Abstract
Point-of-care or point-of-use diagnostics are analytical devices that provide clinically relevant information without the need for a core clinical laboratory. In this review we define point-of-care diagnostics as portable versions of assays performed in a traditional clinical chemistry laboratory. This review discusses five areas relevant to human and animal health where increased attention could produce significant impact: veterinary medicine, space travel, sports medicine, emergency medicine, and operating room efficiency. For each of these areas, clinical need, available commercial products, and ongoing research into new devices are highlighted.
Keywords: Point-of-care, Diagnostics, Space Travel, Sports Medicine, Veterinary, Emergency Care, Operating Room
1. Introduction
Point-of-care (POC) diagnostic tests provide clinically relevant information at the point-of-use, without the need for sample processing or analysis from a remote clinical chemistry laboratory. The blood glucose meter, used for the management of diabetes, and the home pregnancy test (dipstick) are the most popular examples. However, recent advances in microfluidics combined with the decreasing cost and size of advanced electrochemical and optical sensors (Vashist et al., 2015) have broadened the range of applications for POC diagnostics. For example, these advances have made possible the burgeoning field of POC diagnostics for resource-limited settings, such as developing nations. Excellent reviews of POC diagnostic tests for global health have been published recently and thus are not included here (Chin et al., 2011, Yager et al., 2008). In addition to global health, other areas where portability and low cost are key drivers have benefitted from improvements in POC diagnostic technology. In this review, we focus on some of these niche areas that have not been covered extensively in the literature and which have a small market size compared to health diagnostics in developed nations, or where the clinical benefit is still being actively investigated.
First, we briefly review the regulations governing POC diagnostics for human health, as they dictate how the diagnostics are used. Then we review POC diagnostics for veterinary medicine, space travel, sports medicine, emergency care, and operating room applications. A couple of these areas (e.g., veterinary medicine or emergency care) contain a vast number of potentially useful biomarkers that could be tested—enough to warrant a dedicated review article for each. Our goal here is not to exhaustively cover all possible applications within each area, but rather to increase the general awareness of the opportunities available and to focus on selected examples that have the greatest impact.
1.1 CLIA regulation
Healthcare-related diagnostic tests are an integral component of healthcare delivery in the US as they currently have a direct impact on up to 70% of healthcare-related decisions (www.lewin.com, 2005). This impact has been driven by the advances in science and technology of the 20th century. Prior to these advances, decision making was primarily based on patient history and physical examination (Burke, 2000). When these tests were first being used in diagnosis, most of these tests were performed at the side of the patient, and the individual practitioner had a significant amount of autonomy (Moore, 2005). However, the variability in quality of these tests ultimately led to regulation that mandated how these tests were to be performed (Berger, 1999a, b).
This regulation was primarily enforced by the Clinical Laboratory Improvement Amendment (CLIA) of 1988, which established regulatory standards for all human-related laboratories testing for diagnostic purposes (1988). This was, specifically, in response to an alarmingly high number of false negative results from in-house laboratories. Since its enactment, CLIA has required that all labs performing diagnostic tests of human samples must register with the Centers for Medicare and Medicaid Services (CMS). The process of registration is based on the type of testing that is to be performed, and a set of compliance standards are required for the given lab classification. This lab classification is determined by the complexity and clinical significance of the testing to be performed. The resulting CLIA requirements are more stringent for more complicated tests. As outlined in Table 1, diagnostic tests are scored by the FDA using seven independent criteria. These scores are then summed to determine the risk associated with the test. Diagnostic tests with scores of 12 or less are in the moderate-complexity category. Scores higher than that are deemed high-complexity (www.fda.gov, 2015a). CLIA defines waived-tests to be “simple laboratory examinations and procedures that have an insignificant risk of an erroneous result.” Sites that only perform waived tests must still have a CLIA certificate and follow the instructions from the manufacturer (Collopy et al., 2014, www.fda.gov, 2015b.)
Table 1.
Criteria for the categorization of CLIA lab test. After (Collopy, Kivlehan, 2014).
| Criteria | Score of 1 | Score of 3 |
|---|---|---|
| Knowledge | Minimal scientific and technical knowledge required; may be taught on the job | Specialized scientific knowledge required to perform preanalytic, analytic or postanalytic testing |
| Training and experience | Minimal training or limited experience required to perform test | Specialized training is essential or substantial experience necessary for test performance |
| Reagents and materials | Reagents and materials are stable and reliable; they are prepackaged or premeasured with no special handling required | Reagents and materials may be labile and require special handling. Preparation may include manual steps such as volumetric measurements |
| Characteristics of operational steps | Operational steps are either automatically executed or easily controlled | Steps require close monitoring or control; may require special preparation, precise temperature control or procedural steps |
| Calibration, quality control and proficiency testing | Calibration and QC materials are stable and readily available | Calibration, and proficiency materials may be labile |
| Test system troubleshooting | Troubleshooting is automatic | Troubleshooting requires |
| and equipment maintenance | or self-correcting or requires minimal judgment. Maintenance is seldom required and can be easily performed | decision making and direct intervention to solve most problems. Maintenance requires special knowledge and skills |
| Interpretation and judgment | Test processes require minimal judgment or interpretation | Testing processes require extensive judgment, resolution of problems requires extensive interpretation |
These CLIA regulations led clinical practices to outsource many of their diagnostic tests to core laboratories - either regional centers like those operated by LabCorp or local centers within the hospital. This workflow is well-suited for tests where the results are not needed immediately, as the delivery of care would not be changed even if the information was immediate. However, there is a fairly recent push to perform certain tests at the POC (Gubala et al., 2012, John and Price, 2013, McPartlin and O'Kennedy, 2014). Because POC tests are portable, they allow for an expedited workflow (Figure 1) and potentially shorter turnaround times. As a result, they carry the promise of providing the care giver with information at time points that can provide improved delivery of care and reduce the cost. However, to meet regulations, these tests must either be CLIA-waived or users of the devices must meet the associated standards in an attempt to prevent errors (Kost, 2001, Lewandrowski et al., 2011).
Figure 1.
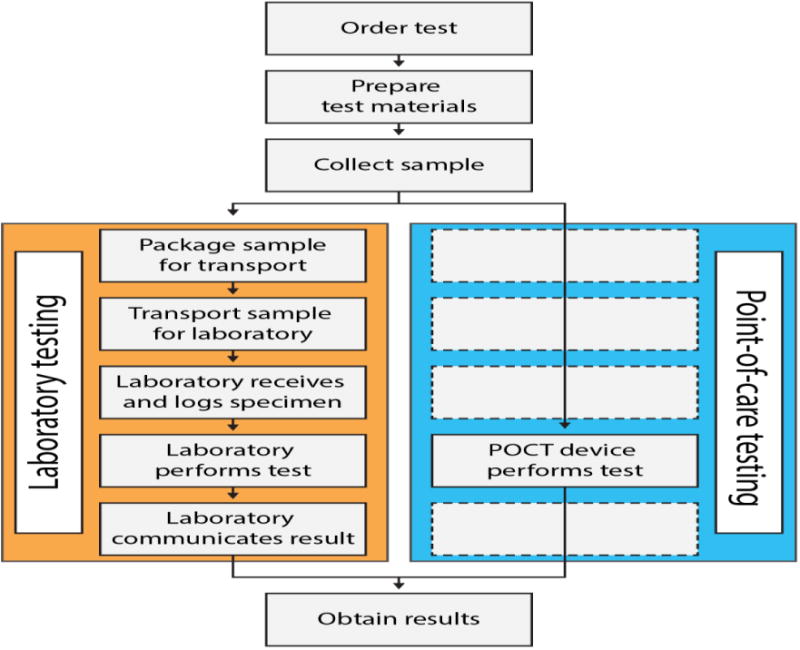
Diagram that highlights the differences in workflow between using traditional laboratory testing and point-of-care testing. From (Larsson et al., 2014).
One example of a CLIA-waived, POC test is the blood glucose meter. With just a drop of blood from a finger-stick, these devices can inform the user of their current blood glucose levels and enable them to better self-regulate their blood glucose values. This self-regulation would not be possible if a blood glucose meter for use at the point-of-care did not exist. As the demand for home-health information continues to grow, additional devices are being developed for home use. The ability to create cost-effective, disposable test cartridges that can store reagents, direct the flow of solutions with passive microfluidics, and automatically read the generated signal leads to POC technologies becoming more user-friendly. In this review we describe POC tests that require CLIA regulation (i.e., those used in the emergency department and the operating room) as well as tests that do not, such as diagnostics for veterinary medicine.
2. Veterinary medicine
The common concerns between human health and animal health have made the development of veterinary diagnostics very appealing, especially for companion animals where the animals are brought to the controlled environment of a clinic. Transport and use of diagnostic tests on the farm increases the level of technical challenge and frequently decreases the price that the market will tolerate. The drivers pushing the development of more diagnostics, in addition to advances in human diagnostics, are the increasing willingness of owners of companion animals to spend money to keep their pets healthy, the increasing concern of customers about antibiotics and transmissible diseases in milk, eggs and meat, and widespread public concern over the spread of diseases through populations of wild and domestic animals.
2.1. Measurements of blood cells and blood chemistry
The first type of portable animal test to become widely available measures blood cell differentials. Originally, the automated cell counter developed by Becton-Dickenson for humans was adapted for animals; several versions of this differential counter are now commercially available as relatively small benchtop systems (ae.g. IDEXX LaserCyte Dx Hematology Analyzer (www.idexx.com, 2015a) and Abaxis Vetscan systems (www.abaxis.com, 2015a)). Abnormal white counts are used as indicators of infection; for example, specialized differential systems evaluate white blood cells in milk as an indicator of bovine mastitis.
Similarly, the automated analyzer for blood gases and electrolytes, first pioneered for humans by i-STAT, has been adapted for animals. Portable blood analyzers are frequently available in small clinics and even in portable veterinary facilities at strenuous equine competitions where maintaining proper electrolyte balance can be challenging. A handheld analyzer for blood gases and electrolyte chemistry is sold by Abaxis (www.abaxis.com, 2015b). The device weighs 0.64 kg, uses 95 μL of blood for the analysis, and performs the assay in 2 minutes. In addition to blood gases, a special cartridge is available to perform assays for cardiac troponin as an indicator of cardiac muscle damage.
2.2. Detection of specific infectious diseases
The adaptation of human diagnostics for specific infectious diseases in animals has been slower, but is well underway. The most frequently used POC tests target diseases of companion animals, especially where the animals are brought to local clinics.
The lateral flow test for heartworm is sold by several companies and widely used in small animal clinics. Other tests based on lateral flow immunoassays are commercially available for dogs and cats (e.g. Abaxis (www.abaxis.com, 2015c)) and include tests for canine parvovirus, Ehrlichia, Giardia, Anaplasma, Lyme disease, and feline leukemia/immunodeficiency virus (Figure 2). The tests take 10-15 minutes to run and no reader is required.
Figure 2.
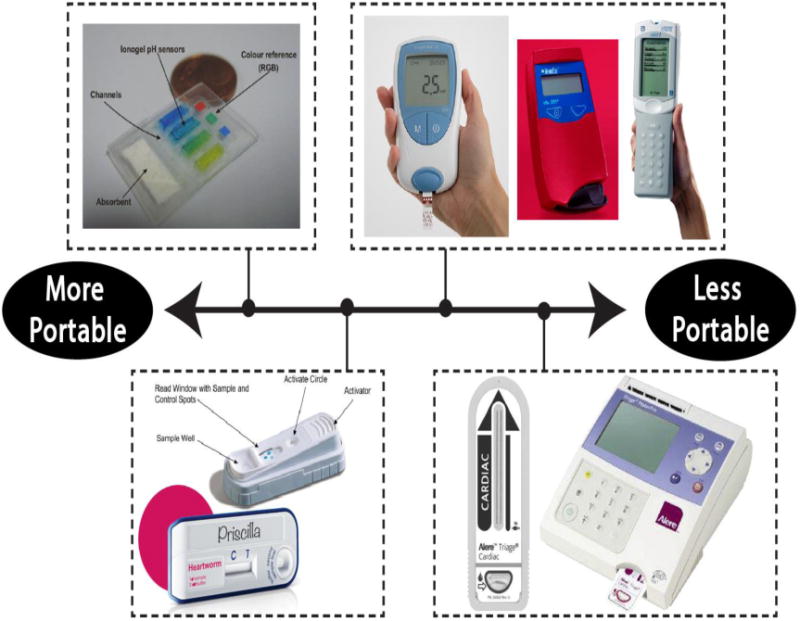
Most portable diagnostic tests can be categorized as a wearable test (top-left), a cartridge-based test that does not need a reader (bottom-left), a cartridge-based test that needs a handheld reader (top-right), or a cartridge-based test that needs a tabletop reader (bottom-right). These subcategories range from being the most portable to the least portable. In the top-left, an absorbent pad wicks sweat to pH sensitive ionogels which change color in response different pH levels. From (Curto, Fay, 2012). In the top-right, examples are shown of the Coaguchek XS System (www.coaguchek.com, 2016), the HemoCue Hb 201+ System (www.hemocue.us, 2016), and the i-STAT portable blood chemistry analyzer (www.abbottpointofcare.com, 2015). The lower-left shows a SNAP test by IDEXX (top) and an Abaxis lateral flow assay for the detection of heartworm disease (bottom). From (O'Connor, Lawrence, 2013) and (www.abaxis.com, 2015e), respectively. The lower-right image shows the Alere Triage system (www.alere.com, 2015).
More sophisticated biosensors for veterinary infectious diseases must address the requirements of device and reagent transport, operational simplicity, low cost, and data presentation in an actionable and useful format. The IDEXX SNAP tests (Figure 2) are based on sandwich immunoassays with added labels and washing to enhance signal discrimination compared to lateral flow tests (www.idexx.com, 2015b). The diagnostic system also includes a 0.66 kg reader that times the assay steps and transmits the data from up to six assays performed simultaneously on a single sample to central record-keeping files. Tests are available for the following pet diseases: heartworm, Ehrlichia, Anaplasma, Lyme disease, feline leukemia virus, feline immunodeficiency virus, Giardia, parvovirus, canine or feline pancreatitis, and feline heart disease. There is also a SNAP test for Leishmaniasis in dogs since dogs often supply the reservoir for disease transmission to humans, but this test is primarily used outside the US (O'Connor et al., 2013).
In addition to tests for dogs and cats, Abaxis sells an FDA-approved lateral flow test for Type A avian influenza that can be used to test chickens, ducks, turkeys, and geese (www.abaxis.com, 2015d). The test uses tracheal, oropharyngeal, or cloacal swabs to collect sample and insert it into the test cartridge. Yes/no answers are available in 15 minutes without a reader and only minimal technical training is required for operation. A SNAP test is also available for testing birds for Influenza A.
During the “bird flu” epidemic with repeated evidence of influenza transmission from birds to animals and people, quite a few of similar lateral flow assays for H5N1 influenza virus were tested in the field, but we could not find evidence that they have been commercially produced, possibly because of limited sensitivity or a cost too high to monitor wild bird populations. Nevertheless, some effort continues. Bai et al. (2012) reported using a portable surface plasmon resonance biosensor incorporating an aptamer (instead of an antibody) that recognized the H5N1 strain in poultry swab samples with a sensitivity ten times better than ELISA in 1.5 hours. The selectivity for the H5N1 compared to the six other influenza strains was excellent. The cost and complexity of the device is still problematic, but the aptamer reagent might provide advantages for other, simpler POC tests.
The detection of Salmonella is of particular interest since it can be transmitted from bird feces, meat, or eggs to humans. While the experiments were performed in the lab, Gauglitz and colleagues demonstrated the use of a portable, low cost reflectometer to analyze sera from chickens infected with Salmonella (Ewald et al., 2015). They demonstrated that the antibodies from different chickens reacted differently against different strains of Salmonella and that C-reactive protein could be simultaneously measured as a generic indicator of the level of inflammation. The reactions required no labels or added reagents after the sample was passed over the chip, and the results were quantitative. Furthermore, the measurements of reactions to two different Salmonella strains and C-reactive protein were performed on a single chip. However, use of the chip and reader still required two syringe pumps and an eight-way valve, so there is still some engineering required to transfer this analytical system to a chicken shed. There are other diseases for which commercial flocks could benefit from point-of-use diagnostics not only because of rapid transmission and high mortality, but also because vaccines may be available. Such diseases include infectious bronchitis virus, infectious bursal disease, and Newcastle disease virus (O'Connor, Lawrence, 2013).
Milk is an attractive sample matrix for testing because of its easy accessibility as well as high value. Liebes et al. (2009) tested milk samples for antibodies to Brucella as an indicator of bovine pathogen exposure. They generated a chemiluminescent signal at the surface of an optical fiber coated with killed Brucella organisms. It was a multistep assay that would be difficult to perform in a barn, but the sensitivity was significantly better than a standard laboratory ELISA. While Brucellosis is of particular concern because it can be transmitted from wild populations of cloven-hooved animals to domestic cattle, most countries require that analyses be performed in a federally accredited laboratory.
Cattle and pigs are often quartered in groups, facilitating transmission of infectious diseases, especially those that are transmitted through aerosols and feces. Frequently, the causative agents are well distributed before an animal becomes symptomatic or there is a very short time between the onset of symptoms and death. Diseases of this type could be better controlled and animals saved if farmers or veterinarians were able diagnose the disease on site. Diseases that cause high mortality include bovine respiratory disease and porcine pseudorabies (O'Connor, Lawrence, 2013).
2.3. Testing to facilitate breeding
Several assays have been developed for detecting ovulation in cattle in order to facilitate artificial insemination. Monitoring progesterone levels in milk has proven to be more effective than monitoring oestrus not only in predicting ovulation, but also for detecting pregnancy and fertility problems (Velasco-Garcia and Mottram, 2003). Competitive immunoassays for progesterone in milk have been demonstrated using ELISA kits, amperometric biosensors, and optical biosensors, but as far as we can tell, a successful commercial product for use in the barn is not yet available.
There is also a market for progesterone testing in horses to document the estrus cycle or to confirm pregnancy. This is particularly important for artificial insemination and embryo transfer procedures. However, it is not clear that a rapid analysis is of significantly more utility than shipment of plasma or serum to a central laboratory, but the opportunity to reduce the cost of a repetitively performed assay could be significant.
A more critical test for horses to be performed at the barn is the test for foal immunoglobulin (IgG). Foals that do not receive maternal immunoglobulins from milk immediately after birth are immunodeficient and highly susceptible to infection unless rapidly treated. IDEXX has developed a SNAP test for this condition (O'Connor, Lawrence, et al., 2013). Another common problem in foals is intoxication with botulinum toxin, and again this is a situation where waiting on transport of a serum sample to a central laboratory has a significant downside in consequent morbidity. On-site tests for botulinum toxin developed by the biodefense community could be adapted to address this need.
2.4. Drug residue analysis
Another area of veterinary application that has received a great deal of attention from researchers is the development of screening tests for drug residues in meat and milk (Sanvicens et al., 2011, Velasco-Garcia and Mottram, 2003). Research labs and companies have been developing tests for antibiotics in milk that can be used in the barn or at the dairy processing facility for over 30 years, and excellent reviews are available (Davis and Higson, 2010; Samsonova et al., 2012; Babington et al., 2012; Danaher and Jordan, 2013). Key issues for continued improvement include elimination of any sample preparation, minimized use of complex equipment, and multiplexed analysis to detect multiple antibiotics simultaneously.
Surface plasmon resonance (SPR) has been used successfully to detect sulfonamides in milk and pig bile at levels below that of standard laboratory methods. The same instrument has also been used to detect illegal growth promoting drugs in calf urine. SPR is attractive because the assays are label-free and multiple tests can be run simultaneously; detection of three different families of antibiotics were measured below regulatory levels. Imaging SPR can detect an even wider array of antibiotic residues in milk using antibody microarrays for immunodetection at levels consistent with regulatory guidelines. However, simpler systems are needed for use in the barn or stockyard.
The portable array biosensor commercialized by mBio Diagnostics (www.mbiodx.com) has been used for simultaneous and quantitative immunoassay of three antibiotics in milk (McGrath et al., 2015). No sample preparation was required, and sensitivities ranged from 0.13 to 2 ng/mL, meeting the European legislated requirements. The device is based on evanescent illumination of a disposable cartridge including fluorescent reagents and planar waveguide modified with capture antibody spots. It simple to use, with sample in-answer out capability, and inexpensive.
Screen-printed amperometric sensors are not quite as sensitive for detecting antibiotics in milk, but much simpler to use. Screen-printed carbon electrodes, similar to those in over-the-counter glucose tests, were used to detect the banned antibiotic clembuterol in bovine hair (Regiart et al., 2013). The assay was a competitive immunoassay that produced an electroactive product and produced excellent sensitivity (0.008 ng/mL), but the sample preparation required two hours and involved multiple steps and hazardous chemicals.
2.5. Future directions
The biggest challenge in getting portable diagnostic tests into the veterinary market is not convincing veterinarians to use them. Once a test is proven to provide a reliable basis on which a veterinarian can make a clinical decision, the hurdles of cost, ease of use, and storage stability are still critical. The cost of the technologies included in portable tests is going down fast. Tests that use glucose meters (Gu et al., 2015) or cell phones (Yu et al., 2015; Ludwig et al., 2015) to analyze milk samples are in the research phase. Fabrication technologies, including cloned reagents, especially synthetic antibodies, ink jet printing of biologics and optoelectronic circuits, organic optics, and roll-to-roll fabrication and assembly of devices will bring the cost down and enable the production of more robust and disposable tests.
Inexpensive methods for measuring DNA or RNA will also fill a major void for veterinary applications, particularly for diagnosis of viral and mycoplasma infections (Dahlhausen, 2010). Neogen Corporation has a range of diagnostic products for food and animal safety based on a 4-plate processing system for detection of pathogens, with results in less than 2 hours. However, it is still a laboratory system requiring a sophisticated user. In addition to genetic characterization for a wide variety of breeding animals where a timely response is less often crucial, mail in samples can currently be tested for bovine diarrhea virus, porcine epidemic diarrhea virus, porcine stress syndrome, porcine reproductive and respiratory syndrome virus, or porcine circovirus type 2 (e.g., (www.neogen.com, 2015)). Other companies, such as Biomerieux sell kits and benchtop devices for tests based on genetic identification of diseases, but they are all intended for use in a laboratory, not in a barn (www.biomerieux-industry.com, 2015). Isothermal amplification approaches may eventually go a long way toward moving tests for pathogens based on oligonucleotide binding out of the lab. The research community has extensive efforts to move oligonucleotide amplification tests onto automated chips, but making the entire analytical system simple and robust remains challenging.
While there are tests for diseases that cause diarrhea or respiratory distress in horses and food animals, nearly all of these tests require shipping a sample to a central laboratory. The time for shipping as well as analysis is a serious problem for animals with life threatening illnesses; usually the veterinarian must start treatment days before the pathogen is identified and initial treatment costs of $5000 for a large animal are not unusual. If the disease is highly infectious, the delay in getting results has ramifications that impact groups of animals and even wide geographic regions if quarantines are not established in time. The market exists, and the opportunity for innovative technologies to find a niche is wide open.
3. Space travel
The amount of time astronauts spend in space has steadily increased in recent decades, first with the establishment of the Mir space station, and more recently with the International Space Station. Unlike earlier low Earth orbit space flights, which were of short duration with potential for a quick return to Earth in the event of a medical emergency, future missions will be much longer in duration: return mission to the moon (14 days), a stay at a moon base (180 days), and human exploration to Mars (395 days). Long missions pose significant challenges to the use of diagnostics for monitoring astronaut health. For example, a mission to Mars prevents the transport of samples to Earth for analysis and also makes impossible shipments of new consumables such as reagents or supplies. As mission lengths continue to increase, the need for diagnostics that detect a wide variety of medical conditions and measure multiple physiological parameters, but are compatible with the rigors of space travel, will continue to grow.
3.1. Human health requirements
The space environment possesses unique health hazards and challenges that are not found on Earth. The key risks for an astronaut are: bone loss, radiation exposure, cardiovascular de-conditioning, muscle loss, and vestibular re-conditioning (Buckey 1999). These conditions begin to develop within days of first entering orbit and progress as the spaceflight duration grows. Some of the effects, such as cardiovascular de-conditioning and muscle loss, can be mitigated through exercise. Research on animals suggests that artificial gravity (e.g., through the use of an on-board short-arm centrifuge) protects agains bone mineral loss and could be beneficial for humans as well (Davis 1999). However, the threat of radiation exposure is considered to be the primary hazard associated with spaceflight (Nicogossian et al., 1994). Completely shielding astronauts from radiation while inside a space vehicle would require prohibitively large masses, so a balance must be struck between acceptably low radiation exposure and vehicle mass (Nachtwey 1991). This same tradeoff between safety and mass must be considered for spacesuits when astronauts are outside a vehicle or shelter (e.g., on the surface of Mars).
In addition to the physiological changes and health hazards caused by the space environment, astronauts are also susceptible to human disease and injury. It is impractical to transport samples to Earth for diagnosis, and in the case of a trip to Mars it will be impossible. Astronauts must have access to the instruments necessary to diagnose and treat a wide range of diseases and injuries. NASA maintains an evolving list of medical conditions that could occur during long-duration space travel in the Exploration Medical Condition List (EMCL) (humanresearchwiki.jsc.nasa.gov, 2015a). There are 88 conditions listed and they range from life-threatening conditions, such as sudden cardiac arrest, to fairly minor conditions such as a skin abrasion. Of these 88 conditions, 25 require fluid specimens for diagnosis, making them ideal candidates for POC analysis. Table 2 summarizes the conditions.
Table 2.
Identified conditions that shall be treatable in space and that require fluid analysis. From the Exploration Medical Capability (ExMC) Evidence Report. After (humanresearchwiki.jsc.nasa.gov, 2015c).
| Medical Conditions | |
|---|---|
| Burns | Pharyngitis |
| Cellulitis | Prostatitis |
| De Novo Cardiac Arrhythmia | Radiation Sickness |
| De Novo Hypertension | Respiratory Infection |
| Decompression Sickness | Seizure |
| Diarrhea | Sepsis |
| Headache | Space Motion Sickness (Space Adaptation) |
| Intra-abdominal Infection | Surgical Treatment |
| Nausea/Vomiting | Toxic Exposure |
| Neck Injury | Urinary Incontinence (Space Adapation) |
| Nephrolithiasis | Urinary Retention (Space Adapatation) |
| Nosebleed (Space Adaptation) | Urinary Tract Infection |
| Osteoporosis | |
All of the conditions listed in Table 2 can be diagnosed by quantifying cells, electrolytes, ions, crystals (for urinalysis), small molecules, or large biomolecules (e.g., proteins) within a fluid specimen. Fluid specimens relevant to human health are saliva, urine, blood, and potable water (to monitor contamination). For example, excessive radiation exposure can be diagnosed by checking for a decrease in peripheral blood lymphocytes and by measuring the expression of cytogenetic markers such as DNA repair gene GADD45 (Blakely et al., 2003; Semkova et al., 1995). Cardiac arrest can be diagnosed by quantifying the levels of a biomolecule, C-reactive protein, in the blood. The instruments for counting cells and measuring the levels of biomarkers are commonly found in core clinical laboratories on Earth. Protein tests, including C-reactive protein, have been reported using lateral flow assays (which may be compatible with use in space) and other biosensors which have not been optimized for zero gravity. Thus, while the base technologies for use on Earth may exist, adapting them for both storage and use in space will be a significant engineering challenge.
3.2. Diagnostic requirements
Space travel poses unique design challenges to portable POC diagnostics. The POC diagnostic must survive transportation to space, where it is exposed to many times the normal gravitational force during launch and severe vibration. Once in orbit, the diagnostic will be in an environment characterized by low humidity, low gravity, elevated radiation levels compared to Earth, and an absence of refrigeration.
Researchers at NASA have developed a list of design principles for the development of microfluidic biomedical diagnostics in space: minimized resource consumption, modularity, long shelf life, and gravitational independence (Nelson 2011). While some of these principles align with recent trends in the development of POC devices for resource-limited settings (e.g., long shelf life), others are unique to space travel.
One of the main design challenges for space-based POC diagnostics is minimizing resource consumption. Resource consumption has two components: the resources consumed to perform the diagnostic and the diagnostic device itself. Most POC diagnostics are disposable because a disposable device limits the chances of cross-contamination and reduces cost During space travel, mass and volume-the “load” associated with a diagnostic-is a key consideration before deployment and must always be minimized. Therefore, the use of disposable pipettes or lancets, and even disposable microfluidic chips, is not optimal. Instead, reversing a trend that is common in the lab-on-a-chip field, diagnostics for space travel should be reusable instead of disposable (Nelson 2011). Minimizing resource consumption also reduces the amount of biohazardous waste generated.
Reusable POC diagnostic devices for space will need to be adaptable to accommodate a wide range of human samples and diagnostic tests. Reusable modules would also save space and minimize the load associated with the diagnostic device. For example, a complete blood count might need to be performed on a sample simultaneously with an analysis of proteins in the serum. This analysis could be accomplished by linking together different recognition modules in series that perform each of the diagnostics (Nelson 2011). On Earth the solution is to have dedicated diagnostics for each type of test. This approach will be impossible in space, so a modular approach provides a more attractive alternative. A modular device that can be disassembled is also easier to clean, which is another design consideration for reusable diagnostic devices suitable for space travel.
The reagents required for the POC diagnostics should have a long shelf life, of up to a few years in the case of a Mars mission without refrigeration(Crucian et al., 2013). Strategies exist to extend the shelf life of antibodies, but alternative recognition elements that are not as susceptible to degradation may extend the shelf life even further.
Lastly, diagnostic devices must be able to function normally in the absence of gravity. This problem is not encountered on Earth and will require the development of novel methods for manipulating fluids within the diagnostic device. For example, while some analytical methods that work on Earth, such as capillary electrophoresis, have been demonstrated in low gravity environments (Culbertson et al., 2005), other methods, such as gravity-driven fluid flow, are impractical. Passive pumping methods that rely on surface tension or surface wetting provide attractive solutions for manipulating fluids because they are insensitive to gravity, contain no moving parts, require no external energy sources, and add minimal weight to the diagnostic device.
Bubbles are another example of an Earth-based problem that will need to be re-examined in the context of microgravity. Bubbles are removed from devices via a bubble trap on Earth. However, these traps rely on gravity and will not work in space. New methods for removing or trapping bubbles can only be developed by studying the behavior of microfluidic devices in low gravity environments.
3.3. Devices deployed on the International Space Station
Only two POC devices have been deployed on the International Space Station (ISS): the i-STAT and the Reflotron (humanresearchwiki.jsc.nasa.gov, 2015b). Both of these diagnostics measure blood chemistry.
The i-STAT is a portable blood chemistry analyzer (Figure 2). It was developed by Abbot Labs in the early 1990s (Erickson and Wilding, 1993) and was the first microfluidic POC device to market. The device has found use in hospitals (Papadea et al., 2002), but because of its small size and low power requirements, it is also well-suited for use in space. The i-STAT was used to measure the blood chemistry of 21 astronauts during five space shuttle missions in the mid-1990s (Smith et al., 1997). In this study, a chip that can measure six analytes was used to measure sodium, potassium, glucose, ionized calcium, pH, and hematocrit. A variety of chips are available for the i-STAT. Analytes are quantified with electrochemical sensors: potentiometry for electrolytes and pH, amperometry for glucose, and conductance for hematocrit measurements. While the i-STAT can provide a reading for hematocrict, it cannot provide detailed blood cell sub-populations. A finger prick is used to collect 85 μL of blood which is then deposited on a cartridge. Analysis takes 120 seconds. The cartridge is inserted into the handheld reader where an on-chip vacuum is used to propel the sample through the chip. Analyses performed on the shuttle agreed well with Earth-based analysis, except for hematocrit. However, others have documented inaccurate hematocrit results in Earth-based clinical use (Erickson and Wilding, 1993, Papadea, Foster, 2002), suggesting these errors are intrinsic to the i-STAT and not a result of microgravity. The i-STAT currently resides on the ISS but is not frequently used because of the 6-month shelf-life of the cartridges (ntrs.nasa.gov, 2015).
The Reflotron IV biochemical analyzer by Boehringer Mannheim has also been tested in space on seventeen cosmonauts over six missions to the Mir station (Markin et al., 1998). The Reflotron IV was used to measure the activity of glutamate-oxalo-acetate transaminase (GOT), glutamate pyruvate transaminase (GPT), creatine kinase (CK), gamma-glutamyltransferase (gamma-GT), and total and pancreatic amylase. The concentration of several analytes was measured as well: hemoglobin, glucose, total bilirubin, uric acid, urea, creatinine, total, high density lipoproteins (HDL-) and low density lipoproteins (LDL-) cholesterol, and triglycerides. The Reflotron is a table-top instrument and is not as portable as the i-STAT. It uses paper strips and dry chemistry to perform the analysis. An optical readout measures the reflectance of the color after the chemical reaction (Markin, Strogonova, 1998).
While the i-STAT and Reflotron IV have been deployed to the ISS, a recent NASA report highlighted that no one diagnostic device has the capability to perform a full blood analysis (ntrs.nasa.gov, 2015.). To address these unmet needs, other devices are currently being developed and validated in space or in low-gravity environments for eventual use on long-duration space missions. We highlight some of these efforts below.
3.4. Ongoing research
One effort focused on developing a microfabricated flow cytometer is the Hemocue, which performs differential blood cell counts (Crucian et al., 2013). Although the device was not originally designed for use in space, its small footprint, low power requirements, and ease of use make it attractive for cytometry in space. A 10 μL sample of blood from a finger prick is passively aspirated into a cartridge which contains lysing reagent and a nuclear stain. The cartridge is inserted into the device where it is imaged via a CCD and the blood cell counts are determined from cell morphology and staining. The total analysis time is 3 minutes 20 seconds. Hemocue was validated in parabolic flight (temporary microgravity) and was found to be suitable for use in space. The Hemocue produced results consistent with a reference cytometer on the ground. Blood sample aspiration is not affected by low gravity nor is the assay.
Another device that has been tested in parabolic flight and aboard the ISS is the fiber optic-based flow cytometer Microflow1 (Cohen et al., 2008, Dubeau-Laramée et al., 2014). Cells flow in a capillary tube perpendicular to a 488 nm laser diode excitation source. Reflected and scattered light is captured by optical fibers sitting perpendicular to both the capillary and excitation light. The system can detect the fluorescent stain phycoerythrin-Cy5 and side scatter. The system can enumerate leukocyte sub-populations via immunophenotyping and can also quantify cytokine levels using fluorescent bead-based assays (IL-2, IL-4, TNFalpha, IFNgamma). The system compared favorably to ground-based FACS array cytometer (BD Biosciences). While initial cytometry results are encouraging, samples were prepared on the ground before launch or before parabolic flight. The device produced cell counts comparable to the ground-based FACS. It was able to successfully discriminate granulocytes, monocytes, CD4+ lymphocytes, and CD4‒ lymphocytes in a 40 μL blood sample. The system does not need sheathing fluid because the excitation laser illuminates the width of the capillary, which helps reduce the amount of reagent needed and the amount of biohazardous waste generated. Optical alignment, a potential drawback for using these devices in a high vibration environment such as space flight, is also eliminated because of the illumination strategy. It takes 50 seconds for the device to analyze the sample. Results indicated that Microflow1 performance did not degrade in a microgravity environment.
A group from CalTech has tested a microfabricated flow cytometer in microgravity during parabolic flight (Shi et al., 2009). The authors demonstrated leukocyte count and two-part differential (lymphocyte vs non-lymphocyte) counts from a blood sample. DNA and RNA in the leukocytes are stained with acridine orange. The fluorescence intensity is used to differentiate lymphocytes from non-lymphocytes. Non-lymphocytes typically contain high levels of RNA, which shows up as a brighter fluorescence intensity in the device (Steinkamp et al., 1973). Small dimension channels within the device physically constrain the cells and eliminate the need for sheath flow. Total sample volume was 50 μL or less. Results are comparable to a commercial hemocytometer. The researchers demonstrated that microgravity from parabolic flight does not negatively affect the counts. There are a few drawbacks to the device. Samples were collected and loaded before testing in microgravity. Also, samples required processing before into the device loading: blood was diluted 10× before injection. The unit is self-contained but requires a laptop for readout. A mini peristaltic pump is used to move the blood sample.
Another recent research effort recognizes the need to combine both molecular analysis and cellular analysis into a single device (Straume et al., 2013). As part of a NASA/Sandia joint effort, the authors aim to create a portable device that can perform these analyses and also analyze molecules present in breath.
Another application area for POC testing is monitoring microorganisms (bacteria and fungi) within spacecraft. Microorganisms in spacecraft have been monitored for years by NASA and in Russia (Maule et al., 2009). Bacteria growing on surfaces within the ISS have been characterized, with the most common strains in air and on surfaces being Staphylococcus and Bacillus spp (Van Houdt et al., 2012). Bacteria and fungi are currently monitored by pressing agar “contact slides” onto a surface and observing culture growth over five days (Maule et al., 2009). This potential health hazard, not covered by the Exploration Medical Condition List, is another health-related application that requires POC testing. As a result, one group has begun testing on a new device, the Lab-on-a-chip Application Development Portable Test System (LOCAD-PTS) (Maule et al., 2009, Morris et al., 2012). This device is used to monitor bacterial colonies that grow on surfaces within the habitat. The assay detects lipopolysaccharides (endotoxins) present in the cell wall of gram-negative bacteria using a limulus amebocyte lysate (LAL) assay (Maule et al., 2009). Testing takes 15 minutes, compared to the previous standard which took five days. A more recent version of the device also detects beta-glucan, a component of fungi cell walls, and lipoteichoic acid (LTA), a component of gram-positive bacterial cell walls. The devices have been tested in the ISS and detected a greater occurrence of potentially dangerous (i.e., endotoxin producing) bacteria than was previously documented. The device is a handheld spectrophotometer with a built-in pump that moves the liquid via vacuum. Samples are deposited on a cartridge which is then inserted into the reader. Endotoxin units (EU) are displayed on the device readout. A custom sample collector that serves as a swab and pipette was used to collect sample and then deposit them, suspended in 25 μL of water, onto the cartridge. The device contains a built-in control.
3.5. Future directions
Even now, the iStat represents the state-of-the-art technology for POC diagnostics in space. However, promising advances are being made in portable diagnostics that can provide a range of analytical capabilities for long-term space travel. While the detection of key analytes has been demonstrated, additional assays that can detect a larger number of analytes still need to be adapted for use in space. Likewise, cell counts have been demonstrated in microfabricated flow cytometers, but a larger number of cell types must be included to give the devices the necessary medical value for long-term space travel.
Despite impressive recent technological advances, much work remains before a reusable and versatile POC diagnostic suitable for long-term space travel is deployed. Not only must new technologies be developed, but a greater understanding of the effects of microgravity on biological and physical systems is needed. In particular, our understanding of the effects of long-term microgravity on the human body and the appropriate biomarkers to quantify those effects remains incomplete. The behavior of fluids in microgravity environments and the development of devices that can effectively manipulate fluids in such environments also requires further study. Continued research and development during short and long duration space flights will address these deficiencies.
4. Sports medicine
To date, POC diagnostics have found limited application in sports medicine. However, POC applications in sports seem likely in the future because of the need for clinical diagnoses in a portable format and in potentially remote locations. The most likely candidates for POC diagnostics are monitoring athletes for doping and for diagnosing injury.
4.1. Doping
The death of an Olympic cyclist in 1960 motivated athletic authorities to establish rules for doping in cycling, and since the 1960's efforts to monitor doping have continued to evolve (Catlin et al., 2008). In 1999 the International Olympic Committee established the World Anti-Doping Agency (WADA) which published standardized definitions of doping and established testing procedures in 2004 with the World Anti-Doping Code (Mazzei et al., 2014, www.wada-ama.org, 2015). WADA offers a comprehensive definition of doping: the attempt of an athlete to improve performance by using a substance or method. WADA may include a substance on the “prohibited list” if it meets any two of three criteria: (1) the substance or method has the potential to enhance sport performance, (2) the use of the substance or method represents an actual or potential health risk to the athlete, and (3) WADA determines the use of a substance or method is against the spirit of the sport (www.wada-ama.org, 2015). The broad definition of doping causes a wide variety of substances to be prohibited, ranging from common doping agents like steroids to less obvious substances like insulin. In addition to small molecules, increasing blood cell counts is considered doping. The wide range of abused substances or methods means that no one POC detection method is ideal.
Many analytical techniques can be used to test for doping: gas chromatography, immunoassay, gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), isotope ratio mass spectrometry (IRMS) (Catlin et al., 2008). GC-MS and LC-MS are the de facto standards; however, miniaturized GC-MS and LC-MS systems remain at the research stage and currently available clinical instruments are unsuitable for point-of-care analysis. Immunoassays have a role to play in doping control, but their main drawback is that they lack the selectivity to distinguish between endogenous steroids and exogenous steroids with similar structure. To date, they are not widely used to test for doping in athletes, but they may have a role for selecting athletes at the point-of-use who should undergo further testing. For a thorough review of affinity-based biosensors used to detect doping agents, see (Mazzei et al., 2014). In addition to testing for molecules in an athlete's blood, flow cytometry can be used to test for increased numbers of red blood cells. Point-of-care devices for counting blood cells have been developed, but they have yet to be applied to doping control. One unexplored POC technique for testing doping is capillary electrophoresis (Harrison, 2013). CE is more portable than the gold standard methods now used, and it can be adapted to detect small molecules such as hormones to large biologicals, like proteins. It could also be adapted to check cell counts.
4.2. Physiological monitoring
The monitoring of athlete health and consequent adaptations in training intensity could benefit from the development of POC analytical devices. Sweat is a readily accessible sample matrix that contains a wealth of potentially useful biomarkers, as well as providing a readout of hydration. Hyponatremia, a sodium concentration < 135 mEq/L, can result from drinking excessive water during endurance sports and is potentially fatal. Marathon runners are particularly susceptible--in the 2002 Boston Marathon, 13% of runners suffered from hyponatremia (Almond et al., 2005).
While one could measure sodium levels in sweat directly, e.g., with ISFETS (Bezegh et al., 1988), it is easier to measure pH, which is a surrogate for sodium levels. Portable sensors for measuring sweat pH have been developed, as shown in Figure 2 (Curto et al., 2012). In this sensor, researchers created a wearable “barcode” that indicated pH via the colors of the bars. An absorbent pad was used to wick sweat from the skin and transport it to the barcode. Wearable textile sensors have also been developed to measure physiological conditions from sweat (Coyle et al., 2009). In these textiles, pH sensitive dye was immobilized into a small patch of fabric and optical sensors were mounted near the patch. Another sensor uses a smartphone to measure the change in color of a commercially available pH paper once sweat is placed on it (Oncescu et al., 2013).
Blood lactate is another potentially useful metabolite to monitor during training. The level of lactate can tell an athlete if he or she is training in the correct/optimal zone and at the appropriate intensity. The i-STAT can be used to measure blood lactate but there are other analyzers specific to blood lactate that are less expensive (e.g., Accusport). The i-STAT and Accusport lactate analyzers produced similar results for blood lactate for samples taken at rest, during moderate exercise, and during maximal exercise (Dascombe et al., 2007). However, others have found that the portable analyzers, and the Accusport in particular, do not agree with laboratory-based lactate analyzers (Buckley et al., 2003). Erroneous lactate measurements can be problematic because their readings are used to establish the intensity of workouts.
Other useful serum biomarkers for monitoring workout intensity are creatine kinase (CK) and lactate dehydrogenase (LDH) (Brancaccio et al., 2008). Increased CK levels are a sign that muscles have been exercised outside of normal metabolic range (i.e., exercise intensity exceeds muscle capacity), but very high levels of CK can also be indicative of muscle injury (Mougios, 2007). Therefore, CK monitoring may be useful for monitoring the intensity of training sessions (Brancaccio, Maffulli, 2008). Serum levels of LDH increase after exercise, too. The level of increase depends on exercise intensity and the fitness level of the individual. CK levels can be used to monitor muscle recovery after strenuous exercise and LDH levels can be used to gauge muscle response after training (Brancaccio et al., 2008). A miniaturized capillary electrophoresis device that measures LDH activity has been developed (Zhuang et al., 2007), but not for use as a POC diagnostic. Creatine kinase assays have been developed because the CK isoform CK-MB is a potentially useful biomarker for detecting myocardial infarction (Brancaccio et al., 2008).
4.3. Traumatic brain injury
It is estimated that approximately 300,000 sports-related concussions occur in the United States annually (Thurman et al., 1998). The definition and assessment of a sports concussion is defined by the Zurich Consensus statement, which was designed to be used by physicians and sports trainers (McCrory et al., 2009). A preliminary evidence-based diagnosis is performed at the point-of-care using a Sports Concussion Assessment Tool (SCAT2) card, which contains a checklist and questionnaire for use by medical professionals. Further analyses, away from the point-of-care, are also subjective: neuroimaging and psychological evaluation. Biomarkers offer an objective measure and many have been studied possible links to concussions or mild-to-moderate traumatic brain injury: S100B, neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), ubiquitin carboxy-terminal hydrolase L1 (UCHL1), and myelin basic protein (MBP) (Jeter et al., 2013). Other candidate markers have been suggested (Shan et al., 2015). However, the efficacy of these markers in diagnosing concussion remains unknown, and it is unlikely that any single biomarker is sufficient to diagnose traumatic brain injury (North et al., 2012). For example, some biomarkers (e.g., S100B) do not easily cross the blood brain barrier, leading to inconsistent levels of protein in serum and cerebrospinal fluid.
Multiple lateral flow immunoassays have been demonstrated for detecting biomarkers (North et al., 2012). These immunoassays can detect common biomarkers such as the protein S100B, glial fibrillary acidic protein (GFAP), and neuron-specific enolase (NSE). They use sera as the sample input. More recently, microfluidic devices have begun to show promise as POC assays for biomarkers of traumatic brain injury. For example, Apori et al. (2011) demonstrated a capillary electrophoresis device for the detection of S100B and C-reactive protein in human cerebral spinal fluid.
The challenges for detecting biomarkers are twofold. The first major challenge is establishing that biomarkers can accurately represent traumatic brain injury. So far, definitive evidence is lacking. Second, questions remain about which sample has greater diagnostic value: cerebral spinal fluid or serum. Serum is more accessible, but the biomarker levels tend to be lower (as much as 10×). On the other hand, cerebral spinal fluid can hardly be collected in a locker room. At any rate, the infrastructure exists to create an assay for traumatic brain injury biomarkers, but the clinical evidence must catch up.
4.4. Future directions
The diagnostic needs most relevant to sports medicine lend themselves to a portable format. Detection of doping has historically been performed using clinical laboratory equipment, such as mass spectrometry. As advances in microfabricated mass spectrometry systems continue, these systems will move out of core lab facilities and evolve into portable POC devices that can be used at a sporting event. Another challenge will be the creation of adaptable diagnostic devices that can evolve as doping strategies change. In the case of antibody-based assays, this means developing new and specific antibodies for doping agents that may change frequently or that recognize a related biomarker within the body. In the case of MS systems, it means looking for new chemical signatures in a sample.
Significant research into POC assays for traumatic brain injury has been driven mainly by military research funding because of the critical need to assess concussive injury in military personnel. Most POC methods have been focused on developing rapid and sensitive assays for specific biomarkers in serum or cerebrospinal fluid. However, there is still debate about which biomarkers, or groups of biomarkers, contain the highest diagnostic value. Continued research will shed new light on the role that each play in diagnosing traumatic brain injury. Furthermore, it is likely that assessment of traumatic brain injury at the point-of-care will include a psychological assessment in addition to the quantification of biomarkers.
Lastly, challenges remain in POC physiological monitoring for sports applications. Diagnostic tests are used to monitor athlete training, and in particular gauging muscle injury as a result of over-training. However, as with traumatic brain injury, there is debate about the most appropriate biomarkers. Further research is needed to identify the effects of exercise on the body, and in particular the role it plays in changing biomarker levels.
5. Emergency department
The role of the emergency department (ED) in the delivery of healthcare has evolved over the years. Whereas the ED was originally responsible for emergency cases alone, today, the ED is the location where a large number of Americans (28%) receive their primary healthcare. In 2010, emergency departments in the US managed 130 million encounters (Pitts et al., 2010). Unfortunately, the emergency medicine setting is one of the clinical settings with the highest cost, and this cost is rising. Between 2003 and 2011, the average cost of a visit to the ED rose from $560 to over $1300 (a 240% increase) (Schuur et al., 2014). As a result of the increased rate of usage, the ED is becoming overcrowded (Kanzaria et al., 2015). This crowding leads to the ED being stressed beyond its current capabilities, making it necessary to triage patients to best use available resources. Even so, there are increased waiting times, a lack of beds, and an increased frequency of patients leaving the ED without treatment.
There are many efforts underway to improve the care and lower the cost in the ED. One of these is to expedite the diagnostic process with cost-effective, POC tests (Fermann and Suyama, 2002). For diseases where the turnaround time is the limiting factor, the implementation of such POC testing has been shown to improve healthcare delivery (Rooney and Schilling, 2014). Here we focus on POC testing that is used to diagnose adverse cardiac events in the emergency department. Other analytes that are commonly measured in the ER include glucose, urine, pregnancy hormones, chemistry panels, antibodies against HIV, hemoglobin, D-dimer, lipids, coagulation markers, and drugs of abuse.
Cardiovascular disease is the leading cause of death in the US. There are many reviews about cardiac biomarkers and the biosensors used for their detection (Ahmad et al., 2012, Braunwald, 2008, Collinson et al., 2015, Qureshi et al., 2012). Biomarkers that have been used include troponin, BNP, CK-MB, myoglobin, D-dimer, and C-reactive protein. The biomarkers have shown differences in the cutoff values that should be used for diagnosis between men and women (Daniels and Maisel, 2015). Additional protein biomarkers, such as sCD40 and copeptin, have recently been identified that provide opportunities for future high-impact diagnostic tests (Jaffe et al., 2006, Maisel and Choudhary, 2012). Additional studies identify micro-RNAs as ideal biomarkers for diagnostics (D'Alessandra et al., 2010, Ji et al., 2009). Here we briefly describe POC tests used in the emergency room to diagnose myocardial infarction (troponin, CK-MB, and myoglobin) and acute heart failure (BNP) as examples of biosensors useful in the ED niche.
5.1. Myocardial infarction: (troponin, CK-MB, myoglobin)
Chest pain is a common symptom of people who attend the ED (∼5%) (Fothergill et al., 1993). This is a common symptom associated with acute myocardial infarctions (AMI), but the majority of patients who present with chest pain do not have AMI. Because it is essential to deliver treatment to patients with a recent AMI, it is essential to quickly diagnose those who present with chest pain (Reichlin et al., 2012). An electrocardiogram can identify ST-segment elevation myocardial infarction (STEMI), but there are cases of acute myocardial infarction without elevated ST-segment (NSTEMI). In these cases, diagnosis is made based on measuring the cardiac biomarkers associated with myocardial necrosis – most notably troponin (Sabatine et al., 2002, Thygesen et al., 2010).
There are three tissue-specific troponin isoforms (I, T, and C). Troponin C is not used in the diagnosis of cardiac injury because it is shared by slow-twitch skeletal muscles. However, cardiac troponin I (cTnI) and cardiac troponin T (cTnT) are used in such diagnostic tests. These concentrations rise 4–6 hours after the onset of symptoms, and they peak at 18–24 hours (Babuin and Jaffe, 2005). Antibodies have been generated with specificity for the cTnI and cTnT isoforms and incorporated into assays for those targets. Elevated levels of cTnI and cTnT indicate cardiac injury, and the American College of Cardiology has included elevated levels of cardiac troponin (I or T) in the criteria for AMI (Antman et al., 2000). The time-dependent concentration profiles are similar for the two isoforms, providing similar clinical information about AMI. The cut-off point for troponin levels that suggest AMI has been defined as the 99th percentile of a reference control group (3 standard deviations above the mean). However, new assays have been developed with improved or “high” sensitivity, and there is new work being done to identify the actual concentrations that are associated with AMI (Apple and Collinson, 2012, Giannitsis et al., 2010, Keller et al., 2009, Mahajan and Jarolim, 2011, Reichlin et al., 2009, Saunders et al., 2011).
Prior to the use of troponin as a biomarker, creatine kinase (CK) and the MB fraction of creatine kinase (CK-MB) were used to diagnose an AMI (Saenger and Jaffe, 2008; Christenson and Azzazy, 1998). However, because of the abundance of CK-MB in skeletal muscle, the assay specificity is limited and has been replaced by assays for troponin (Panteghini, 1998). However, tests for CK-MB are still very useful for diagnosis of re-infarction and are included in many multiplexed diagnostic panels. The CK-MB also uses the 99th percentile cut-off criterion. While myoglobin has been disproven as a definitive biomarker for AMI diagnosis, the myoglobin level in the bloodstream rises much faster than CK-MB or troponin do, and myoglobin assays are still useful in screening (Apple et al., 1999, Kim et al., 2014b).
To expedite diagnosis, many EDs have successfully introduced POC devices to test for cTnI (Bingisser et al., 2012, Friess and Stark, 2009). However, while there are many different devices currently on the market that are capable of making this measurement (Yang and Min Zhou, 2006), there are currently no CLIA-waived tests for troponin (www.accessdata.fda.gov, 2015). The majority of the tests for troponin are of moderate complexity. Many studies have been performed that show the time to diagnosis is faster when POC testing is used (Goodacre et al., 2011). Electrocardiogram testing and POC devices measuring cardiac troponin I levels have been used in a moving ambulance and showed good correlation to tests that were performed in the ED (Venturini et al., 2013).
Lee et al. (2013) recently introduced a centrifugally actuated POC testing system for cardiac troponin I. This microfluidic system performed a sandwich immunoassay with gold nanoparticles, and the fluid manipulation was controlled via centrifugation. It was shown to quantify physiological levels of cTnI in different clinical fluids (plasma and whole blood), and the coefficient of variation was less than 10% from 10 pg/mL to 25 ng/mL. It also showed excellent correlation to the system used in the clinical laboratory.
5.2. Acute heart failure: natriuretic peptide testing
Heart failure is the inability of the ventricles to either fill with or eject blood and needs to be treated as quickly as possible. In emergency medicine, patients experiencing heart failure typically present with shortness of breath (i.e., dyspnea). However, this symptom is associated with other causes such as asthma, pneumonia, and obstructive pulmonary disease. Therefore, treatment depends on diagnosing the underlying cause. This places an incredible burden on EDs (Peacock et al., 2010, Storrow et al., 2014).
One diagnostic biomarker that has shown potential to be effective in diagnosing heart failure is B-type natriuretic peptide (Cowie et al., 2003). B-type natriuretic peptide (BNP, originally termed brain natriuretic peptide) is synthesized from the myocytes in the ventricles and secreted in response to myocardial stretch. Atrial natriuretic peptide (ANP) is synthesized by the myocytes in the atria and C-type natriuretic peptide (CNP) is produced by the vascular endothelial cells. These peptides effectively lower the extracellular fluid volume and blood pressure throughout circulation (O'Donoghue and Braunwald, 2010).
POC tests for BNP and the amino-terminal co-metabolite (NT-proBNP) have often been used to assist in the determination of underlying cause (Clerico et al., 2012, Mayo et al., 2006). For emergency care patients, this test should be performed within one hour of blood collection (Carpenter et al., 2012). Because timely diagnosis and treatment of acute heart failure result in improved outcomes, this is a valuable target for POC applications. BNP POC testing may also find future use in risk stratification and guiding therapeutic decisions of acute coronary syndromes, like AMI (Sabatine et al., 2002). Other diagnostic tools to diagnose the etiology of dyspnea include electrocardiogram, chest x-ray, and cardiopulmonary ultrasound (Gallard et al., 2015, Pirozzi et al., 2014).
The majority of the commercially available tests for BNP are deemed to have moderate complexity. Currently, the only commercially available CLIA-waived test for BNP is the Alere Triage MeterPro (Figure 2). The Alere Triage system is a fluorescent POC system which comprises a portable reader (Triage Meter) and the assay cartridge for the given analyte(s) of interest. The system is based on a fluorescence immunoassay, so the portable meter is an optical system that excites the region of interest with a laser diode (670 nm) and measures the fluorescence emission (760 nm) with a silicon photodiode. Upon loading the sample, the test cartridge filters red blood cells and directs the sample through various zones via capillary action toward the capture spots that are being interrogated. One zone includes antibody-coated latex particles that recognize one epitope of the analyte of interest. These particles achieve a large Stokes shift by incorporating a pair of fluorescent dyes that undergo Fӧrster Resonance Energy Transfer (FRET) (Koshy and Buechler, 2013). The test can accept whole blood, plasma, or urine, and has an assay time of approximately 15 minutes. In addition to BNP, the Alere Triage system has quantitative tests for D-dimer, myoglobin, CK-MB, and troponin I and qualitative tests for various drug screenings (www.alere.com, 2015).
5.3. Future directions
Future advances in POC diagnostics in the emergency room are first and foremost tied to an improved understanding of the biology. For many diseases, the biomarkers used in the diagnosis have continued to evolve (e.g., AMI). In other diseases, groups have yet to identify suitable sets of biomarkers that provide actionable diagnostic information. In addition, there is a growing awareness that the concentrations of the biomarkers are likely different among different patient groups, and guidelines for normal and abnormal levels need to be well established for gender, age, and race. As new biomarkers and panels of biomarkers are correlated with disease states for different groups, diagnostic tests will be developed to quantify those biomarkers in the appropriate setting. Because of the current, established workflow in a majority of the hospitals in the US, these diagnostic tests are typically first developed for use in the clinical laboratory. However, for emergency applications where immediate results could enable more efficient treatment, these diagnostic tests could be converted to a POC format.
Pressing applications that could significantly benefit from POC tests in the emergency room include diagnostics to distinguish ischemic stroke from hemorrhagic stroke and those that distinguish severe cases of sepsis. In the case of stroke, the faster identification of an ischemic stroke could allow the tissue plasminogen activator to be delivered to degrade the culprit clot and restore blood flow. The time between the initial onset of the stroke to the initiation of thrombolysis is inversely related to the probability of disability-free recovery. Sepsis – a whole-body inflammatory response to an infection – can quickly progress to septic shock and needs to be treated early to improve chances for survival. Many emergency departments have protocols to diagnose and treat sepsis quickly. However, approximately 25% of the cases that present with systemic inflammatory response syndrome actually have sepsis, leading to the over-administration of broad-spectrum antibiotics. The identification of biomarker panels that can improve the sensitivity and specificity for stroke and sepsis would immediately be useful in a POC format for the emergency room.
6. Operating room
The operating room is another location where POC diagnostic tests can significantly improve healthcare delivery. Surgeries are associated with a very high cost due to a number of factors, and many of these factors are a function of time (Fischer, 1999, Ismail et al., 2015, Macario, 2010). Certain diagnostic tests have been shown to be useful in the perioperative setting to improve patient outcomes. Point-of-care testing has been shown to decrease the turnaround time of such tests, which can potentially improve patient care and decrease costs (Kost, 1995). There are a few good reviews for POC testing in the operating room (Rhee and Kahn, 2010, Salem et al., 1991). Here we briefly describe the use of diagnostic tests for coagulation monitoring and hemoglobin monitoring in the perioperative setting. Other commonly used POC tests in the operating room include blood gas analyzers (De Koninck et al., 2012, Poesen et al., 2013, Schlebusch et al., 2001) and glucose monitoring for patients with diabetes (Aldam et al., 2014, Rebel et al., 2012, Rice et al., 2010).
6.1. Coagulation monitoring
Coagulation is a normal process of the body by which blood forms clots, and it is triggered by the rupturing of endothelium. Exposure of the blood to certain extravascular factors induces the clotting cascade to initiate clot formation (Davie et al., 1991). Natural anticoagulant mechanisms ensure that this clotting cascade is not induced under normal conditions, but there are many disorders associated with these mechanisms that increase the risk of clotting (Esmon, 1987). In response to these disorders, many people are placed on anticoagulant therapy, with drugs such as warfarin or heparin, to decrease their risk for an adverse clotting event (e.g., heart attack and stroke) (Hirsh, 1991, Hirsh et al., 1998, Hirsh et al., 2003). However, the dosage of the anticoagulant therapy needs to be personalized for each person to minimize the risk associated with clot formation and hemorrhaging. The personalized therapy is often determined with the use of coagulation monitoring. These tests are frequently performed using standard laboratory tests. Plasma is typically used, and it takes 30–60 minutes until results are available. For locations like the emergency or operating room, this turnaround time is not suitable (Faraoni et al., 2013). In response, various POC tests have been developed for coagulation monitoring, but no single technique provides information for all aspects of the complex clotting process. There are many comprehensive reviews on the subject (Harris et al., 2013).
There are several techniques used in portable tests for coagulation monitoring, and most of them are versions of the traditional laboratory tests. This includes activated clotting time (Bittl, 2005), thrombelastography, rotational thromboelastometry (Mallett and Cox, 1992, Saner, 2014, Thölking et al., 2015), and platelet function (Agarwal et al., 2015, Petricevic et al., 2015). Many of the in vitro diagnostic tests attempt to extract information on certain pathways of the clotting cascade, primarily extrinsic and intrinsic pathways. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are the most frequently used tests to determine functionality of the blood coagulation system. Currently, the only CLIA-waived devices for coagulation monitoring measure the PT. These PT values are commonly converted into an internationally normalized ratio (INR) to enable the comparison of PT values between analytical methods. Devices that are currently waived include the CoaguChek XS Plus from Roche (Figure 2), Coag-sense, and Hemosense INR. Some of these are available for use at the home, and studies have shown that their use increases the time that the patient's INR is in the targeted, therapeutic range for the given anticoagulant (Okuyama et al., 2014).
Many groups have compared the use of POC tests with laboratory analyzers directly (Herbstreit et al., 2010). Peña et al. (2012) recently compared the PT/INR results with a POC device (i-STAT) and a central laboratory coagulation analyzer (Tcoag MDA II analyzer), and they showed that it compares well when performed by phlebotomists or nurses. Bardakci et al. (2013) compared the INR value of patients with CoaguChek XS handheld coagulation analyzer from Roche Diagnostics with the automatic analyzer Sysmex CA-7000 from Siemens for the purposes of open–heart valve surgery. These patients had been administered vitamin K antagonists with low molecular weight heparin. Patients with diabetes mellitus were excluded from the study, because previous studies showed some deviations of POC INR values compared to standard methods in diabetic patients. Preoperative and postoperative measurements on the POC test correlated strongly with the conventional methods of laboratory testing (Bardakci et al., 2013).
Additional research efforts are designing improved devices for use at the point-of-care. Dudek et al. (2010) developed a single-use microfluidic device to measure fibrinogen in human plasma, requiring only 15 μL of plasma. Micropillars in the device induced capillary pressure which induced flow through dried thrombin reagent, converting soluble fibrinogen to insoluble fibrin. The insoluble fibrin induces a clot, causing the flow of the plasma within the microfluidic device to stop. Within five minutes, the fibrinogen concentration can be determined by measuring the distance traveled (Dudek et al., 2010). Li et al. (2014) have recently developed a simple paper-based lateral flow device for blood coagulation screening. Using the capillary pressure of the paper to wick the whole blood, the changes of viscosity due to coagulation could be identified by tracking the rate that the fluid imbibes (Li et al., 2014). Cakmak et al. (2015) have recently developed a multiplexed MEMS-based sensing cartridge that can test for both PT and aPTT in plasma. Microfluidic channels were functionalized with dried reagent to add factors to the plasma as the fluid passively flows following loading. The time-dependent viscosity affects the oscillation of a microcantilever that is measured remotely with a laser. By having both measurements of a single sample, both pathways of the clotting cascade (intrinsic and extrinsic) are described (Cakmak et al., 2015). Other groups are developing fluorogenic sensors that become fluorescent upon exposure to heparin to quantify the heparin concentration (Cao et al., 2014, Cheng et al., 2013, Gu et al., 2012).
6.2. Hemoglobin monitoring
Hemoglobin monitoring is often used alongside coagulation monitoring to determine the extent of blood loss during surgery. In the case of extreme blood loss, the first-line therapy is the transfusion of red blood cells to mitigate the risk of hypoxia associated with the lost blood. However, transfusions are associated with severe side effects (Hogervorst et al., 2014), and unnecessary transfusions have been shown to increase patient morbidity (Giraud et al., 2013).
Evidence-based practice has shown that there is not a ‘one-value for all’ for the hemoglobin measurement where the treatment is considered beneficial to the patient and demographics, comorbidities, and physiologic variables need to be considered. However, as a general rule, transfusion has been shown to be useful at a hemoglobin concentration less than 7 g/dL, and it is not needed at concentrations greater than 10 g/dL in adults. Normal values range from 12 to 17 g/dL. The American Association of Blood Banks (AABB) guidelines recommend that perioperative transfusion at a hemoglobin concentration less than 8 g/dL (Carabini et al., 2015). Groups have shown that the monitoring of hemoglobin reduces the frequency of blood transfusions with no negative impact on patient safety (O'Reilly, 2012).
Because hemoglobin values can change dramatically in the OR, the shorter turnaround times of POC tests for hemoglobin have been found to be useful. These POC diagnostic tests are either minimally invasive (require small volumes of blood) or are non-invasive. There are many CLIA-waived tests for hemoglobin. The HemoCue Hb201+ is a CLIA-waived, minimally-invasive system that requires 10 μL of sample to generate a reading in less than one minute (Figure 2). It can use capillary blood from a finger-stick and performs a modified azide methemoglobin reaction with reagents that are stored on chip to elicit a hemoglobin dependent color change (Skelton et al., 2013). Non-invasive systems for hemoglobin monitoring include systems that use multi-wavelength co-oximetry (Radical-7 and Pronto-7 from Masimo) and occlusion spectroscopy (NBM-200 from Orsense). Both of these types of systems have been shown to perform well in the intensive care and emergency departments as well as operating rooms (Kim et al., 2014a).
6.3. Ongoing research
In addition to the development of POC diagnostics for cardiac applications, another area of research is the development of POC diagnostic tests to quantify hormones during the surgical removal of the respective endocrine glands. Here, we detail the history of using intraoperative parathyroid hormone monitoring to guide surgery and highlight ongoing developments toward a POC diagnostic test. Similar POC diagnostic tests could also apply to other hormones of the endocrine system like insulin, ACTH, growth hormone, and gastrin.
Parathyroid hormone (PTH) is an 84 amino acid peptide (9.4 kDa) that is involved in calcium regulation (Potts, 2005). It is secreted by the parathyroid glands located in the neck and its receptor is located in several tissues including the kidney and bone. PTH stimulates these tissues to increase calcium levels in the circulation (Fraser et al., 2013). These glands are hyperfunctioning in disorders such as primary hyperparathyroidism, and the only definitive cure is achieved by a parathyroidectomy (Callender and Udelsman, 2014, Khan, 2013, Yu et al., 2011). Because the circulating half-life of PTH is short (<5 minutes), the circulating concentrations of PTH are expected to dramatically decrease upon removal of all affected glands (Bieglmayer et al., 2006). Intraoperative parathyroid hormone monitoring has demonstrated clinical utility in guiding surgeons to whether all culprit parathyroid glands have been removed (Johnson et al., 2001). While there is work being done to determine whether this monitoring is needed for all, it has been shown to decrease the rates of re-hospitalization for many patients (Hwang et al., 2010, Stalberg et al., 2006). Intraoperative monitoring has been found useful during the removal of other endocrine glands as well (Sokoll et al., 2004).
There are various criteria that have been used to predict successful cure for IOPTH monitoring, but the Miami criterion is the most widely known (Carneiro et al., 2003). This states that successful cure can be predicted if there is a 50% drop in baseline PTH after 10 minutes of gland removal (Irvin Iii et al., 1994). Many different rapid IOPTH tests were developed to have the required sensitivity and assay time to allow for practical use in surgical management (Carneiro and Irvin, 2002, Goodman, 2005, Skugor et al., 2010, Vieira et al., 2006). The majority of these assays use a sandwich immunoassay with capture and detection antibodies that recognize unique epitopes of PTH. Many of these assays have been compared with one another, and they have shown some key differences (Cole et al., 2007, La'ulu and Roberts, 2010, Santini et al., 2004). The differences in reported values between assays can potentially be attributed to the different recognition elements used in the diagnostic tests. These have varying cross-reactivities to the heterogeneous mixture of metabolized fragments in circulation (Gao and D'Amour, 2005, Silverman and Yalow, 1973). This is further complicated because the longer fragments have a significantly longer circulating half-life than the 1-84 PTH (Vieira et al., 2009).
Assay incubation times range from 10–30 minutes, and the majority of institutions send samples to the core clinical lab for analysis (Hortin and Carter, 2002). The transport times can lead to lengthy turnaround times – up to an hour in length. Other institutions have used satellite testing close to the operating room to improve turnaround time, but these tests require an instrument and a laboratory technician to comply with CLIA as all tests to date are either moderate or high-complexity tests (Wians et al., 2000). Normal PTH values range from 10–70 pg/mL (1-7 pM) (Kratz et al., 2004). In primary hyperparathyroidism, baseline PTH values can be higher than 1500 pg/mL. Groups have also explored more complicated mathematical algorithms and models to enable more accurate predictions of cure (Bieglmayer et al., 2002, Richards et al., 2011, Udelsman et al., 2014). Other groups have used the PTH assay as a replacement for frozen sectioning (Conrad et al., 2006, Guarda, 2004).
In an attempt to develop improved PTH tests for the point-of-care in the operating theater, there have been some recent efforts to develop new diagnostic devices (Dittmer et al., 2008, Özcan and Sezgintürk, 2015, Sayikli ŞimŞek et al., 2015). Perhaps most notably, Philips has developed an intraoperative PTH assay based on their handheld magnotech technology (Bruls et al., 2009, Jarrige et al., 2011). This test uses a disposable, self-contained cartridge that contains all assay reagents -including magnetic nanoparticles functionalized with a monoclonal antibody that recognizes the N-terminus (1-34) of PTH - in dry form. Magnetic forces transport particles to the surface, increasing the concentration of analyte near the recognition elements (antibodies that recognize C-terminus (39-84) of PTH) at the surface. These forces are then used to remove unbound particles. The bound nanoparticles are transduced with the principle of frustrated total internal reflection. The assay requires <25 μL of sample (EDTA-treated plasma) and takes eight minutes. Philips has also developed a cardiac troponin test using this technology (Dittmer et al., 2010).
6.4. Future directions
A primary role of all POC diagnostic tests is to expedite the process of obtaining test results, but the operating room has a unique set of requirements for such a test. Moving POC diagnostics into the operating room reduces the sample-to-answer time by eliminating sample transport to a central laboratory, which can reduce the time that a patient is open and/or under anesthesia; any reduction in the actual time required to perform the diagnostic test is additionally advantageous. Inside an operating room there is limited space with many workers, and each of these workers has a specific role with a list of responsibilities. New POC tests would most likely need to be CLIA-waived to be used in the operating room without additional personnel, so it must be simple and have a low risk for error.
Because of these characteristics, any biomarker information measured with a POC diagnostic during surgery needs to provide information essential to a successful surgery and the patient's recovery thereafter. The tests should offer immediate feedback relevant to the treatment while the surgery is in process. Therefore, POC diagnostics should either be used to track the overall health of the patient or to monitor the progress of the surgery. Because of the transient nature of surgery, tracking the rates of change in biomarker concentrations might offer operating room personnel key insights on the how the body is responding to surgery. Sequential measurements could be performed with a POC diagnostic and the portable reader could use predefined algorithms to anticipate problems or validate success. In addition, adding test results to the permanent medical record may provide information important for post-operative care.
7. Conclusions
Most commercially available portable point-of-use tests for specialty applications rely on lateral flow tests, real-time PCR, electrochemical monitors, or simple microfluidic cassettes inserted into a reader. The rapid progress in miniaturization of optical devices offers optical readout systems comparable in cost and size to electrochemical glucose-type readers; making readers using cell phone components and using cell phones as readers are approaches already in the research and development phase. For making even smaller and disposable diagnostic devices, organic light emitting diodes and photodiodes can be incorporated into inexpensive, credit-card sized tests in the same substrate as the electronics and microfluidics. While still a step behind the optics and electronics, fluidic components are being tested that will facilitate more complex sample processing and signal amplification without the need for relatively large pumps and valves. Increased automation, improved ability to distinguish signal from background, and user sophistication are three factors that open the doors to new tests for generating information not available in the past outside of a laboratory.
The drivers for the development of new tests vary with the particular application. Veterinary POC diagnostics will continue to expand to diseases that are currently unmonitored or only diagnosed in central laboratories. Infectious diseases are high value targets, especially if they affect food or companion animals or if the disease is transmissible from animals to people. New optoelectronic materials, robust recognition molecules, integrated components, and automation are all critical features that will open new opportunities for veterinary diagnostics. In the last few years, the availability of portable infrared spectrometers has begun to foster the exploration of spectroscopy as a rapid method for measuring changes in tissues, milk and clinical fluids (e.g. Santos et al., 2013; Samara et al., 2014); this is only the tip of the iceberg in what may be feasible using handheld imaging and spectroscopy tools.
The renewed interest in long-term space flight demands the development of next-generation POC modular diagnostics that are reusable and capable of diagnosing a wide variety of medical conditions. It also demands further research into the behavior of fluids in microgravity and a re-thinking of how to manipulate fluids in such an environment. For example, recent advances in paper fluidics-based diagnostics provides a possible approach for performing assays in microgravity environment (Martinez et al., 2010). In addition, more research is needed on the long-term effects of space on human physiology. As these fundamental studies improve our understanding of the effect of microgravity on human health, new POC diagnostics can be developed to monitor the vital physiological parameters to maintain optimal health for astronauts.
Even though doping testing continues to rely on traditional lab-scale technology such as mass spectrometry, a portable POC diagnostic could be valuable in testing athletes during a competition. Continued advances in miniaturization will produce portable mass spectrometry systems that could meet this need. Indeed, portable mass spectrometers are already commercially available for military and first-responder applications (e.g., the M908 from 908 Devices, Boston, MA) and adapting these devices to non-emergency applications would be straightforward. Simpler tests, such as amplified immunoassays, have the potential to address the need for a POC test for traumatic brain injury; however, the clinical information is first required to determine which biomarkers are most appropriate to measure.
Lastly, emergency and operating room medicine will continue to evolve. To date the development of these POC tests has been rather haphazard—a clinician, scientist or engineer realizes a particular need and formulates an approach to address that need. A comprehensive analysis of what POC diagnostics would most expedite patient care in the ED could focus corporate attention on adapting existing clinical laboratory tests to simple, single-use formats to solve problems that are high impact, even if not high profile. Tests in the operating room currently focus on monitoring the impact of anesthesia and general physical parameters. As pharmacoengineering produces new approaches for drug delivery in concert with surgical procedures, biochemical monitors during surgery must become more sophisticated. Keeping the devices used to perform such assays as simple as possible is essential to their use.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- www.abaxis.com. VetScan VS2. [Accessed 2015 September 27];2015a Available from: http://www.abaxis.com/veterinary/products/vs2.html.
- www.abaxis.com. VetScan i-STAT 1 Handheld Anaylzer. [Accessed 2015 September 27];2015b Available from: http://www.abaxis.com/veterinary/products/istat.html.
- www.abaxis.com. Abaxis Products. [Accessed 2015 September 27];2015c Available from: http://www.abaxis.com/veterinary/products/
- www.abaxis.com. VetScan Avian Influenza Rapid Test. [Accessed 2015 September 27];2015d Available from: http://www.abaxis.com/veterinary/products/avian-influenza-rapid-test.html.
- www.abaxis.com. Abaxis Rapid Tests. [Accessed 2015 September 28];2015e Available from: http://www.abaxis.com/veterinary/products/rapid-tests.html.
- www.abbottpointofcare.com. i-STAT Handheld. [Accessed 2015 September 27];2015 Available from: https://www.abbottpointofcare.com/products-services/istat-handheld.
- www.accessdata.fda.gov. CLIA- Clinical Laboratory Improvement Amendments - Searchable Database. [Accessed 2015 September 27];2015 Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfclia/search.cfm.
- www.alere.com. Alere Triage System Tests. [Accessed 2015 September 27];2015 Available from: http://www.alere.com/au/en/product-details/alere-triage-system-tests.html.
- www.biomerieux-industry.com. Adiavet. [Accessed 2015 September 27];2015 Available from: http://www.biomerieux-industry.com/veterinary-diagnostics/adiavet-real-time-pcr-kits-for-animal-diseases-diagnostic.
- www.coaguchek.com. Coaguchek XS System. [Accessed 2016 January 27];2016 Available from: http://www.coaguchek.com/coaguchek_patient/en/home/products/xs-system.html.
- www.fda.gov. CLIA Categorizations. [Accessed 2015 September 27];2015a Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm39329.htm.
- www.fda.gov. Recommendations: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices. [Accessed 2015 September 27];2015b Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm079632.htm.
- www.hemocue.us. HemoCue Hb 201+ System. [Accessed 2016 January 27];2016 Available from: http://www.hemocue.us/∼/media/hemocue-images/hemocue_us-images/pdf/hemoglobin--lit1056--hb-201product-sheet.pdf?la=en-US.
- humanresearchwiki.jsc.nasa.gov. Exploration Medical Condition List. [Accessed 2015 September 27];2015a Available from: https://humanresearchwiki.jsc.nasa.gov/images/6/62/EMCL_RevC_2013.pdf.
- humanresearchwiki.jsc.nasa.gov. [Accessed 2015 September 27];ExMC Gap Report 4.05. 2015b Available from: https://humanresearchwiki.jsc.nasa.gov/index.php?title=4.05.
- humanresearchwiki.jsc.nasa.gov. Exploration Medical Capability (ExMC) Evidence Report. [Accessed 2015 September 27];2015c Available from: https://humanresearchwiki.jsc.nasa.gov/index.php?title=Exploration_Medical_Capability_(ExMC)_Evide nce_Report.
- www.idexx.com. LaserCyte Dx Hematology Analyzer. [Accessed 2015 September 27];2015a Available from: https://www.idexx.com/small-animal-health/products-and-services/lasercyte-dx-analyzer.html.
- www.idexx.com. SNAP Pro Mobile Device. [Accessed 2015 September 27];2015b Available from: https://www.idexx.com/small-animal-health/products-and-services/snap-pro-mobile-device.html.
- www.lewin.com. The Value of Diagnostics, Innovation, Adoption and Diffusion into Health Care. 2005. [Accessed 2015 September 27];2005 Available from: http://www.lewin.com/~/media/Lewin/Site_Sections/Publications/ValueofDiagnostics.pdf.
- www.neogen.com. Genomic Solutions for Swine. [Accessed 2015 September 27];2015 Available from: http://www.neogen.com/Genomics/Swine.html.
- ntrs.nasa.gov. Portable Diagnostics Technology Assessment for Space Missions. [Accessed 2015 September 27];2015 Available from: http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20100011008.pdf.
- www.wada-ama.org. World Anti-Doping Agency. [Accessed 2015 September 27];2015 Available from: https://www.wada-ama.org/
- Public Law 100-578. United States of America; 1988. Clinical Laboratory Improvement Amendments of 1988; pp. 2903–15. [Google Scholar]
- Agarwal S, Johnson RI, Shaw M. Preoperative point-of-care platelet function testing in cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2015;29:333–41. doi: 10.1053/j.jvca.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Fiuzat M, Felker GM, O'Connor C. Novel biomarkers in chronic heart failure. Nature Reviews Cardiology. 2012;9:347–59. doi: 10.1038/nrcardio.2012.37. [DOI] [PubMed] [Google Scholar]
- Aldam P, Levy N, Hall GM. Perioperative management of diabetic patients: new controversies. British Journal of Anaesthesia. 2014;113:906–9. doi: 10.1093/bja/aeu259. [DOI] [PubMed] [Google Scholar]
- Almond CSD, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, et al. Hyponatremia among runners in the Boston Marathon. The New England Journal of Medicine. 2005;352:1550–6. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- Antman E, Bassand JP, Klein W, Ohman M, Lopez Sendon JL, Rydén L, et al. Myocardial infarction redefined - A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee f or the redefinition of myocardial infarction. Journal of the American College of Cardiology. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- Apori AA, Herr AE. Homogeneous Immunosubtraction Integrated with Sample Preparation Enabled by a Microfluidic Format. Analytical Chemistry. 2011;83:2691–8. doi: 10.1021/ac103219x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple FS, Christenson RH, Valdes R, Jr, Andriak AJ, Berg A, Duh SH, et al. Simultaneous rapid measurement of whole blood myoglobin, creatine kinase MB, and cardiac troponin I by the triage cardiac panel for detection of myocardial infarction. Clinical Chemistry. 1999;45:199–205. [PubMed] [Google Scholar]
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clinical Chemistry. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- Babington R, matas S, Marco MP, Galve R. Current bioanalytical methods for detection of penicillins. Analytical and Bioanalytical Chemistry. 2012;403:1549–66. doi: 10.1007/s00216-012-5960-4. [DOI] [PubMed] [Google Scholar]
- Babuin L, Jaffe AS. Troponin: The biomarker of choice for the detection of cardiac injury. Canadian Medical Association Journal. 2005;173:1191–202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Wang R, Hargis B, Lu H, Li Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors (Switzerland) 2012;12:12506–18. doi: 10.3390/s120912506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardakci H, AltintaŞ G, Çiçek OF, Kervan U, Yilmaz S, Kaplan S, et al. Comparison of the CoaguChek XS handheld coagulation analyzer and conventional laboratory methods measuring international normalised ratio (INR) values during the time to therapeutic range after mechanical valve surgery. Journal of Cardiac Surgery. 2013;28:254–7. doi: 10.1111/jocs.12100. [DOI] [PubMed] [Google Scholar]
- Berger D. A brief history of medical diagnosis and the birth of the clinical laboratory. Part 2--Laboratory science and professional certification in the 20th century. MLO: medical laboratory observer. 1999a;31:32–38. [PubMed] [Google Scholar]
- Berger D. A brief history of medical diagnosis and the birth of the clinical laboratory. Part 3--Medicare, government regulation, and competency certification. MLO: medical laboratory observer. 1999b;31:40–44. [PubMed] [Google Scholar]
- Bezegh K, Bezegh A, Black PG, Janata J. Integrated probe for sweat analysis. Journal of Clinical Laboratory Analysis. 1988;2:16–8. [Google Scholar]
- Bieglmayer C, Kaczirek K, Prager G, Niederle B. Parathyroid hormone monitoring during total parathyroidectomy for renal hyperparathyroidism: Pilot study of the impact of renal function and assay specificity. Clinical Chemistry. 2006;52:1112–9. doi: 10.1373/clinchem.2005.065490. [DOI] [PubMed] [Google Scholar]
- Bieglmayer C, Prager G, Niederle B. Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy. Clinical Chemistry. 2002;48:1731–8. [PubMed] [Google Scholar]
- Bingisser R, Cairns C, Christ M, Hausfater P, Lindahl B, Mair J, et al. Cardiac troponin: A critical review of the case for point-of-care testing in the ED. American Journal of Emergency Medicine. 2012;30:1639–49. doi: 10.1016/j.ajem.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Bittl JA. The truth about activated clotting time measurements. Catheterization and Cardiovascular Interventions. 2005;65:338–9. doi: 10.1002/ccd.20415. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Miller AC, Grace MB, McLeland CB, Luo L, Muderhwa JM, et al. Radiation biodosimetry: applications for spaceflight. Advances in Space Research : The Official Journal of the Committee on Space Research (COSPAR) 2003;31:1487–93. doi: 10.1016/s0273-1177(03)00085-1. [DOI] [PubMed] [Google Scholar]
- Brancaccio P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports medicine. Clinics in Sports Medicine. 2008;27:1-18–vii. doi: 10.1016/j.csm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Biomarkers in heart failure. New England Journal of Medicine. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- Bruls DM, Evers TH, Kahlman JAH, Van Lankvelt PJW, Ovsyanko M, Pelssers EGM, et al. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab on a Chip. 2009;9:3504–10. doi: 10.1039/b913960e. [DOI] [PubMed] [Google Scholar]
- Buckey J. Preparing for mars: the physiologic and medical challenges. European Journal of Medical Research. 1999;4:353–6. [PubMed] [Google Scholar]
- Buckley JD, Bourdon PC, Woolford SM. Effect of measuring blood lactate concentrations using different automated lactate analysers on blood lactate transition thresholds. Journal of Science and Medicine in Sport. 2003;6:408–21. doi: 10.1016/s1440-2440(03)80267-0. [DOI] [PubMed] [Google Scholar]
- Burke MD. Laboratory medicine in the 21st century. American Journal of Clinical Pathology. 2000;114:841–6. doi: 10.1309/TH8P-1CAL-9K3G-VFTM. [DOI] [PubMed] [Google Scholar]
- Cakmak O, Ermek E, Kilinc N, Bulut S, Baris I, Kavakli IH, et al. A cartridge based sensor array platform for multiple coagulation measurements from plasma. Lab on a Chip. 2015;15:113–20. doi: 10.1039/c4lc00809j. [DOI] [PubMed] [Google Scholar]
- Callender GG, Udelsman R. Surgery for primary hyperparathyroidism. Cancer. 2014;120:3602–16. doi: 10.1002/cncr.28891. [DOI] [PubMed] [Google Scholar]
- Cao Y, Shi S, Wang L, Yao J, Yao T. Ultrasensitive fluorescence detection of heparin based on quantum dots and a functional ruthenium polypyridyl complex. Biosensors and Bioelectronics. 2014;55:174–9. doi: 10.1016/j.bios.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Carabini LM, Navarre WJ, Ault ML, Bebawy JF, Gupta DK. A comparison of hemoglobin measured by co-oximetry and central laboratory during major spine fusion surgery. Anesthesia and Analgesia. 2015;120:60–5. doi: 10.1213/ANE.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Carneiro DM, Irvin GL., III New point-of-care intraoperative parathyroid hormone assay for intraoperative guidance in parathyroidectomy. World Journal of Surgery. 2002;26:1074–7. doi: 10.1007/s00268-002-6675-z. [DOI] [PubMed] [Google Scholar]
- Carneiro DM, Solorzano CC, Nader MC, Ramirez M, Irvin GL, Iii, Udelsman R, et al. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: Which criterion is the most accurate? Surgery. 2003;134:973–81. doi: 10.1016/j.surg.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Carpenter CR, Keim SM, Worster A, Rosen P. Brain natriuretic peptide in the evaluation of emergency department dyspnea: Is there a role? Journal of Emergency Medicine. 2012;42:197–205. doi: 10.1016/j.jemermed.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin DH, Fitch KD, Ljungqvist A. Medicine and science in the fight against doping in sport. Journal of Internal Medicine. 2008;264:99–114. doi: 10.1111/j.1365-2796.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- Cheng TT, Yao JL, Gao X, Sun W, Shi S, Yao TM. A new fluorescence “switch on” assay for heparin detection by using a functional ruthenium polypyridyl complex. Analyst. 2013;138:3483–9. doi: 10.1039/c3an00242j. [DOI] [PubMed] [Google Scholar]
- Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nature Medicine. 2011;17:1015–9. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- Christenson RH, Azzazy HME. Biochemical markers of the acute coronary syndromes. Clinical Chemistry. 1998;44:1855–64. [PubMed] [Google Scholar]
- Clerico A, Zaninotto M, Prontera C, Giovannini S, Ndreu R, Franzini M, et al. State of the art of BNP and NT-proBNP immunoassays: The CardioOrmoCheck study. Clinica Chimica Acta. 2012;414:112–9. doi: 10.1016/j.cca.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Cohen LY, Vernon M, Bergeron MG. New molecular technologies against infectious diseases during space flight. Acta Astronautica. 2008;63:769–75. [Google Scholar]
- Cole DEC, Webb S, Chan PC. Update on parathyroid hormone: New tests and new challenges for external quality assessment. Clinical Biochemistry. 2007;40:585–90. doi: 10.1016/j.clinbiochem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Collinson PO, Garrison L, Christenson RH. Cardiac biomarkers - A short biography. Clinical Biochemistry. 2015;48:197–200. doi: 10.1016/j.clinbiochem.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Collopy KT, Kivlehan SM, Snyder S. What's the point of point-of-care testing? Understanding the potential of this evolving capability. EMS World. 2014;43:34–42. [PubMed] [Google Scholar]
- Conrad DN, Olson JE, Hartwig HM, Mack E, Chen H. A Prospective Evaluation of Novel Methods to Intraoperatively Distinguish Parathyroid Tissue Utilizing a Parathyroid Hormone Assay. Journal of Surgical Research. 2006;133:38–41. doi: 10.1016/j.jss.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Cowie MR, Jourdain P, Maisel A, Dahlstrom U, Follath F, Isnard R, et al. Clinical applications of B-type natriuretic peptide (BNP) testing. European Heart Journal. 2003;24:1710–8. doi: 10.1016/s0195-668x(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Coyle S, Morris D, Lau KT, Diamond D, Taccini N, Costanzo D, et al. Textile sensors to measure sweat pH and sweat-rate during exercise. 3rd International Conference on Pervasive Computing Technologies for Healthcare - Pervasive Health 2009, PCTHealth; 2009. [Google Scholar]
- Crucian B, Quiriarte H, Guess T, Ploutz-Snyder R, McMonigal K, Sams C. A Miniaturized Analyzer Capable of White-Blood-Cell and Differential Analyses During Spaceflight. Laboratory Medicine. 2013;44:304–12. [Google Scholar]
- Culbertson CT, Tugnawat Y, Meyer AR, Roman GT, Ramsey JM, Gonda SR. Microchip separations in reduced-gravity and hypergravity environments. Analytical Chemistry. 2005;77:7933–40. doi: 10.1021/ac051198e. [DOI] [PubMed] [Google Scholar]
- Curto VF, Fay C, Coyle S, Byrne R, O'Toole C, Barry C, et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sensors And Actuators B-Chemical. 2012:171–172. 1327–34. [Google Scholar]
- Dahlhausen B. Future veterinary diagnostics. Journal of Exotic Pet Medicine. 2010;19:117–32. doi: 10.1053/j.jepm.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. European Heart Journal. 2010;31:2765–73. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher M, Jordan K. Identification of existing and emerging chemical residue contamination concerns in milk. Irish Journal of Agricultural and Food Research. 2013;52:173–83. [Google Scholar]
- Daniels LB, Maisel AS. Cardiovascular biomarkers and sex: The case for women. Nature Reviews Cardiology. 2015;12:588–96. doi: 10.1038/nrcardio.2015.105. [DOI] [PubMed] [Google Scholar]
- Dascombe BJ, Reaburn PRJ, Sirotic AC, Coutts AJ. The reliability of the i-STAT clinical portable analyser. Journal of Science and Medicine in Sport. 2007;10:135–40. doi: 10.1016/j.jsams.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Davis F, Higson SPJ. Label-free immunochemistry approach to detect and identify antibiotics in milk. Pediatric Research. 2010;67:476–80. doi: 10.1203/PDR.0b013e3181d61c0c. [DOI] [PubMed] [Google Scholar]
- Davis J. Medical issues for a mission to Mars. Aviation, Space, and Environmental Medicine. 1999;70:162–8. [PubMed] [Google Scholar]
- De Koninck AS, De Decker K, Van Bocxlaer J, Meeus P, Van Hoovels L. Analytical performance evaluation of four cartridge-type blood gas analyzers. Clinical Chemistry and Laboratory Medicine. 2012;50:1083–91. doi: 10.1515/cclm-2011-0685. [DOI] [PubMed] [Google Scholar]
- Dittmer WU, de Kievit P, Prins MWJ, Vissers JLM, Mersch MEC, Martens MFWC. Sensitive and rapid immunoassay for parathyroid hormone using magnetic particle labels and magnetic actuation. Journal of Immunological Methods. 2008;338:40–6. doi: 10.1016/j.jim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Dittmer WU, Evers TH, Hardeman WM, Huijnen W, Kamps R, de Kievit P, et al. Rapid, high sensitivity, point-of-care test for cardiac troponin based on optomagnetic biosensor. Clinica Chimica Acta. 2010;411:868–73. doi: 10.1016/j.cca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Dubeau-Laramée G, Rivière C, Jean I, Mermut O, Cohen LY. Microflow1, a sheathless fiber-optic flow cytometry biomedical platform: demonstration onboard the international space station. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2014;85:322–31. doi: 10.1002/cyto.a.22427. [DOI] [PubMed] [Google Scholar]
- Dudek MM, Lindahl TL, Killard AJ. Development of a point of care lateral flow device for measuring human plasma fibrinogen. Analytical Chemistry. 2010;82:2029–35. doi: 10.1021/ac902763a. [DOI] [PubMed] [Google Scholar]
- Erickson KA, Wilding P. Evaluation of a novel point-of-care system, the i-STAT portable clinical analyzer. Clinical Chemistry. 1993;39:283–7. [PubMed] [Google Scholar]
- Esmon CT. The regulation of natural anticoagulant pathways. Science. 1987;235:1348–52. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Ewald M, Fechner P, Gauglitz G. A multi-analyte biosensor for the simultaneous label-free detection of pathogens and biomarkers in point-of-need animal testing. Analytical and Bioanalytical Chemistry. 2015;407:4005–13. doi: 10.1007/s00216-015-8562-0. [DOI] [PubMed] [Google Scholar]
- Faraoni D, Savan V, Levy JH, Theusinger OM. Goal-directed coagulation management in the perioperative period of cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2013;27:1347–54. doi: 10.1053/j.jvca.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Fermann GJ, Suyama J. Point of care testing in the emergency department. Journal of Emergency Medicine. 2002;22:393–404. doi: 10.1016/s0736-4679(02)00429-8. [DOI] [PubMed] [Google Scholar]
- Fischer SP. Cost-effective preoperative evaluation and testing. Chest. 1999;115:96S–100S. doi: 10.1378/chest.115.suppl_2.96s. [DOI] [PubMed] [Google Scholar]
- Fothergill NJ, Hunt MT, Touquet R. Audit of patients with chest pain presenting to an accident and emergency department over a 6-month period. Archives of Emergency Medicine. 1993;10:155–60. doi: 10.1136/emj.10.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser WD, Colston KW, Stevenson JC. Bone and calcium metabolism. The Immunoassay Handbook. 2013:705–20. [Google Scholar]
- Friess U, Stark M. Cardiac markers: A clear cause for point-of-care testing. Analytical and Bioanalytical Chemistry. 2009;393:1453–62. doi: 10.1007/s00216-008-2573-z. [DOI] [PubMed] [Google Scholar]
- Gallard E, Redonnet JP, Bourcier JE, Deshaies D, Largeteau N, Amalric JM, et al. Diagnostic performance of cardiopulmonary ultrasound performed by the emergency physician in the management of acute dyspnea. American Journal of Emergency Medicine. 2015;33:352–8. doi: 10.1016/j.ajem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Gao P, D'Amour P. Evolution of the parathyroid hormone (PTH) assay - Importance of circulating PTH immunoheterogeneity and of its regulation. Clinical Laboratory. 2005;51:21–9. [PubMed] [Google Scholar]
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical Chemistry. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- Giraud B, Frasca D, Debaene B, Mimoz O. Comparison of haemoglobin measurement methods in the operating theatre. British Journal of Anaesthesia. 2013;111:946–54. doi: 10.1093/bja/aet252. [DOI] [PubMed] [Google Scholar]
- Goodacre S, Bradburn M, Fitzgerald P. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial: A randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technology Assessment. 2011;15:iii–xi. doi: 10.3310/hta15230. [DOI] [PubMed] [Google Scholar]
- Goodman WG. The evolution of assays for parathyroid hormone. Seminars in Dialysis. 2005;18:296–301. doi: 10.1111/j.1525-139X.2005.18405.x. [DOI] [PubMed] [Google Scholar]
- Gu CM, Lan t, Shi HC, Lu Y. Portable detection of melamine in milk using a personal glucose meter based on an in vitro selected structure-switching aptamer. Analtyical Chemistry. 2015;87:7676–82. doi: 10.1021/acs.analchem.5b01085. [DOI] [PubMed] [Google Scholar]
- Gu X, Zhang G, Zhang D. A new ratiometric fluorescence detection of heparin based on the combination of the aggregation-induced fluorescence quenching and enhancement phenomena. Analyst. 2012;137:365–9. doi: 10.1039/c1an15874k. [DOI] [PubMed] [Google Scholar]
- Guarda LA. Rapid intraoperative parathyroid hormone testing with surgical pathology correlations: The “chemical frozen section”. American Journal of Clinical Pathology. 2004;122:704–12. doi: 10.1309/RU5L-DXWR-1QNQ-A5WY. [DOI] [PubMed] [Google Scholar]
- Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE. Point of care diagnostics: Status and future. Analytical Chemistry. 2012;84:487–515. doi: 10.1021/ac2030199. [DOI] [PubMed] [Google Scholar]
- Harris LF, Castro-López V, Killard AJ. Coagulation monitoring devices: Past, present, and future at the point of care. TrAC - Trends in Analytical Chemistry. 2013;50:85–95. [Google Scholar]
- Harrison CR. Role of capillary electrophoresis in the fight against doping in sports. Analytical chemistry. 2013;85:6982–7. doi: 10.1021/ac302821x. [DOI] [PubMed] [Google Scholar]
- Herbstreit F, Winter EM, Peters J, Hartmann M. Monitoring of haemostasis in liver transplantation: Comparison of laboratory based and point of care tests. Anaesthesia. 2010;65:44–9. doi: 10.1111/j.1365-2044.2009.06159.x. [DOI] [PubMed] [Google Scholar]
- Hirsh J. Drug therapy: Heparin. New England Journal of Medicine. 1991;324:1565–74. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114:445S–69S. doi: 10.1378/chest.114.5_supplement.445s. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology foundation guide to warfarin therapy. Circulation. 2003;107:1692–711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Rosseel P, Van Der Bom J, Bentala M, Brand A, Van Der Meer N, et al. Tolerance of intraoperative hemoglobin decrease during cardiac surgery. Transfusion. 2014;54:2696–704. doi: 10.1111/trf.12654. [DOI] [PubMed] [Google Scholar]
- Hortin GL, Carter AB. Intraoperative parathyroid hormone testing: Survey of testing program characteristics. Archives of Pathology and Laboratory Medicine. 2002;126:1045–9. doi: 10.5858/2002-126-1045-IPHT. [DOI] [PubMed] [Google Scholar]
- Hwang RS, Morris LF, Ro K, Park S, Ituarte PHG, Hong JC, et al. A selective, bayesian approach to intraoperative PTH monitoring. Annals of Surgery. 2010;251:1122–6. doi: 10.1097/SLA.0b013e3181dd4ee1. [DOI] [PubMed] [Google Scholar]
- Irvin GL, Iii, Prudhomme DL, Deriso GT, Sfakianakis G, Chandarlapaty SKC. A new approach to parathyroidectomy. Annals of Surgery. 1994;219:574–81. doi: 10.1097/00000658-199405000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I, Wolff S, Gronfier A, Mutter D, Swantröm LL. A cost evaluation methodology for surgical technologies. Surgical Endoscopy and Other Interventional Techniques. 2015;29:2423–32. doi: 10.1007/s00464-014-3929-4. [DOI] [PubMed] [Google Scholar]
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease. The present and the future. Journal of the American College of Cardiology. 2006;48:1–11. doi: 10.1016/j.jacc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- Jarrige V, Nieuwenhuis JH, Van Son JPHF, Martens MFWC, Vissers JLM. A fast intraoperative PTH point-of-care assay on the Philips handheld magnotech system. Langenbeck's Archives of Surgery. 2011;396:337–43. doi: 10.1007/s00423-010-0733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, Dash PK. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. Journal of Neurotrauma. 2013;30:657–70. doi: 10.1089/neu.2012.2439. [DOI] [PubMed] [Google Scholar]
- Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clinical Chemistry. 2009;55:1944–9. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- John AS, Price CP. Economic evidence and point-of-care testing. Clinical Biochemist Reviews. 2013;34:61–74. [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Doherty G, Lairmore T, Moley JF, Brunt LM, Koenig J, et al. Evaluation of the performance and clinical impact of a rapid intraoperative parathyroid hormone assay in conjunction with preoperative imaging and concise parathyroidectomy. Clinical Chemistry. 2001;47:919–25. [PubMed] [Google Scholar]
- Kanzaria HK, Hoffman JR, Probst MA, Caloyeras JP, Berry SH, Brook RH. Emergency physician perceptions of medically unnecessary advanced diagnostic imaging. Academic Emergency Medicine. 2015;22:390–8. doi: 10.1111/acem.12625. [DOI] [PubMed] [Google Scholar]
- Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. New England Journal of Medicine. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- Khan AA. Medical management of primary hyperparathyroidism. Journal of Clinical Densitometry. 2013;16:60–3. doi: 10.1016/j.jocd.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lilot M, Murphy LSL, Sidhu KS, Yu Z, Rinehart J, et al. Accuracy of continuous noninvasive hemoglobin monitoring: A systematic review and meta-analysis. Anesthesia and Analgesia. 2014a;119:332–46. doi: 10.1213/ANE.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Kim TK, Oh SW, Hong SC, Mok YJ, Choi EY. Point-of-care fluorescence immunoassay for cardiac panel biomarkers. Journal of Clinical Laboratory Analysis. 2014b;28:419–27. doi: 10.1002/jcla.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy TI, Buechler KF. The Triage® system. The Immunoassay Handbook. 2013:541–4. [Google Scholar]
- Kost GJ. Guidelines for point-of-care testing: Improving patient outcomes. American Journal of Clinical Pathology. 1995;104:S111–S27. [PubMed] [Google Scholar]
- Kost GJ. Preventing medical errors in point-of-care testing: Security, validation, performance, safeguards, and connectivity. Archives of Pathology and Laboratory Medicine. 2001;125:1307–15. doi: 10.5858/2001-125-1307-PMEIPO. [DOI] [PubMed] [Google Scholar]
- Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Laboratory reference values. New England Journal of Medicine. 2004;351:1548–63. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- La'ulu SL, Roberts WL. Performance characteristics of six intact parathyroid hormone assays. American Journal of Clinical Pathology. 2010;134:930–8. doi: 10.1309/AJCPLGCZR7IPVHA7. [DOI] [PubMed] [Google Scholar]
- Larsson A, Greig-Pylypczuk R, Huisman A. The state of point-of-care testing: A european perspective. Upsala Journal of Medical Sciences. 2014;120:1–10. doi: 10.3109/03009734.2015.1006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Jung J, Hahn YK, Kim SK, Lee Y, Lee J, et al. A centrifugally actuated point-of-care testing system for the surface acoustic wave immunosensing of cardiac troponin i. Analyst. 2013;138:2558–66. doi: 10.1039/c3an00182b. [DOI] [PubMed] [Google Scholar]
- Lewandrowski K, Gregory K, MacMillan D. Assuring quality in point-of-care testing: Evolution of technologies, informatics, and program management. Archives of Pathology and Laboratory Medicine. 2011;135:1405–14. doi: 10.5858/arpa.2011-0157-RA. [DOI] [PubMed] [Google Scholar]
- Li H, Han D, Pauletti GM, Steckl AJ. Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab on a Chip - Miniaturisation for Chemistry and Biology. 2014;14:4035–41. doi: 10.1039/c4lc00716f. [DOI] [PubMed] [Google Scholar]
- Liebes Y, Amir L, Marks RS, Banai M. Immobilization strategies of Brucella particles on optical fibers for use in chemiluminescence immunosensors. Talanta. 2009;80:338–45. doi: 10.1016/j.talanta.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Ludwig SKJ, Tokarski C, Lang SN, van Ginkel LA, Zhu HY, Ozcan A, Nielen MWF. Calling biomarkers in milk using a protein microarray on your smartphone. PLOS One. 2015;10:0134360. doi: 10.1371/journal.pone.0134360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. What does one minute of operating room time cost? Journal of Clinical Anesthesia. 2010;22:233–6. doi: 10.1016/j.jclinane.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350–4. doi: 10.1161/CIRCULATIONAHA.111.023697. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Choudhary R. Biomarkers in acute heart failure-state of the art. Nature Reviews Cardiology. 2012;9:478–90. doi: 10.1038/nrcardio.2012.60. [DOI] [PubMed] [Google Scholar]
- Mallett SV, Cox DJA. Thrombelastography. British Journal of Anaesthesia. 1992;69:307–13. doi: 10.1093/bja/69.3.307. [DOI] [PubMed] [Google Scholar]
- Markin A, Strogonova L, Balashov O, Polyakov V, Tigner T. The dynamics of blood biochemical parameters in cosmonauts during long-term space flights. Acta Astronautica. 1998;42:247–53. doi: 10.1016/s0094-5765(98)00121-0. [DOI] [PubMed] [Google Scholar]
- Martinez A, Phillips S, Nie Z, Cheng CM, Carrilho E, Wiley B, et al. Programmable diagnostic devices made from paper and tape. Lab on a Chip. 2010;10:2499–540. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- Maule J, Wainwright N, Steele A, Monaco L, Morris H, Gunter D, et al. Rapid culture-independent microbial analysis aboard the International Space Station (ISS) Astrobiology. 2009;9:759–75. doi: 10.1089/ast.2008.0319. [DOI] [PubMed] [Google Scholar]
- Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. Journal of Emergency Medicine. 2006;31:201–10. doi: 10.1016/j.jemermed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Mazzei F, Antiochia R, Botrè F, Favero G, Tortolini C. Affinity-based biosensors in sport medicine and doping control analysis. Bioanalysis. 2014;6:225–45. doi: 10.4155/bio.13.308. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on Concussion in Sport–the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. South African Journal of Sports Medicine. 2009;21:36–46. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- McGrath TF, McClintock L, Dunn JS, Husar GM, Lochhead MJ, Sarver RW, et al. Development of a rapid multiplexed assay for the direct screening of anitmicrobial residues in raw milk. Analytical and Bioanalytical Chemistry. 2015;407:4459–4472. doi: 10.1007/s00216-015-8526-4. [DOI] [PubMed] [Google Scholar]
- McPartlin DA, O'Kennedy RJ. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Review of Molecular Diagnostics. 2014;14:979–98. doi: 10.1586/14737159.2014.960516. [DOI] [PubMed] [Google Scholar]
- Moore RE. An historical perspective on the clinical diagnostic laboratory. Molecular Diagnostics: For the Clinical Laboratorian. 2005:3–10. [Google Scholar]
- Morris HC, Damon M, Maule J, Monaco LA, Wainwright N. Rapid culture-independent microbial analysis aboard the international space station (ISS) stage two: quantifying three microbial biomarkers. Astrobiology. 2012;12:830–40. doi: 10.1089/ast.2012.0863. [DOI] [PubMed] [Google Scholar]
- Mougios V. Reference intervals for serum creatine kinase in athletes. British Journal of Sports Medicine. 2007;41:674–8. doi: 10.1136/bjsm.2006.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtwey D. Radiological health risks for exploratory class missions in space. Acta Astronautica. 1991;23:227–31. doi: 10.1016/0094-5765(91)90122-l. [DOI] [PubMed] [Google Scholar]
- Nelson E. Design principles for microfluidic biomedical diagnostics in space. In: Reza Fazel., editor. Biomedical Engineering -From Theory to Applications. 2011. pp. 131–56. [Google Scholar]
- Nicogossian A, Huntoon C, Pool S, editors. Space Physiology and Medicine. 3rd. Philadelphia, Pa: Lea and Febiger; 1994. [Google Scholar]
- North SH, Shriver-Lake LC, Taitt CR, Ligler FS. Rapid analytical methods for on-site triage for traumatic brain injury. Annual Review of Analytical Chemistry. 2012;5:35–56. doi: 10.1146/annurev-anchem-062011-143105. [DOI] [PubMed] [Google Scholar]
- O'Connor TP, Lawrence J, Andersen P, Leathers V, Workman E. Immunoassay applications in veterinary diagnostics. The Immunoassay Handbook. 2013:623–45. [Google Scholar]
- O'Donoghue M, Braunwald E. Natriuretic peptides in heart failure: Should therapy be guided by BNP levels? Nature Reviews Cardiology. 2010;7:13–20. doi: 10.1038/nrcardio.2009.197. [DOI] [PubMed] [Google Scholar]
- O'Reilly M. Considerations for evaluating the accuracy of hemoglobin monitoring. Anesthesiology. 2012;117:429–30. doi: 10.1097/ALN.0b013e31825d95d6. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Matsuo M, Matsuo H, Sakaguchi Y, Takai H, Horiguchi Y, et al. Introduction of point-of-care testing in Japanese outpatient clinics is associated with improvement in time in therapeutic range in anticoagulant-treated patients. Circulation Journal. 2014;78:1342–8. doi: 10.1253/circj.cj-13-1256. [DOI] [PubMed] [Google Scholar]
- Oncescu V, O'Dell D, Erickson D. Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab on a Chip. 2013;13:3232–7. doi: 10.1039/c3lc50431j. [DOI] [PubMed] [Google Scholar]
- Özcan HM, Sezgintürk MK. Detection of parathyroid hormone using an electrochemical impedance biosensor based on PAMAM dendrimers. Biotechnology Progress. 2015;31:815–22. doi: 10.1002/btpr.2060. [DOI] [PubMed] [Google Scholar]
- Panteghini M. Diagnostic application of CK-MB mass determination. Clinica Chimica Acta. 1998;272:23–31. doi: 10.1016/s0009-8981(97)00249-0. [DOI] [PubMed] [Google Scholar]
- Papadea C, Foster J, Grant S, Ballard SA, Cate Iv JC, Southgate WM, et al. Evaluation of the i-STAT Portable Clinical Analyzer for point-of-care blood testing in the intensive care units of a University Children's Hospital. Annals of Clinical and Laboratory Science. 2002;32:231–43. [PubMed] [Google Scholar]
- Peacock WF, Braunwald E, Abraham W, Albert N, Burnett J, Christenson R, et al. National heart, lung, and blood institute working group on emergency department management of acute heart failure: Research challenges and opportunities. Journal of the American College of Cardiology. 2010;56:343–51. doi: 10.1016/j.jacc.2010.03.051. [DOI] [PubMed] [Google Scholar]
- Peña JA, Lewandrowski KB, Lewandrowski EL, Gregory K, Baron JM, Van Cott EM. Evaluation of the i-STAT point-of-care capillary whole blood prothrombin time and international normalized ratio: Comparison to the Tcoag MDAII coagulation analyzer in the central laboratory. Clinica Chimica Acta. 2012;413:955–9. doi: 10.1016/j.cca.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Petricevic M, Kopjar T, Biocina B, Milicic D, Kolic K, Boban M, et al. The Predictive Value of Platelet Function Point-of-Care Tests for Postoperative Blood Loss and Transfusion in Routine Cardiac Surgery: A Systematic Review. Thoracic and Cardiovascular Surgeon. 2015;63:2–20. doi: 10.1055/s-0034-1378191. [DOI] [PubMed] [Google Scholar]
- Pirozzi C, Numis FG, Pagano A, Melillo P, Copetti R, Schiraldi F. Immediate versus delayed integrated point-of-care-ultrasonography to manage acute dyspnea in the emergency department. Critical Ultrasound Journal. 2014;6:5. doi: 10.1186/2036-7902-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts SR, Carrier ER, Rich EC, Kellermann AL. Where americans get acute care: Increasingly, it's not at their doctor's office. Health Affairs. 2010;29:1620–9. doi: 10.1377/hlthaff.2009.1026. [DOI] [PubMed] [Google Scholar]
- Poesen K, De Prins M, Van Den Berghe G, Van Eldere J, Vanstapel F. Performance of cassette-based blood gas analyzers to monitor blood glucose and lactate levels in a surgical intensive care setting. Clinical Chemistry and Laboratory Medicine. 2013;51:1417–27. doi: 10.1515/cclm-2012-0848. [DOI] [PubMed] [Google Scholar]
- Potts JT. Parathyroid hormone: Past and present. Journal of Endocrinology. 2005;187:311–25. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Gurbuz Y, Niazi JH. Biosensors for cardiac biomarkers detection: A review. Sensors and Actuators, B: Chemical. 2012:171–172. 62–76. [Google Scholar]
- Rebel A, Rice MA, Fahy BG. The accuracy of point-of-care glucose measurements. Journal of Diabetes Science and Technology. 2012;6:396–411. doi: 10.1177/193229681200600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regiart M, Fernández-Baldo MA, Spotorno VG, Bertolino FA, Raba J. Ultra sensitive microfluidic immunosensor for determination of clenbuterol in bovine hair samples using electrodeposited gold nanoparticles and magnetic micro particles as bio-affinity platform. Biosensors and Bioelectronics. 2013;41:211–7. doi: 10.1016/j.bios.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. New England Journal of Medicine. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Archives of Internal Medicine. 2012;172:1211–8. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- Rhee AJ, Kahn RA. Laboratory point-of-care monitoring in the operating room. Current Opinion in Anaesthesiology. 2010;23:741–8. doi: 10.1097/ACO.0b013e32834015bd. [DOI] [PubMed] [Google Scholar]
- Rice MJ, Pitkin AD, Coursin DB. Glucose measurement in the operating room: More complicated than it seems. Anesthesia and Analgesia. 2010;110:1056–65. doi: 10.1213/ANE.0b013e3181cc07de. [DOI] [PubMed] [Google Scholar]
- Richards ML, Thompson GB, Farley DR, Grant CS. An optimal algorithm for intraoperative parathyroid hormone monitoring. Archives of Surgery. 2011;146:280–5. doi: 10.1001/archsurg.2011.5. [DOI] [PubMed] [Google Scholar]
- Rooney KD, Schilling UM. Point-of-care testing in the overcrowded emergency department - Can it make a difference? Critical Care. 2014;18 doi: 10.1186/s13054-014-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: Simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–3. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- Saenger AK, Jaffe AS. Requiem for a heavyweight: The demise of creatine kinase-MB. Circulation. 2008;118:2200–6. doi: 10.1161/CIRCULATIONAHA.108.773218. [DOI] [PubMed] [Google Scholar]
- Salem M, Chernow B, Burke R, Stacey J, Slogoff M, Sood S. Bedside diagnostic blood testing: Its accuracy, rapidity, and utility in blood conservation. Journal of the American Medical Association. 1991;266:382–9. doi: 10.1001/jama.266.3.382. [DOI] [PubMed] [Google Scholar]
- Samara EM, Ayadi M, Aljumaah RS. Feasibility of utilising an infrared-thermographic technique for early detection of subclinical mastitis in dairy camels (Camelus dromedarius) Journal of Dairy Research. 2014;81:38–45. doi: 10.1017/S0022029913000605. [DOI] [PubMed] [Google Scholar]
- Samsonova JV, Cannavan A, Elliott CT. A critical review of screening methods for the detection of chloramphenicol, thiamphenicol and florfenicol residues in foodstuffs. 2012;42:50–78. [Google Scholar]
- Saner FH. Rotational thrombelastometry: A step forward to safer patient care? Critical Care. 2014;18 doi: 10.1186/s13054-014-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini SA, Carrozza C, Vulpio C, Capoluongo E, Luciani G, Lulli P, et al. Assessment of parathyroid function in clinical practice: Which parathyroid hormone assay is better? Clinical Chemistry. 2004;50:1247–50. doi: 10.1373/clinchem.2003.030759. [DOI] [PubMed] [Google Scholar]
- Santos PM, Pereira ER, Rodriguez-Saona LE. Application of hand-held and portable infrared spectrometers in bovine milk analysis. Journal of agricultural and food chemistry. 2013;61:1205–11. doi: 10.1021/jf303814g. [DOI] [PubMed] [Google Scholar]
- Sanvicens N, Mannelli I, Salvador JP, Valera E, Marco MP. Biosensors for pharmaceuticals based on novel technology. TrAC - Trends in Analytical Chemistry. 2011;30:541–53. [Google Scholar]
- Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayikli ŞimŞek Ç, Nur Sonuç Karaboʇa M, Sezgintürk MK. A new immobilization procedure for development of an electrochemical immunosensor for parathyroid hormone detection based on gold electrodes modified with 6-mercaptohexanol and silane. Talanta. 2015;144:210–8. doi: 10.1016/j.talanta.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Schlebusch H, Paffenholz I, Zerback R, Leinberger R. Analytical performance of a portable critical care blood gas analyzer. Clinica Chimica Acta. 2001;307:107–12. doi: 10.1016/s0009-8981(01)00440-5. [DOI] [PubMed] [Google Scholar]
- Schuur JD, Carney DP, Lyn ET, Raja AS, Michael JA, Ross NG, et al. A top-five list for emergency medicine a pilot project to improve the value of emergency care. JAMA Internal Medicine. 2014;174:509–15. doi: 10.1001/jamainternmed.2013.12688. [DOI] [PubMed] [Google Scholar]
- Semkova JV, TsP D, YuN M, Koleva RT, Baynov PT, Tomov BT, et al. Proposal for a new radiation dose control system for future manned space flights. Acta Astronautica. 1995;36:629–38. doi: 10.1016/0094-5765(95)00152-2. [DOI] [PubMed] [Google Scholar]
- Shan R, Szmydynger-Chodobska J, Warren OU, Mohammad F, Zink BJ, Chodobski A. A new panel of blood biomarkers for the diagnosis of mild traumatic brain injury/concussion in adults. Journal of Neurotrauma. 2015:150625110426006–9. doi: 10.1089/neu.2014.3811. [DOI] [PubMed] [Google Scholar]
- Shi W, Zheng S, Kasdan HL, Fridge A, Tai YC. Leukocyte count and two-part differential in whole blood based on a portable microflow cytometer. TRANSDUCERS 2009 - 15th International Conference on Solid-State Sensors, Actuators and Microsystems; 2009; pp. 616–9. [Google Scholar]
- Silverman R, Yalow RS. Heterogeneity of parathyroid hormone. Clinical and physiologic implications. Journal of Clinical Investigation. 1973;52:1958–71. doi: 10.1172/JCI107380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton VA, Wijayasinghe N, Sharafudeen S, Sange A, Parry NS, Junghans C. Evaluation of point-of-care haemoglobin measuring devices: A comparison of Radical-7™ pulse co-oximetry, HemoCue® and laboratory haemoglobin measurements in obstetric patients. Anaesthesia. 2013;68:40–5. doi: 10.1111/anae.12039. [DOI] [PubMed] [Google Scholar]
- Skugor M, Gupta M, Navaneethan SD. Evolution and current state of assays for measuring parathyroid hormone. Biochemia Medica. 2010;20:221–8. [Google Scholar]
- Smith SM, Davis-Street JE, Fontenot TB, Lane HW. Assessment of a portable clinical blood analyzer during space flight. Clinical Chemistry. 1997;43:1056–65. [PubMed] [Google Scholar]
- Sokoll LJ, Wians FH, Jr, Remaley AT. Rapid intraoperative immunoassay of parathyroid hormone and other hormones: A new paradigm for point-of-care testing. Clinical Chemistry. 2004;50:1126–35. doi: 10.1373/clinchem.2003.030817. [DOI] [PubMed] [Google Scholar]
- Stalberg P, Sidhu S, Sywak M, Robinson B, Wilkinson M, Delbridge L. Intraoperative parathyroid hormone measurement during minimally invasive parathyroidectomy: Does it “value-add” to decision-making? Journal of the American College of Surgeons. 2006;203:1–6. doi: 10.1016/j.jamcollsurg.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Steinkamp JA, Romero A, Van Dilla MA. Multiparameter cell sorting: identification of human leukocytes by acridine orange fluorescence. Acta Cytologica. 1973;17:113–7. [PubMed] [Google Scholar]
- Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, et al. The burden of acute heart failure on U.S. emergency departments. JACC: Heart Failure. 2014;2:269–77. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume T, Loftus D, Li J, Coleman M, Davis C, McMonigal K, et al. Biomarker-detection technologies for comprehensive medical diagnosis during deep-space missions. Recent Patents on Space Technology. 2013;3:13–23. [Google Scholar]
- Thölking G, Mesters R, Dittrich R, Pavenstädt H, Kümpers P, Reuter S. Assessment of hemostasis after plasma exchange using rotational thrombelastometry (ROTEM) PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0130402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Branche CM, Sniezek JE. The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. The Journal of head trauma rehabilitation. 1998;13:1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. European Heart Journal. 2010;31:2197–204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- Udelsman R, Donovan P, Shaw C. Cure predictability during parathyroidectomy. World Journal of Surgery. 2014;38:525–33. doi: 10.1007/s00268-013-2327-8. [DOI] [PubMed] [Google Scholar]
- Van Houdt R, Mijnendonckx K, Leys N. Microbial contamination monitoring and control during human space missions. Planetary and Space Science. 2012;60:115–20. [Google Scholar]
- Vashist S, Luppa P, Yeo L, Ozcan A, Luong J. Emerging technologies for next-generation point-of-care testing. Trends in Biotechnology. 2015;33:692–705. doi: 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Velasco-Garcia MN, Mottram T. Biosensor technology addressing agricultural problems. Biosystems Engineering. 2003;84:1–12. [Google Scholar]
- Venturini JM, Stake CE, Cichon ME. Prehospital point-of-care testing for troponin: are the results reliable? Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors. 2013;17:88–91. doi: 10.3109/10903127.2012.717166. [DOI] [PubMed] [Google Scholar]
- Vieira JGH, Kunii I, Nishida S. Evolution of PTH assays. Arquivos Brasileiros de Endocrinologia e Metabologia. 2006;50:621–7. doi: 10.1590/s0004-27302006000400007. [DOI] [PubMed] [Google Scholar]
- Vieira JGH, Kunii IS, Ohe MN, Carvalho AB. Heterogeneity of carboxyl-terminal parathyroid hormone circulating forms in patients with hyperparathyroidism due to end stage renal disease. Arquivos Brasileiros de Endocrinologia e Metabologia. 2009;53:1074–8. doi: 10.1590/s0004-27302009000900003. [DOI] [PubMed] [Google Scholar]
- Wians FH, Jr, Balko JA, Hsu RM, Byrd W, Synder WH., III Intraoperative vs central laboratory PTH testing during parathyroidectomy surgery. Laboratory Medicine. 2000;31:616–21. [Google Scholar]
- Yager P, Domingo G, Gerdes J. Point-of-care diagnostics for global health. Annual Review of Biomedical Engineering. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- Yang Z, Min Zhou D. Cardiac markers and their point-of-care testing for diagnosis of acute myocardial infarction. Clinical Biochemistry. 2006;39:771–80. doi: 10.1016/j.clinbiochem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Yu L, Shi ZZ, Fang C, Zhang YY, Liu YS, Li CM. A disposable lateral flow-through strip for smartphone-camera to quantitatively detect alkaline phosphatase activity in milk. Biosensors and Bioelectronics. 2015;69:307–315. doi: 10.1016/j.bios.2015.02.035. [DOI] [PubMed] [Google Scholar]
- Yu N, Leese GP, Smith D, Donnan PT. The natural history of treated and untreated primary hyperparathyroidism: The parathyroid epidemiology and audit research study. QJM. 2011;104:513–21. doi: 10.1093/qjmed/hcq261. [DOI] [PubMed] [Google Scholar]
- Zhuang GS, Liu J, Jia CP, Jin QH, Zhao JL, Wang HM. Microchip-based capillary electrophoresis for determination of lactate dehydrogenase isoenzymes. Journal of Separation Science. 2007;30:1350–6. doi: 10.1002/jssc.200600506. [DOI] [PubMed] [Google Scholar]


