Abstract
Osteoporosis is often underdiagnosed and undertreated. Screening of post-menopausal women for clinical risk factors and/or low bone mineral density (BMD) has been proposed to overcome this. Digital X-ray radiogrammetry (DXR) estimates hand BMD from standard hand X-ray images and have shown to predict fractures and osteoporosis. Recently, digital radiology and the internet have opened up the possibility of conducting automated opportunistic screening with DXR in post-fracture care or in combination with mammography. This study compared the performance of DXR with FRAX® and DXA in discriminating major osteoporotic fracture (MOF) (hip, clinical spine, forearm or shoulder), hip fracture and femoral neck osteoporosis. This prospective cohort study was conducted on 5278 women 65 years and older in the Study of Osteoporotic Fractures (SOF) cohort. Baseline hand X-ray images were analyzed and fractures were ascertained during 10 years of follow up. Age-adjusted area under receiver operating characteristic curve (AUC) for MOF and hip fracture and for femoral neck osteoporosis (DXA FN BMD T-score ≤ −2.5) was used to compare the methods. Sensitivity to femoral neck osteoporosis at equal selection rates was tabulated for FRAX and DXR. DXR-BMD, FRAX (no BMD) and lumbar spine DXA BMD were all similar in fracture discriminative performance with an AUC around 0.65 for MOF and 0.70 for hip fractures for all three methods. As expected femoral neck DXA provided fracture discrimination superior both to other BMD measurements and to FRAX. AUC for selection of patients with femoral neck osteoporosis was higher with DXR-BMD, 0.76 (0.74–0.77), than with FRAX, 0.69 (0.67–0.71), (p<0.0001). In conclusion, DXR-BMD discriminates incident fractures to a similar degree as FRAX and predicts femoral neck osteoporosis to a larger degree than FRAX. DXR shows promise as a method to automatically flag individuals who might benefit from an osteoporosis assessment.
Keywords: Bone mineral density, osteoporosis, digital X-ray radiogrammetry, FRAX, dual-energy X-ray absorptiometry
1. Introduction
Fragility fractures represent a major public health and economic burden in the European Union and United States [1,2]. There are cost-effective pharmacologic interventions available [1,3,4], but the cost-risk-benefit profile heavily favors treating only those who have the highest risk for fractures.
The gold standard for selecting those who would benefit from anti-osteoporotic intervention is bone mineral density (BMD) measurement by dual energy X-ray absorptiometry (DXA) of the femoral neck and/or total hip and lumbar spine [5,6]. However, due to cost, workflow and accessibility not all eligible women are evaluated with central DXA [1,7]. More accessible and lower cost techniques for identifying individuals who would benefit from anti-osteoporotic intervention or further evaluation by central DXA, if available, might improve patient care [1,8].
Besides central DXA an increased risk for fracture can be identified based on measurements at a variety of peripheral bone sites including heel, radius, metacarpals and phalanges; by a variety of technologies including DXA, quantitative ultrasound, radiographic absorptiometry and radiogrammetry. Performance varies between measurement sites and technology, but the primary disadvantage of all peripheral measurements is a weaker ability to discriminate hip fractures than DXA BMD measured in the femoral neck.
Besides BMD, there exist form-based tools with clinical risk factors for fracture. One such tool, embraced by the World Health Organization (WHO) and frequently cited in national guidelines, is the FRAX® online tool [9]. Since patient clinical risk factors can be collected and the form filled in at the point of care without a BMD value, FRAX is highly accessible and is low cost.
Digital X-ray radiogrammetry (DXR) is a software technique to estimate bone mineral density in the hand (DXR-BMD). DXR estimates BMD through an automated radiogrammetric analysis; cortical thickness, width and porosity, of the three middle metacarpal bones in a standard hand radiograph [10]. DXR was first introduced in the late nineties in a hardware device and later as a software workstation but like all peripheral BMD systems of the time it eventually failed and never reached widespread use. Today however, the advents of digital radiology systems, electronic medical records and the internet have fundamentally changed the conditions and enable a large degree of automation and efficiency. DXR can be performed on hand images acquired with any digital X-ray machine including those used for digital mammography. This means that DXR can be opportunistically integrated in existing mammography screening workflows with small impact on the workflow [11], or used to automatically process all forearm fracture images [12].
Previous studies have shown DXR-BMD measurements to predict hip, spine and other fractures to a similar degree as other peripheral BMD measurements [12–15]. Furthermore, DXR-BMD did also predict osteoporosis as measured by central DXA [15–17]. In healthcare workflows where a patient already is at an X-ray machine, e.g. at mammography screening, suspected fracture or rheumatoid arthritis evaluation, DXR could be an alternative or a complement to FRAX in identifying individuals at increased risk for fracture requiring evaluation for possible intervention. To our knowledge there are currently no published studies that include both FRAX and DXR.
The purpose of this study was to evaluate and compare the performance of DXR, FRAX and DXA in discriminating major osteoporotic fracture (MOF) (hip, clinical spine, forearm or shoulder), hip fracture and femoral neck osteoporosis.
2. Material and Methods
2.1. Subjects and Clinical Assessments
From 1986 to 1988, 9704 Caucasian women 65 years or older were recruited for participation in the prospective Study of Osteoporotic Fractures (SOF). Women were recruited from population-based listings in 4 regions of the United States [18]. Details of this cohort have been published previously [18].
Briefly, at the baseline visit, radiographs of the non-dominant distal forearm and hand, thoracic spine and lumbar spine were acquired. Surviving participants were invited to a second examination between 1989 and 1990 that included measurement of femoral neck and lumbar spine BMD by DXA. In total, 7963 women had technically adequate femoral neck BMD measurements. Of these, 6252 had provided data for all clinical risk factors in FRAX. Finally, of these, 5278 women had a technically adequate baseline hand radiograph for DXR-BMD measurement available and are the subject of this analysis.
The institutional review board at each center approved the study protocol, and written informed consent was obtained from all participants.
2.2. Clinical Risk Factors
Participants completed a questionnaire and were interviewed at the baseline examination about ethnicity, history of fracture since age of 50, parental history of hip fracture, physician diagnosis of rheumatoid arthritis, use of oral glucocorticoids, smoking status and alcohol intake. Measurements of body height and weight were acquired.
2.3. Confirmation of fractures
After baseline, participants were contacted every 4 months, by postcard or telephone, to enquire about recent fractures. More than 98% of these follow-up contacts were completed. Reported fractures were confirmed by review of radiology reports. Ten years was selected as censoring horizon to match that of the FRAX tool.
2.4. Central Bone Mineral Density
The BMD of the lumbar spine and the proximal femur including the femoral neck (FN) subregion were measured by means of DXA (QDR 1000, Hologic, Waltham, Massachusetts). Details regarding the measurement and quality control methods have been published previously [18].
2.5. Digital X-ray Radiogrammetry
Automated DXR (OneScreen, Sectra Osteoporosis Package, Sectra AB, Linköping, Sweden) was used to calculate BMD (DXR-BMD, g/cm2) in the metacarpals. The technique has been described in more detail previously [10,13,19] and will be only briefly summarized here.
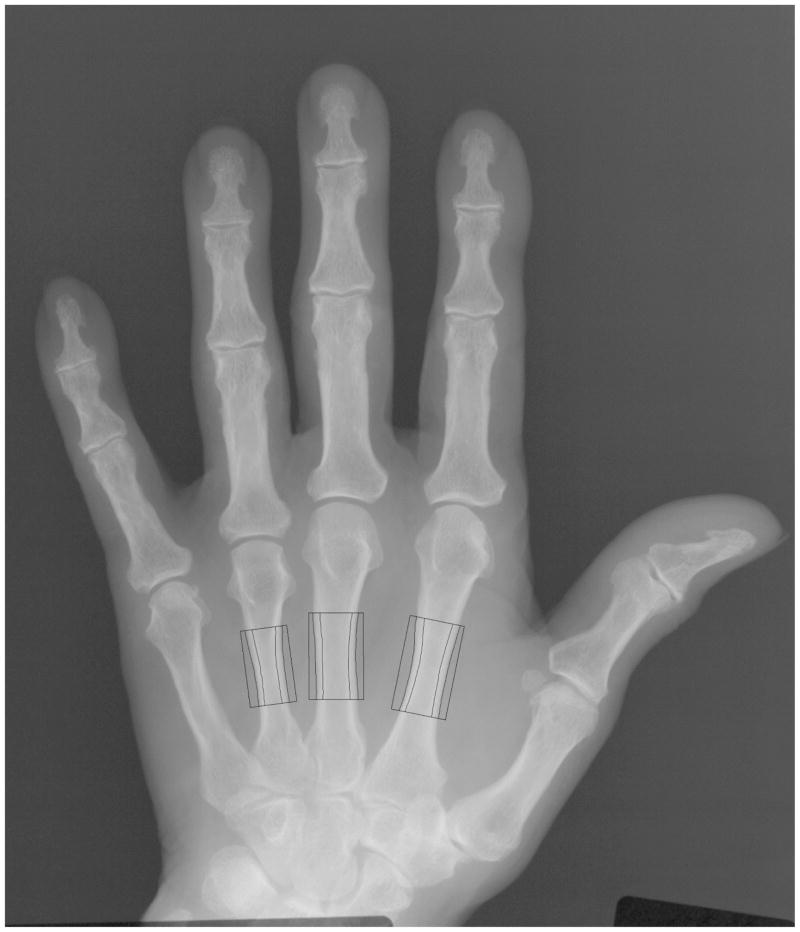
DXR is an automated digital version of the traditional technique of radiogrammetry [20]. A plain digital hand radiograph is sent to a computer. The system automatically locates measurement regions around the diaphyses of metacarpals two, three and four respectively, Figure 1. It determined the average cortical thickness (Ti) and bone width (Wi) individually for each metacarpal i, and the bone volume per projected area (VPAi) was computed assuming a cylindrically shaped bone:
Figure 1.
Digital X-ray radiogrammetry measurement regions on a hand X-ray image.
The system computed the combined VPA of the three middle metacarpals as a weighted average:
The system estimated the estimated three-dimensional porosity P, the fraction of the cortical bone that is not occupied by bone [21], and finally output DXR-BMD as
where c is an empirical density constant to calibrate the DXR-BMD so that the absolute DXR-BMD value best corresponds to the mid-distal forearm region BMD, as assessed by DXA [22]. DXR-BMD has previously been found to have a coefficient of variation of 0.28% [23].
The effective radiation dose of a DXR examination is that of the hand X-ray, in the order of < 0.001 mSv [24]. This level of radiation is similar or lower than a DXA examination and is generally considered negligible.
All DXR-BMD analyses were performed automated and without knowledge of DXA BMD or other patient data.
2.6. FRAX® tool
This analysis used the FRAX tool [9] (Version 3.0, US Caucasian). The FRAX tool included the following: age, sex, weight, height, fracture history, parental history of hip fracture, smoking status, use of oral glucocorticoid, presence of rheumatoid arthritis, presence of disorders strongly associated with osteoporosis (type I diabetes mellitus, osteogenesis imperfecta in adults, untreated long-standing hyperthyroidism, premature menopause (<45 years), chronic malnutrition or malabsorption and chronic liver disease), alcohol intake. The FRAX algorithm provided four fracture probabilities for each subject: The 10-year probability of MOF and the 10-year probability of hip fracture, each calculated with or without femoral neck BMD data.
2.7. Statistical Analysis
Only women with data on clinical risk factors for the calculation of FRAX 10-year probabilities, total hip BMD and DXR-BMD were included in the analysis. Receiver operating characteristic curve analysis was used to compare methods for discriminating fracture risk and for predicting femoral neck osteoporosis. A ranked risk method was used to tabulate observed performance of discrimination methods at equal selection rate. The tables provide data for performance comparison, threshold selection and input data for cost effect estimations for prescreening selection to central DXA by FRAX or by DXR-BMD.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The original analysis plan is available in full on the SOF Online website [25], analysis plan #812. All source data is available for download through the SOF website [25].
3. Results
A total of 5278 women were included in the analysis. Average (SD) age was 71 (5) years and 1797 (34%) had a history of fracture since age of 50. The average (SD) femoral neck BMD, lumbar spine BMD, and DXR-BMD were 0.647 (0.111), 0.854 (0.169), and 0.485 (0.059) g/cm2, respectively. The mean (SD) time between baseline (FRAX and DXR) and the central DXA measurement was 2.1 (0.2) years. Measurement of DXR-BMD was successful in 99.73% of available hand images and failed in 0.27% of cases (primarily due to missing anatomy e.g. prosthesis in measurement region). In 0.02% of cases the automated DXR system failed to invalidate an invalid measurement. Compared with the 4426 women in the SOF cohort excluded from this analysis due to missing data needed for calculation of FRAX model probabilities (primarily parental history of hip fracture), central DXA or DXR-BMD, the 5278 women included in the analytical cohort were, on average, slightly younger (mean age 71.3 vs. 72.1 years, p<0.001) and less likely to report poor to fair health status (15.3% vs. 18.7%, p<0.001) or prior history of fracture (34.1% vs. 40.8%, p<0.001). However, mean body mass index (26.6 vs. 26.4, p=0.06) and femoral neck BMD (0.647 vs. 0.652, p=0.07) were similar between the two groups.
During ten years of follow up, 873 women (17.1%) suffered a major osteoporotic fracture and of these 323 women (6.1%) suffered a hip fracture, table 1. As expected, the percentage of women who had fractures increased with older age.
Table 1.
Number of women who suffered a fracture during 10 years of follow-up
| Age at baseline | Hip fracture (N = 323, 6.1%)a | Major osteoporotic fracture b (N = 873, 17.1%) a |
|---|---|---|
| 65–69 (N = 2343) | 68 (2.9%) | 293 (12.9%) |
| 70–74 (N = 1674) | 104 (6.2%) | 277 (17.2%) |
| 75–79 (N = 847) | 87 (10.3%) | 190 (23.5%) |
| 80+ (N = 414) | 64 (15.5%) | 113 (28.5%) |
After excluding 19 unconfirmed hip fractures, 184 unconfirmed major osteoporotic fractures.
Clinical spine, hip, forearm or shoulder fracture.
The DXA FN BMD AUC statistic for MOF and hip fracture was 0.68 and 0.75 respectively, table 2. Corresponding AUC statistics for DXA lumbar spine BMD, DXR-BMD and FRAX (no BMD, FRAX MOF and FRAX hip respectively) were similar to each other at 0.65, 0.69; 0.65, 0.69 and 0.64, 0.70 respectively. DXR-BMD + prior fracture and DXR-BMD + FRAX had near identical AUC for fractures at 0.67 for MOF and 0.71 for hip fracture.
Table 2.
Discrimination of major osteoporotic fracture (MOF), hip fracture and femoral neck osteoporosis (DXA FN BMD T-score ≤ −2.5). Area under the curve (AUC) determined from receiver operating characteristic (ROC) analysis. Mean and 95 % confidence interval.
| AUC MOFa 10 years | AUC hip fracture 10 years | AUC femoral neck osteoporosis | |
|---|---|---|---|
| Age alone | 0.59 (0.57, 0.61) | 0.68 (0.65, 0.71) | 0.64 (0.61, 0.65) |
| Age + DXR-BMD | 0.65 (0.63, 0.67) | 0.69 (0.66, 0.72) | 0.76 (0.74, 0.77) |
| Age + DXA FN BMD | 0.68 (0.66, 0.70) | 0.75 (0.72, 0.77) | - |
| Age + FRAX (no BMD) | 0.64 (0.61, 0.65) | 0.70 (0.67, 0.73) | 0.69 (0.67, 0.71) |
| Age + DXA L2-L4 BMD | 0.65 (0.63, 0.67) | 0.69 (0.65, 0.71) | - |
| Age + DXR-BMD + FRAX | 0.67 (0.65, 0.69) | 0.71 (0.68, 0.74) | - |
| Age + DXR-BMD + prior fracture | 0.67 (0.65, 0.69) | 0.71 (0.67, 0.73) | - |
| Age + DXA FN BMD + FRAX | 0.69 (0.67, 0.70) | 0.76 (0.73, 0.78) | - |
Clinical spine, hip, forearm or shoulder fracture.
BMD: bone mineral density; DXA FN: femoral neck BMD by DXA; DXR: metacarpal BMD estimated by digital X-ray radiogrammetry
Age-adjusted AUC for selection of patients with femoral neck osteoporosis (DXA FN BMD T-score ≤ −2.5, ≤ 0.558 g/cm2) was higher for DXR-BMD, 0.76 (0.74–0.77); than for FRAX MOF, 0.69 (0.67–0.71) or age alone, 0.64 (0.61–0.65), (p<0.0001 for both), table 2. AUC for FRAX hip was similar as FRAX MOF (data not shown).
The 10-year major osteoporotic fracture rate, 10-year hip fracture rate, sensitivity to femoral neck osteoporosis (FRAX and DXR-BMD) and corresponding thresholds have been tabulated in table 3. The whole population in 5-year groups has been ranked in deciles according to each of DXA FN BMD, FRAX (hip, no BMD), FRAX (MOF, no BMD) and DXR-BMD. The threshold columns list the level at which the cumulative percentage of the population in the age group was reached with each respective selection method. The columns under incident fractures show for each of the methods how many percent of the patients with a measurement below the corresponding threshold suffered an incident fracture within 10 years from baseline. The sensitivity to DXA FN columns list the observed sensitivity achieved with the corresponding threshold. A corresponding table, supplement table 4, with only the subset of the population that had a previous fracture is provided in the online supplement.
Table 3.
Ten year incidence of fracture by DXA FN BMD, FRAX (no BMD) and DXR-BMD and sensitivity to DXA FN T-score ≤ −2.5 of FRAX (no BMD) and DXR-BMD.
| Cumulative percentage of population | Threshold | Major osteoporotic fracture 10 year (%) | Hip fracture 10 year (%) | Sensitivity to DXA FN T-score ≤ −2.5 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DXA FN (g/cm2) | FRAX hip (%) | FRAX MOF (%) | DXR (g/cm2) | DXA FN | FRAX | DXR | DXA FN | FRAX | DXR | FRAX hip | FRAX MOF | DXR | |
| 65–69 years, N = 2343 | |||||||||||||
| 0% | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10% | 0.539 | 6.8 | 24.6 | 0.433 | 24.8 | 21.6 | 24.2 | 5.9 | 6.8 | 5.1 | 26 | 18 | 25 |
| 20% | 0.582 | 4.5 | 19.3 | 0.456 | 21.8 | 19.7 | 21.6 | 4.9 | 5.6 | 3.4 | 39 | 38 | 43 |
| 30% | 0.611 | 3.5 | 17.5 | 0.472 | 22.1 | 19.1 | 21.1 | 6.1 | 4.0 | 3.7 | 53 | 49 | 58 |
| 40% | 0.639 | 2.9 | 15.4 | 0.488 | 20.0 | 18.1 | 19.6 | 5.2 | 4.1 | 3.5 | 62 | 57 | 70 |
| 50% | 0.663 | 2.4 | 12.1 | 0.500 | 18.7 | 17.4 | 18.0 | 4.8 | 4.1 | 3.2 | 73 | 65 | 79 |
| 60% | 0.689 | 2.0 | 10.8 | 0.515 | 17.1 | 16.2 | 16.1 | 4.1 | 4.0 | 3.1 | 83 | 80 | 85 |
| 70% | 0.715 | 1.7 | 10.1 | 0.532 | 15.4 | 15.0 | 15.2 | 3.7 | 3.5 | 3.2 | 89 | 89 | 91 |
| 80% | 0.753 | 1.4 | 9.4 | 0.551 | 14.7 | 14.1 | 14.4 | 3.4 | 3.4 | 3.2 | 94 | 94 | 97 |
| 90% | 0.818 | 1.1 | 8.7 | 0.578 | 13.6 | 13.7 | 13.6 | 3.2 | 3.2 | 2.9 | 99 | 98 | 99 |
| 100% | 1.270 | 0.4 | 5.0 | 0.678 | 12.9 | 12.9 | 12.9 | 2.9 | 2.9 | 2.9 | 100 | 100 | 100 |
| 70–74 years, N = 1674 | |||||||||||||
| 0% | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10% | 0.515 | 12.8 | 31.0 | 0.414 | 34.0 | 25.3 | 29.6 | 15.2 | 12.7 | 12.6 | 18 | 19 | 24 |
| 20% | 0.551 | 9.0 | 24.3 | 0.437 | 29.2 | 24.4 | 27.8 | 12.7 | 12.0 | 10.5 | 34 | 31 | 40 |
| 30% | 0.576 | 7.0 | 21.9 | 0.453 | 26.2 | 23.3 | 25.0 | 10.8 | 9.2 | 9.2 | 44 | 43 | 55 |
| 40% | 0.606 | 5.9 | 19.6 | 0.467 | 25.4 | 21.6 | 22.5 | 10.5 | 9.2 | 8.2 | 56 | 55 | 69 |
| 50% | 0.633 | 4.8 | 16.6 | 0.482 | 23.1 | 20.6 | 21.4 | 9.2 | 8.7 | 8.0 | 68 | 62 | 81 |
| 60% | 0.657 | 4.0 | 14.1 | 0.496 | 21.7 | 20.3 | 20.1 | 8.3 | 7.7 | 7.4 | 77 | 73 | 87 |
| 70% | 0.682 | 3.3 | 12.8 | 0.511 | 20.7 | 19.0 | 19.8 | 7.8 | 7.4 | 7.0 | 84 | 84 | 93 |
| 80% | 0.718 | 2.8 | 11.9 | 0.529 | 19.7 | 18.2 | 19.0 | 7.3 | 7.1 | 6.8 | 92 | 93 | 96 |
| 90% | 0.777 | 2.3 | 10.9 | 0.557 | 18.8 | 17.7 | 18.2 | 6.8 | 6.5 | 6.5 | 97 | 97 | 98 |
| 100% | 1.084 | 0.8 | 6.3 | 0.669 | 17.2 | 17.2 | 17.2 | 6.2 | 6.2 | 6.2 | 100 | 100 | 100 |
| 75–79 years, N = 847 | |||||||||||||
| 0% | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10% | 0.500 | 21.9 | 38.2 | 0.395 | 40.2 | 31.7 | 37.2 | 21.4 | 16.7 | 15.5 | 15 | 15 | 19 |
| 20% | 0.533 | 15.2 | 30.6 | 0.414 | 37.1 | 29.2 | 31.0 | 19.0 | 14.2 | 13.0 | 28 | 29 | 35 |
| 30% | 0.558 | 11.7 | 27.9 | 0.433 | 34.2 | 30.5 | 29.5 | 18.2 | 14.2 | 13.0 | 43 | 44 | 49 |
| 40% | 0.586 | 9.7 | 25.5 | 0.447 | 32.1 | 27.8 | 27.3 | 15.9 | 14.5 | 11.8 | 57 | 57 | 62 |
| 50% | 0.606 | 8.2 | 22.2 | 0.458 | 31.7 | 28.3 | 25.9 | 16.5 | 13.7 | 11.6 | 67 | 64 | 69 |
| 60% | 0.631 | 7.3 | 19.1 | 0.471 | 29.9 | 27.9 | 26.6 | 14.5 | 12.6 | 10.5 | 74 | 77 | 78 |
| 70% | 0.659 | 6.2 | 17.2 | 0.484 | 28.2 | 26.5 | 27.0 | 13.5 | 12.2 | 10.5 | 84 | 84 | 86 |
| 80% | 0.693 | 5.2 | 15.6 | 0.504 | 27.0 | 25.0 | 25.4 | 12.2 | 11.9 | 10.2 | 91 | 91 | 93 |
| 90% | 0.743 | 4.2 | 14.0 | 0.539 | 25.1 | 24.0 | 24.7 | 10.9 | 10.8 | 10.4 | 95 | 95 | 98 |
| 100% | 1.298 | 0.8 | 5.4 | 0.637 | 23.5 | 23.5 | 23.5 | 10.3 | 10.3 | 10.3 | 100 | 100 | 100 |
| 80+ years, N = 414 | |||||||||||||
| 0% | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10% | 0.472 | 27.6 | 45.7 | 0.377 | 61.5 | 31.6 | 39.5 | 41.5 | 22.0 | 24.4 | 12 | 13 | 16 |
| 20% | 0.499 | 21.0 | 37.1 | 0.395 | 43.6 | 28.6 | 33.3 | 24.4 | 18.3 | 17.1 | 26 | 23 | 30 |
| 30% | 0.529 | 16.7 | 33.9 | 0.409 | 41.4 | 28.0 | 36.1 | 25.2 | 17.1 | 18.5 | 37 | 38 | 43 |
| 40% | 0.551 | 14.2 | 31.9 | 0.420 | 39.9 | 32.1 | 35.2 | 25.3 | 17.7 | 17.6 | 48 | 52 | 56 |
| 50% | 0.577 | 12.2 | 29.3 | 0.432 | 38.6 | 34.2 | 36.0 | 23.8 | 19.4 | 18.4 | 59 | 61 | 67 |
| 60% | 0.608 | 11.0 | 25.1 | 0.449 | 35.3 | 34.5 | 34.3 | 21.4 | 19.8 | 18.5 | 71 | 69 | 76 |
| 70% | 0.633 | 9.7 | 22.3 | 0.461 | 33.5 | 35.7 | 33.1 | 19.4 | 19.0 | 18.0 | 85 | 81 | 84 |
| 80% | 0.670 | 8.5 | 21.0 | 0.482 | 31.3 | 32.9 | 30.3 | 18.4 | 18.4 | 16.1 | 93 | 93 | 90 |
| 90% | 0.721 | 7.4 | 19.4 | 0.507 | 29.6 | 30.3 | 29.7 | 16.7 | 16.7 | 15.9 | 98 | 98 | 95 |
| 100% | 1.074 | 4.5 | 14.5 | 0.677 | 28.5 | 28.5 | 28.5 | 15.5 | 15.5 | 15.5 | 100 | 100 | 100 |
BMD: bone mineral density; DXA FN: femoral neck BMD by DXA; DXR: BMD by digital X-ray radiogrammetry; MOF: major osteoporotic fracture (clinical spine, hip, forearm or shoulder fracture)
4. Discussion
This prospective study is the first to directly compare the performance of clinical risk factors (FRAX) and automated DXR in identifying patients with osteoporosis and those at increased risk for fracture. In this population-based cohort of older community-dwelling women, fracture prediction was similar between the two methods, while the DXR method had substantially higher sensitivity than FRAX to discern those with femoral neck osteoporosis.
Our results are in general agreement with previous published studies that femoral neck DXA BMD is a stronger predictor of hip fractures than clinical risk factors alone [26–28] or other BMD sites including DXR-BMD [13–15,29], whereas non-hip major osteoporotic fractures are predicted to a similar degree by other BMD sites including lumbar spine DXA BMD and DXR-BMD. Our results are also in agreement with expectations based on previous studies individually using FRAX [26,30] or DXR-BMD [16,17] to select high risk individuals for confirmation with hip DXA.
Combining clinical risk factors and DXR-BMD increased discriminatory performance relative to fractures compared to using either method alone. The combined fracture prediction performance was also similar to that of DXR-BMD + prior fracture, which indicates that in a post-fracture workflow, adding the complexity of collecting additional clinical risk factors might add only marginally to discrimination performance.
Although area under receiver operating characteristic curves is a widely used method for relative comparison of different screening methods, they are not straightforward to use for threshold selection or as input for cost-effect comparisons in a two-step screening process. Table 3 and supplement table 4 are provided to serve as input when estimating screening performance at equal selection rates with different selection methods, to serve as input for threshold selection and as to serve as input for various cost-effect calculations. For example, a selection threshold for referral to central DXA of 15% FRAX MOF risk (current national guideline in Sweden [31]) would, looking at the threshold values, apply to approximately 40% of women in ages 65–69. To yield a similar portion of women when DXR-BMD is used for screening selection, a threshold of 0.490 g/cm2 would be required. With those thresholds, DXR-BMD captured 70% of all women in the population ages 65–69 that had osteoporosis while a FRAX hip based strategy captured 62% and the FRAX MOF based strategy captured 57%. At the same time the percentage of women who suffered incident fractures was similar between each of the selected populations. However, table 3 applies for the unselected general population. In practice, there will always be multiple pathways to an osteoporosis assessment (fracture liaison services, indications related to specific drugs and diseases) that interact with FRAX and DXR-BMD to different degrees. The population that can be considered for screening is the survival population. Thus, if a substantial portion of the at-risk population is referred to osteoporosis assessment through other pathways, care must be taken to properly estimate the characteristics of the survival population. In addition to table 3 and supplement table 4, all source data in this study is available for download through the SOF website [25].
Overall, the older age groups showed a similar pattern as ages 65–69. The difference between the methods was further pronounced in the 70–74 age group and smaller in the oldest age groups, 75–79 and 80+, where the prevalence of osteoporosis was higher.
The tables were constructed with 5-year-agespans to have sufficient number of fractures and cases. However, the fracture incidence rate is higher at the high end of the age span than at the low end. Table 3 applies for the unselected general population and supplement table 4 applies for the subpopulation of only individuals with a prior fracture. The former is intended for guidance in general age-based population screening and the later in screening of only people with a prior fracture, and by approximation, guidance in post-fracture workflows. Further guidance and examples how table 3 and supplement table 4 can be used are presented in the online supplement.
At a technical availability rate of DXR-BMD of 99.73%, the management burden of individuals without a measurement should be minor in relation the entire screening program. The false automatic validation rate was 0.02%. Thus, relying on solely the automatic validation would not have affected the fracture prediction performance or the osteoporosis prediction performance of DXR-BMD.
This study has a number of strengths, including the size of the cohort, the comprehensive set of measurements and the duration and completeness of follow-up. The study also has limitations, with a cohort consisting of only Caucasian women over 65 and lacking data for men and younger women. Another limitation is that besides rheumatoid arthritis, there are 6 specific conditions associated with secondary osteoporosis that compose an additional component in FRAX. Data on these 6 conditions were not collected in SOF. However these conditions are uncommon in healthy older women.
The recent expiration of patents for alendronic acid and zoledronic acid has made case finding a larger part of the total cost of osteoporosis management. Initiatives such as fracture liaison services and the UK NOGG guidelines [32] have aimed to lower the cost of case finding in order to increase the cared for population. Despite these efforts and the reduced cost for treatment, the uptake of anti-osteoporotic treatment has stagnated or even decreased in many countries [1].
In summary, DXR-BMD without additional clinical risk factors discriminated fractures to a similar degree as FRAX and as lumbar spine DXA BMD. DXR-BMD predicted femoral neck osteoporosis to a larger degree than FRAX. The current analysis provides input data for cost and performance comparisons between DXR-BMD and FRAX based single tier and two-tier screening with confirmation by central DXA, as well as with single tier screening by central DXA. The data indicate that in a healthcare setting where an individual is already at a digital X-ray machine, such as at mammography screening or after a fracture, automated DXR-BMD could be an efficient and effective method to flag patients who might benefit from an osteoporosis assessment. Results require confirmation in other studies.
Supplementary Material
Highlights.
A prospective population-based comparison of DXR, FRAX and DXA in 5385 women.
DXR-BMD and FRAX predicted fractures to a similar degree.
FN DXA BMD predicted hip fractures to a greater degree than DXR-BMD and FRAX.
DXR predicted osteoporosis by FN DXA to a substantially greater degree than FRAX.
Automated DXR shows promise as selection tool in specific opportunistic workflows.
Acknowledgments
This project received funding from Sectra AB, Linköping, Sweden. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Abbreviations
- AUC
area under curve
- BMD
bone mineral density
- DXA
dual energy X-ray absorptiometry
- DXR
digital X-ray radiogrammetry
- FN
femoral neck
- MOF
major osteoporotic fracture
- SD
standard deviation
- SOF
the Study of Osteoporotic Fractures
- VPA
volume per area
- WHO
world health organization
Investigators in the Study of Osteoporotic Fractures Research Group
San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): SR Cummings (principal investigator), DC Bauer (co-investigator), DM Black (co-investigator), W Browner (co-investigator), PM Cawthon (co-investigator), N Lane (co-investigator), MC Nevitt (co-investigator), C McCulloch (co-investigator), A Schwartz (co-investigator), KL Stone (co-investigator), G Tranah (co-investigator), K Yaffe (co-investigator), R Benard, T Blackwell, L Concepcion, D Evans, S Ewing, C Fox, R Fullman, SL Harrison, M Jaime-Chavez, D Kriesel, W Liu, L Lui, L Palermo, N Parimi, K Peters, M Rahorst, C Schambach, J Ziarno.
University of Maryland: MC Hochberg (principal investigator), R Nichols (clinic coordinator), S Link.
University of Minnesota: KE Ensrud (principal investigator), S Diem (co-investigator), M Homan (co-investigator), P Van Coevering (program coordinator), S Fillhouer (clinic director), N Nelson (clinic coordinator), K Moen (assistant program coordinator), K Jacobson, M Forseth, R Andrews, S Luthi, Atchison, L Penland-Miller.
University of Pittsburgh: JA Cauley (principal investigator), LH Kuller (co-principal investigator), JM Zmuda (co-investigator), L Harper (project director), L Buck (clinic coordinator), M Danielson (project administrator), D Cusick, A Flaugh, M Gorecki, C Newman.
The Kaiser Permanente Center for Health Research, Portland, Oregon: T Hillier (principal investigator), K Vesco (co-investigator), K Pedula (co-investigator), J Van Marter (project director), M Summer (clinic coordinator), A MacFarlane, J Rizzo, K Snider, J Wallace.
Footnotes
Author contributions:
Johan Kälvesten: Study concept and design, acquisition of data, interpretation of data, preparation of manuscript.
Li-Yung Lui: Study design, data analysis, interpretation of data, preparation of manuscript. Performed all statistical analyses and accept responsibility for the integrity of the data analysis.
Torkel B. Brismar: Study design, interpretation of data, preparation of manuscript.
Steven R. Cummings: Study concept and design, acquisition of data, interpretation of data, preparation of manuscript.
5.1. Conflicts of Interest
Johan Kälvesten is an employee of Sectra AB, Linköping, Sweden. Li-Yung Lui, Torkel B. Brismar and Steven R. Cummings declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker DJ, Yun H, Kilgore ML, et al. Health services utilization after fractures: evidence from Medicare. J Gerontol A Biol Sci Med Sci. 2010 Sep;65(9):1012–20. doi: 10.1093/gerona/glq093. [DOI] [PubMed] [Google Scholar]
- 3.Zethraeus N, Borgström F, Ström O, Kanis JA, Jönsson B. Cost-effectiveness of the treatment and prevention of osteoporosis--a review of the literature and a reference model. Osteoporos Int. 2007 Jan;18(1):9–23. doi: 10.1007/s00198-006-0257-0. [DOI] [PubMed] [Google Scholar]
- 4.Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011 Mar;22(3):967–82. doi: 10.1007/s00198-010-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013 Oct-Dec;16(4):455–66. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Bates DW, Black DM, Cummings SR. Clinical use of bone densitometry: clinical applications. JAMA. 2002 Oct 16;288(15):1898–900. doi: 10.1001/jama.288.15.1898. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Laster A, Becker DJ, et al. The geographic availability and associated utilization of dual-energy X-ray absorptiometry (DXA) testing among older persons in the United States. Osteoporos Int. 2009 Sep;20(9):1553–61. doi: 10.1007/s00198-008-0821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hans DB, Kanis JA, Baim S, et al. FRAX(®) Position Development Conference Members. Joint Official Positions of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(®). Executive Summary of the 2010 Position Development Conference on Interpretation and use of FRAX® in clinical practice. J Clin Densitom. 2011 Jul-Sep;14(3):171–80. doi: 10.1016/j.jocd.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.FRAX® online tool. http://www.shef.ac.uk/FRAX.
- 10.Rosholm A, Hyldstrup L, Backsgaard L, Grunkin M, Thodberg HH. Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int. 2001;12(11):961–9. doi: 10.1007/s001980170026. [DOI] [PubMed] [Google Scholar]
- 11.Wilczek ML, Nielsen C, Kälvesten J, Algulin J, Brismar TB. Mammography and Osteoporosis Screening-Clinical Risk Factors and Their Association With Digital X-Ray Radiogrammetry Bone Mineral Density. J Clin Densitom. 2015 Jan-Mar;18(1):22–9. doi: 10.1016/j.jocd.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Wilczek ML, Kälvesten J, Algulin J, Beiki O, Brismar TB. Digital X-ray radiogrammetry of hand or wrist radiographs can predict hip fracture risk--a study in 5,420 women and 2,837 men. Eur Radiol. 2013 May;23(5):1383–91. doi: 10.1007/s00330-012-2706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002 May;13(5):358–65. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 14.Bach-Mortensen P, Hyldstrup L, Appleyard M, Hindsø K, Gebuhr P, Sonne-Holm S. Digital x-ray radiogrammetry identifies women at risk of osteoporotic fracture: results from a prospective study. Calcif Tissue Int. 2006 Jul;79(1):1–6. doi: 10.1007/s00223-005-0260-z. [DOI] [PubMed] [Google Scholar]
- 15.Vasireddy S. Dissertation. University of Sheffield; 2010. Metacarpal Radiographic Indices in the assessment of bone strength and fracture risk. [Google Scholar]
- 16.Boonen S, Nijs J, Borghs H, Peeters H, Vanderschueren D, Luyten FP. Identifying postmenopausal women with osteoporosis by calcaneal ultrasound, metacarpal digital X-ray radiogrammetry and phalangeal radiographic absorptiometry: a comparative study. Osteoporos Int. 2005 Jan;16(1):93–100. doi: 10.1007/s00198-004-1660-z. [DOI] [PubMed] [Google Scholar]
- 17.Dhainaut A, Rohde GE, Syversen U, Johnsen V, Haugeberg G. The ability of hand digital X-ray radiogrammetry to identify middle-aged and elderly women with reduced bone density, as assessed by femoral neck dual-energy X-ray absorptiometry. J Clin Densitom. 2010 Oct-Dec;13(4):418–25. doi: 10.1016/j.jocd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990 Feb 2;263(5):665–8. [PubMed] [Google Scholar]
- 19.Black DM, Palermo L, Sørensen T, et al. A normative reference database study for Pronosco X-posure System. J Clin Densitom. 2001;4(1):5–12. doi: 10.1385/jcd:4:1:05. [DOI] [PubMed] [Google Scholar]
- 20.Barnett E, Nordin BE. The radiological diagnosis of osteoporosis: a new approach. Clin Radiol. 1960;11:166–74. doi: 10.1016/s0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- 21.Laval-Jeantet AM, Bergot C, Carroll R, Garcia-Schaefer F. Cortical bone senescence and mineral bone density of the humerus. Calcif Tissue Int. 1983;35:268–72. doi: 10.1007/BF02405044. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen JT, Andersen PB, Rosholm A, Bjarnason NH. Digital X-ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Physiol. 2000;20:330–5. doi: 10.1046/j.1365-2281.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoff M, Haugeberg G, Kvien TK. Hand bone loss as an outcome measure in established rheumatoid arthritis: 2-year observational study comparing cortical and total bone loss. Arthritis Res Ther. 2007;9(4):R81. doi: 10.1186/ar2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008 Jul;248(1):254–63. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 25.Study of Osteoprotic Fractures Online. University of California; San Fransisco: [Accessed 16 December 2015]. http://sof.ucsf.edu/ [Google Scholar]
- 26.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007 Aug;18(8):1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 27.Ensrud KE, Lui LY, Taylor BC, et al. Study of Osteoporotic Fractures Research Group. A comparison of prediction models for fractures in older women: is more better? Arch Intern Med. 2009 Dec 14;169(22):2087–94. doi: 10.1001/archinternmed.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005 Jul;20(7):1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 29.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993 Jan 9;341(8837):72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 30.Short CE, Shaw SG, Fisher MJ, Gilleece YC, Walker-Bone K. Comparison of peripheral forearm DXA and clinical risk factor screening using FRAX® to assess the risk of HIV-associated low bone mass: a cross-sectional study. Arch Osteoporos. 2014;9(1):181. doi: 10.1007/s11657-014-0181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Board of Health and Welfare. Nationella riktlinjer för rörelseorganens sjukdomar 2012. National Board of Health and Welfare; Stockholm, Sweden: 2012. p. 24. [Google Scholar]
- 32.Compston J, Cooper A, Cooper C, et al. National Osteoporosis Guideline Group (NOGG) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009 Feb 20;62(2):105–8. doi: 10.1016/j.maturitas.2008.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.