but 5-year survival was still only 50% or less in the most recent treatment period (2004–2011). We identified subgroups with a higher risk of death from the disease, including those aged 10 to 39 years, uninsured patients, those who received initial care at hospitals not affiliated with NCI-designated cancer centres and those of black race/ethnicity. At the population-based level, strategies to address the high burden of AML, especially among adolescents and young adults, may include wider insurance coverage and treatment at specialized cancer centres.
ACKNOWLEDGEMENTS
The authors thank Shawky Matta (CPIC) for cancer registry expertise, and Keith A. Laycock (St. Jude) for expert review of the manuscript. This work was supported by Children with Cancer UK (RA); Cancer Center Support (CORE) Grant P30 CA021765–30 from the National Institutes of Health (NIH) (RCR), and ALSAC (RCR); and the California Department of Public Health as part of the mandated statewide cancer reporting program (California Health and Safety Code Section 103885) and the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) under contracts HHSN261201000140C awarded to the Cancer Prevention Institute of California (THMK, DYL), HHSN261201000035C awarded to the University of Southern California, and HHSN261201000034C awarded to the Public Health Institute; and the by Center for Disease Control and Prevention’s National Program of Cancer Registries, under agreements U55/CCR921930–02 awarded to the Public Health Institute and U58DP003862–01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Public Health, the NCI, the Centers for Disease Control and Prevention, or their contractors and subcontractors is neither intended nor should be inferred.
Footnotes
CONFLICT OF INTEREST DISCLOSURE: The authors declare no conflict of interests
AUTHOR CONTRIBUTIONS
R Abrahão, RC Ribeiro and THM Keegan designed the study, and R Abrahão led the writing and review of the manuscript. R Abrahão performed the statistical analyses and RH Keogh and DY Lichtensztajn advised on and reviewed the statistical analyses. RH Keogh, RC Ribeiro, DY Lichtensztajn, R Marcos-Gragera, BC Medeiros, MP Coleman and THM Keegan participated in the interpretation of data and drafting and critical review of the manuscript. All authors read and approved the final manuscript. R Abrahão had full access to all of the data in the study and takes responsibility for the decision to submit the manuscript for publication.
REFERENCES
- Abrahão R, Ribeiro RC, Medeiros BC, Keogh RH, Keegan TH. Disparities in early death and survival in children, adolescents and young adults with acute promyelocytic leukemia in California. Cancer. 2015a;121:3990–3997. doi: 10.1002/cncr.29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahão R, Lichtensztajn DY, Ribeiro RC, Marina NM, Keogh RH, Marcos-Gragera R, Glaser SL, Keegan TH. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988-2011: A population-based observational study. Pediatric Blood & Cancer. 2015b;62:1819–1825. doi: 10.1002/pbc.25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplenc R, Alonzo TA, Gerbing RB, Smith FO, Meshinchi S, Ross JA, Perentesis J, Woods WG, Lange BJ, Davies SM. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A, Barr R. Cancer in Young Adults 20 to 39 Years of Age: Overview. Seminars in Oncology. 2009;36:194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Dahman B, Jin Y, Shickle LM, Ginder GD. Acute myeloid leukemia: how the uninsured fare. Cancer. 2011;117:4772–4778. doi: 10.1002/cncr.26095. [DOI] [PubMed] [Google Scholar]
- Brenner H, Gefeller O, Hakulinen T. Period analysis for 'up-to-date' cancer survival data: theory, empirical evaluation, computational realisation and applications. EJC. 2004;40:326–335. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Burnett AK. The treatment of AML: current status and novel approaches. Hematology (Amsterdam, Netherlands) 2005;10 Suppl 1:50–53. doi: 10.1080/10245330512331389773. [DOI] [PubMed] [Google Scholar]
- Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, Wheatley K, Hunter A, Russell N. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. Journal of Clinical Oncology. 2013;31:1293–1301. doi: 10.1200/JCO.2011.40.5977. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Halman LJ, Koniaris LG, Cassileth PA, Rosenblatt JD, Cheung MC. Effects of poverty and race on outcomes in acute myeloid leukemia. American Journal of Clinical Oncology. 2011;34:297–304. doi: 10.1097/COC.0b013e3181dea934. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Meshinchi S, Davies SM. Transplantation for AML in children. Biology of Blood and Marrow Transplantation. 2012;18:S33–39. doi: 10.1016/j.bbmt.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WE, Crews KR, Pui CH. A health-care system perspective on implementing genomic medicine: pediatric acute lymphoblastic leukemia as a paradigm. Clinical Pharmacology and Therapeutics. 2013;94:224–229. doi: 10.1038/clpt.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sanchez-Perez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5-a population-based study. Lancet Oncology. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- Glaser SL, Clarke CA, Chang ET, Yang J, Gomez SL, Keegan TH. Hodgkin lymphoma incidence in California Hispanics: Influence of nativity and tumor Epstein-Barr virus. Cancer Causes and Control. 2014;25:709–725. doi: 10.1007/s10552-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins WB, Lo FFK. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: Evidence from the SEER database 1988-2008. Cancer Causes and Control. 2012;23:737–743. doi: 10.1007/s10552-012-9943-8. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- Hann IM, Webb DK, Gibson BE, Harrison CJ. MRC trials in childhood acute myeloid leukaemia. Annals of Hematology. 2004;83 Suppl 1:S108–112. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, Wheatley K, de Graaf SS, van den Berg E, Burnett AK, Gibson BE. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. Journal of Clinical Oncology. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- Ho PA, Alonzo TA, Gerbing RB, Pollard J, Stirewalt DL, Hurwitz C, Heerema NA, Hirsch B, Raimondi SC, Lange B, Franklin JL, Radich JP, Meshinchi S. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children's Oncology Group. Blood. 2009;113:6558–6566. doi: 10.1182/blood-2008-10-184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibe K, Tsukimoto I, Ohno R. Clinicopathologic characteristics of leukemia in Japanese children and young adults. Leukemia. 2001;15:1256–1261. doi: 10.1038/sj.leu.2402194. [DOI] [PubMed] [Google Scholar]
- Lim JY, Bhatia S, Robison LL, Yang JJ. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer. 2014;120:955–962. doi: 10.1002/cncr.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Basch E. Big data infrastructure for cancer outcomes research: implications for the practicing oncologist. Journal of Oncology Practice. 2015;11:207–208. doi: 10.1200/JOP.2015.004432. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Dover DC, Li S, Yasui Y, Ness KK, Mertens AC, Neglia JP, Sklar CA, Robison LL, Davies SM. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: a report from the Childhood Cancer Survivor Study. Cancer. 2008;112:2071–2079. doi: 10.1002/cncr.23405. [DOI] [PubMed] [Google Scholar]
- Ofran Y, Rowe JM. Acute myeloid leukemia in adolescents and young adults: challenging aspects. Acta Haematologica. 2014;132:292–297. doi: 10.1159/000360200. [DOI] [PubMed] [Google Scholar]
- Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiology, Biomarkers and Prevention. 2015a;24:344–349. doi: 10.1158/1055-9965.EPI-14-0963. [DOI] [PubMed] [Google Scholar]
- Patel MI, Ma Y, Mitchell BS, Rhoads KF. Age and Genetics: How Do Prognostic Factors at Diagnosis Explain Disparities in Acute Myeloid Leukemia? American Journal of Clinical Oncology. 2015b;38:159–164. doi: 10.1097/COC.0b013e31828d7536. [DOI] [PubMed] [Google Scholar]
- Percival ME, Tao L, Medeiros BC, Clarke CA. Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer. 2015;121:2004–2012. doi: 10.1002/cncr.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard-Jones K, Pieters R, Reaman GH, Hjorth L, Downie P, Calaminus G, Naafs-Wilstra MC, Steliarova-Foucher E. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. The Lancet Oncology. 2013;14:e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. New England Journal of Medicine. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981-2005. Cancer. 2009;115:4973–4979. doi: 10.1002/cncr.24548. [DOI] [PubMed] [Google Scholar]
- Pulte D, Redaniel MT, Brenner H, Jeffreys M. Changes in survival by ethnicity of patients with cancer between 1992-1996 and 2002-2006: is the discrepancy decreasing? Annals of Oncology. 2012;23:2428–2434. doi: 10.1093/annonc/mds023. [DOI] [PubMed] [Google Scholar]
- Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98:222–229. doi: 10.3324/haematol.2012.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzouk BI, Estey E, Pounds S, Lensing S, Pierce S, Brandt M, Rubnitz JE, Ribeiro RC, Rytting M, Pui CH, Kantarjian H, Jeha S. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106:2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- Redig AJ, Janne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. Journal of Clinical Oncology. 2015;33:975–977. doi: 10.1200/JCO.2014.59.8433. [DOI] [PubMed] [Google Scholar]
- Ribeiro RC. Advances in treatment of de-novo pediatric acute myeloid leukemia. Current Opinion in Oncology. 2014;26:656–662. doi: 10.1097/CCO.0000000000000136. [DOI] [PubMed] [Google Scholar]
- Robbins AS, Lerro CC, Barr RD. Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer. 2014;120:1212–1219. doi: 10.1002/cncr.28568. [DOI] [PubMed] [Google Scholar]
- Rosenberg AR, Kroon L, Chen L, Li CI, Jones B. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer. 2014;121:1279–1286. doi: 10.1002/cncr.29187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H. Childhood acute myeloid leukaemia. British Journal of Haematology. 2012;159:259–276. doi: 10.1111/bjh.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Lensing S, Razzouk BI, Pounds S, Pui CH, Ribeiro RC. Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatric Blood & Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled Clinical Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, Habdank M, Spath D, Morgan M, Benner A, Schlegelberger B, Heil G, Ganser A, Dohner H. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. New England Journal of Medicine. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Schultz KA, Chen L, Chen Z, Kawashima T, Oeffinger KC, Woods WG, Nicholson HS, Neglia JP. Health conditions and quality of life in survivors of childhood acute myeloid leukemia comparing post remission chemotherapy to BMT: a report from the children's oncology group. Pediatric Blood & Cancer. 2014;61:729–736. doi: 10.1002/pbc.24881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MA, Peterson B, Dodge RK, Mayer RJ, Moore JO, Lee EJ, Kolitz J, Baer MR, Schiffer CA, Carroll AJ, Vardiman JW, Davey FR, Bloomfield CD, Larson RA, Stone RM. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood. 2004;103:4036–4042. doi: 10.1182/blood-2003-09-3118. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Kowalczyk JR, Agarwal B, Ladenstein R, Fitzgerald E, Barr R, Steliarova-Foucher E, Magrath I, Howard SC, Kruger M, Valsecchi MG, Biondi A, Grundy P, Smith MA, Adamson P, Vassal G, Pritchard-Jones K. New policies to address the global burden of childhood cancers. Lancet Oncology. 2013;14:e125–135. doi: 10.1016/S1470-2045(13)70007-X. [DOI] [PubMed] [Google Scholar]
- Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. 2014;123:3553–3562. doi: 10.1182/blood-2013-07-517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlock K, Meshinchi S. Pediatric acute myeloid leukemia: biology and therapeutic implications of genomic variants. Pediatric Clinics of North America. 2015;62:75–93. doi: 10.1016/j.pcl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, Appelbaum FR, Kantarjian HA, Estey EH. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: A novel paradigm for treatment assignment. Journal of Clinical Oncology. 2011;29:4417–4423. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, Thun MJ. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- Wolfson J, Sun C-L, Kim H, Kang T, Bhatia S. Evaluation of the effect of care at NCI comprehensive cancer centers (NCICCCs) on disparities in outcome within adolescents and young adults (AYAs) with cancer. Journal of Clinical Oncology. 2012;30:609s. abstract 9512. [Google Scholar]
- World Health Organisation . In: International Classification of Diseases for Oncology. third Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Sharon W, editors. World Health Organization; Geneva: 2000. [Google Scholar]
- Yoon JH, Kim HJ, Jeon YW, Lee SE, Cho BS, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. Outcome of allogeneic hematopoietic stem cell transplantation for cytogenetically normal AML and identification of high-risk subgroup using WT1 expression in association with NPM1 and FLT3-ITD mutations. Genes, Chromosomes and Cancer. 2015 doi: 10.1002/gcc.22260. doi: 10.1002/gcc.22260. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes and Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]