Abstract
Protein-based therapeutics have made a significant impact in the treatment of a variety of important human diseases. However, given their intrinsically vulnerable structure and susceptibility to enzymatic degradation, many therapeutic proteins such as enzymes, growth factors, hormones, and cytokines suffer from poor physicochemical/biological stability and immunogenicity that may limit their potential benefits, and in some cases limit their utility. Furthermore, when protein therapeutics are developed for intracellular targets, their internalization and biological activity may be limited by inefficient membrane permeability and/or endosomal escape. Development of effective protein delivery strategies is therefore essential to further enhance therapeutic outcomes to enable widespread medical applications. This review discusses the advantages and limitations of marketed and developmental-stage protein delivery strategies, and provides a focused overview of recent advances in nanotechnology platforms for the systemic delivery of therapeutic proteins. In addition, we also highlight nanoparticle-mediated non-invasive administration approaches (e.g., oral, nasal, pulmonary, and transdermal routes) for protein delivery.
Keywords: nanotechnology, nanoparticle, protein delivery, therapy, non-invasive administration
Graphical abstract
1. Introduction
The development of diverse protein therapeutics has seen an enormous surge over the past 3 decades, such as fully human antibodies, chimeric proteins, and new protein scaffolds capable of binding to —undruggable targets, which has resulted in effective therapies for a myriad of human diseases including diabetes, cancer, infection, and inflammatory diseases. Human insulin, approved by the US Food and Drug Administration (FDA) in 1982, was the first commercially available recombinant therapeutic protein, and has since become the major therapy for diabetes mellitus type I and type II [1]. Ever since, the protein market has been growing dramatically, and many other protein therapeutics, such as PEGINTRON (PegInterferon-α2b for hepatitis C), Fabrazyme (agalsidase beta for Fabry disease), Cotazym (pancrelipase for cystic fibrosis or pancreatic insufficiency), and others [1], have been approved for clinical use. Notably, monoclonal antibodies (mAbs) have represented a promising segment of the protein therapy field since the approval of Muromonab-CD3 in 1986 [2]. The recent clinical validations of immune checkpoint mAbs such as Nivolumab and Pembrolizumab, which target the programmed death-1 (PD-1) receptor, are considered one of the exciting advances in cancer immunotherapy [3]. A study by BCC Research indicates that the global market for bioengineered protein drugs was valued at $151.9 billion in 2013 and is expected to grow to about $222.7 billion in 2019 [4].
Compared with the conventional small-molecular drugs that continue to dominate the overall pharmaceutical market, protein therapeutics offer the advantages of higher specificity, greater activity, and less toxicity [5]. Nevertheless, the high specificity often requires maintaining the structural complexity of proteins, which can make them difficult to modify and/or formulate. Moreover, the susceptibility to enzymatic degradation, short circulation half-lives, and poor membrane permeability pose significant barriers for effective delivery of many therapeutic proteins (e.g., enzymes and cytokines) to targeted disease sites. To achieve high therapeutic performance, these unfavorable intrinsic characteristics of proteins need to be counterbalanced by designing appropriate delivery strategies or platforms. Improper design or formulation of protein drugs can cause degradation, denaturation, and/or aggregation of the protein molecules, potentially causing both immunogenic side effects after administration and loss of pharmacological activity. Approaches such as encapsulation within microparticles, chemical modification with hydrophilic polymers, and recombinant protein engineering have been clinically validated to enhance protein therapeutic efficacy. Despite the continuous launch of successful biological products into the market, the know-how and the technologies for the development of biologic drugs with optimal activity, stability, pharmacokinetic and lack of immunogenicity remain elusive today. Furthermore, while nearly all existing biologic drugs were developed against soluble or extracellular targets, the ability for biologic drug to enter cells and intracellular compartments can significantly broaden their utility for a myriad of exiting targets.
Nanotechnology has demonstrated tremendous promise for medical applications [6,7]. Thus far, dozens of nanomedicines have already been approved for clinical use, and many more are under clinical investigation [8,9]. In particular, nanoparticles such as liposomes, micelles, polymer nanoparticles, and inorganic nanomaterials, which are typically in the range of 10–150 nm in size, have considerable advantages as drug carriers. In protein delivery, nanoparticle technologies can: i) protect proteins from premature degradation or denaturation in biological environment; ii) enhance systemic circulation half-life of proteins with poor pharmacokinetic potteries; iii) control sustained and/or tunable release which can maintain drug concentration in the therapeutic range; and iv) target diseased tissues, cells, and intracellular compartments, thus improving the safety and efficacy of biologic therapeutics. The considerable success of nanoparticle formulations of small-molecules such as doxorubicin (Doxil and Myocet), daunorubicin (DaunoXome), paclitaxel (Abraxane), and amphotericin B (Ambisome) has paved the way for the exploration of nanoparticle technologies for protein delivery [10]. This review summarizes marketed and developmental-stage protein delivery strategies, recent progress in intracellular protein delivery, design considerations of nanoparticle technologies and their advancement on systemic protein delivery, and the application of nanotechnology to develop non-injectable protein therapeutics that can enhance patient satisfaction and compliance.
2. Marketed protein delivery strategies
2.1. Microparticle delivery
Biodegradable polymeric microparticles (1–1000 μm) are promising parenteral depot formulations for long-term protein drug release (from weeks to months), in particular when the maintaining of protein concentration in therapeutic range is required for more than 1 week. They enable sustained release of proteins by both diffusion from the polymer matrix and the degradation/erosion of the polymer [11,12]. One of the most widely used materials for the encapsulation of proteins is poly(lactic-co-glycolic acid) (PLGA), as it is biocompatible, biodegradable with favorable degradation rates, and already approved by the FDA for use in humans [13]. Encapsulation of proteins into polymeric microparticles can be achieved by several methods such as double emulsion (the most widely used technique), single emulsion, phase separation (coacervation), ultrasonic atomization, spray-drying, and microfluidics [13]. Once the proteins are encapsulated into microparticles, their release kinetics depend on the microparticle size, molecular mass of the polymer, degradation rate, charge property of the polymers, ratio of hydrophilicity to hydrophobicity, polydispersity of microparticle size, protein loading amount, as well as the surrounding microenvironment. Currently, there are a number of microparticle protein-delivery formulations (e.g., Trelstar depot) on the market, and various microparticles are under preclinical development for delivering therapeutic proteins such as bone morphogenetic protein-2 [14], insulin [15], recombinant human epidermal growth factor [16], and recombinant human erythropoietin (EPO) [17]. However, these clinically successful microparticle systems may cause blockage of the needle required for administration, and the bioactivity of the released proteins under physiological conditions need to be considered for long-term delivery. Extended protein stability is still challenging, and in addition, degradation and erosion of biodegradable polymers including PLGA can lower the pH inside the microparticles, which can further lead to denaturation of the protein as well as aggregate formation.
2.2. Chemical Modification
Proteins smaller than 70 kDa are mostly cleared from the systemic circulation by glomerular filtration [18]. Chemical modification of proteins with hydrophilic polymers can reduce renal clearance by increasing their molecular weight and/or hydrodynamic dynamic radius. The covalent attachment of polyethylene glycol (PEG) chains to proteins (PEGylation), as a typical example, enhances protein stability and pharmacokinetic (PK) properties, and these benefits have allowed some PEGylated therapeutic proteins (e.g., Adagen, Somavert, Oncaspar, and Naloxegol) to reach the market, with many other examples in various stages of clinical development. [19,20]. Hyperglycosylation can also extend biological half-life and improve stability by increasing the solubility and immunogenicity of proteins. The addition of sugar molecules to a protein is a more natural process than PEGylation, since it is already a part of the endogenous post-translational enzymatic process, and polysaccharides are readily degraded into native glucose molecules [21]. N-glycosylated EPO (Aranesp) has been marketed by Amgen since 2001, and other glycosylated protein drugs are under preclinical and clinical investigation such as polysialylated forms of EPO, granulocyte-colony stimulation factor (G-CSF), and insulin [22]. Although chemical modification prolongs the circulation half-life of proteins, this approach may require complicated synthesis and impart unfavorable conformational changes as well as loss of both biological activity and binding affinity to the target due to steric hindrance and heterogeneity [23]. Such alterations in physicochemical properties leads to the systemic exposure of proteins in order to reach sufficient pharmacological potency, but toxicities related to peak exposure can limit clinical use. Various efforts to maintain protein activity include site-specific modification. For example, chemical ligation of synthetic peptides including levulinyllysine to EPO elicited hematopoietic activity superior to native protein [24]. More recent advances in chemo-selective targeting show that the incorporation of canonical and noncanonical amino acids can enhance selectivity while improving PEG architecture [25].
2.3. Genetic engineering
In addition to chemical modification, genetic constructs and fusion technologies to elevate protein half-life and therapeutic efficacy have been intensively studied. Fc-based fusion proteins composed of an immunoglobin Fc domain genetically linked to the therapeutic protein represent a promising approach, as Fc-fusion can endow a protein with unique effector functions mediated by Fc receptor binding and complement fixation [26]. The neonatal Fc receptor (FcRn)–mediated recycling and transcytosis process extends half-life (e.g., IgG: up to 21 days); in addition, the increased molecular weight of fusion proteins through the size of the Fc-domain (~ 50 kDa) reduces renal clearance [27]. A number of therapeutic proteins based on fusion with the IgG Fc domain have come into clinical use since Fc-fused tumor necrosis factor (TNF) receptor-2 (Enbrel; Amgen/Pfizer) was approved for the treatment of rheumatoid arthritis and plaque psoriasis in 1998, and several other candidates are currently in clinical trials [28]. Recent work on the Fc-fusion technology also focuses on retaining biological activity and binding affinity, which are often decreased after the fusion process [29,30]. Jung et al. included a chaperone‘ protein in Toll-like receptor 4 Fc-fusion to stabilize the desired partner [31]. Newly developed heterodimeric Fc platforms, based on strand-exchange engineered-domain CH3 heterodimers consisting of alternating segments of human IgA and IgG CH3, show multiple specificities within the homodimeric Fc-fusion platform [32]. Utilizing alternative backbones, such as IgA, IgE, and IgM, may also benefit the activity of the fused partner [33–35]. However, concerns remain about the immunogenicity of Fc-fusion proteins, because interactions between the Fc domain and its receptors have multivariable immunological consequences, which might limit their usefulness in the treatment of chronic disease [36]. Other attempts to target FcRn, including albumin fusion (which has direct interaction with FcRn) and genetic engineering of Fc domains have also been reported. A glucagon-like peptide-1 (GLP-1) albumin fusion achieved ~ 5-day half-life and received FDA approval (Albiglutide; GSK) for the treatment of type-2 diabetes [37]. A recombinant polypeptide fusion construct, which consists of an unstructured polypeptide and protein drug, is another example of generic fusion technology capable of extending plasma half-life. Schellenberger et al. developed exenatide-XTEN fusion and demonstrated ~58-fold increased half-life and low immunogenicity in animals, even in the presence of the adjuvant [38]. Still, issues remain in the safety of fusion approaches, in particular fusion with native human proteins, because of cross-reactivity with endogenous homologues, which can affect long-term safety and clearance of subsequent doses [39].
3. Intracellular protein delivery
Although protein therapeutics has the potential to directly restore dysfunctional, lost, or rarely expressed proteins in many types of diseases such as cancer, neurodegenerative disease, inflammatory disease, and genetic disease, current clinical applications are mostly restricted to targets in the vascular compartment or extracellular areas, which are systemically accessible. The main barriers to delivering proteins against intracellular targets are their limited membrane permeability and endosomal entrapment [40]. One strategy for overcoming their poor membrane permeability is synthetic attachment or genetic fusion of proteins with cell-permeable sequences. Cell-penetrating peptides (CPP) like TAT-derived peptides, arginine-rich peptides, and amphiphilic peptide carriers such as Pep-1 are efficient cell-permeable sequences, because they are capable of several theoretical translocation mechanisms such as direct penetration, endocytosis-mediated uptake, and translocation via transitory structure formation; thus they have been used to improve intracellular protein delivery [41]. Jo et al. reported CPP (R7)-conjugated recombinant protein delivery into human stromal cells and showed its potential for manipulation of human mesenchymal stem cells [42]. However, the efficiency of cytosolic access is debatable, since CPP-protein conjugates often tend to concentrate inside the endosome [43,44]. One clinical trial, that of delcasertib (KAI-9803) as a TAT-protein kinase C inhibitor peptide modulator, failed to treat heart tissue damage after artery-opening surgery in a phase IIb study, despite this therapeutic system‘s effective inhibition in an acute myocardial infarction animal model [45,46].
Alternatively, virus-like particles (VLPs) have been identified as a highly adaptable platform to deliver proteins. During the past decade, VLPs have been mainly used as vaccines [47,48] or to deliver foreign proteins by generating nonstructural HIV proteins [49]. Safer versions of these systems based on an avian retrovirus were reported recently, in which the construct used to express the VLPs did not contain the Pol gene, thus avoiding the unwanted integration of virion DNA into the host chromosome in transduced cells. This demonstrated the effective intracellular delivery of biologically active proteins and their cellular responsiveness, such as TNF-related apoptosis-inducing ligand (TRAIL)-induced cell death signaling [50]. However, the use of VLPs for intracellular protein delivery requires further evaluation of immunogenicity and safety by in vivo and clinical studies.
Genetic fusion with zinc-finger motifs has recently been demonstrated as an intracellular delivery technology [51]. In this system, the intrinsic cell-permeability of the Cys2-His2 zinc-finger domain enables protein delivery into a wide variety of primary and transformed mammalian cells primarily through micropinocytosis. Gaj et al. reported that electrostatic interactions between the basic residues of one Arg and five Lys (present on the zinc-finger surface) and negatively charged lipids might stabilize the zinc-finger configuration and facilitate its insertion into the membrane interior, notably improving cytosolic delivery [52]. Nonetheless, except for some specific cases, the lack of cell specificity in protein drugs linked to cell-permeable sequences remains the major drawback to their clinical development. In addition, their unique cell-permeable nature (for example, their cationic properties) in intracellular protein delivery can cause toxicity, especially with chronic use [53].
4. Nanoparticle-mediated protein delivery
4.1. Nanoparticle design considerations
In general, nanoparticles tend to aggregate to minimize surface energy, and such aggregates and their surface properties (e.g., charge and hydrophobicity) can trigger opsonization in the blood after systemic delivery, making nanoparticles more recognizable to biological defense systems such as the mononuclear phagocyte system (MPS) and subjecting them to clearance from the circulation by defense cells, greatly lowering their effectiveness [54]. Moreover, the heterogeneity and highly ionic nature of blood can easily increase nanoparticle aggregation, losing the functionalities of nanoparticle-based drug delivery platforms designed for protein delivery. Therefore, increasing the blood circulation half-life while retaining function is the first goal in developing nanoparticles as protein delivery carriers (Fig 1a). One strategy to avoid non-specific adsorption of opsonins to nanoparticles is surface engineering with coating materials that offer stealth properties. Biocompatible and hydrophilic polymers (e.g., PEG), small organic molecules (e.g., zwitterionic materials), and biomimetic surfaces (e.g. red blood cell membrane, human CD47 or its peptide variant) have been utilized to create stealth nanoparticles [55–57]. The hydrodynamic size of nanoparticles also strongly affects the length of their circulation. Overall, smaller nanoparticles are prone to escape phagocytosis and circulate in the bloodstream longer; in contrast, larger ones (> 200 nm) may be more easily cleared by the liver, spleen, and lung [58,59]. Additionally, for safe and prolonged blood circulation, a neutral-charged surface is preferable to a charged one, because the latter can result in nonspecific internalization after binding with nontargeted cells, or activate plasma complement proteins (e.g., factor H and C3b) [60, 61].
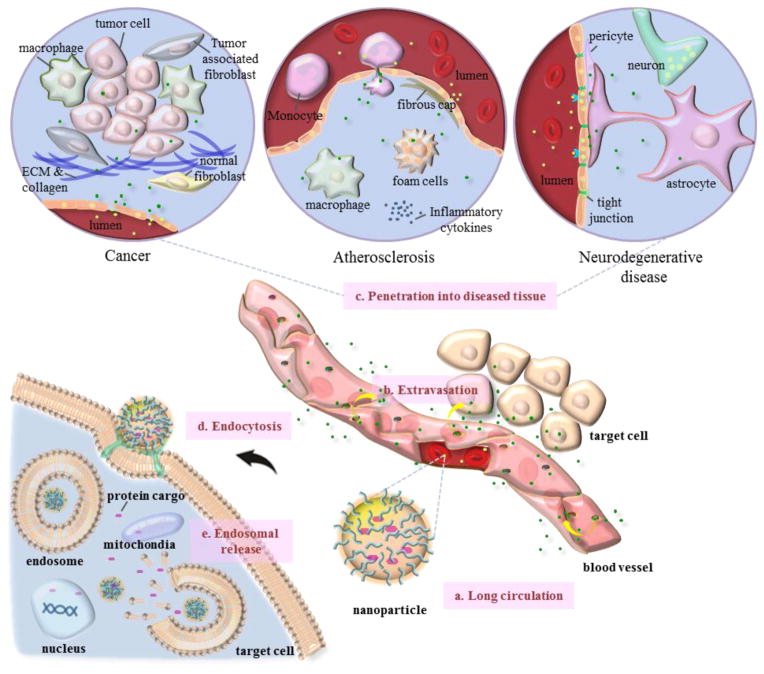
Fig 1. Considerations in designing nanoparticles for protein delivery.
(a) Long circulation: nanoparticles injected into the bloodstream are prone to clearance by the mononuclear phagocyte system (MPS). Surface engineering, hydrodynamic size control, and geometric alterations of the nanoparticles can increase the blood circulation half-life. (b) Extravasation: circulating nanoparticles need to extravasate from the vasculature and accumulate in the diseased tissue. (c) Penetration into diseased tissue: nanoparticles that escape the blood vessel need to penetrate diseased tissues such as those affected by cancer, atherosclerosis, and neurodegeneration, which pose substantial hurdles. (d) Endocytosis: once the nanoparticles arrive at the surface of target cells, they must enter the cells via endocytosis. (e) Endosomal release: nanoparticles that are internalized by the cells must escape from the endosome to release active protein cargo into the desired subcellular compartments, such as the cytosol, mitochondria, or nucleus.
In addition to the consideration of long circulation, systemically delivered nanoparticles face several other physiological barriers such as access to the disease sites and exposure to the targets before exhibiting their biological activity. But fortunately the molecular events underlying several diseases including cancer, atherosclerosis, and neurodegenerative disease give nanoparticles opportunities for access. The rapidly growing endothelium in tumor angiogenesis or the dysfunctional endothelium in atherosclerosis cause large gaps that allow nanoparticles to extravasate from the blood circulation at local sites. In addition, when the nanoparticles are delivered around the tumor region, they are retained locally due to impaired lymphatic drainage. This mechanism is called the enhanced permeability and retention (EPR) effect, and it allows passive targeting and selective accumulation of nanoparticles in these lesions (Fig 1b) [62,63]. In the case of atherosclerosis, nanoparticles in the systemic circulation are extravasated via the leaky neovessels of the vasa vasorum as well as the permeability of the luminal endothelium, and they may associate with circulating cells or cells in the spleen that subsequently migrate to inflammatory areas, which are a major target in atherosclerosis [64]. Most of the microenvironment in human lesions, especially the tumor tissue itself, is remarkably abnormal; however, as vessel permeability is heterogeneous, the blood supply is low, and the blood flow is unevenly distributed, the penetration and distribution of the nanoparticles throughout the lesions via the EPR effect alone is therefore limited (Fig 1c) [65]. Generally, larger nanoparticles travel slowly through the interstitial space and have the benefit of accumulation, but such nanoparticles are also subject to restricted penetration into the tumor mass. In contrast, smaller nanoparticles usually penetrate tumors more rapidly and uniformly than larger ones, but they can also be readily washed out into the blood because of elevated interstitial fluid pressure (IFP). In addition, very small particles (<11 nm) are eliminated quickly by renal and hepatobiliary clearance [66,67]. The inadequate retention of nanoparticles can be improved by attaching targeting ligands such as antibodies, peptides, or other small molecules to their surface, thus increasing their accumulation at diseased sites, which is a form of active targeting [68]. Chauhan et al. suggested vascular normalization, which involves correction of excessive angiogenesis signaling to repair abnormalities in vascular structure and function, and reported improved penetration of the nanoparticles against the tumor through several technologies, including anti-angiogenics, reduction of IFP, and increased perfusion [66, 69–71]. On the other hand, non-spherical (e.g., rod-shaped) nanoparticles can penetrate into diseased areas more rapidly and accumulate at higher levels than size-matched spheres, as the shortest dimension of the nanoparticle determines its penetration [72]. Neurodegenerative disease is an area of particular focus, owing to the difficulty of developing therapeutics that can penetrate the central nervous system (CNS), which requires crossing the blood-brain barrier (BBB), where the brain endothelial cells are connected by tight junctions with an extremely high electrical resistivity. Active transport utilizing overexpressed receptors in the brain such as transferrin and insulin receptors has been considered an attractive approach to increase the penetration of drugs after systemic administration. Since nanoparticles decorated with targeting ligands can penetrate brain capillary endothelial cells through receptor-mediated endocytosis, these targeting strategies for the treatment of CNS diseases holds promise for enabling crossing of the BBB [73].
Once nanoparticles pass through diseased sites and arrive at the surface of the target cells, they must enter the target cells to reach desired subcellular compartments such as the cytosol, the mitochondria, and the nucleus (Fig 1d). Optimization in engineering of surface coating materials and targeting ligands is important for successful endocytosis of nanoparticles [74,75]. Subsequently, they must be able to escape from the endosome to release the active protein cargos at their targets (Fig 1e). Development of stimuli-responsive nanoparticles that respond predictably to cellular environmental conditions such as pH and redox can achieve on-demand‘ release of protein drugs into desired targets [76–78].
4.2. Nanoparticle platforms
4.2.1. Polymeric nanoparticles
Polymeric nanoparticles have been explored as promising drug delivery vehicles for decades, as their molecular architectures/properties can be tuned by changing molecular weight and composition of the repeating unit and residues on the polymer backbone. However, in protein therapeutics, the encapsulation of proteins in the nanoparticles is still challenging, since various organic solvents essential to the preparation of protein-carrying nanoparticles can denature proteins or harm their biological activity; in addition, the loading efficiency of proteins into nanoparticles is generally low. To overcome these difficulties, Wu et al. designed PLGA-polycation (PC) nanoparticles, which can efficiently capture proteins without organic solvents, i.e., in a “green” manner [79]. The PCs were prepared by making an L-arginine-based PC library including NH2 termini (NH2-PC-NH2), and the PCs were conjugated with PLGA-COOH to make diblock copolymers of PLGA-b-PC. The core-shell structure of PLGA-PC nanoparticles was created through the nanoprecipitation method, yielding a size range of 20–80 nm, depending on the PLGA-b-PC composition. Protein loading was achieved by electrostatic interactions between the cationic PLGA-PC nanoparticles and negatively charged proteins such as bovine serum albumin (BSA), insulin, and TNF-α. The complex nanostructures (<200 nm) indicated more than 20 wt% of protein-loading capacity and ~95% encapsulation efficiency and were capable of sustained release of proteins over the course of several weeks. The same research group developed another protein-carrying nanoplatform, thermosponge nanoparticles (TNP), in a green manner (Fig 2a) [80]. They prepared temperature-responsive polymeric nanoparticles composed of negatively charged (PLA-COOH) or positively charged (PLA-NH2) polymer cores and temperature-responsive swelling/deswelling Pluronic shell through nanoprecipitation. The prepared TNPs were around 60 nm, which grew to ~100–140 nm at 4°C due to the expanded Pluronic polymer shell, preparing them to adsorb proteins effectively. The positively charged proteins (IL-10 and erythropoietin) and the negatively charged proteins (insulin and human growth hormone) were loaded into each type of TNP via the electrostatic interaction between proteins and TNPs, as well as the volume expansion of the shell. Upon further change of the temperature from 4°C to 37°C, the shells of the TNPs shrank, compressing the TNPs (<50 nm), which is a favorable size range for in vivo use. As shown in Fig 2b–g, protein-loaded TNPs demonstrated greater half-life than the parent drugs; in addition, IL-10-loaded TNPs (IL-10@TNPs) significantly reduced inflammation in a dinitrofluorobenzene (DNFB)-induced allergic contact-dermatitis (ACD) animal model. The mice treated with IL-10@TNPs had reduced ear swelling, less edema and myeloid infiltration, and lower neutrophil numbers than mice treated with IL-10 alone, indicating that the protein-friendly TNPs are a promising nanoplatform for in vivo therapeutic protein delivery.
Fig 2. Schematic illustration of a TNP platform and in vivo anti-inflammatory efficacy.
(a) The proteins are incorporated into the core of the nanoparticles via electrostatic interaction when the Pluronic shell is expanded, to form a stable complex with polymer after shrinkage of the shell. (b–c) Pharmacokinetics of protein-loaded TNPs. Changes in serum protein levels in mice after intravenous administration of (b) IL-10 and IL-10–loaded TNP, and (c) insulin and insulin–loaded TNP. (d) Therapeutic efficacy of IL-10 and TNPs on ear swelling in a mouse model of ACD at 100 μg IL-10/kg dose via i.v. administration. (e) Total neutrophils in skin at 36 h upon acetone or DNFB challenge. (f,g) Representative histological images of DNFB-treated ears from IL-10 and IL-10@TNP groups. Reprinted with permission from ref 80. Copyright 2014 American Chemical Society.
Stimuli-responsive characteristics of polymers are also attractive for the design of various protein-carrying nanoplatforms, especially for targeting intracellular compartments. At the cellular level, pH-sensitivity can either trigger the release of cargo drugs into late endosomes or lysosomes, or promote the escape of the nanoparticles from acidic organelles to the cytosol. Yan et al. reported pH-responsive nanocapsules that are degraded after endocytosis to release active proteins and enable apoptosis in response to macromolecular substrates [81]. They developed nanocapsules with a single-protein core and thin polymer shell through radical polymerization in the presence of an acryloylated protein solution, a neutral or positively charged vinyl group containing monomers (acrylamide and 2-dimethylaminoethyl methacrylate), and non-degradable or degradable crosslinkers (N,N′-methylene bisacrylamide and glycerol dimethacrylate). The synthesized spherical nanocapsules indicated ~15 nm hydrodynamic size surrounded by ~5 nm of polymeric shell, and their surface charge and degradability were adjusted by varying the ratios of the cationic and neutral monomers, and the amount of non-degradable and degradable crosslinkers, respectively. Proteins such as horseradish peroxidase (HRP) and caspase-3 (CAS), used as the core of the nanocapsules, showed gradual release into the cellular cytosol, because the cationic nanocarriers acted as a proton-sponge‘ within the endolysosomal organelles, leading to osmotic disruption as these compartments became increasingly acidified. Moreover, at 8 h post-injection their biological activity within mice organ sections was significantly greater than that of native proteins, indicating that the polymer shell not only efficiently protects the protein core from proteolysis in vivo, but also enables escape from the endosome to release proteins in a controlled manner. Gu et al. utilized pH-sensitivity in an injectable and acid-degradable polymeric nano-network for the self-regulated delivery of insulin [82]. Oppositely charged acid-degradable nanoparticles consisting of a chemically modified dextran core coated with chitosan or alginate were self-assembled to create a gel-like porous structure. To create a chemically controlled closed-loop system that is able to deliver accurate levels of insulin continuously, glucose oxidase (GOx) and insulin were then encapsulated into the nanoparticles. The nano-network was hydrolyzed into native dextran as the pH decreased as a result of the enzymatic conversion of glucose to gluconic acid in a hyperglycemic condition, and the encapsulated insulin was released by the subsequent degradation of the polymeric matrix. The blood glucose levels of diabetic mice treated with one injection of the nano-network via subcutaneous administration were stably maintained in the normoglycemia range (<200 mg/dL) for up to 10 days, demonstrating the effectiveness of the glucose-mediated release strategy and long-term diabetes management.
Another stimuli-responsive system for in vivo cancer therapy, redox-responsive polymeric nanocapsules, was reported by Zhao and colleagues [83]. This work used apoptin protein (APO), which can induce tumor-specific apoptosis by phosphorylation at Thr-108 (which is absent in normal cells) as the therapeutic protein. The APO was fused with maltose-binding protein (MBP) to make a soluble MBP-APO complex, in which the APO was arranged to be accessible to its protein partners, and in situ polymerization was performed without covalent bonds between the protein cargo and the polymeric shell. The polymeric layer was constructed using redox-responsive cross-linkers containing disulfide bonds (S-S) that are degraded once the nanocapsules are exposed to the reducing environment in the cytoplasm. The hydrodynamic size of the S-S APO nanocapsules was ~75 nm, and the surface had a lightly positive charge of 2.8 mV. Their redox-responsive activity was confirmed by measuring reduced size of the nanocapsules into ~30 nm of MBP-APO preassembles upon treatment with the glutathione (GSH). The S-S APO nanocapsules were highly internalized into target cells while protecting the proteins from degradation or aggregation by the serum proteases or surrounding environment (respectively) and showed selective cytotoxicity in cancer cells such as HeLa and MCF-7 by releasing the MBP-APO into the nucleus. In contrast, the APO nanocapsules remained in the cytosol, indicating that proteins well shielded by the nondegradable polymer shell could not localize into the nucleus. Also, noncancerous human foreskin fibroblast cells treated with the S-S APO nanocapsules did not exhibit nuclear accumulation of the proteins, resulting in ~75% cell viability, whereas all other cancer cells treated with the S-S APO nanocapsules indicated almost 0% viability at the same concentration. The tumor-specific cytotoxic ability of the nanocapsules was further demonstrated in a MCF-7-bearing xenograft animal model. Intratumoral administration of the S-S APO nanocapsules every other day for 10 days caused considerable levels of apoptosis in the tumors as a result of APO-mediated DNA fragmentation, translating into a significant delay in tumor growth. These results suggest that redox-responsive strategies based on nanoparticles have attractive potential as applied to intracellular protein delivery systems for the treatment of disease.
Many neurodegenerative diseases involve accumulation of intracellular aggregates of misfolded protein, and these can be useful targets for therapeutic drug development [84,85]. However, a major barrier to therapeutic protein delivery is crossing the BBB in the neuronal membrane. In response, Hasadsri et al. designed polybutylcyanoacrylate (PBCA) nanoparticles and demonstrated their ability to deliver proteins into neurons and neuronal cell lines [86]. Three different functional cargo proteins [Escherichia coli β–galactosidase (β-gal), small GTPase rhoG, and the mouse anti α–aynuclein monoclonal antibody H3C] were utilized as model proteins. The proteins were loaded into fluorescent PBCA nanoparticles in the presence of polysorbate 80 as a surfactant, and the protein-containing nanoparticle system (~200 nm) showed low-density lipoprotein (LDL) receptor-mediated endocytosis in primary rat hippocampal neurons. All three cargo proteins were delivered to the cytosolic compartment in ~70 % of neuronal cells by the proton-sponge effect after endocytosis and interacted with their cytosolic targets. Interestingly, cells treated with the rhoG-loaded nanoparticles induced neurite outgrowth and differentiation in PC12 cells, which were not observed when the cells were treated with empty nanoparticles or rhoG protein alone. In addition, the amount of neurite outgrowth after 10 days’ treatment with rhoG-loaded nanoparticles was comparable to that in genetically transfected cells with rhoG through conventional lipofectin-based means. These results show that functional proteins delivered via PBCA nanoparticles efficiently interact with cytosolic downstream effectors and control morphological activity in neurons. The authors also suggested the use of PBCA nanoparticles for delivering therapeutic proteins to in vivo brain neurons, since PBCA nanoparticles have been shown to mediate significant transport of chemotherapeutics across the BBB via LDL receptors in live animals [87,88].
Antigen-specific, immune-tolerogenic therapies hold considerable promise for the treatment of allergic asthma, life-threatening food allergies, and autoimmune disease [89]. A major concern in antigen immunotherapy is the ability to maintain durable tolerance in the presence of proinflammatory stimuli caused by tissue stress, injury, or concurrent infection. Recently, Maldonado and colleagues reported an antigen-specific tolerogenic technology that controls both T-cell- and B-cell-mediated immunity using biodegradable nanoparticles [90]. They used PLGA and pegylated polylactic acid (PLA-PEG) as biodegradable polymers, and through double-emulsion, synthesized nanoparticles carrying both antigens (such as OVA protein) and a tolerogenic immunomodulator (rapamyin), yielding tolerogenic nanoparticles (tNPs). Treatment with tNPs through a variety of routes (i.v. and s.c.) efficiently inhibited B-cell activation and antibody responses against antigens administered locally or systemically. Importantly, tNPs were capable of inducing tolerance even when coadministered with free antigen in the presence of potent Toll-like receptor (TLR) agonists, such as CpG or R848, and adjuvants, such as Alum and CTx. The admixture of free rapamycin with NP-encapsulated OVA did not result in immune tolerance. Moreover, NPs loaded with protein antigen without rapamycin were very immunogenic, implying that the biology of both the rapamycin and the antigen are influenced by the context in which they are delivered. The authors suggested that the nanoparticles are selectively endocytosed by antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs) [91], and that the codelivery of antigen and rapamycin provide an ‘instruction set’ by allowing tolerogenic DCs capable of inducing CD4+ Treg [92]. Wilson et al. reported pH-responsive nanoparticle vaccines for enhancing humoral and cellular immune responses [93]. Micellar nanoparticles ~ 30 nm in size were assembled from amphiphilic diblock copolymers with two multifunctional modules. First module was composed of a polycation-rich block that incorporated pyridyl disulfide groups for complexation of oligonucleotide adjuvants (CpG ODN) and conjugation of antigens. Second module was hydrophobic and endosomolytic block for facilitating micelle assembly and cytosolic antigen delivery. The designed polymer micelle carrying both antigen and immunostimulatory adjuvant as dual delivery platform exhibited potent pH-dependent membrane destabilizing activity and significantly enhanced antigen cross-presentation by enhancing intracellular delivery of protein antigen and initiating innate immune response. Mice immunized with dual-delivery nanocarriers synergistically improved both CD8+T cell and CD4+Th1 T cell responses while eliciting a balanced IgG1/IgG2c antibody response.
Although PLGA micro/nanoparticles have been widely applied for protein delivery, the acidic internal environment produced by the hydrolysis of PLGA can be deleterious to proteins [94]. To increase protein stability and to avoid initial burst release in the acidic internal pH environment, Zhou et al. utilized ε–polylysine (ε-PL) as an anti-acidic agent and protein protectant during the preparation step of PLGA nanoparticles for protein delivery [95]. The cationic ε-PL was physically bound with negatively charged proteins such as BSA, and lactosylated PLGA (Lac-PLGA) was incorporated with the complex via nanoprecipitation. The synthesized Lac-PLGA/ε-PL nanoparticles maintained their regular spherical shapes and a relatively neutral pH (>6.0) over 24 days, whereas Lac-PLGA nanoparticles exhibited irregular morphology due to hydrolysis in aqueous medium, and the pH fell below pH 5.8 after 8 days, indicating that introduction of the ε-PL enhanced the stability of the nanoparticles. Importantly, the loaded BSA was slowly released from the Lac-PLGA/ε-PL nanoparticles (~15.8 % release after 32 days), and the released BSA exhibited no significant conformational changes. However, BSA-loaded Lac-PLGA nanoparticles showed an obvious burst release, and the helix content of BSA released from Lac-PLGA nanoparticles decreased to 32.7% because of the protein unfolding, aggregation, and peptide bond damage in an acidic environment. An in vivo pharmacokinetics study further demonstrated the liver-targeting property of the Lac-PLGA/ε-PL nanoparticles, long circulation (half-life: 3.25 days), and sustained release of BSA, suggesting the promise of this nanoparticle system as carrier for negatively charged proteins.
4.2.2. Lipid-based nanoparticles
Lipid-based formulation is another area that has shown great promise for therapeutic proteins [96,97]. Among various lipid-based formulations, classical examples are liposomes, because they are suitable carriers for the delivery of almost any drug independent of its solubility. However, the stability of such a formulation and the release profiles of encapsulated agents are not easily controlled [98]. To overcome these limitations, Kim et al. developed lipid micelle-type nanoparticles that actively target lung cancer and deliver therapeutic proteins into the cytosol of tumor cells. They fabricated lipid nanoparticles using 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), DOPE, and apolipoprotein (APOPA-I), and included DSPE-PEG-Anisamide to enable specific targeting through sigma receptors, which are overexpressed in H460 lung carcinoma [99]. To enhance the permeability of the cytochrome C (cytC, an apoptosis inducer), the membrane-permeable sequence (MPS) peptide was covalently conjugated to the cytC, and the highly lipophilic MPS-cytC was loaded into the lipid layer of the nanoparticles. The synthesized MPS-cytC-NP was 20–30nm in size and had 2–3 mV of surface charge. Notably, the DOPE, incorporated in the MPS-cytC-NP, caused a hexagonal phase of the nanoparticles in the endosome, destabilized the endosomal membrane, and induced cytosomal release of the therapeutic proteins from the nanoparticles in H460 lung cancer cells, resulting in significant greater cellular apoptosis compared to the nanoparticles without MPS or MPS-cytC. The surface decoration of the nanoparticles with the DSPE-PEG-Anisamide enabled strong tumor targeting and highly retarded tumor growth via caspase-9 activation. On the other hand, non-PEGylated nanoparticles exhibited obvious accumulation of proteins in the liver, not the tumor, implying that the surface coating with PEG was necessary to provide the nanoparticles with stealth characteristics.
Park and co-workers developed hybrid platforms integrating advantageous features for combinatorial encapsulation and delivery of therapeutic agents [100]. They designed biodegradable core-shell liposomal polymeric gels (nanolipogels; nLGs) composed of TGF-β inhibitor (SB505124, SB)-complexed cyclodextrins (CDs) and IL-2-encapsulating biodegradable polymers as an immunotherapeutic nanoplatform for cancer treatment. Liposomes were used as nanoscale molds for photo-initiated hydrogel formation of acrylated β-CDs and diacrylated PLA-PEG-PLA in the reservoir. Thus, the hydrophobic SBs, incorporated into β-CDs through the host-guest interaction, are released only on the hydrolysis of the polymer ester groups and subsequently diffused out from the nLGs, enabling sustained release — as compared to the burst-dominated release of SB in the absence of crosslinked CD. The hydrophilic IL-2s were loaded into the polymer-hydrogel space, then released from the nLGs in a manner similar to the SB, implying simultaneous sustained release of both drugs. The bioactivities of the SB and IL-2 were not changed by nLG incorporation, and encapsulation of IL-2 and SB proteins were 80% and 36%, respectively. The nLG-SB+IL-2 (neutral charge, 120 nm), administered intravenously to mice, significantly delayed tumor growth, increased survival of tumor-bearing mice, and inhibited highly aggressive B16 lung metastases as a result of the increased number of natural killer cells in tumors and intratumoral-activated CD8+ T-cell infiltration, suggesting that sustained release of both immunotherapeutic drugs around the tumor activated innate and adaptive immune responses simultaneously.
Cancer immunotherapy aimed at activation of cancer-specific immune responses has received much attention as a promising approach to treatment or prevention [101]. The main goal of this approach is to deliver cancer-specific antigens into APCs such as DCs, in addition to activating them, which is an adjuvant function [102]. To design a highly fusogenic and pH-sensitive membrane-disruptive nanoparticle system for antigen delivery, Yoshizaki et al. also utilized a polymer-liposome hybrid platform [103]. They developed egg-yolk phosphatidylcholine liposomes modified with a pH-sensitive fusogenic polymer, 3-methylglutarylated hyper-branched poly(glycidol) (MGlu-HPG), and loaded antigenic OVA protein to generate a tumor-specific antigen-delivery system (<100 nm), in which antigens are released into the cytosol of DCs and induce activation of tumor-specific cytotoxic T lymphocytes (CTLs). Additionally, the 3,5-didodecyloxybenzamidine (TRX) (a cationic lipid) was incorporated in the liposomal membrane as an adjuvant as well as to lend an efficient pH-sensitivity to the developed antigen-delivery system. The MGlu-HPG-modified liposomes with 30 mol% of TRX showed more intensive content release than the polymer-modified liposomes without TRX at a weakly acidic pH (6.5–6.0), because partially protonated carboxyl groups of the polymer chains interacted with TRX (including liposome membranes) through both hydrophobic and electrostatic interactions, destabilizing the surface of nanoparticles and releasing their contents. In contrast, the partially protonated polymer chains were insufficient to interact with the TRX-free membrane due to the lack of hydrophobicity, demonstrating that the inclusion of the cationic lipid on the surface membrane of the nanoparticles improves pH sensitivity. Furthermore, the TRX-containing nanoparticles were much more likely to be internalized into DCs than the nanoparticles without TRX because of greater recognition of liposomes by the scavenger receptors on DCs. Treatment with the liposomes incorporating cationic lipids up-regulated antigen presentation-involved molecules on DCs through both major histocompatibility complex (MHC) class I and II molecules, promoted production of cytokines such as IFN-γ, and reduced tumor volume significantly more than liposomes without cationic lipids, demonstrating efficient antigen delivery of the liposomes and activation of APCs.
Recently, Wang et al. reported combinatorial design of cationic lipid-like materials (termed lipidoids‘), coupled with reversible chemical protein engineering [104]. Pioneered by Anderson and colleagues, the combinatorial library strategy has been used to generate lipidoids for siRNA delivery [105], and the authors in this report extended this class of materials for use in protein delivery. They developed cationic lipid-based nanoparticles for protein delivery by designing and synthesizing a library of lipidoids through the ring-opening reaction of 1,2-expoxyhexadecane and aliphatic amines with diverse chemical structures. The RNase A and saporin proteins, which have both been used in clinical trials in patients with cancers that are refractory to traditional chemotherapy [106], were modified with cis-aconitic anhydride via lysine residues to obtain a negative charge, allowing them to bond with cationic lipidoids. The modified proteins were acid-labile and chemically reversible in the slightly acidic intracellular environment (e.g., endosome/lysosome), leading to the restoration of their biological activities in the cytosol. All lipidoids displayed low carrier cytotoxicity, with cell viabilities greater than 90%; however, the Lipidoid(EC16-1)-protein complex (~200 nm) showed distinct cell cytotoxicity. The endocytosized EC16-1/FITC/protein nanoparticles efficiently escaped from the endosome/lysosome after entering cells, whereas free proteins exhibited neither cellular internalization nor endosomal release. The EC16-1/protein nanoparticles were further optimized with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), cholesterol, and N-(palmitoyl)sphingosine[succinyl{methoxy(polyethylene glycol)2000}] (C16-mPEG-ceramide) for in vivo protein delivery. In vivo therapeutic evaluation indicated that intravenous injection of EC16-1/saporin nanoparticles remarkably reduced tumor volumes in mice (by 80%), with significantly greater saporin accumulation at tumor sites compared to the control group, demonstrating the effectiveness of this protein delivery platform based on cationic lipidoids.
4.2.3. Inorganic nanoparticles
Inorganic nanoparticles with a variety of unique intrinsic physiochemical properties have also attracted growing interest for use in biomedical applications, including drug delivery, hyperthermia therapy, disease diagnosis, and sensing and/or separation of target biomolecules [107,108]. Above all, mesoporous silica nanoparticles have been extensively studied, because they are inert, non-immunogenic, and modifiable as therapeutic agents, owing to the benefits of cargo-loading efficiency via large surface area and pore volume. Han and co-workers utilized mesoporous silica nanoparticles to load and deliver therapeutic proteasomes, which are known to degrade tau aggregates, a pathological hallmark of Alzheimer‘s disease [109]. They synthesized mesoporous silica nanoparticles with nickel moieties (MSNPN) and developed proteasome-loaded MSNPN (Ptsm-MSNPN) by chelating polyhistidine-tagged proteasomes with the nickel moieties of the nanoparticles. Approximately 37 proteasome holoenzymes were non-covalently incorporated into a single nanoparticle, yielding a proteasome-MSNPN complex ~240 nm in size and with −6.38 mV of charge density. The fluorescence and TEM studies reveal that the Ptsm-MSNPN were internalized into the target cells via both caveolae- and clathrin-mediated endocytosis and were distributed in the cytosol after escaping from the endosomes through the proton-sponge imidazole group of histidine. The proteasomes exogenously transported via the nanoparticles were active and structurally intact in the cytosol, as their activities were restored by the cytosolic environment (pH 7.5) despite the substantial decrease in such activity under endosomal conditions (pH 5.5). To evaluate the therapeutic ability of Ptsm-MSNPN in living cells, cellular tau was modeled using a HEK293-derived cell line that expressed high levels of the longest central-nervous-system isoform of human tau on doxycycline induction. The treatment with Ptsm-MSNPN significantly degraded the overexpressed tau in the cells as compared to the free proteasomes, and decreased the levels of the truncated tau, which is known to be a toxic aggregate. These findings suggest that the direct delivery of exogenous proteasomes using MSNPN can alleviate the cytotoxicity induced by overexpressed tau, recommending it as a beneficial intervention for cells under proteotoxic or oxidative stress.
Gold nanoparticles are also useful materials in biomedical applications, because their surface is easy to modify via thiol group-containing molecules, they are biocompatible, and they have unique spectroscopic properties [110]. Specifically, the large surface area and tunable functionality of gold nanoparticles make them an excellent scaffold for protein delivery [111], while the conformation of proteins is preserved by tailoring the monolayer of the nanoparticles [112]. Tang et al. designed gold nanoparticle-stabilized supramolecular nanocapsules (NPSC) to deliver therapeutic caspase-3 proteins [113]. This research group developed surface-functionalized gold nanoparticles with three domains: 1) polyarginine for membrane translocation, 2) tetraethylene glycol for preventing both non-specific interactions with biomolecules and denaturation of the proteins, and 3) exterior peptide tags (His-Lys-Arg-Lys) for recognition and endosomal buffering. The caspase-3 proteins were then mixed with the gold nanoparticles, and the template emulsions were formed separately by homogenizing gold nanoparticles with linoleic acid and decanoic acid. Finally, combining the solutions completed the supramolecular structure of the NPSCs via hydrogen bonding and electrostatic interactions between the guanidinium moieties of the nanoparticles and the carboxylates of the lipids. This approach provided not only stability for the protein-delivery system, but also provided reversible release properties of the payload through the membrane fusion mechanism. The delivered caspase-3 by the NPSCs caused ~72% of HeLa cells to undergo apoptosis; whereas the NPSCs without caspase-3 or caspase-3 alone elicited apoptosis in less than 10% of cells, demonstrating that the NPSCs enabled cytosolic delivery of caspase-3 in cancer cells by keeping the proteins intact and releasing them into the cytosol in a freely diffusing fashion.
Another example of using gold nanoparticles as a carrier of vascular endothelial growth factor (VEGF) for the treatment of ischemic lesions was reported by Kim et al. [114]. Recombinant human VEGF 165, which has been extensively used in preclinical and clinical trials, was selected as a therapeutic angiogenic agent and conjugated to the surface of the gold nanoparticles via coordinated covalent binding between the gold and the thiol group of cysteine residues in VEGF. The prepared VEGF-conjugated gold nanoparticles were ~124 nm in hydrodynamic diameter. Human microvascular endothelial cells (HUVEC-d) treated with VEGF-conjugated gold nanoparticles proliferated substantially faster than cells without growth factor, and the proliferation ratio was similar to the value in HUVEC-d treated with intact VEGF, demonstrating that the thiol-mediated covalent conjugation of VEGF to the gold nanoparticles does not alter protein activity. These results were further confirmed in a hind-limb ischemia mouse model after the intravenous administration of nanoparticles. Injections of free VEGF and PEGylated gold nanoparticles did not produce results significantly different from the non-treated control group, while VEGF-conjugated gold nanoparticles led to a recovery of blood perfusion over time to 93% of normal tissue, as well as increased blood vessel density, quantified by immunohistochemical staining with CD31. Moreover, nanoparticles <200 nm in diameter accumulated in ischemic muscle, which has permeable vessels, supporting passive targeting of the nanoparticles by the EPR effect.
Jiang et al. reported a graphene-based nanostructure as a cancer-specific protease-mediated programmed co-delivery system integrating cell membrane-associated cytokines and chemotherapeutic agents for combination cancer therapy [115]. To develop the graphene-based nanocarrier, carboxylated graphene oxide (GO) nanosheet incorporating doxorubicin via π-π stacking interaction was connected with furin-cleavable peptide via hetero-bifunctional PEG linker (NH2-PEG-N3), and TNF-related TRAIL was conjugated to the cysteine group of the peptide. Intravenous administration of Cy5.5-labeled TRAIL-conjugated furin-cleavable GO (TRAIL-fGO) into nude mice bearing A549 tumor showed a remarkable tumor targeting ability of the GO nanosheet after 24 h. In addition, TRAIL/DOX-fGO indicated the strongest effect on suppressing tumor growth compared to other groups, suggesting their synergistic antitumor activity by releasing TRAIL and DOX separately in their distinct sites of action, in a site-specific manner.
In recent years, a tremendous amount of research has been devoted to theranostic nanoplatforms capable of co-delivery of diagnostic and therapeutic agents, with the goal of providing therapeutic protocols that are more effective and less toxic for individual patients. Magnetic iron oxide nanoparticles offer an attractive means for remotely directing therapeutic agents to a disease site while simultaneously visualizing drug distribution and predicting therapeutic responses due to their intrinsic magnetic and/or diagnostic capabilities via magnetic resonance imaging (MRI) [60]. Additionally, the surface tunability of magnetic iron oxide nanoparticles makes them multifunctional, allowing their fabrication with a variety of therapeutic or functional moieties. Importantly, magnetic nanoparticle–based theranostic agents can be selectively concentrated at pathological sites through magnetic attraction or in vivo systemic targeting. However, there are few reports on the use of magnetic nanoparticles for delivering proteins. Towards the goal of long-circulating magnetic nanoparticles for potential application as a protein drug-delivery platform, Zhang and co-workers developed starch-coated, PEGylated, and heparin-functionalized iron oxide magnetic nanoparticles (DNPH) and evaluated their tumor-targeting ability in a flank 9L-glioma mouse model [116]. They prepared DNPH by attaching both PEG and heparin to starch-coated iron oxide nanoparticles via EDC/NHS chemistry, and loaded protamine as a model protein, a heparin antidote in clinical use after cardiovascular surgeries, making use of the electrostatic interaction between anionic heparin and cationic protamine to form the stable DNPH-protamine complex (~167 nm and 42 mV). The DNPH displayed a long-circulating half-life of 9.37 h, 37.5-fold longer than that of PEGylated heparin-coated iron oxide magnetic nanoparticles (HP), and were clearly visualized in MRI, where the hyperintense glioma tumor region was darkened after intravenous injection of the DNPH and magnetic targeting, suggesting the potential of DNPH as a protein-delivering theranostic platform.
5. Non-invasive administration approaches
The major parenteral routes for protein delivery are the intravenous (i.v.), intramuscular (i.m.) or subcutaneous (s.c.) injection into the systemic circulation. However, non-invasive methods need to be developed to improve patient satisfaction and compliance and to overcome the limitations associated with needle-based administration. Mucosal (oral, nasal, pulmonary) and transdermal administration are generally painless and simpler than traditional injection technologies (Fig 3a) [117]. However, these alternative routes still face the issue of unpredictable/low bioavailability, limiting protein delivery; this is the result of the extreme conditions (low pH and enzymes) in the gastrointestinal tract, poor penetration of the proteins across mucosal or skin barriers, and/or variable inhalation efficiency. Nanotechnology has been used to address these challenges by developing novel nanoparticle platforms as protein delivery carriers for non-traditional delivery routes.
Fig 3. Non-invasive methods for protein administration using nanoparticles.
(a) Four representative non-invasive alternative routes to parenteral injections (A. nasal delivery, B. oral delivery, C. pulmonary delivery, and D. transdermal delivery). Nanoparticles as protein carriers reach epithelial cells covered with mucus layer after nasal, oral, or pulmonary administration. (b) Mucosal delivery of nanoparticles via routes A, B, and C. (i; receptor-mediated transport, ii; paracellular transport, iii; M cell-mediated transport, iv; adsorption-mediated transport). (c) Transdermal delivery of nanoparticles via route D. (i; intracellular transport, ii; intercellular transport, iii; transport via skin appendages).
5.1. Oral delivery
The oral route is highly patient-friendly, as solid tablets or liquids can simply be non-invasively ingested, increasing patient compliance. However, orally administered proteins must survive in the harsh enzymatic and acidic environments of the gastrointestinal (GI) tract and be transported across the intestinal epithelial barrier before entering the bloodstream to arrive at target sites. Both transcellular absorption and paracellular transport of proteins are made less likely because they are hydrophilic molecules (log P values <0) and larger than 30–50 Å [118], leading to poor absorption of the protein drugs into systemic circulation with low bioavailability. Nanoparticles are promising protective carriers for protein delivery via the oral route, as they can protect proteins from enzymatic and hydrolytic degradation, providing safe transport across epithelial barriers through receptor-mediated transport, adsorption-mediated transport, paracellular transport, and recognition by microfold cells (M cells) in Peyer‘s patches (Fig 3b) [117]. Recently, considerable effort has been devoted to the development of oral protein delivery systems (especially insulin) using nanotechnologies [117]. For example, Sonaje et al. designed a pH-sensitive nanoparticle system composed of chitosan, poly(γ-glutamic acid), insulin, sodium tripolyphosphate, and magnesium sulfate (MgSO4) via the ionic-gelation method and evaluated its pH-dependent insulin-delivery ability with oral administration [119]. To protect insulin from degradation by the acidic environment of the stomach, enteric-coated capsules (Eudragit®S100 and L100-55) were utilized to cover the nanoparticles. X-ray/computed tomography (CT) images of the rats orally administered the nanoparticle-loaded capsules showed that the nanoparticles were intact in the stomach and were delivered to the proximal segment of the small intestine while the enteric coating was dissolved. The apparent permeability (Papp) value of insulin in the nanoparticles at pH 6.6 was significantly greater than that at pH 7.4, implying that positively charged nanoparticles at pH 6.6 interact with negatively charged cells and tight junctions (ZO-1) transiently opened by chitosan to induce paracellular permeability at acidic sections of the intestine, such as the duodenum and portions of the jejunum. At pH 7.4, the nanoparticles became unstable and subsequently disintegrated due to the negligible electrostatic interaction between chitosan and poly(γ-glutamic acid); thus the insulin was released into the intercellular spaces between enterocytes (pH ~ 7.4) and permeated through the paracellular pathway. The relative bioavailability of insulin after oral administration of enteric-coated capsules enclosing nanoparticles was around 20% and elicited an obvious hypoglycemic effect in diabetic rats, suggesting that this pH-sensitive nanoparticle system was promising for the transport of orally administered insulin into the systemic circulation.
In a similar manner, Shan et al. developed self-assembled nanoparticles that permeate the mucus layer and are absorbed into the epithelial layer to deliver insulin orally by inducing phased change of the nanostructure [120]. Penetratin, one of the most promising CPPs, was mixed with negatively charged insulin to make nanocomplex (NC) cores (148 nm), which were spontaneously assembled with a dissociable hydrophilic N-(2-hydroxypropyl) methacrylamide copolymer (pHPMA) derivatives via electrostatic interaction to form the self-assembled nanoparticles (175 nm). The Papp values across the mucus layer based on the accumulative amount of diffusion within 3 h indicated that one of the self-assembled nanoparticles (NPs-1) permeated the mucus layer via a favorable ratio of electrostatic and/or hydrophobic interaction and repulsion between the nanoparticles and mucus layer. In addition, the amount of internalized insulin after treatment with NPs-1 in the mucus layer mimicking the HT29-MTX-E12 cell line was significantly higher than after treatment with free insulin and NC, demonstrating the mucus layer-penetration ability of the NPs-1. A fluorescence resonance energy transfer (FRET) analysis revealed that the pHPMA coating on the nanoparticles was dissociable, and this process happened much more quickly in mucus than in PBS for internalizing into the epithelium by exposing the CPP of the NC core. Oral administration of NPs-1 generated a prominent hypoglycemic response with maximal 50% of blood glucose level reduction, while the administration of free insulin failed to reduce glucose levels in diabetic rats. The authors suggested that the improved transepithelial transport of NPs-1 was mainly attributable to the better mucus layer permeation capability and more timely exposure of the CPP-rich core for cellular internalization. Moreover, the unchanged transepithelial electrical resistance (TEER) value of cell monolayers throughout the treatment indicates the transcellular pathway of the NPs-1, implying the safe transportation of the insulin without disturbing the integrity of the epithelium.
Another example of nanoparticles that travel the transcytosis pathway for orally administered insulin delivery was reported by Pridgen and co-workers [121]. They utilized the neonatal Fc receptor (FcRn), expressed at levels similar to fetal expression in the apical region of epithelial cells of the adult small intestine, to transport orally administered FcRn-targeting nanoparticles (Fc-NPs) across the intestinal epithelium in rodents. They synthesized PLA-PEG-maleimide block copolymer to make self-assembled nanoparticles and decorated the surface of the nanoparticles with thiol-modified polyclonal IgG Fc fragments via covalent conjugation. Then, insulin-loaded Fc-NPs (Fc-insNPs) were prepared by encapsulating insulin inside the Fc-NPs through nanoprecipitation. FcRn at the apical surface of absorptive epithelial cells caught Fc-NPs and guided bound nanoparticles through transcytosis, avoiding lysosomal degradation. On the basolateral side, after exocytosis the Fc-NPs were released into the lamina propria to enter the systemic circulation, since the binding between FcRn and Fc-NPs was not strong at physiological pH (~7.4). The orally administered Fc-insNPs elicited a more prolonged hypoglycemic response in mice compared with free insulin administered intravenously (15 vs 1.5 hours). Non-targeted insNPs elicited no significant glucose response, nor did they cause a hypoglycemic response in the FcRn knockout mice, suggesting that the benefit gained from using Fc was specifically owing to FcRn.
5.2. Nasal and Pulmonary delivery
Utilizing the respiratory tract, e.g., nasal and pulmonary routes, offers several advantages for protein delivery: i) the local proteolytic activity in both administration routes is relatively low compared to the gastrointestinal tract, ii) it is easy to elicit strong immune responses in both routes through the immunologically active mucosal-associated lymphoid tissue, which contains many lymphoid follicles (B-cell areas), macrophages, and dendritic cells, and iii) these routes require lower doses of drug compared to the oral route [122]. Thus the respiratory tract offers great potential, especially with antigenic protein delivery systems for vaccination. However, the airway epithelium is firmly closed by tight junctions, and the mucus layer covers the upper/central respiratory tracts (e.g. nasal cavity, trachea, bronchi and bronchioles), limiting the uptake of proteins into the respiratory lumen [123]. On the other hand, the tight junctions between the epithelial cells are loose at the distal airway epithelium, just before the alveoli, and there is no mucus barrier, allowing relatively simple access of proteins to the underlying vascular and lymphatic systems [124]. Nanoparticle formulations are attractive ways to enhance delivery of inhaled proteins, since they can improve distribution in the airways owing to the preferable size on local deposition, the capability of regulating drug release rates and transporting protein cargos across the epithelial layer, including the mucosal barrier, while protecting them from denaturation or aggregation.
Nochi et al. developed an intranasal vaccine-delivery system with a nanometer-sized hydrogel (nanogel) consisting of a cationic type of cholesteryl-bearing pullulan (cCHP) [125]. The physically cross-linked nanogels (~ 40 nm) encapsulate proteins within the polymer network by mainly hydrophobic interactions and improve antigen delivery efficacy via interactions with the anionic epithelial cell layer. In this system, a non-toxic receptor-binding fragment of C. botulinum type-A neurotoxin subunit antigen Hc (BoHc/A) was prepared and used as a prototype vaccine antigen. In vivo positron emission tomography (PET)/CT images and histochemical studies clearly showed that intranasally administered cCHP carrying [18F]-labelled BoHc/A effectively penetrated the nasal mucosa through recognition by M cells in the nasopharynx-associated lymphoid tissues (NALT), gradually releasing BoHc/A in the nasal epithelial cells. In addition, intranasal immunization with cCHP-BoHc/A markedly increased the numbers of IgA-committed B cells in the lamina propria and paranasal sinuses. In contrast, intranasally delivered naked BoHc/A not only disappeared from the nasal cavity within 6 h as a result of mucociliary clearance, it also did not cause any immune response. After intraperitoneal (i.p.) challenge with BoNT/A, mice intranasally immunized with cCHP-BoHc/A survived without any clinical signs and were completely protected from the progenitor toxin, whereas those that received BoHc/A alone almost immediately developed neurological signs and died within half a day. Overall results demonstrated that the cCHP nanogel acted as an artificial chaperone, effectively delivering the vaccine antigen into the respiratory immune system via both systemic and mucosal protective immunity in the absence of the mucosal adjuvant. These findings suggest that needle-free vaccination with nanoparticle-mediated intranasal delivery has the potential for protecting against not only airway or systemic infections but also gastrointestinal- or reproductive-tract pathogens. In a similar manner, Keijzer et al. investigated whether antigen-encapsulated nanoparticles modulate immune responses after nasal vaccination in autoimmune disease [126]. They developed three polymeric nanoparticles: PLGA, PLGA-TMC (N-trimethyl chitosan), and TMC-TPP (tri-polyphosphate) nanoparticles, and encapsulated OVA in each one as a model antigen. Nasal vaccination with OVA-loaded nanoparticles (250–500 nm) induced strong local CD4+T-cell proliferation in the NALT and cervical lymph nodes (CLN), whereas low-dose soluble OVA (sOVA) did not induce such T-cell proliferation. Treatment with OVA-loaded PLGA nanoparticles suppressed the OVA-specific delayed-type hypersensitivity response in mice sensitized and challenged with OVA/Incomplete Freund‘s Adjuvant and OVA, respectively, but non-tolerized mice showed a strong increase in ear thickness comparable to mice that received a low dose of sOVA. The enhanced nasal tolerance induction by PLGA treatment was sufficient to suppress chronic and relapsing arthritis, leading to a significant reduction of disease. On the other hand, TMC-TPP led to a systemic OVA-specific B-cell response and remarkably greater humoral immunity locally in the draining CLN, implying that the nanoparticles with different physicochemical characteristics differentially modulate immune response after nasal vaccination, and that appropriate combinations of nanoparticles and antigen are key to designing successful vaccines for inducing a preferred type of immune response.
Recently, an attempt to promote potent effector memory CD8+T cells (TEM)-biased responses via pulmonary vaccination with nanoparticles was reported [127]. Compared to DCs in the NALT, the lung mucosa is densely lined with APCs that can actively collect antigen from across the epithelial barrier for delivery into the draining lymph node (dLNs) [128]. Motivated by such different populations in immune systems, the research group evaluated whether pulmonary vaccination increases antigen transport to APCs and generates robust T-cell responses for protection of mucosal surfaces. Previously, they developed nanoscale capsules with walls composed of stacked lipid bilayers stapled to one another via bilayer-to-bilayer chemical cross-links, interbilayer-crosslinked multilamellar vesicles (ICMVs) designed to have superior stability in physiological conditions compared to traditional liposomes, and optimized ICMV vaccines by incorporating OVA, TLR4 agonist monophosphoryl lipid A (MPLA), and TLR3 agonist polyI:C (polyinosinic-polycytidylic acid) [129]. Pulmonary vaccination with the ICMV not only increased antigen transport to APCs ~60-fold relative to parenteral injection, but also primed CD8+T cells with greater functionality than did equivalent soluble pulmonary vaccines. In particular, TEM was successfully generated with antigen-specific CD8+T cells while persisting at both local mucosal tissues and distal mucosal effector sites, and safety issues such as destructive pathology, systemic inflammation, or clinical signs of distress were not encountered, demonstrating efficient and safe nanocapsule-mediated design of a potent vaccine system.
5.3. Transdermal delivery
Transdermal patches also offer a painless alternative to parenteral injections, but their application is generally limited to small hydrophobic drugs; this is because large or hydrophilic molecules such as proteins are not easily absorbed into the skin because of the very low permeability of the stratum corneum, also called the horny layer [39,130]. The intercellular spaces in the stratum corneum are filled with lipid bilayers (lamellae) consisting of both lipophilic and hydrophilic domains, including ceramides, fatty acids, and cholesterol; thus drug molecules administered by the transdermal route must i) penetrate through corneocytes and intervening lipids (intracellular transport), or ii) pass between corneocytes (intercellular transport), or iii) be transported across skin appendages such as hair follicles and sebaceous glands (skin appendageal transport) to reach target sites (Fig 3c). Choi et al. encapsulated proteins into chitosan-conjugated, Pluronic-based nano-carrier (nanogel) to enhance skin penetration properties and evaluated its transdermal protein delivery efficiency [131]. The nanogel (~70 nm) was prepared via a photo-crosslinking reaction between diacrylated Pluronic F127 and acrylated chitosan, and several proteins such as insulin, BSA, and β-galactosidase were encapsulated into the nanogel by utilizing large-volume transition characteristics of the Pluronic. A Franz-type diffusion experiment with the human cadaver skin indicated that the loaded proteins in the nanogel robustly penetrated the skin layer, including the stratum corneum, whereas the micelle type of nanocarrier or admixture of proteins and chitosan did not achieve such penetration. The results clearly demonstrated that the stable nanogel with chemical cross-linking was critical for effective skin permeation. Additionally, the chitosan-conjugated nanogel showed better transport across the skin than the nanocarrier without the chitosan, owing to the electrostatic interaction between chitosan and the negatively charged stratum corneum and/or the potential of chitosan to loosen the accumulative structure of keratin in the stratum corneum [132]. Enzymatic activity assay of the β-galactosidase delivered by the nanogel indicated that the proteins transported through the skin were biologically active, suggesting that the newly developed Pluronic-based nanogel with small size, flexibility, and reservoir properties could be an excellent carrier for transdermal protein delivery.
Nose et al. reported on gold nanorods (GNRs), which can decrease blood glucose levels via the transdermal route with near-infrared (NIR) light irradiation in diabetic mice [133]. Insulin-loaded GNRs were prepared with the aid of surfactants such as L-195 and isopropyl myristate (IPM) using a modified emulsion method, and the insulin-loaded GNRs were around 228 nm in length. The intact mice skin treated with insulin-loaded GNRs plus NIR light irradiation showed substantially higher penetration than other formulations such as insulin-loaded GNRs without NIR light or insulin-formulated with the surfactants, demonstrating that NIR light absorption of the GNRs improved transdermal penetration of insulin by converting light energy into heat to disrupt skin lipids or to change the size and density of the skin barrier. In vivo applications of transdermal patches containing insulin-loaded GNRs and other formulations placed on diabetic mice further demonstrated their ability to deliver insulin through the skin. Unlike other treatments, the insulin-loaded GNRs plus NIR light irradiation significantly decreased the blood glucose level of diabetic mice at 4 h (to ~58%) and further decreased it to ~15% after another 6 h. On the other hand, subcutaneous administration of insulin rapidly decreased blood glucose levels of mice (to ~22%) at 2 h, but the level immediately increased to ~92%. Overall results showed that the combination of the surfactants and the photothermal effect in the GNRs-based protein carrier enhanced the permeation of insulin through the skin barrier, and the insulin molecules eventually entered the systemic circulation in diabetic mice.
Combinatorial design of nanocapsules and microneedles to deliver vaccine through the skin has also been reported [134]. To transport antigens into the skin, where epidermal and dermal APCs are abundant, degradable polyelectrolyte multilayers (PEMs)-coated microneedles were used. The research group selected Poly-1, a hydrolytically degradable polymer from a class of polyelectrolytes known as poly(β-amino esters) (PBAEs), as a complementary degradable partner for antigen-encapsulating lipid nanocapsules (~240 nm) in multilayers. The (Poly-1/nanocapsules) multilayers were assembled layer-by-layer and deposited in the dorsal ear or flank skin of mice via brief microneedle application, where hydrolytic degradation of Poly-1 over time led to the release of antigen-encapsulating lipid nanocapsules into the surrounding tissue, followed by uptake into local APCs. Confocal imaging analysis revealed that the Poly-1 multilayers were degraded within 24 h, and the dispersed nanocapsules were consistently localized in the APCs, demonstrating that the nanocapsules released from the implanted multilayers were actively being phagocytosed by resident immune cells in the skin. Interestingly, mice immunized in this way demonstrated 10-fold higher IgG2C titers compared to mice vaccinated with free OVA with microneedles or intradermal injection, indicating the design potential for enhanced protection in both infectious disease and cancer vaccines. Recently, Yu et al. reported a similar framework composed of microneedle-array containing glucose-responsive vesicles (GRVs) as a “smart” insulin patch [135]. In the presence of high blood glucose, GOx catalyze glucose oxidation, generating a local hypoxic environment by consuming the dissolved oxygen. Thus, this research group designed hypoxia-sensitive hyaluronic acid vesicles including insulin and GOx to rapidly release insulin by converting the vesicle structure in response to hyperglycemia, and the vesicles were loaded in the tips of a silicone mold to produce a microneedle-array patch as a painless and disposable modality. The blood glucose level in the streptozotocin-induced type I diabetic mice treated with the GRVs quickly declined to around 200 mg/dL within 0.5 h and maintained a normoglycemic state for up to 4 h. In addition, the microchannels on the skin created by the patch recovered within 6 h post-administration, demonstrating a fast and safe glucose-responsive insulin delivery device.
6. Outlook
Since the first nanotherapeutic Doxil (pegylated liposomal doxorubicin) received FDA approval in 1995, the pipeline of nanomedicines for the management of various diseases has greatly expanded [9, 136]. While nanotechnology-based approaches have also shown outstanding potential in protein therapies, the clinical development of protein delivery systems using nanoparticles is still in its early stages, and several challenges remain. First, drug encapsulation needs to be well controlled to maintain the protein cargo properties and to avoid batch-to-batch variability. We now realize that higher protein loading capacity is preferable, but not always necessarily more desirable than lower loading capacity, as the degree of protein loading affects the physicochemical properties of the delivery vehicle and the cargo itself [137]. Second, the loaded protein drugs need to be released from the nanoparticles in a controlled manner while keeping desired concentrations within their target area. Smart design of delivery vehicles such as stimuli-responsive nanoparticles may be needed to facilitate on-demand‘ release. Third, the simplicity of preparation procedures should be considered for future scale-up of production. Fourth, effective intracellular delivery is still an issue, as many nanocarriers cannot efficiently escape into the cytosol from endosomes [138]. Fifth, disease-specific targeting is required to increase drug efficacy, while decreasing toxicity, eventually culminating in personalized medicine,‘ the tailoring of medical treatment to the individual characteristics, needs, and preferences of each patient [139]. Lastly, safety and long-term performance should be fully evaluated in the design of nanoparticles for protein delivery, especially for materials that are not biodegradable. With the continuous emergence of clinically validated nanotechnologies and the advancements of protein delivery strategies, we envision that these barriers will be explicitly addressed and nanotechnology-based protein therapeutics will be brought into the clinic for safer and more effective treatment of important human diseases.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants EB015419 (O.C.F.), R00CA160350 (J.S.), and U54-CA151884 (O.C.F.); the Movember-Prostate Cancer Foundation (PCF) Challenge Award (O.C.F. and J.S.), PCF Young Investigator Award (J.S.) and David Koch-PCF Program in Nanotherapeutics (O.C.F.); and the National Research Foundation of Korea K1A1A2048701 (O.C.F.).
Footnotes
Conflict of interest: O.C.F. discloses financial interest in BIND Therapeutics, Selecta Biosciences and Blend Therapeutics, which are developing nanoparticle therapeutics for medical applications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 2.Leavy O. Therapeutic antibodies: past, present and future. Nat Rev Immunol. 2010;10:297. doi: 10.1038/nri2763. [DOI] [PubMed] [Google Scholar]
- 3.Domling A, Holak TA. Programmed death-1: therapeutic success after more than 100 years of cancer immunotherapy. Angew Chem Int Ed. 2014;53:2–5. doi: 10.1002/anie.201307906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global markets for bioengineered protein drugs. BBC research; 2014. http://www.reportlinker.com/p02354690-summary/Global-Markets-for-Bioengineered-Protein-Drugs.html. [Google Scholar]
- 5.Haschek WM, Rousseaux CG, Wallig MA. Haschek and Rousseaux‘s handbook of toxicologic pathology. 3. Elsevier Inc; 2013. [Google Scholar]
- 6.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 7.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–779. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 9.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 11.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 12.Chiellini F, Piras AM, Errico C, Chiellini E. Micro/nanostructured polymeric systems for biomedical and pharmaceutical applications. Nanomedicine. 2008;3:367–393. doi: 10.2217/17435889.3.3.367. [DOI] [PubMed] [Google Scholar]
- 13.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4:1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Builders PF, Kunle OO, Okpaku LC, Builders MI, Attama AA, Adikwu MU. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur J Pharm Biopharm. 2008;70:777–783. doi: 10.1016/j.ejpb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Han K, Lee KD, Gao ZG, Park JS. Preparation and evaluation of poly(L-lactic acid) microspheres containing rhEGF for chronic gastric ulcer healing. J Control Release. 2001;75:259–269. doi: 10.1016/s0168-3659(01)00400-x. [DOI] [PubMed] [Google Scholar]
- 17.Morlock M, Kissel T, Li YX, Koll H, Winter G. Erythropoietin loaded microspheres prepared from biodegradable LPLG-PEO-LPLG triblock copolymers: protein stabilization and in-vitro release properties. J Control Release. 1998;56:105–115. doi: 10.1016/s0168-3659(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 18.Sassi AB, Nagarkar R, Hamblin P. Novel Approaches and Strategies for Biologics. In: Singh M, Salnikova M, editors. Vaccines and Cancer Therapies. Elsevier Inc; 2015. pp. 199–217. [Google Scholar]
- 19.Burnham NL. Polymers for delivering peptides and proteins. Am J Hosp Pharm. 1994;51:210–218. [PubMed] [Google Scholar]
- 20.Garnock-Jones KP. Naloxegol: a review of its use in patients with opioid-induced constipation. Drugs. 2015;75:419–425. doi: 10.1007/s40265-015-0357-2. [DOI] [PubMed] [Google Scholar]
- 21.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deepagan VG, Thambi T, Ko H, Kang YM, Park JH. Amphiphilic polysialic acid derivatives: synthesis, characterization, and in-vitro cytotoxicity. J Nanosci Nanotechnol. 2013;13:7312–7318. doi: 10.1166/jnn.2013.8089. [DOI] [PubMed] [Google Scholar]
- 23.Pisal DS, Kosolski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochendoerfer GG, Chen SY, Mao F, Cressman S, Traviglia S, Shao H, et al. Design and chemical synthesis of a homogeneous polymer-modified erythropoiesis protein. Science. 2003;299:884–887. doi: 10.1126/science.1079085. [DOI] [PubMed] [Google Scholar]
- 25.Nischan N, Hackenberger CP. Site-specific PEGylation of proteins: recent developments. J Org Chem. 2014;79:10727–10733. doi: 10.1021/jo502136n. [DOI] [PubMed] [Google Scholar]
- 26.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.02.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challa DK, Velmurugan R, Ober RJ, Sally Ward E. FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr Top Microbiol Immunol. 2014;382:249–272. doi: 10.1007/978-3-319-07911-0_12. [DOI] [PubMed] [Google Scholar]
- 28.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012;4:1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fast JL, Cordes AA, Carpenter JF, Randolph TW. Physical instability of a therapeutic Fc fusion protein: domain contributions to conformational and colloidal stability. Biochemistry. 2009;48:11724–11736. doi: 10.1021/bi900853v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in nonhuman primates through an immunoglobulin transport pathway. Proc Natl Acad Sci. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung K, Lee JE, Kim HZ, Kim HM, Park BS, Hwang SI, et al. Toll-like receptor 4 decoy, TOY, attenuates gram-negative bacterial sepsis. PLoS One. 2009;4:e7403. doi: 10.1371/journal.pone.0007403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muda M, Gross AW, Dawson JP, He C, Kurosawa E, et al. Therapeutic assessment of SEED: a new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng Des Sel. 2011;24:447–454. doi: 10.1093/protein/gzq123. [DOI] [PubMed] [Google Scholar]
- 33.Bakema JE, Ganzevles SH, Fluitsma DM, Schilham MW, Beelen RH, et al. Targeting FcαRI on polymorphonuclear cells induces tumor cell killing through autophagy. J Immunol. 2011;187:726–732. doi: 10.4049/jimmunol.1002581. [DOI] [PubMed] [Google Scholar]
- 34.Karagiannis SN, Josephs DH, Karagiannis P, Gilbert AE, Saul L, et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother. 2012;61:1547–1564. doi: 10.1007/s00262-011-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ammann JU, Jahnke M, Dyson MR, Kaufman J, Trowsdale J. Detection of weak receptor-ligand interactions using IgM and J-chain-based fusion proteins. Eur J Immunol. 2012;42:1354–1356. doi: 10.1002/eji.201142151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin D, Golding B, Strome SE, Sauna ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33:27–34. doi: 10.1016/j.tibtech.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Albiglutide Study Group, Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–1886. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schellenberger V, Wang CW, Geething NC, Spink BJ, Campbell A, To W, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nature Biotech. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 39.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellert K, Lamla M, Scheffzek K, Wittig R, Kaufmann D. Enhancing endosomal escape of transduced proteins by photochemical internalization. PLoS One. 2012;7:e52473. doi: 10.1371/journal.pone.0052473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Jo J, Hong S, Choi WY, Lee DR. Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymalstromal cells. Sci Rep. 2014;4:4378. doi: 10.1038/srep04378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 44.Green I, Christison R, Voyce CJ, Bundell KR, Lindsay MA. Protein transduction domains: are they delivering? Trends Pharmacol Sci. 2003;24:213–215. doi: 10.1016/S0165-6147(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 45.Munyendo WL, Lv H, Benza-Ingoula H, Baraza LD, Zhou J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules. 2012;2:187–202. doi: 10.3390/biom2020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyaji Y, Walter S, Chen L, Kurihara A, Ishizuka T, Saito M, et al. Distribution of KAI-9803, a novel δ-protein kinase C inhibitor, after intravenous administration to rats. Drug Metab Dispos. 2011;39:1946–1953. doi: 10.1124/dmd.111.040725. [DOI] [PubMed] [Google Scholar]
- 47.Ramqvist T, Andreasson K, Dalianis T. Vaccination, immune and gene therapy based on virus-like particles against viral infections and cancer. Expert Opin Biol Ther. 2007;7:997–1007. doi: 10.1517/14712598.7.7.997. [DOI] [PubMed] [Google Scholar]
- 48.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 49.Muratori C, Bona R, Federico M. Lentivirus-based virus-like particles as a new protein delivery tool. Methods Mol Biol. 2010;614:111–124. doi: 10.1007/978-1-60761-533-0_7. [DOI] [PubMed] [Google Scholar]
- 50.Kaczmarczyk SJ, Sitaraman K, Young HA, Hughes SH, Chatterjee DK. Protein delivery using engineered virus-like particles. Proc Natl Acad Sci U S A. 2011;108:16998–17003. doi: 10.1073/pnas.1101874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaj T, Guo J, Kato Y, Sirk SJ, Barbas CF. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nature Methods. 2012;9:805–807. doi: 10.1038/nmeth.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaj T, Liu J, Anderson KE, Sirk SJ, Barbas CF., 3rd Protein delivery using Cys2-His2 zinc-finger domains. ACS Chem Biol. 2014;9:1662–1667. doi: 10.1021/cb500282g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weeks BS, Lieberman DM, Johnson B, Roque E, Green M, Loewenstein P, et al. Neurotoxicity of the human immunodeficiency virus type 1 tat transactivator to PC12 cells requires the Tat amino acid 49–58 basic domain. J Neurosci Res. 1995;42:34–40. doi: 10.1002/jnr.490420105. [DOI] [PubMed] [Google Scholar]
- 54.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nano-spheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 55.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, et al. Minimal —self peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2:22–32. [Google Scholar]
- 59.Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 60.Yu MK, Park J, Jon S. Magnetic nanoparticles and their applications in image-guided drug delivery. Drug Deliv Transl Res. 2012;2:3–21. doi: 10.1007/s13346-011-0049-8. [DOI] [PubMed] [Google Scholar]
- 61.Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flogel U, Ding Z, Hardung H, Jander S, Reichmann G. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, et al. Normalization of tumor blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature Nanotech. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi HS, Liu W, Liu F, Nasr K, Misra P, et al. Design Considerations for Tumor-Targeted Nanoparticles. Nature Nanotech. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 69.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JH, Von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264–273. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao C, Ding J, Ma L, Yang C, Zhuang X, et al. Synthesis of thermal and oxidation dual responsive polymers for reactive oxygen species (ROS)-triggered drug release. Polym Chem. 2015;6:738–747. [Google Scholar]
- 77.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 78.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu J, Kamaly N, Shi J, Zhao L, Xiao Z, Hollett G, et al. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew Chem Int Ed Engl. 2014;53:8975–8979. doi: 10.1002/anie.201404766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi WI, Kamaly N, Riol-Blanco L, Lee IH, Wu J, Swami A, et al. A solvent-free thermosponge nanoparticle platform for efficient delivery of labile proteins. Nano Lett. 2014;14:6449–6455. doi: 10.1021/nl502994y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan M, Du J, Gu Z, Liang M, Hu Y, Zhang W, et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53. doi: 10.1038/nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- 82.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao M, Hu B, Gu Z, Joo KI, Wang P, Tang Y. Degradable polymeric nanocapsule for efficient intracellular delivery of a high molecular weight tumor-selective protein complex. Nano Today. 2013;8:11–20. [Google Scholar]
- 84.Windisch M, Wolf H, Hutter-Paier B, Wronski R. The role of alpha-synuclein in neurodegenerative diseases: A potential target for new treatment strategies? Neurodegener Dis. 2008;5:218–221. doi: 10.1159/000113707. [DOI] [PubMed] [Google Scholar]
- 85.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function inneurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hasadsri L, Kreuter J, Hattori H, Iwasaki T, George JM. Functional protein delivery into neurons using polymeric nanoparticles. J Biol Chem. 2009;284:6972–6981. doi: 10.1074/jbc.M805956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–1569. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 88.Méresse S, Delbart C, Fruchart JC, Cecchelli R. Low-density lipoprotein receptor on endothelium of brain capillaries. J Neurochem. 1989;53:340–345. doi: 10.1111/j.1471-4159.1989.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 89.Bluestone JA, Bour-Jordan H. Current and future immunomodulation strategies to restore tolerance in autoimmune diseases. Cold Spring Harb Perspect Biol. 2012;4:a007542. doi: 10.1101/cshperspect.a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112:E156–65. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, et al. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Estey T, Kang J, Schwendeman SP, Carpenter JF. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J Pharm Sci. 2006;95:1626–1639. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 95.Zhou P, An T, Zhao C, Li Y, Li R, Yang R, et al. Lactosylated PLGA nanoparticles containing ε-polylysine for the sustained release and liver-targeted delivery of the negatively charged proteins. Int J Pharm. 2015;478:633–643. doi: 10.1016/j.ijpharm.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 96.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porter CJH, Wasan KM, Constantinides P. Lipid-based systems for the enhanced delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 2008;60:615–616. doi: 10.1016/j.addr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Rev Drug Disc. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 99.Kim SK, Foote MB, Huang L. The targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials. 2012;33:3959–3966. doi: 10.1016/j.biomaterials.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park J, Wrzesinski SH, Stern E, Look M, Criscione J. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palucka AK, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35:8186–8196. doi: 10.1016/j.biomaterials.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 104.Wang M, Alberti K, Sun S, Arellano CL, Xu Q. Combinatorially designed lipid-like nanoparticles for intracellular delivery of cytotoxic protein for cancer therapy. Angew Chem Int Ed Engl. 2014;53:2893–2898. doi: 10.1002/anie.201311245. [DOI] [PubMed] [Google Scholar]
- 105.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iordanov MS, Ryabinina OP, Wong J, Dinh TH, Newton DL, Rybak SM, et al. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res. 2000;60:1983–1994. [PubMed] [Google Scholar]
- 107.Kim T, Hyeon T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology. 2014;25:012001. doi: 10.1088/0957-4484/25/1/012001. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater. 2013;25:3144–3176. doi: 10.1002/adma.201205292. [DOI] [PubMed] [Google Scholar]
- 109.Han DH, Na HK, Choi WH, Lee JH, Kim YK, Won C, et al. Direct cellular delivery of human proteasomes to delay tau aggregation. Nat Commun. 2014;5:5633. doi: 10.1038/ncomms6633. [DOI] [PubMed] [Google Scholar]
- 110.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh P, Yang X, Arvizo R, Zhu ZJ, Agasti SS, Mo Z, et al. Intracellular delivery of a membrane-impermeable enzyme in active form using functionalized gold nanoparticles. J Am Chem Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong R, Fischer NO, Verma A, Goodman CM, Emrick T, Rotello VM. Control of protein structure and function through surface recognition by tailored nanoparticle scaffolds. J Am Chem Soc. 2004;126:739–743. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]
- 113.Tang R, Kim CS, Solfiell DJ, Rana S, Mout R, Velázquez-Delgado EM, et al. Direct delivery of functional proteins and enzymes to the cytosol using nanoparticle-stabilized nanocapsules. ACS Nano. 2013;7:6667–6673. doi: 10.1021/nn402753y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett. 2011;11:694–700. doi: 10.1021/nl103812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang T, Sun W, Zhu Q, Burns NA, Khan SA, et al. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv Mater. 2015;27:1021–1028. doi: 10.1002/adma.201404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Shin MC, David AE, Zhou J, Lee K, He H, et al. Long-circulating heparin-functionalized magnetic nanoparticles for potential applications as a protein drug delivery platform. Mol Phrm. 2013;10:3892–3902. doi: 10.1021/mp400360q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Woitiski CB, Carvalho RA, Ribeiro AJ, Neufeld RJ, Veiga F. Strategies toward the improved oral delivery of insulin nanoparticles via gastrointestinal uptake and translocation. BioDrugs. 2008;22:223–237. doi: 10.2165/00063030-200822040-00002. [DOI] [PubMed] [Google Scholar]
- 119.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 120.Shan W, Zhu X, Liu M, Li L, Zhong J, Sun W, et al. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano. 2015;9:2345–2356. doi: 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- 121.Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dombu CY, Betbeder D. Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 2013;34:516–525. doi: 10.1016/j.biomaterials.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 123.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62:59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 124.Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 125.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 126.Keijzer C, Slütter B, van der Zee R, Jiskoot W, van Eden W, Broere F. PLGA, PLGA-TMC and TMC-TPP nanoparticles differentially modulate the outcome of nasal vaccination by inducing tolerance or enhancing humoral immunity. PLoS One. 2011;6:e26684. doi: 10.1371/journal.pone.0026684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prausnitz MR, Langer R. Transdermal drug delivery. Nature Biotech. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nose K, Pissuwan D, Goto M, Katayama Y, Niidome T, et al. Gold nanorods in an oil-base formulation for transdermal treatment of type 1 diabetes in mice. Nanoscale. 2012;4:3776–3780. doi: 10.1039/c2nr30651d. [DOI] [PubMed] [Google Scholar]
- 132.He W, Guo X, Zhang M. Transdermal permeation enhancement of N-trimethyl chitosan for testosterone. Int J Pharm. 2008;356:82–87. doi: 10.1016/j.ijpharm.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 133.Nose K, Pissuwan D, Goto M, Katayama Y, Niidome T. Gold nanorods in an oil-base formulation for transdermal treatment of type 1 diabetes in mice. Nanoscale. 2012;4:3776–3780. doi: 10.1039/c2nr30651d. [DOI] [PubMed] [Google Scholar]
- 134.DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye M, Kim S, Park K. Isuues in long-term protein delivery using biodegradable microparticles. J Control Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 138.Gu Z, Biswas A, Zhao M, Tang Y. Tailoring nanocarriers for intracellular protein delivery. Chem Soc Rev. 2011;40:3638–3655. doi: 10.1039/c0cs00227e. [DOI] [PubMed] [Google Scholar]
- 139.US FDA. Paving the way for personalized medicine; FDA‘s role in a new era of medical product development. Oct, 2013. [Google Scholar]