Abstract
Various cognitive differences have been reported between consistent and weak handers (Prichard, Propper, & Christman, 2013), but little is known about the neurobiological factors that may be associated with this distinction. The current study examined cortical structural lateralization and corpus callosum volume in a large, well-matched sample of young adults (N = 164) to explore potential neurostructural bases for this hand group difference. The groups did not differ in corpus callosum volume. However, at the global hemispheric level, weak handers had reduced or absent asymmetries for gray and white matter volume, cortical surface area, thickness, and local gyrification, relative to consistent handers. Group differences were also observed for some regional hemispheric asymmetries, the most prominent of which was reduced or absent gyrification asymmetry for weak handers in a large region surrounding the central sulcus and extending into parietal association cortex. The findings imply that variations in handedness strength are associated with differences in structural lateralization, not only in somatomotor regions, but also in areas associated with high level cognitive control of action.
Keywords: handedness, corpus callosum, cortical thickness, gyrification, structural asymmetry
Handedness, its expression, measurement, development, and cognitive correlates, was an abiding interest of M.P. “Phil” Bryden. It was considered in some of his earliest work (Bryden, 1964, 1965) as well as in one of his final papers (Steenhuis & Bryden, 1999). Although much of this work investigated the direction of handedness (left- vs right-handers), Bryden also drew our attention to the importance of the degree of handedness (e.g., Bryden & Steenhuis, 1991; McManus & Bryden, 1992). More recently, genetic studies have identified polymorphisms associated with degree, but not, direction of handedness (Arning et al., 2013). In addition, evidence has been accumulating that groups defined by the strength or degree of handedness also vary in cognitive functioning. Many studies have used questionnaires to dichotomize hand preference, contrasting those with strong, consistent preference for one hand to those with weak or inconsistent hand preferences1 (e.g., Christman et al., 2004; Jasper & Christman, 2005; Sontam, Christman, & Jasper, 2009). Such studies have uncovered a broad array of tasks on which these groups differ, and it appears that the group differences in behavior reflect variations in hand preference rather than performance (Badzakova-Trajkov, Haberling, & Corballis, 2011; Grimshaw, Yelle, Schloger, & Bright, 2008). In a recent review of this work, Prichard et al. (2013) describe an advantage for weak handers over consistent handers across numerous domains: activation of ambiguous words, free recall of words, divergent creativity, number of episodic memories recalled, counterfactual production, belief in evolution over creationism, among others. To our knowledge, no underlying cognitive process has been offered to account for the hand group differences across these disparate domains.
Instead, the fundamental explanatory mechanism, according to Christman and colleagues, is the extent of interhemispheric interaction. It is posited that mixed/weak handers rely on greater crosstalk across hemispheres, and increased access to right hemisphere processes, than do consistent/strong handers (Prichard et al., 2013). Surprisingly, very little neurobiological evidence has been gathered to support this conjecture (but see Davidson & Tremblay, 2013). Instead, degree of handedness is used as a proxy variable for the extent of hemispheric interaction (Lyle & Orsborn, 2011; Sontam & Christman, 2012; Sontam et al., 2009) – that is, such handedness differences are taken as the evidence of variations in interhemispheric interaction. While hemispheric interaction is not an unreasonable hypothesis, it is certainly possible that other neurobiological differences could be associated with the handedness distinction. In particular, degree of cortical lateralization might be expected to vary across these hand groups. Since handedness is itself one overt expression of degree of lateral asymmetry, one could conjecture that those with weaker hand preference might have fewer or smaller functional or structural asymmetries. Some prior findings document reduced functional asymmetry for individuals with weaker hand preference (Bourne, 2008; Dassonville, P., Zhu, X-H., Ugurbil, K., Kim, S-G., & Ashe, J., 1997; Khedr, Hamed, Said, & Basahi, 2002). In addition, variations in the degree of cerebral asymmetry need not necessarily imply differences in hemispheric interaction (or vice versa). If weaker lateralization implies some duplication of function across hemispheres (i.e., redundancy rather than sharing of function - see Bernal & Ardila, 2014), then this need not imply a greater need for crosstalk across hemispheres. Alternatively, if some interhemispheric interaction is inhibitory (see Chiarello & Maxfield, 1996), then increased interhemispheric “interaction” could be associated with greater lateralization of function. Hence, when exploring the bases of handedness differences, it may be useful to seek separate correlates of hemispheric interaction and degree of lateralization, rather than assuming any particular relation between them.
Only a few previous studies have explored neurostructural correlates of the consistent/weak hand dichotomy. In prior studies we have observed that weak and consistent handers do not differ in manual measurements of corpus callosum area (Welcome et al., 2009), in manual asymmetry measurements of the length of planum temporale or Heschl’s gyrus, (Leonard et al., 2009), or in automated measures of surface area asymmetry of perisylvian language-relevant cortex (Chiarello et al., 2013). Another study involving manual measurements reported reduced planum temporale and posterior ascending ramus asymmetries for mixed/weak handers relative to consistent (right) handers (Foundas, Leonard, & Hanna-Pladdy, 2002). Luders et al. (2010), employing automated measurements, found a negative correlation between the degree (absolute value) of hand preference and the thickness of the anterior and posterior midbody of the corpus callosum (weaker hand preference associated with increased callosal thickness). Propper et al. (2010) reported larger left, than right, hemisphere volume of the arcuate fasciculus for consistent right handers, but no asymmetry for inconsistent/weak handers, in a very small sample (10 or fewer participants per group) that utilized diffusion tensor imaging (DTI). These studies do not present a consistent picture of structural associations related to degree of handedness, and each study investigated only a small number of brain regions. No prior study has examined both the corpus callosum and asymmetries across the entire cortex, including measures of cortical thickness, surface area, or extent of gyrification. Each of these morphometric indices has differing neurodevelopmental trajectories and genetic bases (Panizzon et al., 2009; Raznahan et al., 2011), and hence may potentially reveal unique neural signatures of strength/consistency of hand preference.
Although rarely commented on in the current literature, dichotomous comparisons of weak and consistent handers generally involve a confound with sex. As noted by Bryden and others (Tapley & Bryden, 1985; Lai et al., 2014; Papadatou-Patou et al., 2008; Prichard et al., 2013) somewhat more women than men have strong (right) hand preference, while males are somewhat more numerous among weaker (and/or left) handers. For example, among participants in the Biological Substrates for Language Project in our lab (see Chiarello et al., 2009a,b; Welcome et al., 2009), 59% of the females, but only 44% of the males, were consistent handers. Hence, one cannot exclude sex differences as contributors to behavioral differences reported for the hand groups. One approach is to examine the four sex X hand groups (e.g., Welcome et al., 2009). In the current study we examined weak and consistent hand groups that were balanced by sex, to better highlight potential brain differences associated with strength/consistency of handedness.
In the current paper we investigate potential neurostructural correlates of handedness strength/consistency dichotomy using automated measures provided by the FreeSurfer processing pipeline (Dale, Fischl, & Sereno, 1999). We examine plausible structural correlates of both interhemispheric interaction and lateralization: corpus callosum volume (total and subdivisions) and a variety of cortical structure measurements (cortical surface area, thickness, gyrification) for which asymmetries can be measured. For each cortical measure we examined: (1) average values for each hemisphere and hemisphere-wide asymmetries (global measures), and (2) asymmetries within each of 68 regional parcellations (regional measures). If weak handers have increased interhemispheric interaction and/or reduced asymmetries, one might expect some structural differences to be observed (larger callosum and/or reduced cortical asymmetries). Although the callosal measures are theoretically motivated by the increased interhemispheric interaction proposal, because of the broad array of cognitive differences observed between the hand groups (Prichard et al., 2013), it is difficult to predict which specific cortical regions might differ across groups. For this reason, we consider the regional comparisons to be exploratory.
METHOD
Participants
From a larger sample of 200 college students (Chiarello et al., 2009a,b), we selected 82 consistent handers (41 female) and 82 weak handers (41 female). All participants were native English speakers with normal or corrected-to-normal vision. Hand group was determined via scores on the 5-item Bryden (1977, 1982) hand preference questionnaire that yields an index ranging from +1.00 (extreme right handedness) to −1.00 (extreme left-handedness)2. We considered consistent handers to be those who scored either −1.0 or +1.0 on the hand preference questionnaire. This division categorizes groups in a similar manner to that used by Christman and colleagues (see footnote 2 in Chiarello et al., 2013). These individuals reported no use of the nondominant hand for any activity, and four of them (2 male, 2 female) were consistent left-handers. Weak handers’ mean handedness score was .48 (range −.9 to +.9; mean of absolute value = .68). A 5-point scale was used to assess parental education; 5 represented the highest level (post-graduate or professional degree). Scores were averaged across parents to yield the estimate of socio-economic status (SES). The consistent and weak hand groups did not differ by age or parental education (t < 1). The hand groups were also similar in full scale [t(162) = −1.64, p = .10] and performance [t(162) = −1.0] IQ (Wechsler, 1999). However, weak handers had slightly higher verbal IQs, although this group difference was nonsignificant, t(162) = −1.85, p = .07. The participants also received three subtests of the Woodcock Reading Mastery Test – Revised (Woodcock, 1998) – word attack, word identification, and passage comprehension. There were no group differences on any subtest (all p-values > .25). Table 1 contains the means, by group, for these demographic variables, and indicates that the two hand groups were quite well matched.
Table 1.
Demographic and Psychometric Data for the Consistent and Weak Handedness Groups.
| Consistent Handers (N = 82) |
Weak Handers (N = 82) |
|
|---|---|---|
| Mean Age (years) | 21.4 | 21.4 |
| Number Female | 41 | 41 |
| Mean Parental Education (SES) | 3.35 | 3.33 |
| Mean Full Scale IQ | 108.6 | 111.3 |
| Mean Performance IQ | 107.3 | 110.6 |
| Mean Verbal IQ | 107.8 | 109.6 |
| Mean Word Attack (scaled score) | 100.1 | 98.4 |
| Mean Word Identification (scaled score) | 99.8 | 100.3 |
| Mean Passage Comprehension (scaled score) | 108.2 | 108.2 |
Brain Imaging Procedure
Two MRI scans were obtained for each participant on a 1.5-T GE Signa scanner (3-D SPGR, 1.2 mm thick sagittal images, TR 11 ms, TE 2.2 ms, flip angle 25°, field of view 24 cm, acquisition matrix 256 × 256, acquisition time 4.36 min). Cortical reconstruction and volumetric segmentation was performed using the FreeSurfer v 4.5 analysis suite (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999) which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Briefly, processing includes motion correction and coregistration of T1 weighted images, removal of non-brain tissue, automated Talairach transformation, segmentation of deep grey and subcortical white matter volumetric structures, intensity normalization, tessellation of gray and white matter boundaries, automated topology correction, and surface deformation after intensity gradients optimally identify boundaries based on greatest intensity shifts. Manual inspection of the gray/white segmentation for all 328 hemispheres was performed.
A variety of surface based data representations were created using both intensity and continuity information from the entire three-dimensional MR volume. Surface area for an entire hemisphere (global measure) is calculated by adding the surface area of all faces of the triangulated mesh. Global hemispheric measures for thickness and local gyrification (see below) are obtained by averaging the value of each vertex within a hemisphere. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006; Reuter et al., 2012).
Cortical surface area (pial area), and thickness values were automatically extracted for left and right hemispheres by the FreeSurfer software. A 3D local gyrification index (LGI) was computed using the procedures outlined by Schaer et al. (2008). This calculation essentially divides the amount of pial surface by the amount of cortex on a closely fitting outer contour of the brain (hull surface). Computation starts at a given vertex of the tessellated surface and calculates within a given sphere of designated size (25 mm radius) the amount of surface area on the outer hull compared to the total amount of pial surface area. This approach is similar to the 2-D Zilles method of measuring the inner and outer contour of the cortex (Zilles, Armstrong, Schleicher, & Kretschmann, 1988). Larger LGI values indicate greater cortical folding within the radius. LGI for each cortical parcellation is estimated by averaging across all vertices within that parcellation.
During processing, surface images are produced and mapped onto an averaged surface for each hemisphere. These surfaces are used to parcellate the cerebral cortex into units based on gyral and sulcal structure, using the atlas of Destrieux, Fischl, Dale, & Halgren (2010). These parcellations utilize standard anatomical conventions and generally correspond to accepted anatomical/functional units. We examined 68 cortical parcellations that had acceptable concordances with manual measurements (Destrieux et al., 2010). The individual surfaces are nonlinearly warped back into individual subject space prior to analyses (Destrieux et al., 2010). Asymmetries for each parcellation were calculated by subtracting the right measure from the left and dividing by the average, so that leftward asymmetries yielded positive coefficients. Intracranial volume values from FreeSurfer were also extracted to be used as covariates in some analyses.
Corpus callosum volume and segmentation was obtained from the FreeSurfer volume stream (aseg) that divides the callosum into five segments of equal length along the primary eigendirection (long axis) of this structure: anterior, mid-anterior, central, mid-posterior, posterior (Rosas et al., 2010). Measurements were made for a 5 mm lateral extent centered on the mid-sagittal plane. Callosum volume was regressed against total intracranial volume and the residuals were used to obtain callosum volume estimates unbiased by overall brain size. These residuals were used in all statistical analyses involving corpus callosum volume.
RESULTS
Global Brain Measurements
There were no group differences for within hemisphere measurements of total gray volume, total white volume, surface area, thickness, or gyrification (see Supplementary Table 1, all group contrast t’s ≤ 1). Table 2 provides the means, by group, for intracranial volume and corpus callosum area. As indicated by the t-test results in Table 2, the hand groups did not differ in overall brain size nor in any corpus callosum volume measurement. In order to more precisely compare our corpus callosum results to those of Luders et al. (2010), we also computed correlations between the absolute value of the hand preference score and the corpus callosum measurements. No significant correlations were obtained for total callosal volume, nor for any callosal subregion (all r’s < .10).
Table 2.
Mean Values of Intracranial Volume and Corpus Callosum Volume (Total and Subregion) for Consistent and Weak Handers and t-Test of the Group Difference
| Consistent Handers | Weak Handers | t(162) | |
|---|---|---|---|
| Intracranial Volume (mm3) | 1,565,708 | 1,585,299 | −0.69 |
| Total Corpus Callosum (mm3) | 3130 | 3165 | −0.52 |
| Anterior | 839.1 | 848.3 | −0.41 |
| Mid-Anterior | 465.5 | 472.2 | −0.49 |
| Central | 480.5 | 485.2 | −0.30 |
| Mid-Posterior | 446.8 | 445.1 | 0.13 |
| Posterior | 897.6 | 913.8 | −0.75 |
Table 3 includes the global asymmetry means by group, and relevant statistics. In these and further analyses, we report findings from (1) signed asymmetries to test whether there are group differences when the direction of asymmetry is preserved; (2) absolute value of asymmetries to test whether the magnitude of asymmetry differs, regardless of direction. Univariate tests were used to determine whether the reported asymmetries were significantly different from zero for each group. Several group differences in left/right asymmetry were evident (see Table 3). With only one exception, all mean asymmetries were significant. However, in every case, asymmetries were larger for consistent, than for weak, handers. For signed asymmetries, larger rightward asymmetries were observed for consistent handers for both gray matter volume and cortical thickness. When examining absolute values, consistent handers had larger asymmetries for white matter volume, cortical surface area, and gyrification. These data imply some reduction in overall structural asymmetry for weak, relative to consistent, handers. However, for some cortical indices the direction of asymmetry is relevant, while for others the magnitude of asymmetry, independent of direction, is associated with the hand group difference.
Table 3.
Mean Values for the Global Asymmetry Comparisons of Brain Structure for Consistent and Weak Handers and t-Test of the Group Difference (significant asymmetries in bold)
| Consistent Handers | Weak Handers | t(162) | p | |
|---|---|---|---|---|
| Signed Asymmetries | ||||
| Gray volume | −.010 | −.004 | −2.75 | .007 |
| White Volume | −.008 | −.006 | −1.11 | .27 |
| Surface Area | −.003 | −.0002 | −1.50 | .13 |
| Thickness | −.013 | −.009 | −2.18 | .03 |
| Local Gyrification | .009 | .004 | 1.68 | .10 |
| Absolute Value Asymmetries | ||||
| Gray volume | .013 | .011 | 0.93 | .35 |
| White Volume | .013 | .011 | 1.97 | .05 |
| Surface Area | .011 | .008 | 3.25 | .001 |
| Thickness | .015 | .013 | 1.64 | .10 |
| Local Gyrification | .018 | .013 | 2.45 | .02 |
Because our group of weak handers contained more left handed individuals (N = 14) than the consistent hander group (N = 4), one might question whether the current findings truly indicate differences in degree of handedness. To examine this possibility, we dropped all left-handed participants (both consistent and weak handers) from the sample and recalculated the t-tests of the group differences in global asymmetries. As indicated in Supplementary Table 2, the findings were similar to those of the entire sample – weak right handers had smaller global asymmetries than consistent right handers.
We also examined correlations between total corpus callosum volume and the global cortical asymmetries. For signed asymmetries, surface area asymmetries negatively correlated with corpus callosum volume (i.e., greater callosal volume associated with reduced leftward/increased rightward asymmetry), for weak handers, r = −.217, p < .05, but not for consistent handers, r = −.087. No correlations were observed for absolute value asymmetries for either hand group.
To summarize, there was no evidence for group differences in callosum volume measurements, nor for within hemisphere measurements of cortical anatomy when considering global hemisphere-wide indices. However, global asymmetries were reduced for weak, as compared to consistent, handers.
Regional Brain Measurements
Our exploratory analyses used a significance value of .01 (uncorrected) to identify potential regions with differing asymmetries for consistent and weak handers. This cut-off value was selected to reduce the probability of truly spurious findings, while still permitting leeway for exploration.
For surface area asymmetry only one isolated parcellation on the dorsolateral surface of the occipital lobe (superior and transverse occipital sulci) differed by hand group3, t(162) = −2.51, p = .01 (see Supplementary Figure 1). Reliable rightward asymmetries were found for both consistent (−.160) and weak (−.241) handers but the asymmetry was enhanced in the weak hand group. Absolute value asymmetry did not differ between groups for this region. However, the horizontal ramus of the anterior lateral fissure did differ across groups in absolute value asymmetry, t(162) = 2.59, p = .01 with larger asymmetry for consistent than weak handers (see Supplementary Table 3).
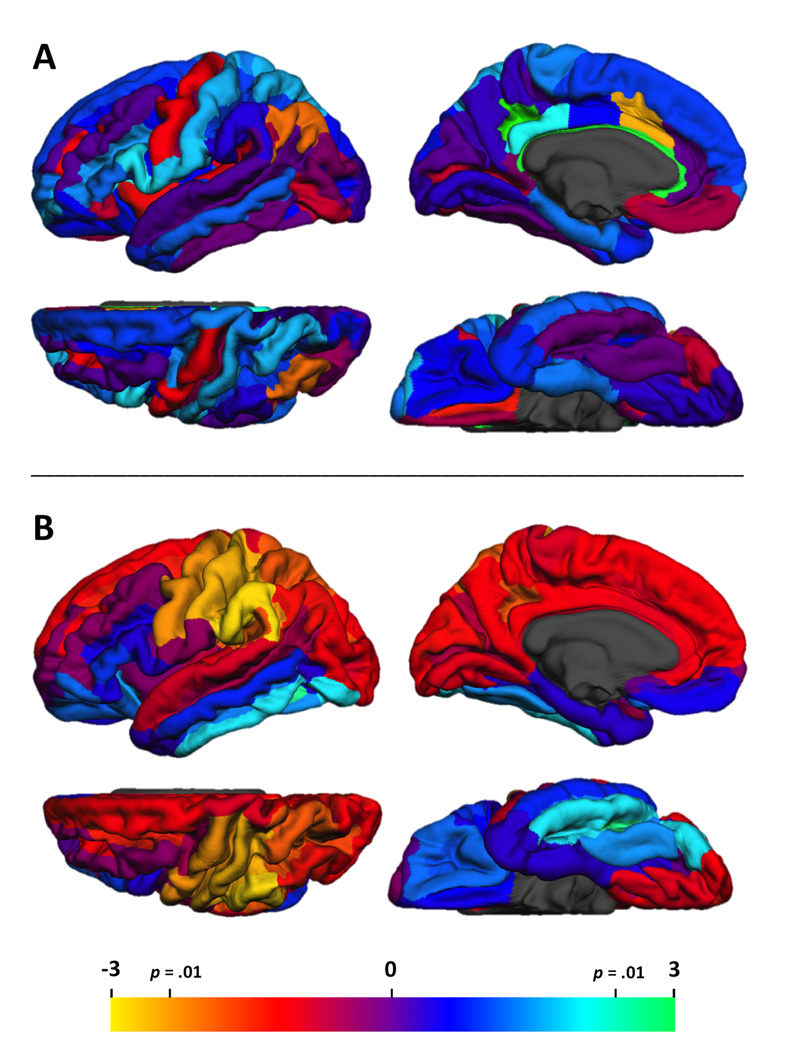
Figure 1 graphically displays the t-contrasts between consistent and weak handers regional signed asymmetries for cortical thickness (A), and local gyrification (B). For cortical thickness signed asymmetry, three regions on the medial surface differed by hand group: midanterior cingulate gyrus/sulcus, t(162) = −2.58, p = .01, pericallosal sulcus, t(162) = 3.1, p < .005, subparietal sulcus, t(162) = 3.61, p < .001. (see Figure 1A)3. Consistent handers had no reliable asymmetries in any of these regions (see Table 4, upper panel). Weak handers, in contrast, had reliable leftward asymmetries for the pericallosal and subparietal regions, and rightward asymmetry for the midanterior cingulate. A 3 (region) X 2 (hemisphere) X 2 (hand group) ANOVA was next conducted to examine whether the differing asymmetries could be attributed to hand group differences in left or right cortical thickness. The 3-way interaction was significant, F(2, 324) = 11.85, p < .0001, and was explored further via separate 2 × 2 analyses for each hemisphere. The right hemisphere analysis indicated that consistent handers had thicker cortex than weak handers across the 3-regions, F(1,162) = 9.51, p < .005 (see Table 5, upper panel). The hand group effects in the left hemisphere varied by region, F(2,324) = 7.85, p < .001.
Figure 1.
Statistical map of the t-test contrast of hand group differences in signed asymmetry for cortical thickness (A) and local gyrification index (B). Hot colors indicate a greater leftward asymmetry for consistent, relative to weak, handers. Findings are projected onto the pial surface of the FreeSurfer average brain.
Table 4.
Regional Signed Asymmetry Means for Cortical Thickness and Local Gyrification (significant asymmetries in bold) Which Differ by Hand Group
| Consistent Handers | Weak Handers | |
|---|---|---|
| Cortical Thickness | ||
| Midanterior Cingulate Gyrus/Sulcus | −.007 | −.034 |
| Pericallosal Sulcus | .013 | .071 |
| Subparietal Sulcus | −.014 | .033 |
| Local Gyrification Index | ||
| Precentral Gyrus | .019 | .005 |
| Central Sulcus | .027 | .013 |
| Postcentral Gyrus | .020 | .006 |
| Postcentral Sulcus | .024 | .008 |
| Supramarginal Gyrus | .028 | .009 |
Table 5.
Mean Cortical Thickness and Local Gyrification Index by Hemisphere for Consistent and Weak Handers.
| Left Hemisphere | Right Hemisphere | |||
|---|---|---|---|---|
| Consistent | Weak | Consistent | Weak | |
| Cortical Thickness | ||||
| Mid-anterior cingulate gyrus/sulcus |
2.95 | 2.86 | 2.97 | 2.96 |
| Pericallosal Sulcus | 2.45 | 2.52 | 2.42 | 2.34 |
| Subparietal Sulcus | 2.44 | 2.44 | 2.47 | 2.36 |
| Local Gyrification Index | ||||
| Precentral Gyrus | 3.26 | 3.26 | 3.20 | 3.24 |
| Central Sulcus | 3.27 | 3.28 | 3.19 | 3.23 |
| Postcentral Gyrus | 3.27 | 3.27 | 3.20 | 3.25 |
| Postcentral Sulcus | 3.23 | 3.25 | 3.16 | 3.23 |
| Supramarginal Gyrus | 3.60 | 3.61 | 3.21 | 3.58 |
None of the hand group thickness asymmetry contrasts remained significant when absolute value asymmetries were examined. One additional region (superior occipital gyrus) differed across groups t(162) = 2.50, p = .01 for absolute value asymmetry, with larger asymmetry for consistent handers (see Supplementary Table 3).
As is evident in Figure 1B, asymmetry for local gyrification differed by hand group for several contiguous parcellations: postcentral gyrus, t(162) = −2.59, p = .01, postcentral sulcus, p = .01, t(162) = −2.58, and supramarginal gyrus, t(162) = −2.81, p < .01. Similar trends were obtained in two adjacent regions, precentral gyrus, t(162) = −2.38, p < .02, and central sulcus, t(162) = −2.4, p < .02. In each of these regions, consistent handers had reliable leftward asymmetries (i.e., greater folding in LH than RH), whereas weak handers had smaller leftward or no asymmetry (see Table 4, lower panel). To explore whether these asymmetry differences were more attributable to right or left hemisphere gyrification differences, we conducted a 5 (region) X 2 (hemisphere) X 2 (hand group) ANOVA on LGI. These analyses revealed a main effect of hemisphere F(1,162) = 42.21, p < .0001, which was modified by a Hand Group X Hemisphere interaction, F(1,162) = 9.86, p < .005. Follow-up analyses indicated that the reduction of asymmetry for weak handers was attributable to group differences in right hemisphere gyrification (see Table 5, lower panel). The hand groups did not differ in LH gyrification in these regions F < 1, but weak handers had significantly greater folding than consistent handers in the RH, F(1,162) = 5.95, p < .02. These effects did not differ over the 5 brain regions as indicated by the absence of a Region X Hemisphere X Hand interaction, F < 1. Finally, there was one region, the lateral occipital temporal sulcus, that had greater leftward gyrification asymmetry for weak, relative to consistent, handers, t(162) = 2.53, p = .01.
These findings indicate increased right hemisphere gyrification and reduced gyrification asymmetry for weak handers in a large region surrounding the central sulcus and extending posteriorly into the parietal cortex. Increased local gyrification can reflect deeper sulci and/or greater number of more superficial sulci. Some prior studies have reported leftward asymmetry for the depth of the central sulcus in right handers, but no asymmetry or rightward asymmetry of this feature for left handers (Amunts et al., 1996, 2000). Because our group of weak handers contained more left handed individuals than the consistent hander group, we dropped all left-handed participants (both consistent and weak handers) from the sample and recalculated the local gyrification asymmetry t-tests of the group differences. All group asymmetry contrasts remained significant (p’s < .01, see Supplementary Table 4, lower panel).
When absolute value asymmetries were examined for local gyrification only the central sulcus region continued to show a significant group difference, t(162) = 2.64, p < .01 (see Supplementary Table 3), with consistent handers having a larger asymmetry than weak handers. Additionally, absolute value asymmetry for the anterior transverse collateral sulcus on inferior temporal surface differed over groups, t(162) = −3.18, p < .01, with greater asymmetry for weak than for consistent handers.
To summarize the exploratory regional asymmetry analyses, there were two major findings. First, several regions on the medial cortical surface had greater thickness asymmetry for weak than for consistent handers. Second, when we examined local gyrification, a large cortical region surrounding the central sulcus and extending into the anterior inferior parietal cortex was considerably less asymmetrical for weak than for consistent handers. This effect was due to increased cortical folding for weak handers within right hemisphere regions.
DISCUSSION
Myriad behavioral differences have been reported between weak and consistent handers, but ideas about neurobiological differences have heretofore been primarily speculative. In the current study, we took a whole-brain approach to investigate potential neurostructural associations of these dichotomous hand preference groups, in a well-matched sample unconfounded by sex differences. Several findings were notable. First, there was no evidence for group differences in corpus callosum volume. Second, when examining global hemisphere asymmetries, weak handers had smaller asymmetries for all measures (total gray, total white, surface area, thickness, local gyrification). Finally, there was preliminary evidence for group differences in regional asymmetry for both cortical thickness and local gyrification. Because each of these effects remained significant when data from left handers were omitted, this suggests that the findings reflect group differences in degree of handedness even when unconfounded by differing numbers of left handed participants. We now consider the implications of each of these findings.
Increased interhemispheric interaction is frequently invoked to account for the broad array of cognitive differences between consistent and weak handers (Lyle & Orsborn, 2011; Sontam & Christman, 2012; Sontam et al., 2009). If cross-hemispheric exchange of information is indeed more frequent or efficient in those with weaker hand preference, then this conjecture could be supported by structural adaptations in the major fiber tract connecting the two hemispheres. To the extent that greater number of axons and/or larger or more myelinated axons in the corpus callosum mediate such increased interhemispheric interaction, and this could be associated with volumetric increases in callosal cross-sectional area. However, in the current large, well-matched sample we observed no hand group differences in total callosal volume, nor in the volume of any callosal subregion. Similar automated callosal measures have revealed differences between other subject groups (e.g., monolinguals vs bilinguals, see Vazquez et al., submitted), implying that our corpus callosum volume measurements are sensitive to some individual differences in behavior. Although negative results should always be treated with caution, the current investigation does not provide support for the view that the wide-ranging behavioral differences between weak and consistent handers should be attributed to increased interhemispheric interaction and increased access to right hemisphere processes (Prichard et al., 2013)4.
The increased hemispheric interaction hypothesis is clearly premised on the view that a major function of interhemispheric cross talk is to facilitate the transfer of information between hemispheres. However, it is also possible that the corpus callosum serves to allow one hemisphere to inhibit processing within the other (Chiarello & Maxfield, 1996). A recent transcranial magnetic stimululation (TMS) study found evidence for more rapid transcallosal inhibition of motor cortex for mixed/weak as compared to strong/consistent handers (Davidson & Tremblay, 2013). It is unclear whether such findings can be generalized to other, more cognitive domains, but if weak handers have increased transcallosal inhibition a potential structural correlate of this might still be greater callosal volume for this group, an effect we did not observe. Nevertheless, DTI measures such as fractional anisotropy could indicate differences in interhemispheric connectivity that are not revealed by volumetric approaches (Davis & Cabeza, 2015), and this should be explored in future investigations of consistent/weak handedness.
The current study did document reduced structural asymmetry for weak, relative to consistent, handers, when measuring across entire hemispheres (i.e., global asymmetries). For measures of gray matter volume and cortical thickness, the direction of asymmetry was important, with consistent handers having larger rightward asymmetries than weak handers. However, for white matter volume, surface area, and local gyrification, the hand groups differed in the size of the asymmetry regardless of direction – consistent handers obtaining larger hemisphere differences than weak handers. These data are consistent with the view that weaker hand preference may reflect reduced cortical asymmetry in a more general sense. However, the group differences in asymmetry, albeit statistically significant, were small. Nevertheless, the fact that the reduced asymmetry was replicated across 5 distinct measures of cortical structure implies that the differences may be a true reflection of variations in structural lateralization. If, as the split brain data suggest, the left and right hemispheres represent at least partially separable information processing systems, then slight lateral biases in cortical organization could have cascading effects on functional outcomes. It is a moot point as to whether these small structural asymmetry differences between consistent and weak handers could account for any of the varied behavioral associations (Prichard et al., 2013). The findings do indicate that weaker hand preference is associated with less neurostructural lateralization at the level of entire hemispheres.
Slight lateral biases at the whole hemisphere level may well conceal a variety of differing regional asymmetries. We therefore conducted exploratory analyses to investigate potential regional differences between consistent and weak handers. Several differences were observed. However, we will focus our discussion on the findings that were similar across several regions, to avoid interpreting what may well be sporadic, chance results.
Although 3 regions on medial cortical surface (pericallosal and subparietal sulci, mid-anterior cingulate) had greater cortical thickness asymmetry in weak, relative to consistent, handers, to our knowledge these regions are not part of a coordinated functional network. In addition, the direction of the increased asymmetry was not consistent across the areas. Hence, we do not assume these medial cortical thickness findings are related. There is little neuroimaging evidence on the functional significance of either the pericallosal or subparietal sulci (but see Richer et al., 1993). The mid-anterior cingulate may well be involved in cognitive control functions (see Shenhav, Botvinick, & Cohen, 2013), but we did not observe similar group differences in thickness asymmetry in other regions known to be involved in cognitive control (e.g., dorsolateral prefrontal cortex). However, the midanterior cingulate cortex has recently been identified as an important region in the cognitive control of movement (Hoffstaedter et al., 2013, 2014). This region was more robustly activated for intentional self-initiated hand movements5, relative to more reactive hand movements whose execution was triggered by an external stimulus (Hoffstaedter et al., 2013). The participants in that study were all right handed, with no information provided about degree of handedness. We found the midanterior cingulate region to have thicker right, than left, cortex only for weak handers. It will be important in future functional imaging research to perform similar motor control studies examining both degree and direction of handedness, and to obtain cortical thickness measures as well. The current findings suggest that strength of handedness may be associated with structural adaptations for regions important for cognitive control of action. In any case, the fact that the three medial regions showed increased asymmetry for weak handers underscores the point that the reduced global thickness asymmetry reported for this group (see Table 3) does not imply that every cortical region is more symmetrical for weak handers.
The most striking hand group difference at the regional level involved a large area extending posteriorly from the precentral gyrus to include the central sulcus, postcentral gyrus and sulcus and the supramarginal gyrus in the parietal lobe. Across these regions, weak handers had little or no asymmetry in local gyrification, whereas consistent handers demonstrated robust leftward asymmetries (i.e., greater gyrification in the left than the right hemisphere). These differing asymmetries were attributable to increased right hemisphere gyrification in the weak handed group. It is notable that there were no group differences in surface area asymmetry in these regions. Hence, the increased right hemisphere gyrification for weak handers does not reflect greater cortical surface, but rather increased local folding relative to surface area (i.e., more cortex buried in the sulci than on the outer cortical hull for weak handers).
Some prior research has documented anatomical differences in the central sulcus related to handedness. Measures of surface area in the hand area along the central sulcus demonstrated greater left than right area for consistent right handers, but no asymmetry for left handers (Foundas, Hong, Leonard, & Heilman, 1998). Amunts et al. (1996) reported deeper left than right central sulci for right handers, but no asymmetry for left handers. The same study obtained histological measures from the hand region of the precentral gyrus of 12 postmortem brains (handedness unknown, presumed to be mainly right handers) and found increased neuropil within the left hemisphere region (Amunts et al., 1996). The authors note that this would support greater intracortical connectivity within motor cortex of the preferred hand. A later study by this group measured the depth of the central sulcus in the hand region and replicated the leftward asymmetry for consistent right handers, but obtained a nonsignificant rightward asymmetry for consistent left handers and no asymmetry for mixed handers (Amunts et al., 2000). Increased local gyrification using the measure employed in the current study reflects the relative extent of buried cortex that could be due to deeper sulci and/or a greater number of more superficial sulci. Our finding of asymmetry differences between consistent and weak handers was obtained even among right handers, suggesting that the gyrification asymmetry is associated with strength of hand preference. Most importantly, our findings indicate that these gyrification differences are not restricted to one region of the central sulcus, but extend posteriorly over a large area from the to include parietal association cortex.
It is perhaps not surprising to find neurostructural differences based on handedness strength in sensorimotor regions. However, the same gyrification difference was obtained in the adjacent supramarginal gyrus, with a similar trend in the superior parietal cortex (see Figure 1B). DTI data indicate that the supramarginal gyrus is densely connected to the pre- and post-central gyri, as well as the inferior frontal and lateral temporal regions (Ruschel et al., 2014). Functional imaging, TMS, and voxel lesion studies implicate the supramarginal gyrus and/or postcentral gyrus in a variety of cognitive functions. This parietal region is important for phonological processing during object naming and reading (Schwartz, Faseyitan, Kim, & Coslett, 2012; Hartwigsen et al., 2010; Sliwinska et al., 2012), and speech reading (Chu et al., 2013). In addition, this region is implicated in knowledge of object functions (Leshinskaya & Caramazza, 2015), tool use (Brandi, Wohlschälger, Sorg, & Hersdörfer, 2014; Vingerhoets, 2008), and visual object decoding (Smith & Goodale, 2015). These functions do not appear to overlap with the various behavioral differences found between consistent and mixed handers (Prichard et al., 2013). However, phonology and functional knowledge of object and tool use both involve high level, relatively abstract processing of information that may have its basis in somato-motor systems. It will be important to determine whether the more bilateral gyrification pattern we observed for weak handers in these areas is associated with variations in higher level “action” functions.
Because the group differences in gyrification could not be attributed to differences in surface area, we should consider the neurobiological factors that could produce cortical folding. Currently, both gray and white matter theories have been proposed. One theory argues that differential tangential surface expansion (faster growth of one region relative to an adjacent one) causes the cortex to fold (Xu et al., 2010; Ronan et al., 2014). Another theory argues that mechanical tension produced by growth of axons acts to pull together strongly interconnected regions, producing cortical folds and thereby reducing wiring length (Van Essen, 1997). An additional white matter theory posits that developing connections in some cortical regions “push” outward in a tangential direction to form gyri (Chen et al., 2013). A recent review notes that gyrification is likely caused by the interaction of cell generation processes and evolving fiber tract connectivity (Zilles, Palomero-Gallagher, & Amunts, 2013). It should also be noted that, although major sulci are present at birth, gyrification continues to increase at least until 2 years of age, and is developmentally distinct from expansion of surface area (Li et al., 2014). This suggests that connectivity differences may play an important role in individual differences in gyrification.
How then to interpret the increased right hemisphere gyrification among weak handers in somatomotor and anterior parietal cortex? One speculative interpretation derives from a recent structural and functional connectivity study that investigated connectivity differences between gyral and sulcal cortex (Deng et al., 2014). This study found that relative to sulci, gyri had greater connectivity to distant cortical regions; connections within sulcal regions tended to be more local with neighboring areas. The study only compared cortex in major sulci to that at the crown of the gyri. However, if the Deng et al. (2014) connectivity results can be extended to more superficial sulci (such as those that occur within gyri), then the current findings could suggest that weak handers have increased local connectivity within the right hemisphere somatomotor/anterior parietal cortex, as compared to consistent handers. Perhaps this local connectivity is more asymmetrical in consistent handers promoting greater reliance on the dominant hand, whereas the more bilateral local connectivity among weak handers is associated with less pronounced hand preference. However, since the same gyrification differences were observed in adjacent association cortex, the hypothesized differences in connectivity could extend to functions less obviously related to hand dominance.
Before concluding, it is important to acknowledge the limitations of the current study. First, our regional analyses were exploratory and should be replicated in a new sample. Second, in order to examine whether there were any neurostructural differences between groups for whom many behavioral difference had been found (Prichard et al., 2013), we adopted a dichotomous approach to handedness. However, strength of hand preference can also be conceptualized as a continuum and a less categorical approach could yield additional insights about structural variations which themselves vary on a continuum. Third, we examined degree of handedness regardless of direction. Since the majority of our participants were right handed, our data cannot speak to issues regarding neural correlates of left handedness. Fourth, structural and functional connectivity data will be needed to provide a more comprehensive picture of brain organization related to strength of hand preference. Our data, except for corpus callosum volume, was restricted to morphometric indices of cortical organization. Fifth, the absence of functional data from our participants is a significant limitation. We cannot determine the functional significance of the structural differences we reported between consistent and weak handers. It is an open empirical question as to whether variations in structural asymmetry between consistent and mixed handers correspond to asymmetry of brain activity. But we note that even if there is such a correspondence, this need not imply group differences in ability, as it is possible that differing types of brain organization could be equally effective. In addition, some research indicates that familial sinistrality may moderate the relationship between handedness strength and functional lateralization or cognitive ability (Mellet et al., 2014; Tzourio-Mazoyer et al., 2010). It will be important to examine the influence of this variable in future studies.
To summarize, we provided evidence for differences in lateral brain structure between consistent and weak handers. At the global hemispheric level, weak handers had reduced or absent asymmetry for every cortical metric we examined (gray and white matter volume, surface area, thickness, and local gyrification). We suggest that these slight biases in cortical organization could provide a substrate for functional differences in lateral brain organization. At a more regional level, some asymmetry differences between hand groups were observed for cortical thickness and local gyrification. These regions were those associated with somatomotor function, as well as with higher level functions relating to the cognitive control of action or to access of action-related information about objects. Although these functions do not overlap with the behavioral differences that originally motivated our study, the findings do suggest that further research into the behavioral correlates of handedness strength should examine the processing of more abstract action-related information.
Phil Bryden, more than any other scientist of his time, understood that handedness was an important dimension of human variation, one worthy of close empirical examination. The current neuroimaging era provides us with many new tools to examine Bryden’s prescient questions. We offer the current study as one small contribution to his enduring legacy.
Supplementary Material
Acknowledgements
This research was supported by NIH grant DC006957 to CC and an NSF Graduate Research Fellowship to DV. We thank Ronald Otto, M.D. for facilitating this research, and Laura K. Halderman, Janelle Julagay, and Suzanne Welcome for assistance with data collection and/or analysis.
Footnotes
Many prior studies use the term mixed hander to refer to individuals with weak or inconsistent hand preference (Chiarello et al., 2009; Christman et al., 2004). The term mixed hander is somewhat problematic as this group includes both those who report use of different hands for different tasks and those who have a consistent, but weak preference for the dominant hand. Here we use the term weak handers as both mixed and weak consistent handers can be considered to have relatively weak hand preference.
The items on this inventory are writing, drawing, throwing a ball, cutting with scissors, and using a toothbrush.
The differences remained significant when left handers were dropped from the sample – see Supplementary Table 4.
Our correlational findings also do not replicate those previously reported by Luders et al. (2010), i.e., we did not find a negative correlation between degree of hand preference and any callosal measurement. We note that because of the distribution of our handedness scores (nearly half of the sample had the same score of +1), the current lack of correlation may not be easily interpretable. In addition, there were several methodological differences between the studies (age and sex distribution of participants, measurement technique).
Participants could choose which hand to respond with and when to respond.
REFERENCES
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Amunts K, Jäncke L, Mohlber H, Steinmetz H, Ziles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Arning L, Ocklenburg S, Schulz S, Ness V, Gerding WM, Hengstler JG, Beste C. PCSK6 VNTR polymorphism is associated with degree of handedness but not direction of handedness. PLOS One. 2013;8:e67251. doi: 10.1371/journal.pone.0067251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badzakova-Trajkov G, Häberling IS, Corballis MC. Magical ideation, creativity, handedness, and cerebral asymmetries: a combined behavioural and fMRI study. Neuropsychologia. 2011;49:2896–2903. doi: 10.1016/j.neuropsychologia.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A. Bilateral representation of language: A critical review and analysis of some unusual cases. Journal of Neurolinguistics. 2014;28:63–80. [Google Scholar]
- Bourne VJ. Examining the relationship between degree of handedness and degree of cerebral lateralization for processing facial emotion. Neuropsychology. 2008;22:350–356. doi: 10.1037/0894-4105.22.3.350. [DOI] [PubMed] [Google Scholar]
- Brandi M-L, Wohlschläger A, Sorg C, Hermsdörfer J. The neural correlates of planning and executing actual tool use. Journal of Neuroscience. 2014;34:13183–13194. doi: 10.1523/JNEUROSCI.0597-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden MP. Tachistoscopic recognition and cerebral dominance. Perceptual and Motor Skills. 1964;19:686. doi: 10.2466/pms.1964.19.3.686. Retrieved from http://www.amsciepub.com/doi/pdf/10.2466/pms.1964.19.3.686. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Tachistoscopic recognition, handedness, and cerebral dominance. Neuropsychologia. 1965;3:1–8. [Google Scholar]
- Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15:617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Laterality: Functional asymmetry in the intact brain. New York: Academic Press; 1982. [Google Scholar]
- Bryden MP, Steenhuis RE. Issues in the assessment of handedness. In: Kitterle FL, editor. Cerebral Laterality: Theory and Research. Hillsdale: Lawrence Erlbaum Associates; 1991. pp. 35–51. [Google Scholar]
- Chen H, Zhang T, Guo L, Kaiming L, Xiang Y, Li L, et al. Coevolution of gyral folding and structural connection patterns in primate brains. Cerebral Cortex. 2013;23:1208–1217. doi: 10.1093/cercor/bhs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Maxfield L. Varieties of interhemispheric inhibition, or how to keep a good hemisphere down. Brain and Cognition. 1996;30:81–108. doi: 10.1006/brcg.1996.0006. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Vazquez D, Felton A, Leonard CM. Structural asymmetry of the insula: Behavioral correlates and individual differences. Brain and Language. 2013;126:109–122. doi: 10.1016/j.bandl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Welcome SE, Halderman LK, Towler S, Julagay J, Otto R, Leonard CM. A large-scale investigation of lateralization in cortical anatomy and word reading: Are there sex differences? Neuropsychology. 2009;23:210–222. doi: 10.1037/a0014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Welcome SE, Halderman LK, Leonard CM. Does degree of asymmetry relate to performance? An investigation of word recognition and reading in consistent and mixed handers. Brain and Cognition. 2009;69:521–530. doi: 10.1016/j.bandc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Christman SD, Propper RE, Dion A. Increased interhemispheric interaction is associated with decreased false memories in a verbal converging semantic associates paradigm. Brain and Cognition. 2004;56:313–319. doi: 10.1016/j.bandc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Chu Y-H, Lin F-H, Chou Y-J, Tsai KW-K, Kuo W-J, Jaaskelainen IP. Effective cerebral connectivity during silent speech reading revealed by functional magnetic resonance imaging. PLoS ONE. 2013;8(11):e80265. doi: 10.1371/journal.pone.0080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu X-H, Ugurbil K, Kim S-G, Ashe J. Functional activation in motor cortex reflects the direction and degree of handedness. Proceedings of the National Academy of Sciences. 94:14015–14018. doi: 10.1073/pnas.94.25.14015. Retrieved from http://www.pnas.org/content/94/25/14015.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, Tremblay F. Hemispheric differences in corticospinal excitability and in transcallosal inhibition in relation to degree of handedness. PLOS One. 2013;8:270286. doi: 10.1371/journal.pone.0070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Cabeza R. Cross-hemispheric collaboration and segregation associated with task difficulty as revealed by structural and functional connectivity. Journal of Neuroscience. 2015;35:8191–8200. doi: 10.1523/JNEUROSCI.0464-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Jiang X, Zhu D, Zhang T, Li K, Guo L, Liu T. A functional model of cortical gyri and sulci. Brain Structure and Function. 2014;219:1473–1491. doi: 10.1007/s00429-013-0581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Hong K, Leonard CM, Heilman KM. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11:65–71. [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: Do right- and left handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Yelle SK, Schoger J, Bright KS. Magical ideation is related to questionnaire but not behavioural measures of handedness. Laterality. 2008;13:22–33. doi: 10.1080/13576500701508539. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR. Phonological decisions require both the left and right supramarginal gyri. Proceedings of National Academy of Sciences. 2010;107:16494–16499. doi: 10.1073/pnas.1008121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. Cerebral Cortex. 2013;23:520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: Evidence from functional connectivity analyses. Human Brain Mapping. 2014;35:2741–2753. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper JD, Christman SD. A neuropsychological dimension for anchoring effects. Journal of Behavioral Decision Making. 2005;18(5):343–369. [Google Scholar]
- Khedr EM, Hamed E, Said A, Basahi J. Handedness and cerebral lateralization. European Journal of Applied Physiology. 2002;87:469–473. doi: 10.1007/s00421-002-0652-y. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy E, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lai A, Serra M, Petretto DR, Masala C, Preti A. Patterns of hand preference in Italian adolescent high-school students. Laterality: Asymmetries of Body, Brain and Cognition. 2014;19:718–744. doi: 10.1080/1357650X.2014.911747. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Chiarello C. Sex, hand preference and brain asymmetry; Presented at Cognitive Neuroscience Society, 16th Annual Meeting; March 24, 2009; San Francisco. [Google Scholar]
- Leshinskaya A, Caramazza A. Abstract categories of functions in anterior parietal lobe. Neuropsychologia. 2015 doi: 10.1016/j.neuropsychologia.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Li G, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. Journal of Neuroscience. 2014;34:4228–4238. doi: 10.1523/JNEUROSCI.3976-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Cherbuin N, Thompson PM, Gutman B, Anstey KJ, Sachdev P, Toga AW. When more is less: Associations between corpus callosum size and handedness lateralization. NeuroImage. 2010;52:43–49. doi: 10.1016/j.neuroimage.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle KB, Orsborn AE. Inconsistent handedness and saccade execution benefit face memory without affecting interhemispheric interaction. Memory. 2011;19:613–624. doi: 10.1080/09658211.2011.595418. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance, and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology. Amsterdam: Elsevier Science Publishers; 1992. pp. 115–144. [Google Scholar]
- Mellet E, Zago L, Jobard G, Crivello F, Petit L, Joliot M, Mazoyer B, Tzourio-Mazoyer N. Weak language lateralization affects both verbal and spatial skills: An fMRI study in 297 subjects. Neuropsychologia. 2014;65:56–62. doi: 10.1016/j.neuropsychologia.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Niebauer CL, Christman SD, Reid SA, Garvey KJ. Interhemispheric interaction and beliefs on our origin: Degree of handedness predicts beliefs in creationism versus evolution. Laterality: Asymmetries of Body, Brain and Cognition. 2004;9(4):433–447. doi: 10.1080/13576500342000266. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Worley E, Neale M, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatou-Pastou M, Martin M, Munafo MR, Jones GV. Sex differences in left-handedness: A meta-analysis of 144 studies. Psychological Bulletin. 2008;134:677–699. doi: 10.1037/a0012814. [DOI] [PubMed] [Google Scholar]
- Prichard E, Propper RE, Christman SD. Degree of handedness, but not direction, is a systematic predictor of cognitive performance. Frontiers in Psychology. 2013;4(9):1–6. doi: 10.3389/fpsyg.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper RE, O’Donnell LJ, Whalen S, Tie Y, Norton IH, Suarez RO, Golby AJ. A combined fMRI and DTI examination of functional lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain and Cognition. 2010;73:85–92. doi: 10.1016/j.bandc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Giedd JN. How does you cortex grow? The Journal of Neuroscience. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansk NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer F, Martinez M, Robert M, Bouvier G, Saint-Hillaire JM. Stimulation of human somatosensory cortex: tactile and body displacement perceptions in medial regions. Experimental Brain Research. 1993;93:173–176. doi: 10.1007/BF00227792. [DOI] [PubMed] [Google Scholar]
- Ronan L, Voets N, Rua C, Alexander-Bloch A, Hough M, Mackay C, et al. Differential tangential expansion as a mechanism for cortical gyrification. Cerebral Cortex. 2014;24:2219–2228. doi: 10.1093/cercor/bht082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Hersch SM. Altered white matter microstructure in the corpus callosum in Huntington’s disease: Implications for cortical “disconnection”. NeuroImage. 2010;49:2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Ruschel M, Knösche TR, Friederici AD, Turner R, Geyer S, Anwander A. Connectivity architecture and subdivision of the human inferior parietal cortex revealed by diffusion MRI. Cerebral Cortex. 2014;24:2436–2448. doi: 10.1093/cercor/bht098. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran J-P. A surface-based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska MW, Khadilkar M, Campbell-Ratcliffe J, Queveno F, Devlin JT. Early and sustained supramarginal gyrus contributions to phonological processing. Frontiers in Psychology. 2012;3:161. doi: 10.3389/fpsyg.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Goodale MA. Decoding visual object categories in early somatosensory cortex. Cerebral Cortex. 2015;25:1020–1031. doi: 10.1093/cercor/bht292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontam V, Christman SD, Jasper JD. Individual differences in semantic switching flexibility: Effects of handedness. Journal of the International Neuropsychological Society. 2009;15(6):1023–1027. doi: 10.1017/S1355617709990440. [DOI] [PubMed] [Google Scholar]
- Sontam V, Christman SD. Semantic organization and handedness: Mixed-handedness is associated with more diffuse activation of ambiguous word associates. Laterality: Asymmetries of Body, Brain and Cognition. 2012;17(1):38–50. doi: 10.1080/1357650X.2010.529450. [DOI] [PubMed] [Google Scholar]
- Steenhuis RE, Bryden MP. The relation between hand preference and hand performance: What you get depends on what you measure. Laterality: Asymmetries of Body, Brain, and Cognition. 1999;4(1):3–26. doi: 10.1080/713754324. [DOI] [PubMed] [Google Scholar]
- Tapley SM, Bryden MP. A group test for the assessment of performance between the hands. Neuropsychologia. 1985;23:215–221. doi: 10.1016/0028-3932(85)90105-8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Petit L, Razafimandimby A, Crivello F, Zago L, Jobard G, et al. Left hemisphere lateralization for language in right-handers is controlled in part by familial sinistrality, manual preference strength, and head size. Journal of Neuroscience. 2010;30:13314–13318. doi: 10.1523/JNEUROSCI.2593-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vazquez D, Ramos AI, Felton A, Greene MR, McDowell A, Hernandez AE, Chiarello C. Bilingualism influences structural indices of interhemispheric organization. doi: 10.1016/j.jneuroling.2016.10.004. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G. Knowing about tools: Neural correlates of tool familiarity and experience. NeuroImage. 2008;40:1380–1391. doi: 10.1016/j.neuroimage.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Welcome SE, Chiarello C, Towler S, Halderman LK, Otto R, Leonard CM. Behavioral correlates of corpus callosum size: Anatomical/behavioral relationships vary across sex/handedness groups. Neuropsychologia. 2009;47:2427–2435. doi: 10.1016/j.neuropsychologia.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test-Revised Normative Update (WRMT- R) Circle Pines, MN: American Guidance Service, Inc.; 1998. [Google Scholar]
- Xu G, Knutsen AK, Dikranian K, Kroenke CD, Bayly PV, Taber LA. Axons pull on the brain, but tension does not drive cortical folding. Journal of Biomedical Engineering. 2010;132:071013. doi: 10.1115/1.4001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends in Neurosciences. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann H-J. The human pattern of gyrification in the cerebral cortex. Anatomy and Embryology. 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.