Abstract
OBJECTIVE
To identify key features differentiating Multiple System Atrophy Cerebellar type (MSA-C) from Idiopathic Late-Onset Cerebellar Ataxia (ILOCA).
METHODS
We reviewed records of patients seen in the Massachusetts General Hospital Ataxia Unit between 1992 and 2013 with consensus criteria diagnoses of MSA-C or ILOCA. 12 patients had Definite MSA-C, 53 had Possible/Probable MSA-C, and 12 had ILOCA.
RESULTS
Autonomic features, specifically urinary urgency, frequency, and incontinence with erectile dysfunction in males, differentiated MSA-C from ILOCA throughout the disease course (p = 0.005). Orthostatic hypotension developed later and differentiated MSA-C from ILOCA (p < 0.01). REM sleep behavior disorder (RBD) occurred early in Possible/Probable MSA-C (p < 0.01). Late MSA-C included pathologic laughing and crying (PLC, p < 0.01), bradykinesia (p = 0.01), and corticospinal findings (p = 0.01). MRI distinguished MSA-C from ILOCA by atrophy of brainstem (p < 0.01) and middle cerebellar peduncles (MCP, p = 0.02). MSA-C progressed faster than ILOCA: by 6 years, MSA-C walker dependency was 100%, ILOCA 33%. MSA-C survival was 8.4 ± 2.5 years. Mean length of ILOCA illness to date is 15.9 ± 6.4 years.
CONCLUSIONS
A sporadic onset, insidiously developing cerebellar syndrome in mid-life, with autonomic features of otherwise-unexplained bladder dysfunction with or without erectile dysfunction in males, and atrophy of cerebellum, brainstem, and MCP points strongly to MSA-C. RBD and postural hypotension confirm the diagnosis. Extrapyramidal findings, corticospinal tract signs and PLC are helpful but not necessary for diagnosis. Clarity in early MSA-C diagnosis can prevent unnecessary investigations and facilitate therapeutic trials.
INTRODUCTION
Multiple system atrophy (MSA) is a late-onset, sporadic neurodegenerative disorder characterized by autonomic failure, parkinsonism, cerebellar ataxia, and pyramidal tract signs.1–3 Mean age of onset is 54 years, with survival ranging 7 to 9 years.4,5 Two clinical subtypes based on the predominant motor feature at presentation are poorly levodopa (L-dopa)-responsive parkinsonism (MSA-P) or cerebellar ataxia (MSA-C). Both subtypes share the neuropathologic hallmark of α-synuclein glial cytoplasmic inclusions accompanied by widespread neurodegeneration in striatonigral (predominant in MSA-P) and olivopontocerebellar (predominant in MSA-C) structures.6–10
Diagnostic criteria for MSA were established by expert consensus in 1999 and revised in 2008.1,11 Definite MSA requires autopsy confirmation of widespread CNS α-synuclein inclusions associated with neurodegeneration. The clinical diagnosis of MSA-C is based on a combination of clinical symptoms, signs and imaging data. Diagnostic certainty is categorized at a level of Possible or Probable MSA-C (Table 1).
Table 1.
Current consensus criteria for the diagnosis of Multiple System Atrophy (MSA), according to Gilman et al. 2008.1
| Definite MSA | Autopsy-confirmed case with neuropathologic evidence of widespread and abundant CNS α-synuclein–positive glial cytoplasmic inclusions in association with striatonigral and/or olivopontocerebellar neurodegeneration |
| Probable MSA | Sporadic, progressive, adult (>30 years) -onset disease characterized by
|
| Possible MSA | Sporadic, progressive, adult (>30 years) -onset disease characterized by
|
Investigations into the clinical course of MSA have focused primarily on MSA-P, possibly reflecting an epidemiologic and selection bias: MSA-P is twice as prevalent as MSA-C in North America and Europe, 12–17 and MSA shares α-synuclein pathology with Parkinson’s disease.18–20 Accordingly, clinical, laboratory, and radiologic approaches have been developed to differentiate Parkinson’s disease from MSA-P.21–23
The symptoms and natural history of MSA-C have received relatively less attention. One recent study24 followed 49 Probable MSA-C patients from 12 US Movement Disorder centers over 5 years, together with 126 Probable MSA-P patients. Median survival of MSA-C from symptom onset was 9.8 years, and severe symptomatic autonomic failure at diagnosis was associated with worse prognosis. MSA-C is more common than MSA-P in the Japanese population. In a study of 230 Japanese MSA patients, cerebellar dysfunction (MSA-C) was evident in 67%, and interval from initial symptom to combined motor and autonomic dysfunction predicted functional deterioration and survival, with a median survival rate of 9 years.25 These authors also noted putaminal rim hyperintensity and pontine hot cross bun sign on MRI,26 with progression of pontine and cerebellar (vermis) atrophy as the disease evolved. In another Japanese cohort of 142 Probable MSA patients, 84% were diagnosed with the cerebellar form at first clinical presentation. Sixty-five of these MSA-C patients were followed for more than 3 years, and parkinsonism became the predominant feature in 25%, overwhelming the cerebellar signs.27
The critical unanswered question for the clinician evaluating a patient with ataxia, however, is how to differentiate MSA-C from the many other causes of adult-onset cerebellar ataxias,28 the differential diagnosis of which includes acquired (including toxins, tumors, and infections), genetic (spinocerebellar ataxias) and sporadic etiologies.29,30 A complete history, examination, and laboratory investigations including magnetic resonance imaging (MRI), can differentiate many disorders from MSA-C, but for the adult patient presenting with an insidious onset, pure or predominantly cerebellar ataxia with no family history or apparent inciting event, distinguishing idiopathic late-onset cerebellar ataxia (ILOCA) from MSA-C early in the disease course remains a major diagnostic challenge.
The term ILOCA was introduced in 1981 for a progressive cerebellar ataxia onset > 20 years with no history of “alcoholism, hypothyroidism, chronic anticonvulsant ingestion, or malignancy”.31 The understanding of ILOCA, also known as sporadic adult-onset ataxia of unknown etiology (SAOA), has evolved with increased knowledge of the genetics and biology of the ataxias,32 and ILOCA is now a diagnosis of exclusion. The problem for the clinician is that ILOCA and MSA-C both present in mid-life, and it has been suggested that both disorders include extra-cerebellar symptoms such as erectile dysfunction, bladder urgency, dysphagia, snoring, restless leg syndrome, and rapid eye movement sleep behavior disorder.33,34 The natural histories of MSA-C and ILOCA are quite different, however, as MSA-C has an average survival of 7 to 9 years,4,5 whereas ILOCA patients can have a normal lifespan.35,36 Making the correct diagnosis early in the course is therefore of paramount importance for patient management, and for research initiatives into early intervention and treatment strategies. A critical shortcoming of many previous studies is the lack of pathologic confirmation of MSA-C patients. In the present study we compare the clinical features of patients with ILOCA against those with Definite (pathologically proven) MSA-C, as well as those with Possible and Probable MSA-C, in order to define the core clinical features necessary and sufficient for the diagnosis of MSA-C. To date this is the largest single center / single investigator study of MSA-C patients (n=65) over the longest period of time (22 years), which compares and contrasts this cohort with patients with ILOCA, includes salient features on brain imaging, and provides neuropathological data for the Definite MSA-C cases.
METHODS
Patient Selection
We reviewed the Massachusetts General Hospital (MGH) Ataxia Unit patient list, a prospective, rolling inventory of >1,500 patients seen in the Unit since 1992, and identified all patients examined between January 1992 and December 2013 who received a clinical diagnosis of MSA-C or ILOCA.
MSA-C
12 patients with a clinical diagnosis of Probable MSA-C came to autopsy at the MGH during the study period and met consensus criteria1 for Definite MSA-C. The genetic work-up of one of these Definite MSA-C cases revealed an expansion of 84 CTG repeats in the SCA 8 gene. This “false-positive” association of an SCA8 expansion in a patient with pathologically proven MSA-C has been reported previously37,38 The trinucleotide expansion in this setting is thought to be clinically silent, although its role in the underlying neurobiology remains uncertain. An additional 53 patients met consensus criteria for Possible MSA-C (n=10) or Probable MSA-C (n=43). None of these patients came to autopsy by December 2013 after the entry into the three study groups was closed, although 4 of those who have died since the study closed were shown to have Definite MSA-C on pathologic examination.
ILOCA
Of the adult patients with cerebellar ataxia, 12 met the following ILOCA inclusion criteria: (i) stable or slowly progressing cerebellar ataxia with age of symptom onset after age 20; (ii) absence of family history of clinically diagnosed ataxia; (iii) known causes of acquired ataxia excluded; (iv) no known genetic etiology as determined by negative findings on clinically indicated, commercially available genetic tests. These included the autosomal dominant spinocerebellar ataxia (SCA) types 1, 2, 3, 5, 6, 7, 8, 10, 12, 13, 17, 28 and dentatorubro-pallidoluysian atrophy (DRPLA), the autosomal recessive ataxias Friedreich’s ataxia (FRDA), ataxia oculomotor apraxia (AOA) types 1 and 2, ataxia with vitamin E deficiency, Marinesco Sjogren syndrome (SIL1), and polymerase gamma related ataxias (POLG1). There was insufficient clinical or imaging indication to test these patients for fragile X tremor ataxia syndrome (FXTAS) or ataxia telangiectasia. Testing for autosomal recessive cerebellar ataxia type 1 (ARCA1) with a high prevalence in the French Canadian population was relevant in only one patient, but the test became available only after the patient’s death from cardiac disease. A number of variants of unknown significance (VUS) were identified in the ILOCA cohort. These included VUS in FRDA, SCA5, SCA13, SCA14, SCA28, POLG1, SIL1, AOA1, AOA2. In the time since this study closed and whole exome sequencing (WES) became available, one patient was shown to have a VUS in the TGM6 gene for SCA 35 thought likely to be pathogenic, along with VUS in the A433V variant of APOB (hypercholesterolemia), the R11c variant of GFAP (Alexander's disease), and P439L variant in the SQSTM1 disorders gene. The presence of these VUS is a matter of ongoing discussion in the ataxia community, and their significance is unclear. Whether ILOCA will in the end be replaced in whole or in part by the new genetic approaches afforded by WES and whole genome sequencing remains to be shown, but for the purposes of comparing these cases with MSA-C, they all fit the diagnostic criteria for ILOCA. Two ILOCA patients also had elevated gliadin antibodies – one has remained stable on a gluten free diet, the other showed no response to dietary restriction.
Table 2 A, B lists age of reported symptom onset, disease duration at time of initial visit, gender distribution, and clinical follow-up time in the Ataxia Unit according to the study group. The population studied here largely reflects a western heritage: of the 77 patients in the study, 68 were Caucasian and 9 were of Asian descent (4 had Chinese ancestry, and 1 each from India, Indonesia, Japan, Korea, and Vietnam). This study was approved by the Human Studies Committee of the Institutional Review Board of the Massachusetts General Hospital.
TABLE 2.
| A: PATIENT DEMOGRAPHICS, BY STUDY GROUP | |||||
|---|---|---|---|---|---|
| Definite MSA-C [n = 12] |
ILOCA [n=12] |
Definite MSA-C vs. ILOCA p |
Possible / Probable MSA-C [n = 53] |
Possible / Probable MSA-C vs. ILOCA p |
|
| Age at symptom onset yrs | 54.7 ± 8.5 | 41.1 ± 14.2 | 0.01 | 56.8 ± 8.7 | < 0.001 |
| Disease duration, initial visit, yrs | 3.4 ± 1.5 | 5.5 ± 3.7 | 0.08 | 4.2 ± 2.7 | 0.14 |
| Male sex, n (%) | 6 (50) | 8 (67) | 0.68 | 32 (60) | 0.75 |
| Clinical follow-up time yrs | 4.5 ± 3.1 | 7.4 ± 7.0 | 0.19 | 2.1 ± 2.2 | < 0.001 |
| B: PATIENT DEMOGRAPHICS, Possible MSA-C vs Probable MSA-C | |||
|---|---|---|---|
| Possible MSA-C [n = 10] |
Probable MSA-C [n=43] |
Possible vs. Probable MSA-C p |
|
| Age at symptom onset, yrs | 54.0 ± 12.2 | 56.9 ± 7.7 | 0.49 |
| Disease duration, initial visit, yrs | 3.7 ± 2.3 | 4.3 ± 2.8 | 0.52 |
| Male sex, n (%) | 7(25) | 25(58) | 0.72 |
| Clinical follow-up time yrs | 1.1 ± 1.3 | 2.3 ± 2.3 | 0.045 |
Baseline characteristics of patients with Definite and Possible/Probable Multiple System Atrophy-Cerebellar type (MSA-C) and Idiopathic Late Onset Cerebellar Ataxia (ILOCA) are compared. Data are presented as mean ± standard deviation and n (%). yrs = years.
The same characteristics are compared between the Possible and Probable MSA-C patients whose diagnosis was not confirmed pathologically.
Data Collection
For each patient meeting inclusion criteria, we reviewed the medical record for the period between the patient’s initial clinic visit (intake visit) and the last exam as of December 2013 (final study visit). The entire clinical record was reviewed, including neurological interviews and examinations, laboratory data, radiology reports, and neuropathology reports.
Clinical history and neurological examination
We reviewed contemporaneously written records for patient-reported history of present illness, past medical history, medication use and toxic exposure, family history, social history, allergies, and comprehensive review of systems. The history was elicited and the neurological examination performed in all cases by the senior author and founding director of the MGH Ataxia Unit (JDS). For this study, the presence or absence in the clinical record of the symptoms and physical examination signs listed in Table 3 were coded. Orthostatic hypotension was assessed with a blood pressure (BP) cuff in the office, with the patient supine and again after 3 minutes of standing. REM sleep behavior disorder was diagnosed clinically, by a history of self or family-member reported motor behavior during sleep. These included sleep-related vocalizations, thrashing, punching, or kicking, and acting out violent or threatening dreams. Symptoms or signs not mentioned in the record were assumed absent.
TABLE 3. Comparison of clinical features in Definite MSA-C, ILOCA, and Possible/Probable MSA-C.
Table shows columns grouped by diagnosis and rows with clinical features examined. Clinical features were coded at intake visit (TABLE 3A) as well as over all clinical visits (cumulative visits, TABLE 3B). For cumulative visits, a feature was counted as present if it was noted at any visit, and absent if it was absent at all visits. Values are shown as absolute number, n, followed by percent (%). For features in which fewer than the total number of patients had values (i.e. systolic blood pressure drop), the number of patients coded is shown in the denominator. Columns with P-values (p) show results of Fisher’s exact test of proportions comparing the specific clinical feature for the groups specified.
| INTAKE VISIT | |||||
|---|---|---|---|---|---|
| Definite MSA-C |
ILOCA |
Definite MSA-C v. ILOCA p |
Possible / Probable MSA-C |
Possible / Probable v. ILOCA p |
|
| Affected examined (n) | 12 | 12 | 53 | ||
| Cerebellar signs | 12 (100) |
12 (100) |
1 | 53 (100) | 1 |
| Gait ataxia | 12 (100) |
12 (100) |
1 | 52 (98) | 1 |
| Lower extremity dysmetria | 10 (83) |
12 (100) |
0.48 | 51 (96) | 1 |
| Upper extremity dysmetria | 12 (100) |
12 (100) |
1 | 51 (96) | 1 |
| Dysarthria | 10 (83) |
10 (83) |
50 (94) | 0.23 | |
| Oculomotor | 11 (92) | 7 (58) | 0.16 | 46 (87) | 0.036 |
| Handwriting affected | 9 (75) | 9 (75) | 1 | 42 (79) | 0.71 |
| Requires Walker | 6 (50) | 1 (8) | 0.07 | 18 (34) | 0.09 |
| Wheelchair bound | 0 (0) | 0 (0) | 1 | 4 (8) | 1 |
| Extrapyramidal signs | 5 (42) | 3 (25) | 0.67 | 19 (36) | 0.74 |
| Bradykinesia | 3 (25) | 1 (8) | 0.59 | 8 (15) | 1 |
| Rigidity | 3 (25) |
2 (17) |
1 | 10 (19) | 1 |
| Resting tremor | 1 (8) | 0 (0) | 1 | 4 (8) | 1 |
| Corticospinal signs | 7 (58) |
3 (25) |
0.21 | 15 (28) | 1 |
| Autonomic features | 10 (83) | 0 (0) | < 0.001 | 46 (87) | < 0.001 |
| Systolic blood pressure drop of at least 30 mm Hg |
5/9 (56) | 0/3 (0) | 0.22 | 16/50 (32) | 0.55 |
| Presyncopal symptoms | 3 (25) | 0 (0) | 0.22 | 16 (30) | 0.029 |
| Urinary urgency or frequency |
6 (50) | 0 (0) | 0.014 | 31 (58) | < 0.001 |
| Urinary incontinence | 7 (58) | 0 (0) | 0.005 | 15 (28) | 0.054 |
| Erectile dysfunction | 5/6 (83) | 0/8 (0) | 0.003 | 26/32 (81) | < 0.001 |
| Other clinical signs and symptoms |
11 (92) |
11 (92) |
1 | 50 (94) | 0.57 |
| Vertigo | 2 (17) | 1 (8) | 1 | 4 (8) | 1 |
| Shortness of breath | 1 (8) | 0 (0) | 1 | 3 (6) | 1 |
| Inspiratory stridor | 1 (8) | 1 (8) | 1 | 1 (2) | 0.34 |
| Loud snoring | 2 (17) | 0 (0) | 0.48 | 6 (11) | 0.58 |
| Dysphagia | 3 (25) | 5 (42) | 0.67 | 16 (30) | 0.50 |
| REM sleep behavior disorder |
1 (8) | 0 (0) | 1 | 23 (43) | 0.005 |
| Fatigue | 3 (25) | 0 (0) | 0.22 | 6 (11) | 0.58 |
| Pathologic Laughing and Crying |
1 (8) | 0 (0) | 1 | 5 (9) | 0.57 |
| Frontal Release Signs | 1 (8) | 0 (0) | 1 | 5 (9) | 0.57 |
| Tinnitus | 2 (17) | 0 (0) | 0.48 | 4 (8) | 1 |
| Mood | 4 (33) | 2 (17) | 0.64 | 14 (26) | 0.71 |
| Constipation | 1 (8) | 0 (0) | 1 | 8 (15) | 0.33 |
| Bowel Urgency / Incontinence |
0 (0) | 1 (8) | 1 | 1 (2) | 0.34 |
| Cold Extremities | 0 (0) | 1 (8) | 1 | 4 (8) | 1 |
| Sensory abnormalities | 1 (8) | 3 (25) | 0.59 | 5 (9) | 0.16 |
| Absent ankle reflexes | 0 (0) | 0 (0) | 1 | 1 (2) | 1 |
| Double vision | 0 (0) | 2 (17) | 0.48 | 8 (15) | 1 |
| Dizziness (not presyncopal) |
1 (8) | 2 (17) | 1 | 14 (26) | 0.71 |
| Cognitive Difficulties | 6 (50) |
6 (50) |
1 | 22 (42) | 0.75 |
| B: CUMULATIVE VISITS | |||||
|---|---|---|---|---|---|
| Definite MSA-C |
ILOCA |
Definite MSA-C v. ILOCA p |
Possible / Probable MSA-C |
Possible / Probable v. ILOCA p |
|
| Affected examined (n) | 12 | 12 | 53 | ||
| Cerebellar signs | 12 (100) | 12 (100) | 1 | 53 (100) | 1 |
| Gait ataxia | 12 (100) | 12 (100) | 1 | 53 (100) | 1 |
| Lower extremity dysmetria | 12 (100) | 12 (100) | 1 | 52 (98) | 1 |
| Upper extremity dysmetria | 12 (100) | 12 (100) | 1 | 52 (98) | 1 |
| Dysarthria | 12 (100) | 11 (92) | 1 | 51 (96) | 0.46 |
| Oculomotor | 12 (100) | 11 (92) | 1 | 51 (96) | 0.46 |
| Handwriting affected | 12 (100) | 9 (75) | 0.22 | 46 (87) | 0.38 |
| Requires Walker | 12 (100) | 4 (33) | 0.001 | 39 (74) | 0.015 |
| Wheelchair bound | 12 (100) | 3 (25) | < 0.001 | 18 (34) | 0.74 |
| Extrapyramidal signs | 9 (75) | 6 (50) | 0.4 | 37 (70) | 0.31 |
| Bradykinesia | 7 (58) | 1 (8) | 0.011 | 17 (32) | 0.15 |
| Rigidity | 6 (50) | 4 (33) | 0.68 | 24 (45) | 0.53 |
| Resting tremor | 4 (33) | 3 (25) | 1 | 17 (32) | 0.74 |
| Corticospinal signs | 10 (83) | 3 (25) | 0.012 | 22 (42) | 0.34 |
| Autonomic features | 12 (100) | 5 (42) | 0.005 | 51 (96) | < 0.001 |
| Systolic blood pressure drop of at least 30 mm Hg |
8/11 (73) | 0/6 (0) | 0.009 | 34 (64) | 0.004 |
| Presyncopal symptoms | 9 (75) | 0 (0) | < 0.001 | 29 (55) | < 0.001 |
| Urinary urgency or frequency |
10 (83) | 4 (33) | 0.036 | 44 (83) | 0.001 |
| Urinary incontinence | 12 (100) | 1 (8) | < 0.001 | 35 (66) | < 0.001 |
| Erectile dysfunction | 6/6 (100) | 0/8 (0) | < 0.001 | 27/32 | < 0.001 |
| Other clinical signs and symptoms |
12 (100) | 12 (100) | 1 | 52 (98) | 1 |
| Vertigo | 2 (17) | 3 (25) | 1 | 5 (9) | 0.16 |
| Shortness of breath | 2 (17) | 1 (8) | 1 | 8 (15) | 1 |
| Inspiratory stridor | 2 (17) | 1 (8) | 1 | 4 (8) | 1 |
| Loud snoring | 4 (33) | 2 (17) | 0.64 | 13 (25) | 0.78 |
| Dysphagia | 9 (75) | 6 (50) | 0.40 | 28 (53) | 1 |
| REM sleep behavior disorder |
3 (25) | 0 (0) | 0.22 | 31 (58) | < 0.001 |
| Fatigue | 4 (33) | 7 (58) | 0.41 | 11 (21) | 0.014 |
| Pathologic Laughing and Crying |
7 (58) | 0 (0) | 0.005 | 13 (25) | 0.10 |
| Frontal Release Signs | 5 (42) | 1 (8) | 0.16 | 11 (21) | 0.44 |
| Tinnitus | 2 (17) | 1 (8) | 1 | 5 (9) | 1 |
| Mood | 7 (58) | 5 (42) | 0.68 | 27 (51) | 0.75 |
| Constipation | 7 (58) | 1 (8) | 0.027 | 17 (32) | 0.15 |
| Bowel Urgency / Incontinence |
0 (0) | 3 (25) | 0.22 | 9 (17) | 0.68 |
| Cold Extremities | 0 (0) | 2 (17) | 0.48 | 10 (19) | 1 |
| Sensory abnormalities | 3 (25) | 4 (33) | 1 | 7 (13) | 0.19 |
| Absent ankle reflexes | 0 (0) | 0 (0) | 1 | 2 (4) | 1 |
| Double vision | 3 (25) | 5 (42) | 0.67 | 18 (34) | 0.74 |
| Dizziness (not presyncopal) | 7 (58) | 5 (42) | 0.68 | 24 (45) | 1 |
| Cognitive Difficulties | 7 (50) | 9 (75) | 0.67 | 26 (49) | 0.12 |
| Imaging | |||||
| Cerebellar Atrophy | 11/11 (100) |
9/10 (90) |
0.48 |
26/27 (96) |
0.47 |
| Cerebellar Peduncle Atrophy |
7/11 (64) |
1/10 (10) |
0.024 |
11/27 (41) |
0.12 |
| Brainstem Atrophy | 8/11 (73) |
1/10 (10) |
0.008 |
21/27 (78) |
<0.001 |
Imaging
11 Definite MSA-C, 10 ILOCA, and 27 Possible and Probable MSA-C patients who underwent Magnetic Resonance Imaging (MRI) for clinical indications at a Partners Healthcare hospital during the 22-year study period, January 1992 to December 2013, had images available for review. We personally reviewed all these images – T1-weighted, T2-weighted, fluid attenuated inversion recovery (FLAIR) sequences, and, when available, MPRAGE (magnetization-prepared rapid acquisition with gradient echo) sequences. The imaging data included in our analyses were derived from the clinical MRI reports generated by the physicians in the MGH Department of Radiology. We documented whether these reports described cerebellar atrophy, middle cerebellar peduncle (MCP) atrophy, and brainstem atrophy - including volume loss in the pons and medulla. We analyzed only the comments of the radiologists that were consistently present in all reports, and since a note regarding the presence or absence of the hot cross bun sign was not included in all reports, we did not incorporate this finding in our analysis. The remaining Definite MSA-C case, 2 ILOCA patients, and 26 Possible / Probable MSA-C patients were imaged at outside facilities, and whereas the studies were all personally reviewed, the reports for these scans (when available) were not included in the present analysis.
Pathology
Neuropathological evaluation of all Definite MSA-C patients was performed according to standard procedures by senior faculty in the MGH Department of Neuropathology. Eleven of the 12 cases that met pathologic criteria for Definite MSA-C came to autopsy with a clinical diagnosis of Probable MSA-C. There was clinical uncertainty about one patient seen 15 years ago whose course ended prematurely following a motor vehicle accident. The neuropathological examination focused specifically on the search for distinctive pathological hallmarks of MSA-C. Autopsy reports on these 12 cases were reviewed for mention of volume loss, neuronal loss, gliosis, white matter attenuation, and/or α-synuclein inclusions. We regarded these findings as present if they were noted in the finalized neuropathology report.
Data Analysis
The frequency of clinical signs and symptoms and imaging features at initial visit (intake), and across all study visits (cumulative) were compared across the three study groups – ILOCA, Definite MSA-C, and Possible/Probable MSA-C. For the cumulative category, a feature was counted as present if it was noted at any visit, and absent if it was absent at all visits. To determine whether a feature distinguished between the different groups, p values were calculated using Fisher’s Exact method. Survival analysis was conducted using the Kaplan-Meier method. Group averages were compared using student t-test of means. A significance threshold of α < 0.05 was used.
RESULTS
Study Population
Demographics and baseline clinical characteristics of the patient groups are compared in Table 2A. Definite MSA-C patients had a later age of symptom onset compared to ILOCA patients (MSA-C, 54.7 ± 8.5 years, [35.0 – 66.8] vs. ILOCA, 41.1 ± 14.2 years, [20.1 – 69.4]; mean ± standard deviation, [range]; p < 0.05). The Possible/Probable MSA-C group had an age of onset symptom onset of 56.8 ± 8.7 years, [35.3 – 79.8], similar to the Definite MSA-C group (p > 0.05) and also significantly later than ILOCA (p < 0.001).
There were no group differences for gender ratio (ILOCA 8/12 males, Definite MSA-C 6/12 males, Possible/Probable MSA-C 32/53 males) (p > 0.05), or time from symptom onset to initial visit in the MGH Ataxia Unit (Definite MSA-C 3.4 ± 1.5 years, ILOCA 5.5 ± 3.7 years, Possible/Probable MSA-C 4.2 ± 2.7 years) (p > 0.05). Clinical follow-up time in the Ataxia Unit did not differ between the Definite MSA-C group (4.5 ± 3.1 years, [0.3 – 10.1]) and the ILOCA group (7.4 ± 7.0 years, [0.8 – 21.7]) (p > 0.05). Follow-up time for the Possible/Probable MSA-C group was significantly shorter (2.1 ± 2.2 years, [0 – 9.8]) when compared to both the Definite MSA-C and ILOCA groups (p < 0.05), as many of the patients in the Possible/Probable group are still relatively early in their disease course. Variability in length of follow-up time reflects the fact that some patients were seen in consultation once or twice, while others were followed regularly over a period of many years. No patient in any of our study groups was lost to follow-up.
Table 2B compares the demographics of patients with Possible and Probable MSA-C. Age of symptom onset, disease duration, and gender ratios did not differ between the groups, although clinical follow-up time was shorter for the Possible group as they were still in the earlier stages of their course.
Comparison of Clinical Features
Table 3 compares the clinical features of Definite MSA-C, Possible/Probable MSA-C, and ILOCA patients at their intake visit (Table 3A) and over cumulative visits (Table 3B). All MSA-C and ILOCA patients presented (by definition) with a cerebellar motor syndrome, including some combination of gait ataxia, appendicular dysmetria usually with impaired hand and finger dexterity as with handwriting and typing, dysarthria, and oculomotor abnormalities. The prevalence of individual cerebellar findings was not different between the study groups either at intake visit or over cumulative visits.
Clinical features of pathologically-confirmed, Definite MSA-C vs. ILOCA
Autonomic features were very reliable for differentiating MSA-C from ILOCA (p = 0.005). Urinary urgency or frequency differentiated the Definite MSA-C and ILOCA groups both at intake visit and throughout the disease course (p < 0.05). Similarly, urinary incontinence distinguished between Definite MSA-C and ILOCA at intake visit (p < 0.005) and throughout the disease course (p < 0.001). All Definite MSA-C patients exhibited urinary incontinence by their final visit. Erectile dysfunction was present in five of the six males with Definite MSA-C at intake visit and in all MSA-C males by their final visit. Erectile dysfunction was not present in the ILOCA group (p < 0.001).
Postural hypotension was a prominent feature of the Definite MSA-C group: 56% of patients dropped their systolic blood pressure (SBP) > 30 mm Hg from lying to standing at initial visit and 73% did so by their final visit. Of 9 Definite MSA-C patients with orthostatic blood pressure measured at intake visit, the drop in SBP from lying to standing after 3 minutes was 30.0 ± 18.2 mm Hg (mean ± stdev). In the 11 Definite MSA-C patients with orthostatic measures over their cumulative visits, the average peak drop in SBP from lying to standing was 39.2 ± 18.0 mm Hg. The majority of these patients were symptomatic, complaining of presyncopal symptoms by their final clinic visit. In contrast, in the 3 ILOCA patients in whom orthostatic blood pressure changes were measured at initial visit, the mean drop in SBP was 3.3 ± 5.8 mm Hg. In the 6 ILOCA patients who had blood pressure measurements by their final clinic visit, the SBP decline was 3.7 ± 4.3 mm Hg.
Extrapyramidal signs (bradykinesia, rigidity, resting tremor) and corticospinal tract findings (hyperreflexia, extensor plantar responses) did not differentiate between the two groups at intake visit. However, by final study visit, compared to only a minority of ILOCA patients, the majority of Definite MSA-C patients exhibited both bradykinesia (7/12, p = 0.01) and corticospinal tract signs (10/12, p = 0.01).
Additional symptoms and signs that have been reported previously in MSA-C and that were examined in our study include vertigo, shortness of breath, stridor, loud snoring, dysphagia, REM sleep behavior disorder (RBD), fatigue, pathologic laughing and crying (PLC), frontal release signs, tinnitus, mood symptoms, constipation, bowel urgency and incontinence, cold extremities, sensory abnormalities, absent ankle reflexes, double vision, dizziness, and cognitive difficulties. Of these, only two reached significance in differentiating Definite MSA-C from ILOCA: PLC affected 58% (7/12) of patients with Definite MSA-C but none of the ILOCA patients (p = 0.005), and constipation affected 58% (7/12) of patients with Definite MSA-C and only one patient with ILOCA (p = 0.027). Inspiratory stridor was noted in 2 of the 12 patients in the Definite MSA-C cohort, and there was no significant difference at the group level in stridor frequency (1 of 12 ILOCA cases had stridor). Cognitive difficulties on examination manifested principally as executive dysfunction, and were similar in both groups (50% affected in Definite MSA-C, 75% affected in ILOCA).
On MRI the prevalence of cerebellar atrophy did not differ between the groups (100% of the 11 Definite MSA-C patients, 90% of the 10 ILOCA cases). In contrast, compared to the ILOCA group, the MSA-C group demonstrated atrophy in the brainstem (p = 0.008) and MCP (p = 0.02).
Clinical features of clinically-diagnosed, Possible/Probable MSA-C vs. ILOCA
The prevalence of clinical features in the 53 patients with Possible (n = 10) and Probable MSA-C (n= 43) were compared with the ILOCA cohort (Table 3). As with the other groups, cerebellar motor findings required for the clinical diagnosis affected 100% of the Possible/Probable MSA-C patients on their initial visit.
Autonomic features were pervasive, and significantly differentiated the Possible/Probable MSA-C group from the ILOCA group, affecting 87% of the MSA-C cases at initial visit (p < 0.001), and 96% in the cumulative visits (p < 0.001). Compared to ILOCA, 58% of the Possible/Probable MSA-C group reported urinary urgency or frequency at their initial visit (p < 0.001), and 83% cumulatively (p = 0.001). Urinary incontinence was present in 28% at initial visit (p = 0.054), and 66% cumulatively (p < 0.001). Erectile dysfunction in males was reported in 81% at first visit (p < 0.001), and 84% cumulatively (p < 0.001). Orthostatic measures were performed in 50 of the 53 Possible/Probable patients at intake visit and demonstrated an average SBP drop of 22.8 ± 19.3 mm Hg. By final visit, all 53 patients had their orthostatic signs recorded and had an average SBP decline of 35.1 ± 15.6 mm Hg. A SBP drop of greater than 30 mm Hg was present in 32% at initial visit and in 64% cumulatively (p = 0.004). Presyncopal symptoms were present in 30% at initial visit and in 55% cumulatively.
Extrapyramidal signs were present in 36% of the Possible/Probable MSA-C group at initial visit and 70% cumulatively. Corticospinal tract findings increased from 28% initially to 42% cumulatively. Unlike in the Definite MSA-C group, these values were not significantly different from those in the ILOCA group.
Whereas the frequency of REM sleep behavior disorder did not differ significantly between our ILOCA and Definite MSA-C patients, RBD occurred with greater frequency in the Possible/Probable MSA-C cohort than the ILOCA group at both intake (p = 0.005) and over cumulative visits (p < 0.001). A history of RBD was elicited in 43% of this Possible/Probable MSA-C cohort during their initial visit, and in 58% of the group by their most recent clinical visit, a significant difference from the ILOCA cases, not one of whom developed RBD.
Pathologic laughing and crying was reported in the history or noted during the office examination in 9% of the Possible/Probable MSA-C patients at intake, and in 25% by cumulative visit. Even though no ILOCA patient developed PLC, this frequency of PLC in the Possible/Probable MSA-C group did not reach significance compared to ILOCA (p = 0.1). This is in contrast to the Definite MSA-C group in which PLC did occur with significantly greater frequency than in ILOCA.
Additional clinical symptoms and signs that occurred with some frequency by final visit in the Possible/Probable MSA-C group included loud snoring (25%), dysphagia (53%), mood symptoms (51%), constipation (32%), diplopia (34%), dizziness (45%), and cognitive difficulty (49%), but there was no significant difference between the prevalence of these features compared to the ILOCA group. Fatigue was reported more frequently in ILOCA compared to the Possible/Probable MSA-C patients by the cumulative visit (p = 0.01). Notably, these MSA-C patients were earlier in their course than the Definite MSA-C patients in whom the frequency with which fatigue was reported was no different than in the ILOCA cohort.
On imaging, cerebellar atrophy occurred in both the Possible/Probable MSA-C and ILOCA groups, with no significant difference between them (Table 3B). In contrast, the MSA-C cohort was distinguished from ILOCA by the presence of brainstem atrophy (78% MSA-C vs. 10% ILOCA, p < 0.001). Middle cerebellar peduncle atrophy on MRI was reported in 41% of the Possible/Probable MSA-C cases (11 of 27 patients), but this did not reach statistical significance (p = 0.1) when compared with the ILOCA group, in which 1 out of the 10 MRI reports described MCP atrophy.
Clinical features of Possible/Probable MSA-C vs. Definite MSA-C
Comparison of the clinical features reported in the Definite MSA-C and the Possible/Probable MSA-C groups revealed one diagnostically critical difference and two expected differences.
REM sleep behavior disorder was reported in 43% of Possible/Probable MSA-C patients vs. 8% in Definite MSA-C patients (p = 0.04) at intake visit, and cumulatively, RBD was present in 58% of Possible/Probable MSA-C patients versus 25% of the Definite MSA-C group (p = 0.05). Notably, in some patients, RBD predated the development of cerebellar ataxia by a matter of years.
Pathologic laughing and crying and urinary incontinence became more prevalent as the disease progressed into its later stages. PLC was present in 25% of the Possible/Probable MSA-C patients cumulatively, versus 58% of the Definite MSA-C patients cumulatively (p = 0.04). Urinary incontinence was present in 66% of the Possible/Probable MSA-C group cumulatively compared to 100% of the Definite MSA-C group cumulatively (p = 0.03).
Clinical features of Possible MSA-C vs. Probable MSA-C
In a subgroup analysis of Possible and Probable MSA-C patients at Intake Visit (Table 4A), there was a higher prevalence in the Probable group of systolic blood pressure drop > 30 mmHg (as expected according to published Consensus criteria), erectile dysfunction, and corticospinal tract signs. In the cumulative visits the Probable group was more reliant on walkers and wheelchairs (Table 4B). All other parameters were similar between the two groups.
TABLE 4. Comparison of clinical features in Possible MSA-C vs. Probable MSA-C.
The clinical features are compared at (A) intake visit and (B) cumulative visit, as in Table 3, in Possible and Probable MSA-C patients whose diagnosis was not confirmed pathologically.
| A Possible MSA-C vs Probable MSA-C: Intake Visit | |||
|---|---|---|---|
| Possible MSA-C |
Probable MSA-C |
Possible MSA-C v. Probable MSA-C p |
|
| Affected examined (n) | 10 | 43 | |
| Cerebellar signs | 10 (100) | 43 (100) | 1 |
| Gait ataxia | 10 (100) | 42 (98) | 1 |
| Lower extremity dysmetria | 9 (90) | 42 (98) | 0.34 |
| Upper extremity dysmetria | 9 (90) | 42 (98) | 0.34 |
| Dysarthria | 9 (90) | 41 (95) | 0.47 |
| Oculomotor | 9 (90) | 37 (86) | 1 |
| Handwriting affected | 9 (90) | 33 (77) | 0.67 |
| Requires Walker | 2 (20) | 16 (37) | 0.46 |
| Wheelchair bound | 0 (0) | 4 (9) | 1 |
| Extrapyramidal signs | 3 (30) | 16 (37) | 1 |
| Bradykinesia | 1 (10) | 7 (16) | 1 |
| Rigidity | 2 (20) | 8 (19) | 1 |
| Resting tremor | 0 (0) | 4 (9) | 1 |
| Corticospinal signs | 0 (0) | 15 (35) | 0.046 |
| Autonomic features | 5 (50) | 41 (95) | 0.002 |
| Systolic blood pressure drop of at least 30 mm Hg |
0/9 (0) | 16/41 (39) | 0.043 |
| Presyncopal symptoms | 1 (10) | 15 (35) | 0.25 |
| Urinary urgency or frequency | 5 (50) | 26 (60) | 0.72 |
| Urinary incontinence | 1 (10) | 14 (33) | 0.25 |
| Erectile dysfunction | 2/7 (29) | 24/25 (96) | < 0.001 |
| Other clinical signs and symptoms | 9 (90) | 41 (95) | 0.47 |
| Vertigo | 1 (10) | 3 (7) | 1 |
| Shortness of breath | 0 (0) | 3 (7) | 1 |
| Inspiratory stridor | 0 (0) | 1 (2) | 1 |
| Loud snoring | 1 (10) | 5 (12) | 1 |
| Dysphagia | 1 (10) | 15 (35) | 0.25 |
| REM sleep behavior disorder | 4 (40) | 19 (44) | 1 |
| Fatigue | 1 (10) | 5 (12) | 1 |
| Pathologic Laughing and Crying | 0 (0) | 5 (12) | 0.57 |
| Frontal Release Signs | 0 (0) | 5 (12) | 0.57 |
| Tinnitus | 1 (10) | 3 (7) | 1 |
| Mood | 3 (30) | 11 (26) | 1 |
| Constipation | 2 (20) | 6 (14) | 0.64 |
| Bowel Urgency / Incontinence | 0 (0) | 1 (2) | 1 |
| Cold Extremities | 1 (10) | 3 (7) | 1 |
| Sensory abnormalities | 0 (0) | 5 (12) | 0.57 |
| Absent ankle reflexes | 0 (0) | 1 (2) | 1 |
| Double vision | 1 (10) | 7 (16) | 1 |
| Dizziness (not presyncopal) | 4 (40) | 10 (23) | 0.43 |
| Cognitive Difficulties | 4 (40) | 18 (42) | 1 |
| B Possible MSA-C vs Probable MSA-C: Cumulative Visits | |||
|---|---|---|---|
| Possible MSA-C |
Probable MSA-C |
Possible MSA-C v. Probable MSA-C p |
|
| Affected examined (n) | 10 (100) | 43 (100) | |
| Cerebellar signs | 10 (100) | 43 (100) | 1 |
| Gait ataxia | 9 (90) | 43 (100) | 0.19 |
| Lower extremity dysmetria | 9 (90) | 43 (100) | 0.19 |
| Upper extremity dysmetria | 9 (90) | 43 (100) | 0.19 |
| Dysarthria | 9 (90) | 42 (98) | 0.34 |
| Oculomotor | 9 (90) | 42 (98) | 0.34 |
| Handwriting affected | 9 (90) | 36 (84) | 1 |
| Requires Walker | 4 (40) | 35 (81) | 0.014 |
| Wheelchair bound | 0 (0) | 18 (42) | 0.011 |
| Extrapyramidal signs | 5 (50) | 32 (74) | 0.15 |
| Bradykinesia | 1 (10) | 16 (37) | 0.14 |
| Rigidity | 5 (50) | 19 (44) | 1 |
| Resting tremor | 1 (10) | 16 (37) | 0.14 |
| Corticospinal signs | 1 (10) | 21 (49) | 0.034 |
| Autonomic features | 8 (80) | 43 (100) | 0.033 |
| Systolic blood pressure drop of at least 30 mm Hg |
0 (0) | 34 (79) | 0.0001 |
| Presyncopal symptoms | 4 (40) | 25 (58) | 0.48 |
| Urinary urgency or frequency | 8 (80) | 36 (84) | 1 |
| Urinary incontinence | 4 (40) | 31 (72) | 0.07 |
| Erectile dysfunction | 3/7 (43) | 24/25 (96) | 0.004 |
| Other clinical signs and symptoms | 9 (90) | 43 (100) | 0.19 |
| Vertigo | 2 (20) | 3 (7) | 0.23 |
| Shortness of breath | 0 (0) | 8 (19) | 0.33 |
| Inspiratory stridor | 0 (0) | 4 (9) | 1 |
| Loud snoring | 1 (10) | 12 (28) | 0.42 |
| Dysphagia | 3 (30) | 25 (58) | 0.16 |
| REM sleep behavior disorder | 5 (50) | 26 (60) | 0.72 |
| Fatigue | 1 (10) | 10 (23) | 0.67 |
| Pathologic Laughing and Crying | 0 (0) | 13 (30) | 0.10 |
| Frontal Release Signs | 0 (0) | 11 (26) | 0.10 |
| Tinnitus | 1 (10) | 4 (9) | 1 |
| Mood | 4 (40) | 23 (53) | 0.48 |
| Constipation | 2 (20) | 15 (35) | 0.47 |
| Bowel Urgency / Incontinence | 1 (10) | 8 (19) | 1 |
| Cold Extremities | 1 (10) | 9 (21) | 0.67 |
| Sensory abnormalities | 0 (0) | 7 (16) | 0.32 |
| Absent ankle reflexes | 0 (0) | 2 (5) | 1 |
| Double vision | 1 (10) | 17 (40) | 0.14 |
| Dizziness (not presyncopal) | 6 (60) | 18 (42) | 0.48 |
| Cognitive Difficulties | 4 (40) | 22 (51) | 0.73 |
| Imaging | |||
| Cerebellar Atrophy | 4/4 (100) | 22/23 (96) | 1 |
| Cerebellar Peduncle Atrophy | 1/4 (25) | 10/23 (43) | 0.62 |
| Brainstem Atrophy | 4/4 (100) | 17/23 (74) | 0.55 |
Disease progression in MSA-C vs. ILOCA
Disease progression was faster in patients with MSA-C than those with ILOCA. 50% of patients with Definite MSA-C were dependent on walking aids (walker or wheelchair) during their initial visit to the Ataxia Unit (an average of 3 years into their disease course), compared to only 8% of patients with ILOCA. By 6 years into their disease course, 100% of the Definite MSA-C patients were dependent on walking aids compared to 33% of ILOCA patients.
Mean survival in the Definite MSA-C group was 8.4 ± 2.5 years, [5 – 13] (mean ± SD, [range]) from symptom onset. Cause of death in all cases of Definite MSA-C was either established by general autopsy findings as cardiorespiratory failure such as bronchiectasis, pneumonia, empyema, aspiration pneumonia, and acute cardiac decompensation, or presumed cardiorespiratory failure according to clinical scenario. One Definite MSA-C patient died from complications following a motor vehicle accident. There were no instances of sudden death in this cohort, either at the conclusion of the study (12 deceased) or in the time since the study was closed (an additional 4 deaths of the Probable MSA-C cases). Life-sustaining interventions such as endotracheal intubation for prolonged respiratory support were not used in any case, although one had a speaking tracheotomy to manage inspiratory stridor. One patient used continuous positive airway pressure ventilation at night to manage sleep apnea. Two had nutrition maintained with endoscopically placed gastrostomy feeding tubes, two had suprapubic cystostomies and two had indwelling urinary catheters for urinary retention.
One patient with ILOCA passed away during the study period from known pre-existing cardiovascular disease unrelated to his ataxia. Another patient passed away after the study end date from adenocarcinoma of unknown primary coming on 16 years after the onset of ataxia. All other ILOCA patients are still living 18 months following completion of the study at the time of submission of this manuscript, a mean length of illness, from symptom onset to date, of 15.9 ± 6.4 years, [6 – 26].
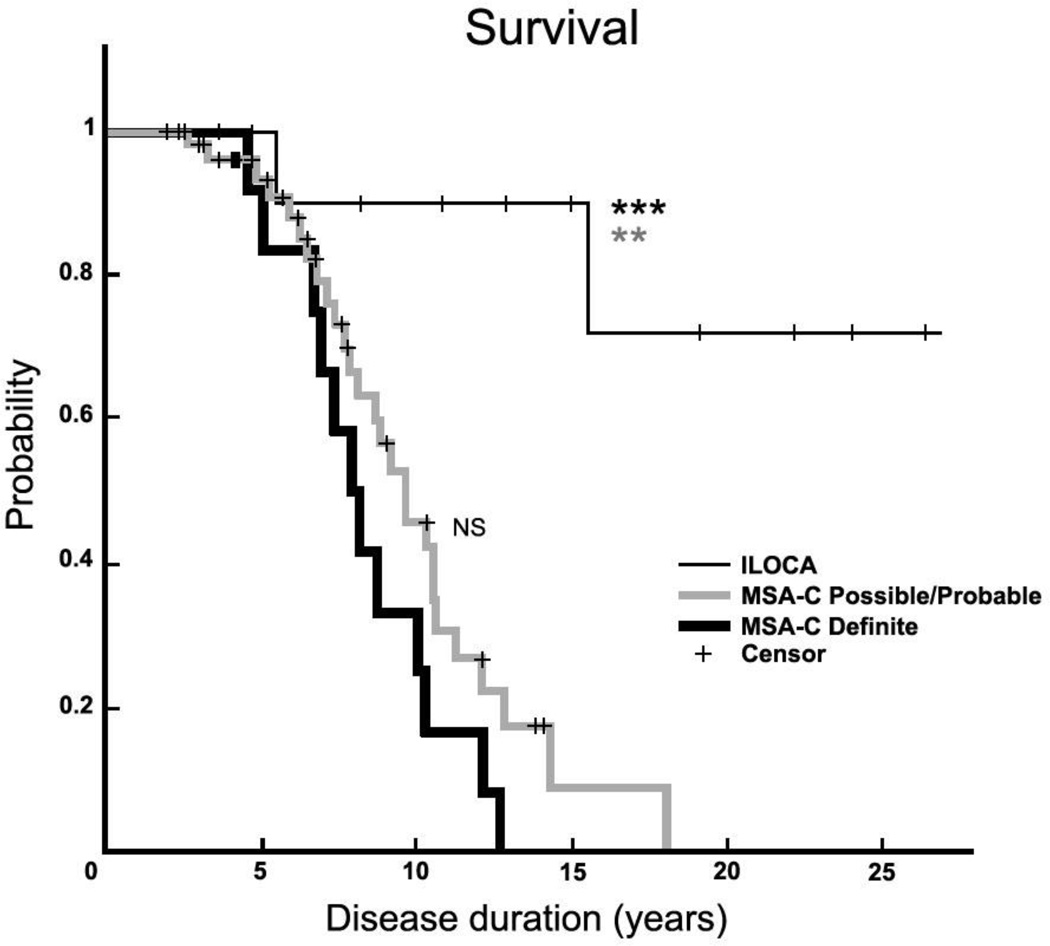
Of the 53 patients in the Possible/Probable MSA-C group, 29 (54.7%) have passed away. This Possible/Probable cohort had an average survival of 8.8 ± 3.4 years [3 – 18], very similar to that of the Definite MSA-C cohort (p = 0.74). By log-rank test, the Kaplan-Meier survival curves of the ILOCA group differed from both the Definite MSA-C (p < 0.001) and the Possible/Probable MSA-C (p = 0.001) groups (Figure 1). There was no difference between the survival curves of the Definite and Possible/Probable MSA-C cohorts (p = 0.15).
Figure 1. Survival plots in MSA-C and ILOCA.
Kaplan-Meier graph showing survival in patients with ILOCA (n=12, thin black line), Possible/Probable MSA-C (n=53, gray line), and Definite MSA-C (n=12, thick black line). Disease duration denotes years from symptom onset. NS, there is no significant difference (p = 0.15, log-rank test) between survival of Definite MSA-C (median survival 8.1 years) and Possible/Probable MSA-C (median survival 8.6 years). Patients with ILOCA have significantly longer survival than either patients with Definite MSA-C (p<0.001) *** or patients with Possible/Probable MSA-C (p=0.001) **. + denotes censored data points.
Pathology
The neuropathology reports of the Definite MSA-C patients are summarized in Table 5. All had severe pathology involving the white matter and cortex of the cerebellum and brainstem (basis pontis, MCP, and inferior olivary nuclei). Basal ganglia were next most affected, followed by the thalamus. Findings included volume loss, neuronal loss, gliosis, and white matter attenuation. All cases had abundant oligodendroglial cytoplasmic inclusions (GCI). The cerebellum and brainstem contained GCIs in all 11 cases. GCIs were prominent also in the cerebral cortex, subcortical white matter and internal capsule even in the absence of overt neuronal loss or gliosis. They were noted in the basal ganglia and thalamus in about one-third of cases. Other findings noted included hypertensive cerebrovascular disease in four patients. Neurofibrillary tangles (NT) consistent with Braak stage II/VI were seen in one 67-year-old man, and stage III/VI in a 73-year-old man.
TABLE 5. Neuropathology of Definite MSA-C patients.
The neuropathology reports of the Definite MSA-C patients are summarized. Data are presented as the absolute number of cases demonstrating pathology in this anatomical region (N) compared to the total number of cases in which these areas were available for review (T), as well as percent (%) involvement. Brainstem includes midbrain, pons, medulla, and middle cerebellar peduncles. n/a = not applicable
| Brain Area | Volume Loss N / T (%) |
Neuronal Loss N / T (%) |
Gliosis N / T (%) |
White Matter Attenuation N / T (%) |
α-synuclein inclusions N / T (%) |
|---|---|---|---|---|---|
| Cerebral Cortex | 2/11 (18) | 1/11 (9) | 2/11 (18) | n/a | 7/10 (70) |
| Subcortical White Matter | 1/11 (9) | n/a | 3/11 (17) | 2/11 (18) | 6/10 (60) |
| Hippocampus | 0/11 (0) | 2/11 (18) | 1/11 (9) | 1/10 (10) | 5/11 (45) |
| Amygdala | 0/11 (0) | 1/10 (10) | 2/11 (18) | 0/10 (0) | 1/11 (9) |
| Internal Capsule | 2/9 (22) | n/a | 3/10 (30) | 2/8 (25) | 8/8 (100) |
| Basal Ganglia | 4/11 (36) | 5/11 (45) | 6/12 (50) | 1/10 (10) | 3/11 (27) |
| Thalamus | 0/12 (0) | 2/11 (18) | 3/11 (27) | 1/11 (9) | 4/11 (36) |
| Cerebellum | 12/12 (100) | 12/12 (100) | 12/12 (100) | 12/12 (100) | 11/11(100) |
| Brainstem | 12/12 (100) | 12/12 (100) | 12/12 (100) | 12/12 (100) | 11/11 (100) |
| Spinal cord | 0/6 (0) | 1/6 (17) | 1/5 (20) | 2/6 (33) | 2/4 (50) |
| Sympathetic and dorsal root ganglia | 0/2 (0) | 1/2 (50) | 1/2 (50) | 0/2 (0) | 1/2 (50) |
Four of the 53 patients in the Possible/Probable MSA-C group died in 2014. All autopsies were performed at the MGH, and the findings met diagnostic consensus criteria for Definite MSA-C. However, these four cases were analyzed with the Possible/Probable cohort, as the entry into the study groups was closed for analysis in December 2013.
DISCUSSION
MSA-C and ILOCA, separate and distinct neurological disorders
Earlier reports questioned whether ILOCA and MSA-C are separate clinical entities, suggesting that ILOCA represents a slow variant of MSA-C.35,36 These prior reports lack pathologic confirmation. Our results, derived from a comparison of pathologically-confirmed Definite and consensus criteria Possible/Probable MSA-C patients with those clinically diagnosed with ILOCA, indicate that MSA-C and ILOCA are indeed distinct neurological entities.
Average age of symptom onset (AO) for our MSA-C cohorts was in the mid-fifties, Definite MSA-C 54.7 ± 8.5 years, Possible/Probable MSA-C 56.8 ± 8.7 years, similar to previously published results.4,5,39 Our ILOCA patients had an earlier AO of 41.1 ± 14.2 years. The range of AO was broad for both diseases, approximately 35 to 67 years for MSA-C, and 20 to 69 years for ILOCA. Abele and colleagues report that the median AO of ILOCA was 56 years in their 2002 study,35 and 47 years in their 2007 study.33 These differing AO may reflect the fact that the understanding of ILOCA continues to evolve as new genetic findings sculpt out genetic-ataxia syndromes from what was previously regarded as idiopathic ataxia.38–48 Even in our own ILOCA cohort, the finding of possibly pathogenic VUS in dominant ataxia genes (SCA5, 13, 14, 28, 35) in five cases is intriguing, but not yet established with certainty.
The natural histories of MSA-C and ILOCA are markedly different. Our MSA-C patients were dependent on walking aids by 6 years, compared to only one-third of ILOCA patients. A previous study found that half the patients with ILOCA were still ambulatory after 11 years.35 Mean survival in our Definite MSA-C group was 8.4 ± 2.5 years, similar to previously reported results.4,5,49 With the exception of two ILOCA patients who died from diseases unrelated to ataxia, all are alive and actively clinically followed in the Ataxia Unit with a current follow-up time of 7.8 ± 7.3 years [1.2 – 21.7]. The later age of onset of MSA-C patients and the more rapid disease progression compared to those with ILOCA, support the idea that these are in fact separate diagnoses.
A fresh perspective on diagnostic criteria for MSA-C
There were no significant differences between the clinical features and survival curves of the Definite MSA-C group (confirmed at autopsy) and the Possible/Probable group (clinical diagnosis, confirmed as Definite by autopsy in two individuals after study closure). This finding provides strong independent support for the clinical utility of the current consensus criteria for MSA-C.1 This is further supported by the comparison of the clinical features of our Possible and Probable MSA-C groups, in which the only differences reflected those required for the diagnostic distinction (the >30 mmHg systolic blood pressure drop), and the more advanced stage of the Probable group who had corticospinal findings, reliance on walker and wheelchair, and a higher incidence of erectile dysfunction.
Confusion in earlier reports has led to the view that the clinical features of MSA-C and ILOCA overlap and are complex to disentangle. The reality appears quite different. Our results differentiate between clinical features that are specific to MSA-C as opposed to those that occur also in ILOCA, and offer insights into the symptoms and signs necessary and sufficient for the early diagnosis of MSA-C.
Autonomic dysfunction is the single most important, reliable, and statistically significant feature differentiating MSA-C from ILOCA in the adult patient with sporadic-onset cerebellar ataxia. This is in line with recent reports that severe and progressive generalized autonomic failure combined with clinical phenotype is highly predictive of MSA (MSA-P and MSA-C)50 and that generalized autonomic failure and early bladder catheterization are independent predictors of mortality.49 In our cohort, urinary frequency, urinary incontinence, and erectile dysfunction in male patients were early and lasting features in patients with MSA-C. In contrast, patients with ILOCA did not develop urinary incontinence, although in the later stages of their long course there was a tendency to develop urinary frequency or urgency. Neurogenic orthostatic hypotension with a systolic blood pressure drop of > 30 mmHg accompanied by presyncopal symptoms developed in MSA-C patients, but not in ILOCA. It is important to note that orthostasis did not differentiate MSA-C from ILOCA patients at their initial visit. Thus, orthostasis is not a prerequisite for the diagnosis of MSA-C in its early stages, but rather may develop over time. Similarly, extrapyramidal signs and corticospinal tract signs did not differentiate early MSA-C from ILOCA, but emerged later in the disease course.
REM sleep behavior disorder (RBD), in the Possible/Probable MSA-C cohort, was present in 43% at initial visit, sometimes predating the development of ataxia, and was present by final visit in 58% of cases. This was a highly significant difference from the ILOCA cohort, none of whom developed RBD.
REM sleep behavior disorder did not reach significance when comparing Definite MSA-C to ILOCA. The discrepancy between the reported lower prevalence of RBD in Definite MSA-C (8% and 25% at intake and cumulative visits, respectively) and the high prevalence in Possible/Probable MSA-C most likely reflects ascertainment bias and highlights the need to ask about specific signs and symptoms in the clinical history. We began evaluating Definite MSA-C cases in 1992. The occurrence of RBD early in the course of MSA51–53 and other synucleinopathies such as Lewy Body Dementia54 was not recognized in the literature at that time and may not have been specifically sought in the clinical encounter. Patients do not generally volunteer this information. Rather, patients and their families need to be asked directly about the presence or absence of RBD, because we show here that its presence in a patient with sporadic ataxia points strongly to a diagnosis of MSA-C.
Pathologic laughing and crying was common in Definite MSA-C, developing in 58% of patients cumulatively, an even higher prevalence than the 36% we reported previously.54 This is consistent with the suggestion that lesions in the corticopontocerebellar fiber pathways (damaged in MSA-C but preserved in ILOCA) may underlie the pathophysiology of this syndrome.
We did not routinely perform laryngoscopic examinations looking for vocal cord dysfunction, but inspiratory stridor was reported by our patients or their family members, and detected on clinical examination. Stridor is a recognized clinical manifestation in MSA15, 52,55–59 and is a component of the current consensus criteria1. It occurred in 6 of the 65 MSA-C patients in our cohort (9.2%; cumulative visits), and in 1 of the 12 ILOCA cases (8%), which compares with prior reports of stridor frequency from 9%57 to as high as 61% in all cases of MSA, including both cerebellar and Parkinsonian forms.1,57–60 In our series, stridor did not distinguish MSA from ILOCA, possibly reflecting the limited number of ILOCA cases. Data on the incidence of inspiratory stridor in MSA-C versus MSA-P are not available to our knowledge, and it is possible that there is a difference in incidence of stridor in these two forms of the illness. Evaluation of this question will require a larger cohort that includes patients with MSA-C and those with MSA-P.
Half the patients examined in our MSA-C cohort (both Definite and Possible/Probable groups) reported depressed mood and performed below expectation on tests of mental state function, notably on tests of executive control such as phonemic fluency, verbal working memory, and spontaneous recall of newly learned information, with memory retrieval impaired more than storage. These features are in line with observations from recent studies of cognition in MSA-C,61 occurred with similar frequency in both MSA-C and ILOCA cohorts, and are consistent with the cerebellar cognitive affective syndrome.62,63 They are thus likely to reflect dysfunction of the cerebellum in these disorders, and are not specific to MSA-C. We note, however, the presence on autopsy of hypertensive cerebrovascular disease in four Definite MSA-C patients, and early stage neurofibrillary tangle deposition in two (albeit in hippocampal sector CA2 > CA1 which is characteristic of Lewy Body disease more than Alzheimer’s disease64). It is possible that these findings may be relevant in the domains of cognition, but the findings observed were not confined to older individuals, and cognitive impairments were documented in both the MSA-C and ILOCA cohorts.
The imaging findings that brainstem (mostly pontine) and MCP atrophy are specific for MSA-C versus ILOCA have been reported previously65,66 and are helpful diagnostically. Findings cited as hallmarks for MSA are the hyperintense putaminal rim67–68 and the hot-cross bun sign69–71 – a cruciform pattern of hypertensity in the basis pontis, both seen on T2-weighted MRI sequences. Neither of these findings is specific for MSA, however,72,73 and both emerge well after symptom onset, limiting their role in early diagnosis.74 Advanced imaging techniques such as MR-spectroscopy and diffusion tensor imaging are being studied in MSA,75–78 but it remains to be shown whether simple office-based radiographic measurements can provide further support for the clinical diagnosis.
Limitations of the study
Our study is the largest, single site, single investigator, longitudinal follow-up study to date of Possible, Probable and Definite MSA-C patients (n=65). Nevertheless, the findings are limited by the relatively small number of Definite MSA-C and ILOCA patients (12 in each group). Larger clinico-pathologic studies to validate our findings are warranted, and this is likely to require a multicenter approach. Despite these relatively small numbers of patients, we were able to define the clinical features of MSA-C in an unbiased manner, provide pathologic confirmation of 12 patients with Definite MSA-C (and a further 4 after the study concluded), and the findings were statistically robust.
This was, for the most part, a retrospective study, although 20 patients have been followed prospectively in the Ataxia Unit since the analysis of these data commenced in mid-2012. All the clinical data were collected by one investigator. This allows for uniformity of approach, and limits variability in clinical examination technique and interpretation of findings in the office/bedside setting. It should be noted, however, that not all patients were asked the identical set of questions at each encounter.
Ascertainment bias affected the prevalence of selected clinical features in MSA-C, as discussed for RBD and PLC. This highlights the need to inquire directly about these features in the patient interview. We did not study adrenergic, sudomotor, cardiovagal or thermoregulatory functioning. The use of laboratory-defined composite autonomic severity scores have recently been shown to be predictive in MSA.50 However, we were struck by the diagnostic power of simple, office-based approaches, including measures of supine and standing SBP, and targeted questioning about autonomic and other symptoms (bladder, erectile function, RBD, PLC). The laboratory measures are valuable, but not mandatory.
We lack pathologic confirmation of the ILOCA control cases, who have long, often normal, life-spans. It was not possible to obtain autopsies on the one ILOCA patient who succumbed to unrelated cancer, or the other who died of cardiac causes. Pathologic examination confirmed the Definite MSA-C diagnosis in four of the Possible/Probable MSA-C cases after the study end-date, and the similarity of the survival curves and the clinical features of the Definite MSA-C and Possible/Probable MSA-C groups supports the accuracy of the clinical diagnosis.
The brain MRI reports that we analyzed were not obtained at the same stage of the illness in all cases. It remains to be determined whether measures of pontine and MCP atrophy can be used to enhance early clinical diagnosis.
CONCLUSIONS
The diagnosis of MSA-C
Our analysis of a single-clinician, greater than two decade, longitudinal follow up of 65 patients with MSA-C (12 Definite, 53 Possible/Probable) and 12 ILOCA patients advances our understanding of the manifestations, diagnosis, and natural history of both MSA-C and ILOCA. It helps to confirm, clarify, and simplify the current consensus criteria for MSA-C, and it does so with the old-fashioned clinical neurology method, aided by a brain imaging study. The conclusion is straightforward. An adult in midlife who presents with a sporadic, insidious-onset cerebellar ataxia with accompanying autonomic features of otherwise-unexplained urinary bladder issues (frequency, urgency, or incontinence) with or without otherwise-unexplained erectile dysfunction in males, with evidence of volume loss in the cerebellum, basis pontis, and middle cerebellar peduncles on brain imaging, likely has MSA-C. When combined with postural hypotension or REM sleep behavior disorder, the clinical diagnosis of MSA-C is assured. Extrapyramidal, corticospinal signs, and PLC evolve later in the disease course, but may not be helpful early on. This pattern looks distinctly different than the hereditary ataxias and the long list of the other causes of cerebellar ataxia.79,80 Whereas sleep disorders have been identified in other ataxias,81 these are not accompanied by the prerequisite diagnostic features of MSA-C as described in the consensus criteria and identified and confirmed in our study. Such diagnostic clarity empowers the clinician to rely upon clinical skills and conventional MRI to make the diagnosis. The results of our investigation demonstrate that it is possible to diagnose MSA-C early and accurately, and distinguish it from other causes of ataxia, by eliciting a history that differentiates acute, subacute, and slowly evolving ataxias, obtaining a thorough symptom-based personal and family history, and performing a neurological examination designed to detect the cardinal features of the disorder. This is the prerequisite for disease-modifying therapeutic trials, some of which are now on the horizon.82 We note that whereas genetic analysis including the use of whole exome sequencing represents a watershed development in the diagnosis and management of the cerebellar ataxias,83 clinical genetic testing is not necessary when the patient has MSA-C. The MSA-C diagnosis, as we show in this study, is made at the bedside, supported by the appropriate findings on anatomical brain imaging (preferably MRI). That there is a possible complex genetic basis to MSA has already been shown,84,85 but those research findings have not yet impacted clinical care or decision making.
Acknowledgments
The authors gratefully acknowledge helpful discussions regarding statistical analysis with Hang Lee, PhD of the MGH Department of Biostatistics, the assistance of Jason MacMore, BA, and the clinical care and coordination of the patients in this study by Marygrace Neal, MA. The neuropathological studies were performed, and reports generated, by Dr. E. Tessa Hedley-Whyte and Dr. Matthew P. Frosch of the MGH Department of Neuropathology. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. This work was supported in part by the Birmingham Foundation and the MINDlink Foundation. This study is dedicated to our patients in the MGH Ataxia Unit and their families, without whose commitment to research into MSA-C and related ataxias this study could not have been performed.
Footnotes
Potential conflicts of interest
The authors report no other potential conflicts of interest.
REFERENCES
- 1.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn N. Multipe system atrophy- the nature of the beast. Journal of Neurology, Neurosurgery, and psychiatry. 1989;(Suppl):78–89. doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenning GK, Stefanova N. Recent developments in multiple system atrophy. J Neurol. 2009;256(11):1791–1808. doi: 10.1007/s00415-009-5173-8. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern. Med. 1994;33(6):321–325. doi: 10.2169/internalmedicine.33.321. [DOI] [PubMed] [Google Scholar]
- 5.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Movement Disorders. 2008;23(2):294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 6.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) Journal of the Neurological Sciences. 1989;94(1–3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 1998;249(2–3):180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 8.Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44(3):415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Crowther RA, Jakes R, et al. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 10.Fellner L, Wenning GK, Stefanova N. Models of Multiple System Atrophy. Curr Top Behav Neurosci. 2013 doi: 10.1007/7854_2013_269. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. 1999:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 12.May S, Gilman S, Sowell BB, et al. Potential outcome measures and trial design issues for multiple system atrophy. Movement Disorders. 2007;22(16):2371–2377. doi: 10.1002/mds.21734. [DOI] [PubMed] [Google Scholar]
- 13.Geser F, Seppi K, Stampfer-Kountchev M, et al. The European Multiple System Atrophy-Study Group (EMSA-SG) J Neural Transm. 2005;112(12):1677–1686. doi: 10.1007/s00702-005-0328-y. [DOI] [PubMed] [Google Scholar]
- 14.Gilman S, May SJ, Shults CW, et al. The North American Multiple System Atrophy Study Group. J Neural Transm. 2005;112(12):1687–1694. doi: 10.1007/s00702-005-0381-6. [DOI] [PubMed] [Google Scholar]
- 15.Wenning GK, Ben Shlomo Y, Magalhaes M, et al. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117(Pt 4):835–845. doi: 10.1093/brain/117.4.835. [DOI] [PubMed] [Google Scholar]
- 16.Köllensperger M, Geser F, Ndayisaba J-P, et al. Presentation, diagnosis, and management of multiple system atrophy in Europe: Final analysis of the European multiple system atrophy registry. Movement Disorders. 2010;25(15):2604–2612. doi: 10.1002/mds.23192. [DOI] [PubMed] [Google Scholar]
- 17.Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson DW. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2(8):1–15. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joutsa J, Gardberg M, Röyttä M, Kaasinen V. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat. Disord. 2014;20(8):804–814. doi: 10.1016/j.parkreldis.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Roncevic D, Palma J-A, Martinez J, et al. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm. 2014;121(5):507–512. doi: 10.1007/s00702-013-1133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallelunga A, Ragusa M, Di Mauro S, et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson's disease and Multiple System Atrophy. Front Cell Neurosci. 2014;8:156. doi: 10.3389/fncel.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jecmenica-Lukic M, Petrovic IN, Pekmezovic T, Kostic VS. Clinical outcomes of two main variants of progressive supranuclear palsy and multiple system atrophy: a prospective natural history study. J Neurol. 2014;261(8):1575–1583. doi: 10.1007/s00415-014-7384-x. [DOI] [PubMed] [Google Scholar]
- 23.Sako W, Murakami N, Izumi Y, Kaji R. The difference in putamen volume between MSA and PD: Evidence from a meta-analysis. Parkinsonism Relat. Disord. 2014;20(8):873–877. doi: 10.1016/j.parkreldis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015 Jul;14(7):710–719. doi: 10.1016/S1474-4422(15)00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002 May;125(Pt 5):1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 26.Kasahara S, Miki Y, Kanagaki M, Kondo T, Yamamoto A, Morimoto E, Okada T, Ito H, Takahashi R, Togashi K. “Hot cross bun” sign in multiple system atrophy with predominant cerebellar ataxia: a comparison between proton density-weighted imaging and T2-weighted imaging. Eur J Radiol. 2012;81:2848–2852. doi: 10.1016/j.ejrad.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Yabe I, Soma H, Takei A, Fujiki N, Yanagihara T, Sasaki H. MSA-C is the redominant clinical phenotype of MSA in Japan: analysis of 142 patients with probable MSA. J Neurol Sci. 2006 Nov 15;249(2):115–121. doi: 10.1016/j.jns.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 28.Lin DJ, Hermann KL, Schmahmann JD. Multiple system atrophy of the cerebellar type: clinical state of the art. Movement Disorders. 2014;29(3):294–304. doi: 10.1002/mds.25847. [DOI] [PubMed] [Google Scholar]
- 29.Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol. 2010;9(1):94–104. doi: 10.1016/S1474-4422(09)70305-9. [DOI] [PubMed] [Google Scholar]
- 30.Manto M, Gruol D, Schmahmann J, et al. Handbook of the Cerebellum and Cerebellar Disorders. Springer; 2012. [Google Scholar]
- 31.Harding AE. “Idiopathic” late onset cerebellar ataxia. A clinical and genetic study of 36 cases. Journal of the Neurological Sciences. 1981;51(2):259–271. doi: 10.1016/0022-510x(81)90104-0. [DOI] [PubMed] [Google Scholar]
- 32.Lin DJ, Hermann KL, Schmahmann JD. Multiple System Atrophy of the Cerebellar Type: Clinical State of the Art. Movement Disorders. 2014;(29 Suppl 3):294–304. doi: 10.1002/mds.25847. [DOI] [PubMed] [Google Scholar]
- 33.Abele M, Minnerop M, Urbach H, et al. Sporadic adult onset ataxia of unknown etiology. J Neurol. 2007;254(10):1384–1389. doi: 10.1007/s00415-007-0556-1. [DOI] [PubMed] [Google Scholar]
- 34.Jecmenica-Lukic M, Poewe W, Tolosa E, Wenning GK. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11(4):361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- 35.Abele M, Burk K, Schols L, et al. The aetiology of sporadic adult-onset ataxia. Brain. 2002;125(Pt 5):961–968. doi: 10.1093/brain/awf107. [DOI] [PubMed] [Google Scholar]
- 36.Gilman S, Little R, Johanns J, et al. Evolution of sporadic olivopontocerebellar atrophy into multiple system atrophy. Neurology. 2000;55(4):527–532. doi: 10.1212/wnl.55.4.527. [DOI] [PubMed] [Google Scholar]
- 37.Factor SA, Qian J, Lava NS, et al. False-positive SCA8 gene test in a patient with pathologically proven multiple system atrophy. Ann Neurol. 2005;57(3):462–463. doi: 10.1002/ana.20389. [DOI] [PubMed] [Google Scholar]
- 38.Munhoz RP, Teive HA, Raskin S, Werneck LC. CTA/CTG expansions at the SCA 8 locus in multiple system atrophy. Clinical Neurology and Neurosurgery. 2009;111(2):208–210. doi: 10.1016/j.clineuro.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Wenning GK, Kraft E, Beck RW, et al. Cerebellar Presentation of Multiple System Atrophy. Movement Disorders. 1997;12(1):115–117. doi: 10.1002/mds.870120121. [DOI] [PubMed] [Google Scholar]
- 40.Schols L, Szymanski S, Peters S, et al. Genetic background of apparently idiopathic sporadic cerebellar ataxia. Hum. Genet. 2000;107(2):132–137. doi: 10.1007/s004390000346. [DOI] [PubMed] [Google Scholar]
- 41.Kerber KA, Jen JC, Perlman S, Baloh RW. Late-onset pure cerebellar ataxia: differentiating those with and without identifiable mutations. Journal of the Neurological Sciences. 2005;238(1–2):41–45. doi: 10.1016/j.jns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Brussino A, Gellera C, Saluto A, et al. FMR1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology. 2005;64(1):145–147. doi: 10.1212/01.WNL.0000148723.37489.3F. [DOI] [PubMed] [Google Scholar]
- 43.Wardle M, Majounie E, Muzaimi MB, et al. The genetic aetiology of late-onset chronic progressive cerebellar ataxia. A population-based study. J Neurol. 2009;256(3):343–348. doi: 10.1007/s00415-009-0015-2. [DOI] [PubMed] [Google Scholar]
- 44.Margolin DH, Kousi M, Chan Y-M, et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N. Engl. J. Med. 2013;368(21):1992–2003. doi: 10.1056/NEJMoa1215993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce SB, Walsh T, Chisholm KM, et al. Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am. J. Hum. Genet. 2010;87(2):282–288. doi: 10.1016/j.ajhg.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieber DS, Vafai SB, Horton LC, et al. Atypical case of Wolfram syndrome revealed through targeted exome sequencing in a patient with suspected mitochondrial disease. BMC Med. Genet. 2012;13:3. doi: 10.1186/1471-2350-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieber DS, Calvo SE, Shanahan K, et al. Targeted exome sequencing of suspected mitochondrial disorders. Neurology. 2013;80(19):1762–1770. doi: 10.1212/WNL.0b013e3182918c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieber DS, Hershman SG, Slate NG, et al. Next generation sequencing with copy number variant detection expands the phenotypic spectrum of HSD17B4-deficiency. BMC Med. Genet. 2014;15:30. doi: 10.1186/1471-2350-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figueroa JJ, Singer W, Parsaik A, et al. Multiple system atrophy: Prognostic indicators of survival. Movement Disorders. 2014;29(9):1151–1157. doi: 10.1002/mds.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iodice V, Lipp A, Ahlskog JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. Journal of Neurology, Neurosurgery, and psychiatry. 2012;83(4):453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iranzo A, Santamaría J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65(2):247–252. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 52.Muntean M-L, Sixel-Döring F, Trenkwalder C. No Difference in Sleep and RBD between Different Types of Patients with Multiple System Atrophy: A Pilot Video-Polysomnographical Study. Sleep Disord. 2013;2013:258390. doi: 10.1155/2013/258390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghorayeb I, Bioulac B, Tison F. Sleep disorders in multiple system atrophy. J Neural Transm. 2005;112(12):1669–1675. doi: 10.1007/s00702-005-0348-7. [DOI] [PubMed] [Google Scholar]
- 54.Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42–47. doi: 10.1055/s-2006-956754. [DOI] [PubMed] [Google Scholar]
- 55.Parvizi J, Joseph J, Press DZ, Schmahmann JD. Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Movement Disorders. 2007;22(6):798–803. doi: 10.1002/mds.21348. [DOI] [PubMed] [Google Scholar]
- 56.Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson's disease--a retrospective comparison. Acta Neurol. Scand. 1995;91(2):98–102. doi: 10.1111/j.1600-0404.1995.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 57.Jecmenica-Lukic M, Poewe W, Tolosa E, Wenning GK. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11:361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- 58.Silber MH, Levine S. Stridor and death in multiple system atrophy. Mov Disord. 2000 Jul;15(4):699–704. doi: 10.1002/1531-8257(200007)15:4<699::aid-mds1015>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi M, Arai K, Asahina M, Hattori T. Laryngeal stridor in multiple system atrophy. Eur Neurol. 2003;49(3):154–159. doi: 10.1159/000069077. [DOI] [PubMed] [Google Scholar]
- 60.Wenning GK, Ben-Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995;58:160–66. doi: 10.1136/jnnp.58.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stankovic I, Krismer F, Jesic A, et al. Cognitive impairment in multiple system atrophy: A position statement by the neuropsychology task force of the MDS multiple system atrophy (MODIMSA) study group. Movement Disorders. 2014;29(7):857–867. doi: 10.1002/mds.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 63.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 64.Iseki E, Takayama N, Marui W, et al. Relationship in the formation process between neurofibrillary tangles and Lewy bodies in the hippocampus of dementia with Lewy bodies brains. Journal of the Neurological Sciences. 2002;195(1):85–91. doi: 10.1016/s0022-510x(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 65.Burk K, Globas C, Wahl T, et al. MRI-based volumetric differentiation of sporadic cerebellar ataxia. Brain. 2004;127(Pt 1):175–181. doi: 10.1093/brain/awh013. [DOI] [PubMed] [Google Scholar]
- 66.Bürk K, Bühring U, Schulz JB, et al. Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Arch Neurol. 2005;62(6):981–985. doi: 10.1001/archneur.62.6.981. [DOI] [PubMed] [Google Scholar]
- 67.Konagaya M, Konagaya Y, Iida M. Clinical and magnetic resonance imaging study of extrapyramidal symptoms in multiple system atrophy. Journal of Neurology, Neurosurgery, and psychiatry. 1994;57(12):1528–1531. doi: 10.1136/jnnp.57.12.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naka H, Ohshita T, Murata Y, et al. Characteristic MRI findings in multiple system atrophy: comparison of the three subtypes. Neuroradiology. 2002;44(3):204–209. doi: 10.1007/s00234-001-0713-7. [DOI] [PubMed] [Google Scholar]
- 69.Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. Journal of Neurology, Neurosurgery, and psychiatry. 1998;65(1):65–71. doi: 10.1136/jnnp.65.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54(3):697–702. doi: 10.1212/wnl.54.3.697. [DOI] [PubMed] [Google Scholar]
- 71.Kasahara S, Miki Y, Kanagaki M, et al. “Hot cross bun” sign in multiple system atrophy with predominant cerebellar ataxia: a comparison between proton density-weighted imaging and T2-weighted imaging. Eur J Radiol. 2012;81(10):2848–2852. doi: 10.1016/j.ejrad.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 72.Lee W-H, Lee C-C, Shyu W-C, et al. Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR Am J Neuroradiol. 2005;26(9):2238–2242. [PMC free article] [PubMed] [Google Scholar]
- 73.Burk K, Skalej M, Dichgans J. Pontine MRI hyperintensities (“the cross sign”) are not pathognomonic for multiple system atrophy (MSA) Movement Disorders. 2001;16(3):535. doi: 10.1002/mds.1107. [DOI] [PubMed] [Google Scholar]
- 74.Horimoto Y, Aiba I, Yasuda T, et al. Longitudinal MRI study of multiple system atrophy - when do the findings appear, and what is the course? J Neurol. 2002;249(7):847–854. doi: 10.1007/s00415-002-0734-0. [DOI] [PubMed] [Google Scholar]
- 75.Prakash N, Hageman N, Hua X, et al. Patterns of fractional anisotropy changes in white matter of cerebellar peduncles distinguish spinocerebellar ataxia-1 from multiple system atrophy and other ataxia syndromes. Neuroimage. 2009;(47 Suppl 2):T72–T81. doi: 10.1016/j.neuroimage.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 76.Lirng J-F, Wang P-S, Chen H-C, et al. Differences between spinocerebellar ataxias and multiple system atrophy-cerebellar type on proton magnetic resonance spectroscopy. PLoS ONE. 2012;7(10):e47925. doi: 10.1371/journal.pone.0047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oz G, Iltis I, Hutter D, et al. Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum. 2011;10(2):208–217. doi: 10.1007/s12311-010-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boesch SM, Wolf C, Seppi K, et al. Differentiation of SCA2 from MSA-C using proton magnetic resonance spectroscopic imaging. J. Magn. Reson. Imaging. 2007;25(3):564–569. doi: 10.1002/jmri.20846. [DOI] [PubMed] [Google Scholar]
- 79.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 80.Manto M-U, Pandolfo M. The Cerebellum and Its Disorders. Cambridge University Press; 2002. [Google Scholar]
- 81.Pedroso JL, Braga-Neto P, Felício AC, et al. Sleep disorders in cerebellar ataxias. Arq Neuropsiquiatr. 2011;69(2A):253–257. doi: 10.1590/s0004-282x2011000200021. [DOI] [PubMed] [Google Scholar]
- 82.Kuzdas-Wood D, Stefanova N, Jellinger KA, et al. Towards translational therapies for multiple system atrophy. Prog. Neurobiol. 2014;118:19–35. doi: 10.1016/j.pneurobio.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fogel BL, Lee H, Deignan JL, et al. Exome sequencing in the clinical diagnosis of sporadic or familial cerebellar ataxia. JAMA Neurol. 2014;71(10):1237–1246. doi: 10.1001/jamaneurol.2014.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scholz SW, Houlden H, Schulte C, et al. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol. 2009;65(5):610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in Familial and Sporadic Multiple-System Atrophy. N. Engl. J. Med. 2013;369(3):233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]