Summary
Reasons for performing study
Although exertional rhabdomyolysis (ER) is common in Arabian horses, there are no dedicated studies describing histopathological characteristics of muscle from Arabian horses with ER.
Objectives
To prospectively identify distinctive histopathological features of muscle from Arabian endurance horses with a history of ER (pro-ER) and to retrospectively determine their prevalence in archived samples from Arabian horses with exertional myopathies (retro-ER).
Study design
Prospective and retrospective histopathological description.
Methods
Middle gluteal muscle biopsies obtained from Arabian controls (n = 14), pro-ER (n = 13) as well as archived retro-ER (n = 25) muscle samples previously classified with type 2 polysaccharide storage myopathy (15/25), recurrent exertional rhabdomyolysis (7/25) and no pathology (3/25) were scored for histopathology and immunohistochemical staining of cytoskeletal proteins. Glutaraldehyde-fixed samples (2 pro-ER, one control) were processed for electron microscopy. Pro-ER and retro-ER groups were compared with controls using Mann–Whitney U and Fisher's exact tests.
Results
Centrally located myonuclei in mature myofibres were found in significantly more (P<0.05) pro-ER (12/13) and retro-ER (21/25) horses than controls (4/14). Degenerating myofibres were not evident in any biopsies. Retro-ER horses had amylase-resistant polysaccharide (6/25, P<0.05) and higher scores for cytoplasmic glycogen, rimmed vacuoles and rod-like bodies. A few control horses (3/14) and significantly (P<0.05) more pro-ER (12/13) and retro-ER (18/25) horses had disrupted myofibrillar alignment and large desmin and αβ-crystallin positive cytoplasmic aggregates. Prominent Z-disc degeneration and focal myofibrillar disruption with regional accumulation of β-glycogen particles were identified on electron microscopy of the 2 pro-ER samples.
Conclusions
In a subset of Arabian horses with intermittent episodes of exertional rhabdomyolysis, ectopic accumulation of cytoskeletal proteins and Z-disc degeneration bear a strong resemblance to a myofibrillar myopathy. While many of these horses were previously diagnosed with type 2 polysaccharide storage myopathy, pools of glycogen forming within disrupted myofibrils appeared to give the false appearance of a glycogen storage disorder.
Keywords: horse, desmin, myofilaments, intermediate filaments, exertional rhabdomyolysis
Introduction
Endurance horses, primarily Arabian horses, suffer from a high incidence of exertional myopathy, with 12% of endurance horse-owners reporting a history of exertional rhabdomyolysis (ER) [1]. Exertional rhabdomyolysis is definitively diagnosed by measuring elevations in serum muscle enzymes such as creatine kinase (CK) and aspartate transaminase (AST) in proximity to the exercise stimulus [2]. Although ER is classically characterised by muscle stiffness and pain, markedly increased serum CK activity can occur after exercise in endurance horses in the absence of signs of muscle stiffness [1,3,4]. To further complicate the diagnosis of exertional myopathy, muscle pain can occur in endurance horses in the absence of abnormal elevations in serum CK activity [2]. Thus, serum CK and AST activities alone provide valuable but limited diagnostic information with regard to the basis for muscle disorders in this breed.
Exertional rhabdomyolysis has been proposed to originate from extrinsic and intrinsic causes [1,5]. Extrinsic causes in Arabian horses include dehydration and metabolic derangements that develop with the prolonged and demanding nature of endurance exercise, particularly in horses with insufficient conditioning [3,6–8]. Some elite and well-conditioned endurance racing Arabians, however, repeatedly develop ER, which suggests that intrinsic muscular causes of chronic ER also exist [3,4,9]. Deficits in glycogen metabolism arising from polysaccharide storage myopathy (PSSM) and deficits in myoplasmic calcium release linked to recurrent ER are proposed but as yet unsubstantiated causes of ER in Arabians [2,10,11]. A recent study of 101 endurance Arabians, including 88 Arabian and Arabian-cross horses, did not identify the R309H glycogen synthase 1 (GYS1) mutation responsible for type 1 PSSM in any of the horses that were evaluated [1]. Abnormal polysaccharide, including amylase-resistant polysaccharide, however, has been identified in Arabian horses with ER suggesting that Arabians suffer from another form of PSSM [1,10,11]. Forms of PSSM not associated with the GYS1 mutation have been termed type 2 PSSM but their basis is unknown [12]. Clearly there is a pressing need for a better method for characterising myopathies beyond measurement of serum CK activity and routine muscle histology in order to identify the basis for intrinsic muscle disorders in Arabian horses.
The current study evaluated serum CK activity and used histopathology, immunohistochemistry and electron microscopy to identify distinctive histopathological characteristics of muscle biopsies from Arabian endurance horses with a history of ER. We first prospectively compared histopathology in gluteal muscle samples from Arabian endurance horses with a history of ER with samples from environmentally matched Arabian horses without a history of ER. We then retrospectively determined the prevalence of the identified distinctive histopathological features in a second population of Arabian horses with a history of ER that had previously had a muscle biopsy sample submitted to the Neuromuscular Diagnostic Laboratory at the University of Minnesota (NMDL) in order to confirm the prevalence and significance of these findings.
Materials and methods
Horses
Control and prospective ER
Endurance riders in the Pacific Northwestern USA were contacted in 2013 and 2014 to identify Arabian endurance horses with and without a history of ER. Inclusion criteria for the prospective ER (pro-ER) horses were: Arabian or Arabian-cross breeding, R309H GYS1 mutation negative, a history of at least one episode of ER confirmed by veterinary evaluation and where possible (n = 9 of 13 horses) at least one record of serum or plasma CK >4000 u/l or AST activity >1000 u/l. Clusters of closely related horses were avoided by excluding sires or dams of control or pro-ER horses and by not including more than one pair of full and one pair of half siblings in the study. Control horses (n = 14) were each located on the same farm as a pro-ER horse (12/14), had similar selection criteria for relatedness and had no history of ER during >3 years of endurance training. Prior to obtaining the 20 muscle biopsy samples in 2014, plasma samples were obtained for assessment of CK activity at rest (n = 10 pro-ER and n = 8 controls).
Retrospective ER
The database of the NMDL was searched to identify muscle biopsies from Arabian or Arabian-cross horses that had been submitted because of a history of at least one episode of ER and that had high-quality muscle biopsy samples available to be recut for histological evaluation. All Arabian-cross horses and horses with amylase-resistant polysaccharide were tested and found to be negative for the R309H GYS1 mutation. At the time of initial biopsy submission to the NMDL, the diagnosis provided was normal muscle biopsy (n = 3), type 2 PSSM (n = 15), based on the presence of subsarcolemmal or cytoplasmic aggregates of periodic acid-Schiff (PAS)-positive material, and recurrent ER (n = 7) based on centrally located myonuclei and normal PAS staining.
Muscle analysis
Muscle biopsy
Percutaneous needle biopsy samples were obtained from pro-ER and control horses using a standardised site in the middle gluteal muscle [13]. Muscle tissue was divided into 3–4 aliquots, fixed in formalin, snap frozen in liquid nitrogen, prepared for frozen histopathology and placed in glutaraldehyde. For frozen section muscle histopathology in 2013, samples (n = 7) were rolled in talcum powder and then frozen in liquid nitrogen. To reduce freeze artefacts, samples obtained in 2014 (n = 20) were immediately oriented in cross section on cork and placed in cassettes that were kept on ice packs until the sample could later be frozen in methylbutane suspended in liquid nitrogen. Histochemical samples were frozen within 24 h of sampling and all frozen samples were stored at −80°C.
Retrospective ER (retro-ER) muscle samples were obtained by needle biopsy of the gluteal muscle (n = 3) similarly to pro-ER horses or were surgically excised by referring veterinarians from the semimembranosus/tendinosus (n = 22) muscle and shipped on ice packs to the NMDL. Upon arrival within 48 h of biopsy, samples were oriented in cross section on cork, frozen in methylbutane suspended in liquid nitrogen and then stored at −80°C until analysis.
Muscle glycogen concentrations
For pro-ER and control horses muscle glycogen concentrations were determined in snap frozen muscle biopsies that had been stored at −80°C until analysis. Glycogen concentrations were also determined in archived muscle samples from retro-ER horses that had amylase-resistant polysaccharide (n = 6). For comparison with shipped retro-ER samples, muscle glycogen concentrations were also analysed in the next consecutive archived shipped sample with a diagnosis of normal muscle and with a diagnosis of type 1 PSSM confirmed by genetic analysis. Glycogen concentration was assayed fluorometrically as glucose residues [14].
Histochemistry
Muscle sections 8 μm thick were sectioned on a cryostat and stained with haematoxylin and eosin, modified Gomori trichrome, PAS, amylase-PAS, and oil red O [15]. Muscle biopsies were evaluated by one experienced histopathologist (S.J.V.) blinded to the case/control status for the presence of excessive fibre size variation, acute myodegeneration, macrophage infiltration, regenerative fibres, vacuoles and lipid accumulation. A scoring system was used at ×20 magnification to grade central nuclei, anguloid atrophy, subsarcolemmal vacuoles and aggregates of cytoplasmic and subsarcolemmal glycogen based on: 0 = not present, 1 = present in approximately 10% of fibres in the biopsy, 2 = present in approximately 11–25% of fibres in the sample, 3 = present in more than 25% of fibres in the sample.
Formalin-fixed sections
Formalin-fixed tissue was paraffin-embedded within 96 h of biopsy. Sections 4 μm thick were stained with PAS, counterstained with haematoxylin and eosin and scored for a wavy pattern of disrupted myofibrillar alignment using the same scoring system described for frozen sections.
Desmin immunohistochemistry
Because perturbations in myofibrillar alignment were noted in PAS stains of ER muscle biopsies, all formalin-fixed and frozen muscle biopsies underwent immunohistochemical (IHC) staining for desmin, a cytoskeletal intermediate filament that maintains parallel alignment of sarcomeres at the Z-disc. Detailed methods are provided in the online supporting information (Supplementary Item 1).
Analysis of desmin stains
A scoring system was used to grade the extent of sarcolemmal desmin staining. Staining that was inconsistently present around the sarcolemma of muscle fibres was graded as 1, present in a thin line around most of the sarcolemma of fibres was graded as 2 and present around the entire sarcolemma of most fibres in a thick line was graded as 3. Cytoplasmic aggregates of desmin positive material were also assessed. Muscle fibres that contained at least 2 aggregates of cytoplasmic desmin that were at least 50% of the size of myonuclei were counted as positive and the number of such fibres was counted for the entire muscle section. A desmin score was assigned based on: 0 = not present, 1 = present in 1–2 fibres, 2 = present in 3–6 fibres, 3 = present in 7–10 fibres, 4 = present in >10 fibres. A maximum of 30 positive fibres was counted. The proportion of horses with abnormal desmin staining was calculated as the percentage of horses with desmin score ≥1.
Additional immunohistochemistry
To further characterise the abnormal aggregates of desmin, IHC staining for additional cytoskeletal proteins αβ-crystallin, nebulin and dystrophin was performed on 4 pro-ER horses with numerous cytoplasmic desmin aggregates and 4 controls without desmin aggregates. Detailed methods are provided in Supplementary Item 1.
Electron microscopy
Cubes of approximately 5 mm2 were dissected from fresh muscle samples and placed in 2.5% glutaraldehyde in 0.1 mol/l sodium cacodylate buffer. Due to the substantial expense of electron microscopy, samples from one control and 2 ER horses were selected for thin sectioning. Detailed methods are provided in Supplementary Item 1.
Data analysis
The scores for anguloid atrophy, central nuclei, subsarcolemmal vacuoles, aggregates of cytoplasmic and subsarcolemmal glycogen, disrupted alignment and desmin were compared for frozen sections between controls and either pro-ER or retro-ER samples using a Mann–Whitney U test for nonparametric scores. Mann–Whitney U testing was also used for comparisons of cytoplasmic and subsarcolemmal glycogen, disrupted alignment and desmin staining in formalin-fixed samples. The proportion of either pro-ER or retro-ER samples with anguloid atrophy, central nuclei, subsarcolemmal vacuoles, aggregates of cytoplasmic and subsarcolemmal glycogen, disrupted alignment and desmin with scores ≥1 were compared with control horses using a Fisher's exact test. A Spearman r correlation coefficient was calculated for age vs. desmin positive fibres. Muscle glycogen concentrations were compared between control and pro-ER horses with a t test and among retro-ER horses, negative controls and PSSM disease controls using a one way ANOVA and Tukey's multiple comparison test. Data are presented as mean ± s.d. Significance was set at a P value of <0.05.
Results
Horses
The control group of horses consisted of 14 Arabians, including 4 mares and 10 geldings with a mean age of 11.4 ± 6.1 years. The pro-ER group consisted of 10 Arabians, 2 Arabian-Saddlebred crosses and one Arabian-Paint cross with 7 mares, 5 geldings, one stallion and a mean age of 15.8 ± 6.1 years. Median resting plasma CK activity at the time of biopsy was 264 ± 97 u/l (maximum 490 u/l) in control horses and 246 ± 100 u/l (maximum 458 u/l) in pro-ER horses. The retro-ER group consisted of 18 Arabians and 7 Arabian crosses (Warmblood, Morgan, Tennessee Walker, Pinto, Quarter Horse) including one stallion, 16 mares and 7 geldings (one unreported) with a mean age of 10.7 ± 4.5 years. Ten horses were used for endurance racing, 3 horses for pleasure riding and, for 10 horses, a specific use was not provided. Serum CK and AST activities were reported for 14/25 retro-ER horses without reference to time of sampling relative to exercise or obtaining the biopsy sample. The median reported serum CK activity in this group was 1516 u/l (range 282–42,000 u/l) and AST 1503 u/l (range 288–2262 u/l).
Muscle glycogen concentrations
Muscle glycogen concentrations were not significantly different between pro-ER (136 ± 30 mmol/kg wet weight) and control horses (145.1 ± 32 mmol/kg) (P = 0.5). Muscle glycogen in retro-ER horses (124 ± 46 mmol/kg) was not significantly different from shipped samples from horses with no observed muscle pathology (101.8 ± 25 mmol/kg) whereas glycogen concentrations in shipped type 1 PSSM horses (172 ± 55 mmol/kg) were significantly higher than in shipped samples from horses with no observed muscle pathology (P = 0.006).
Muscle histopathology
Control horses
Four of 14 control horses had centrally displaced nuclei in mature muscle fibres (Table 1). No other histopathology was identified.
TABLE 1.
The histopathological scores and prevalence of histopathological features of frozen muscle biopsy samples from control Arabians and Arabian horses with a history of exertional rhabdomyolysis selected prospectively or retrospectively
| Central nuclei | Anguloid atrophy | Subs. vacuoles | Glycogen |
Amylase resistant polysaccharide | |||
|---|---|---|---|---|---|---|---|
| n | Subs. | Cytop. | |||||
| Score | |||||||
| Controls | 14 | 0.5 ± 1.0 | 0.54 ± 0.69 | 0.1 ± 0.3 | 0.7 ± 0.7 | 0.2 ± 0.4 | 0 |
| Pro-ER | 13 | 1.4 ± 0.6* | 0.54 ± 0.63 | 0.3 ± 0.6 | 0.5 ± 0.8 | 0.5 ± 0.6 | 0 |
| Retro-ER | 25 | 1.1 ± 0.8* | 1.26 ± 1.85* | 0.7 ± 0.8** | 0.8 ± 0.9 | 1.4 ± 0.9*** | 0.3 ± 0.6* |
| Prevalence (score ≥1) | |||||||
| Controls | 14 | 4 (29%) | 6 (43%) | 1 (7%) | 7 (50%) | 5 (36%) | 0 |
| Pro-ER | 13 | 12 (92%)** | 6 (46%) | 3 (23%) | 4 (31%) | 7 (54%) | 0 |
| Retro-ER | 25 | 21 (84%)** | 19 (76%)*** | 12 (48%)* | 13 (52%) | 21 (84%)* | 6 (24%) |
Scores are means ± s.d. Pro-ER = prospective exertional rhabdomyolysis group; Retro-ER= retrospective exertional rhabdomyolysis group; Subs. = subsarcolemmal; Cytop. = cytoplasmic.
Significantly different from controls in same column at P<0.05.
Significantly different from controls in same column at P<0.01.
Significantly different from controls in same column at P<0.001.
Pro-ER horses
There were significantly more pro-ER horses with centrally displaced nuclei in mature myofibres than controls (Table 1, Fig 1a). The scores and proportion of horses with anguloid atrophy, subsarcolemmal vacuoles and subsarcolemmal and cytoplasmic aggregates of glycogen were similar between control and pro-ER horses (Table 1). Degenerating muscle fibres and amylase-resistant polysaccharide were not evident in any pro-ER biopsies. Regenerating small basophilic muscle fibres were present in the muscle of one pro-ER horse.
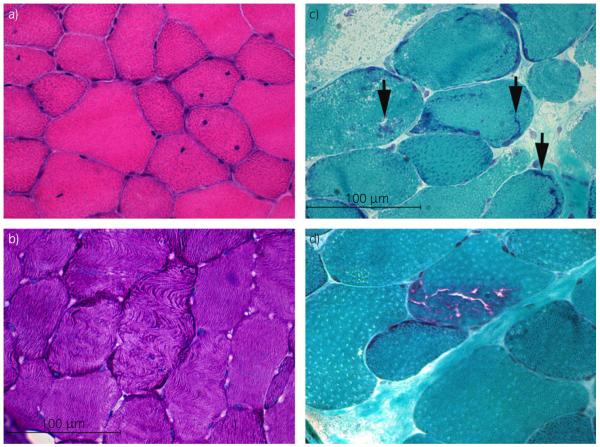
Fig 1.
a) Cross section of gluteal muscle from a pro-ER horse (prospective exertional rhabdomyolysis) containing numerous fibres with one or more centrally displaced nuclei (haematoxylin and eosin ×40). b) Cross section of gluteal muscle from a pro-ER horse demonstrating fibres with a wavy disrupted pattern of myofibre alignment (periodic acid-Schiff (PAS) ×40). c) Cross section of semimembranosus muscle from a retro-ER (retrospective exertional rhabdomyolysis) horse showing abnormal amorphous basophilic material in several fibres (arrows) (modified Gomori trichrome ×40). d) Cross section of semimembranosus muscle from a retro-ER horse demonstrating rimmed vacuoles within a muscle fibre (modified Gomori trichrome ×40).
Scores for a wavy pattern of disrupted myofibrillar alignment were higher in pro-ER than control horses (Table 2, Fig 1b). Significantly more pro-ER horses had a disrupted pattern of myofibrillar alignment than controls (Table 2). Disrupted alignment was seen in 3 more control horses and 2 more pro-ER horses in formalin-fixed compared with frozen samples (Table 2).
TABLE 2.
Scores and prevalence of disrupted myofibre alignment and cytoplasmic aggregates of desmin in frozen and formalin-fixed muscle biopsy samples from control Arabians and Arabian horses with a history of exertional rhabdomyolysis (ER) selected prospectively and in frozen sections of ER horses selected retrospectively
| Disrupted alignment |
Desmin |
||||
|---|---|---|---|---|---|
| n | Frozen | Formalin | Cytop. frozen | Cytop. formalin | |
| Score | |||||
| Controls | 14 | 0.4 ± 0.8 | 0.8 ± 0.9 | 0.7 ± 1.5 | 0.6 ± 1.5 |
| Pro-ER | 13 | 1.2 ± 0.9* | 1.9 ± 0.9* | 2.8 ± 1.3** | 2.2 ± 1.6** |
| Retro-ER | 25 | 1.1 ± 1.0* | NP | 1.9 ± 1.5** | NP |
| Prevalence (score ≥1) | |||||
| Controls | 14 | 4 (29%) | 7 (50%) | 3 (22%) | 3 (22%) |
| Pro-ER | 13 | 10 (77%)* | 12 (92%)* | 12 (92%)** | 11 (85%)** |
| Retro-ER | 25 | 15 (60%) | NP | 18 (72%)** | NP |
Disrupted alignment was assessed in both frozen and formalin fixed sections. Scores are means ± s.d. Pro-ER = prospective exertional rhabdomyolysis group; Retro-ER= retrospective exertional rhabdomyolysis group; Cytop.= cytoplasmic; NP = not performed.
Significantly different from controls in same column at P<0.05.
Significantly different from controls in same column at P<0.01.
Retro-ER horses
Retro-ER horses had significantly more mature myofibres with centrally displaced nuclei than controls (Table 1). Degenerating muscle fibres were not evident. Regenerating small basophilic muscle fibres were present in 2/25 (8%) retro-ER muscle samples. Scores for anguloid atrophy were higher for retro-ER than control horses but the proportion of horses with anguloid atrophy was not different between retro-ER and control horses (Table 1). Scores for, and the proportion of horses with, subsarcolemmal vacuoles and cytoplasmic glycogen were higher in retro-ER than control horses (Table 1). Subsarcolemmal glycogen scores and proportions were similar between retro-ER and control horses (Table 1). Retro-ER horses had histopathological features that were not noted in pro-ER samples. Rod-like bodies were noted in a few fibres in the Gomori trichrome stain of 5 retro-ER horses (Fig 1c). Rimmed vacuoles were identified in a few myofibres of 3/25 retro-ER horses in the Gomori trichrome stain (Fig 1d). The 3 horses with rimmed vacuoles and 3 additional horses had PAS-positive amylase-resistant polysaccharide inclusions within a few muscle fibres in the biopsy sample (<3 fibres/sample).
Scores for disrupted myofibrillar alignment were higher in retro-ER than control horses; however, the proportion of horses with disrupted alignment did not differ between retro-ER and control horses (Table 2).
Immunohistochemistry
Control horses
Aggregates of cytoplasmic desmin were identified in myofibres of 3 Arabian control horses, 2 of which also had centrally displaced nuclei (Table 2).
Pro-ER
Significantly more pro-ER horses had aggregates of cytoplasmic desmin in myofibres than control horses in frozen (Table 2, Fig 2a and b) and formalin-fixed sections (Table 2). Scores for cytoplasmic desmin were significantly higher in pro-ER compared with control horses in (Table 2, Fig 2a). Cytoplasmic desmin scores ≥1 were found in 9 Arabians and 2 Arabian crosses out of the 13 total pro-ER horses. Desmin positive aggregates did not correspond to PAS positive inclusions (Fig 3a–d). In contrast with aggregates of desmin in mature myofibres (Fig 3d and e), regenerating fibres noted in one pro-ER horse had dense desmin staining of the entire fibre (Fig 3f). Scores for subsarcolemmal desmin were not different between pro-ER and control horses in frozen or formalin-fixed sections (frozen: pro-ER 2.0 ± 0.3, controls 1.7 ± 0.4; formalin: pro-ER 1.4 ± 0.9, controls 1.5 ± 0.4).
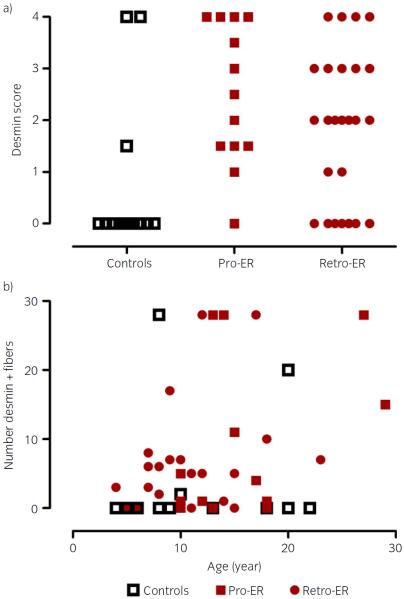
Fig 2.
a) The cytoplasmic desmin score for control, prospective exertional rhabdomyolysis (pro-ER) and retrospective ER (retro-ER) horses. For control and pro-ER horses scores are the average of formalin and frozen desmin stains. For retro-ER horses only frozen sections were available to score. b) The relationship between the number of muscle fibres with cytoplasmic desmin aggregates and the age of the horse. The maximum number of desmin positive fibres counted was 30. Age does not appear to be correlated with the number of desmin positive fibres.
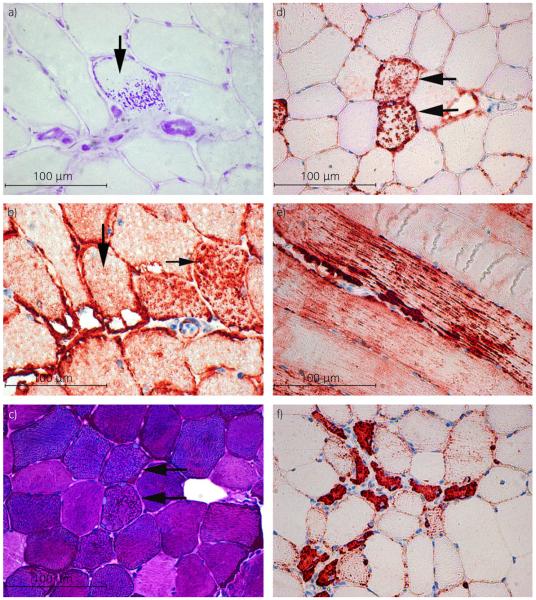
Fig 3.
a) Cross section of semimembranosus muscle from a retrospective exertional rhabdomyolysis (retro-ER) horse stained with amylase periodic acid-Schiff (PAS) demonstrating amylase-resistant abnormal PAS positive material (arrow). (PAS ×40). b) Serial frozen section demonstrating that myofibres with abnormal PAS positive material (vertical arrow) do not contain desmin positive aggregates (horizontal arrow) (desmin immunohistochemistry ×40). c) Cross section of gluteal muscle from a prospective ER (pro-ER) horse demonstrating normal staining for glycogen (PAS ×40). d) Serial formalin-fixed section of the same muscle shown in (c) showing that muscle fibres with desmin positive aggregates have normal glycogen staining (horizontal arrows in (b) and (c); desmin immunohistochemistry ×40). e) Longitudinal section of a formalin-fixed gluteal muscle from a pro-ER horse showing aggregates of cytoplasmic desmin throughout the length of a mature muscle fibre (desmin immunohistochemistry ×20). f) Cross-section of formalin-fixed gluteal muscle from a pro-ER horse showing small anguloid regenerating fibres with centrally located nuclei that have a uniform dark desmin stain (desmin immunohistochemistry ×40).
Retro-ER
Retro-ER horses had higher scores for, and prevalence of, cytoplasmic desmin aggregates than control horses (Fig 3a). Retro-ER horses had aggregates of cytoplasmic desmin in 18/25 (72%) of muscle biopsies (Table 2, Fig 3a). Aggregates of cytoplasmic desmin were found in 13 Arabian and 5 Arabian-cross horses. Small regenerating fibres noted in 2 horses had a uniform dense desmin stain. Scores for subsarcolemmal desmin were not different between retro-ER (1.6 ± 0.7) and control horses (1.8 ± 0.3).
There was a significant low positive correlation (r = 0.30, P = 0.03) between age and the number of fibres with aggregates of cytoplasmic desmin in pro-ER and retro-ER horses (Fig 3b).
Characterisation of desmin aggregates
Aggregates of desmin occurred in type 2A and 2B(x) fibres but not type 1 muscle fibres. Nicotinamide adenine dinucleotide (NADH) stains for mitochondria were normal. A proportion of desmin positive aggregates stained positively for αβ-crystallin (Fig 4a and b) but desmin positive aggregates did not stain positively for nebulin or dystrophin (Fig 4c and d).
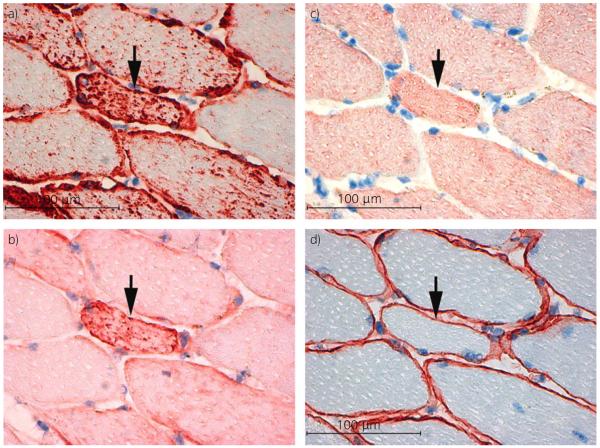
Fig 4.
Serial cross sections of frozen gluteal muscle from a prospective exertional rhabdomyolysis (pro-ER) horse. a) Desmin (×40). b) αβ-Crystallin (×40). c) Nebulin (×40). d) Dystrophin (×40). Note that aggregates within a desmin positive fibre (arrow) stain positively for αβ-crystallin (arrow) but not nebulin or dystrophin.
Electron microscopy
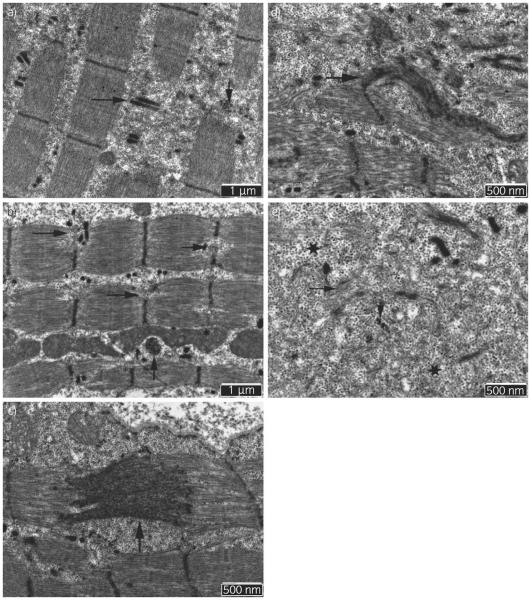
Many regions of myofibres in the 2 pro-ER horses were similar to the control sample. However, in some areas of the sections of both pro-ER horses, focal disruptions of sarcomeres were observed that were not present in the control horse. Within these regions, T-tubules with intact adjacent terminal cisternae, remnants of Z-disc proteins and a few degenerating myeloid bodies were present along with a large accumulation of β-glycogen particles (Fig 5a). Some sarcomeres of pro-ER horses had evidence of Z-disc degeneration ranging from partial disruption (Fig 5b), to streaming (Fig 5c), to complete lysis (Fig 5d). One of the 2 pro-ER horses had flag-like semi-dense extensions of the Z-discs that were oriented perpendicularly to the Z-disc (Fig 5d). Within myofibres, regions of β-glycogen accumulation were intertwined with degenerated Z-disc material and degenerating myofilaments (Fig 5e). Occasional deposits resembling lipofuscin were evident under the sarcolemma in both pro-ER samples but not the control sample.
Fig 5.
Electron microscopy of gluteal muscle from 2 prospective exertional rhabdomyolysis (pro-ER) horses. a) Segments of myofibres demonstrating breaks in sarcomeres through the Z-disc and remnants of Z-discs (vertical arrow). Breaks contain junctional sarcoplasmic reticulum and T-tubules (horizontal arrow) as well as accumulations of β-glycogen particles (×20,000). b) Partial disruption of Z-discs is apparent in numerous sarcomeres of the same pro-ER horse as (a) (horizontal arrows) and a membranous whirl near mitochondria (vertical arrow) (×20,000). c) Marked Z-disc streaming (vertical arrow) (×30,000) in a second pro-ER horse. d) Marked myofibrillar disarray and Z-disc degeneration with a flag-like semi-dense extension of the Z-disc in the second horse (×30,000). e) Degenerated Z-disc material (vertical arrow), myofilaments (horizontal arrow) and β-glycogen particles (stars) in the gluteal muscle of the second pro-ER horse.
Discussion
Many of the horses with ER in the current study were previously diagnosed with type 2 PSSM or recurrent ER based on history and the presence (type 2 PSSM) or absence (recurrent ER) of PAS positive aggregates in muscle biopsies. Although the diagnostic term `type 2 PSSM' has implied a glycogen storage disorder, analysis of muscle samples did not support this supposition because muscle glycogen concentrations were normal. Furthermore, an additional study of the metabolic response to exercise of 10 of the Arabian horses with a history of ER that were also included in the present study found normal metabolic responses to exercise (McKenzie E.C., Eyrich L., Payton M.E., Valberg S.J., unpublished observations). In contrast, Quarter Horses with type 1 PSSM have higher lactate concentrations during submaximal exercise, as well as >1.8-fold higher muscle glycogen concentrations than healthy controls [16]. The results of the current study suggest an alternative explanation for the appearance of increased cytoplasmic glycogen in a subset of Arabian horses susceptible to ER. That is, that the distribution of glycogen shifts with aggregation of cytoplasmic β-glycogen among breaks in sarcomeres because of structural defects in the skeletal muscle cytoskeleton and Z-disc. Amylase resistance could develop in a few fibres as a potential result of entanglement with Z-disc remnants and degenerating myofilaments. While not conclusive at this point, the possibility that type 2 PSSM is not an underlying glycogenosis but rather a reflection of structural defects in myofibrils and Z-discs warrants further investigation.
The Z-disc is a highly specialised multiprotein complex and a `hot spot' for mutations and muscle diseases [17,18]. In addition to anchoring the thin filaments of the myofibril, it serves as the interface of the sarcomere and the cytoskeleton with complex signalling functions within the cell [17]. The histopathological and ultrastructural findings in the present study suggest that a subset of Arabian horses susceptible to ER have a specific myopathy characterised by disruption of the Z-disc and secondary ectopic accumulation of desmin and αβ-crystallin. Desmin is one of the primary cytoskeletal proteins at the Z-disc; it interlinks and tethers myofibrils and further connects them to the cell membrane, myonuclei and mitochondria, whereas αβ-crystallin serves as a chaperone for desmin and other Z-disc proteins, ensuring their normal folding and protecting them from stress-induced damage [17,18]. Ectopic expression of multiple Z-disc associated proteins, a pathological pattern of disintegration of the Z-disk, foci of myofibril dissolution and accumulation of myofibrillar degradation products are characteristic of a group of inherited disorders in man called desmin-associated myopathies or myofibrillar myopathies (MFM) [19–21]. The streaming Z-discs and flag-like semidense extensions oriented perpendicularly to the Z-disc found in the 2 Arabian horses with ER in the current study were remarkably similar to ultrastructural features of human MFM [22]. Other histopathological features common to muscle samples from human MFM patients and a subset of Arabian horses in the current study include the presence of very few necrotic or regenerating muscle fibres, the presence of small lakes of PAS-positive material and the presence of a few fibres with rod-like material in Gomori trichrome stains [20].
One of the classic histopathological features in EM reported in human patients with MFMs that was not found in Arabian horses in the current study is a large accumulation of granulofilamentous material or filamentous bundles [22]. The number of equine muscle fibres evaluated by electron microscopy in the present study was less than 20 and ectopic desmin aggregates were only found in a small number of fibres per biopsy, making it very possible to miss examining fibres with the most ectopic protein expression. Other features not present in the Arabian horses include hyaline or spheroid bodies in Gomori trichrome stains and extensive accumulation of autophagic rimmed vacuoles [18,19,21]. Muscle involvement in human MFM cases can be highly selective, for example, affecting the semitendinosus but not the semimembranosus muscle [18]. In the present study, abnormal desmin accumulation was found in both the semimembranosus and gluteal muscles; however, it is possible that other muscle groups that were not sampled in the current study may be more severely affected.
The clinical presentation of dominantly inherited MFM in man is very heterogeneous, with the age at diagnosis ranging from 21 to 82 years [21]. Arabian horses with suspected MFM were mature, with their mean age (11–15 years) being twice that reported for horses diagnosed with type 1 PSSM at initial muscle biopsy (mean age 6–7 years) [21,23]. Primary symptoms of human MFM include slowly progressive weakness, paraesthesia, muscle wasting, muscle stiffness, aching, cramps, dyspnoea and dysphagia; skeletal muscle, cardiac muscle or both may be involved [21]. Prognosis for patients with MFM can be highly variable depending on the genetic mutation and ranges from shortened lifespan due to cardiomyopathy to a normal lifespan with a gradually progressive weakness of limb and potentially trunk and neck muscles [20,21]. Muscle stiffness, pain or cramps and, in some cases, muscle atrophy were features of MFM in Arabian horses. Cardiac disease was not suspected in any horse. Differences between Arabian ER and human MFM include the lack of slowly progressive debilitating weakness in Arabian ER and the presence of intermittent rhabdomyolysis in horses, which is not a reported feature of human MFM cases [18]. Development of chronic ER limited the number of competitive races for some horses in the present study but, even in retirement, all horses were able to perform trail rides. Serum CK activities are usually normal or mildly increased in many human MFM patients. One review of 63 MFM cases reported that serum CK activity was within the reference interval in 33 patients and increased up to 7-fold above the upper reference limit in the remaining patients [21]. In the present study, a 7-fold increase above the upper reference limit would correspond to a CK of 4431 u/l, which is well above the plasma CK measured in pro-ER horses and almost 3 times higher than the median CK of retro-ER horses. Myofibrillar myopathy has been reported in a 1-year-old dog that had severe debilitating weakness and a serum CK activity of 4127 u/l [24]. Thus, the intermittent nature of the serum CK elevations characteristic of many Arabian ER cases could fall within the range of reported serum CK with MFM cases, particularly considering that the amount of exercise that Arabian horses experienced is probably far in excess of anything human patients with MFM would voluntarily perform. It is apparent that, on a day-to-day basis, both the clinical presentation and histopathological features of the subset of Arabian horses with suspected MFM in the present study were milder than those reported in human and canine MFMs [20,21,24]. Since 3 of the control horses were found to have myofibres with abnormal desmin accumulation (and central nuclei) and many of the older Arabian horses with suspected MFM were still being ridden, a suspected MFM is potentially common in Arabian horses and not necessarily as debilitating a disease in horses as it is in man or dogs.
An alternative explanation for ectopic accumulation of cytoskeletal proteins including desmin in Arabian horses with ER is increased expression in response to the mechanical stresses induced by strenuous exercise. Desmin immunohistochemistry, however, has been studied in rats subjected to excessive eccentric exercise [25]. Rather than an increase, eccentric exercise resulted in diminished desmin IHC staining. Several days after strenuous exercise, immunoblots showed an increase in the amount of desmin in muscle but aggregates of desmin are not apparent in IHC stains [25]. Furthermore, muscle disorders characterised by progressive degeneration, such as Duchenne muscular dystrophy, are not characterised by desmin aggregates [26]. Finally, many of the horses in the current study were lightly exercised and all pro-ER horses had been rested for at least 24–48 h before exercise. Thus, Z-disc degeneration and ectopic expression of desmin in the present study does not appear to be the result of preceding strenuous exercise.
Desmin positive immunostaining has been reported in 2 previous studies of equine muscle. In the first study, a horse with severe rhabdomyolysis had diffusely dark desmin staining of small regenerating myofibres [27]. A similar uniformly dense staining pattern was noted in a few myofibres of 3 horses in the present study and these fibres corresponded to small basophilic regenerating fibres that had large central nuclei with prominent nucleoli. The uniform dense staining pattern of regenerating fibres was distinct from the abnormal desmin positive aggregates found in mature myofibres of numerous ER horses in the present study. In a second IHC study aggregates of desmin, myoglobin and amylase-resistant polysaccharide were reported to co-localise in muscle fibres of horses with type 1 PSSM [12]. The aggregates were thought to reflect unrelated muscle proteins wrapped up in the large filamentous polyglucosan bodies associated with type 1 PSSM. We closely evaluated sequential PAS and desmin stains in the present study and determined that desmin positive aggregates did not correspond to aggregates of PAS positive material. Further, horses in the present study did not have the GYS1 mutation causative for type 1 PSSM. Thus, cytoskeletal aggregates that were desmin and αβ-crystallin positive, and nebulin and dystrophin negative, appear to represent a primary rather than a secondary pathological finding.
In conclusion, the results of this study introduce the novel possibility that a subset of Arabian and Arabian-cross horses with a history of exertional myalgia and intermittent elevations in serum CK activity have an underlying myofibrillar myopathy caused by weakness in Z-discs and resulting in ectopic proliferation of cytoskeletal proteins. Aggregation of glycogen within disrupted myofibrils may have given the appearance of a glycogen storage disorder such as type 2 PSSM. Immunohistochemical staining for aggregates of cytoskeletal proteins such as desmin could be a valuable addition to muscle biopsy screening of horses with myopathies.
Supplementary Material
Acknowledgements
Owners and referring veterinarians of horses that participated in this study are gratefully acknowledged.
Source of funding This work was funded by the Morris Animal Foundation (D14EQ-021).
Footnotes
Authors' declaration of interests In order to rule out type 1 PSSM, horses in this study were genotyped to ensure they did not possess the mutation. To prevent any potential bias genotyping was done in the veterinary diagnostic laboratory and the presence or absence of the mutation was identified by personnel in that laboratory. All samples were negative. This is in compliance with the conflict of interest management plan generated by the University of Minnesota due to S.J. Valberg being one of the patent holders of the GYS1 genetic test for PSSM.
Ethical animal research All procedures were approved by the Oregon State University Institutional Animal Care and Use Committee as well as the University of Minnesota Animal Care and Use Committee. All clients involved in this study signed an informed consent document prior to participation.
Authorship E.C. McKenzie, S.J. Valberg and C.J. Finno developed the study design. J. Shivers and N.E. Barnes contributed to methods development and implementation. E.C. McKenzie, S.J. Valberg and L.V. Eyrich performed data collection and along with C.J. Finno contributed to data interpretation and manuscript preparation. All authors provided final approval for the submitted version of the manuscript.
Supporting Information Additional Supporting Information may be found in the online version of this article at the publisher's website:
Supplementary Item 1: Supplementary methods information.
References
- 1.Wilberger MS, McKenzie EC, Payton ME, Rigas JD, Valberg SJ. Prevalence of exertional rhabdomyolysis in endurance horses in the Pacific Northwestern United States. Equine Vet. J. 2015;47:165–170. doi: 10.1111/evj.12255. [DOI] [PubMed] [Google Scholar]
- 2.Valberg SJ. Diseases of muscle. In: Smith BP, editor. Large Animal Internal Medicine. 5th edn. Elsevier; St Louis, Missouri: 2015. pp. 1276–1308. [Google Scholar]
- 3.Schott HC, Marlin DJ, Geor RJ, Holbrook TC, Deaton CM, Vincent T, Dacre K, Schroter RC, Jose-Cunilleras E, Cornelisse CJ. Changes in selected physiological and laboratory measurements in elite horses competing in a 160 km endurance ride. Equine Vet. J. 2006;38(Suppl. 36):37–42. doi: 10.1111/j.2042-3306.2006.tb05510.x. [DOI] [PubMed] [Google Scholar]
- 4.Barnes A, Kingston J, Beetson S, Kuiper C. Endurance veterinarians detect physiologically compromised horses in a 160 km ride. Equine Vet. J. 2010;42(Suppl. 42):6–11. doi: 10.1111/j.2042-3306.2010.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Valberg SJ. Muscling in on the cause of tying-up. Proc. Am. Ass. Equine Practnrs. 2012;58:85–123. [Google Scholar]
- 6.Fielding CL, Magdesian KG, Rhodes DM, Meier CA, Higgins JC. Clinical and biochemical abnormalities in endurance horses eliminated from competition for medical complications and requiring emergency medical treatment: 30 cases (2005–2006) J. Vet. Emerg. Crit. Care. 2009;19:473–478. doi: 10.1111/j.1476-4431.2009.00441.x. [DOI] [PubMed] [Google Scholar]
- 7.Schott HC, McGlade KS, Molander HA, Leroux AJ, Hines MT. Body weight, fluid, electrolyte, and hormonal changes in horses competing in 50- and 100-mile endurance rides. Am. J. Vet. Res. 1997;58:303–309. [PubMed] [Google Scholar]
- 8.Muñoz A, Riber C, Trigo P, Castejon F. Muscle damage, hydration, electrolyte balance and vasopressin concentrations in successful and exhausted endurance horses. Pol. J. Vet. Sci. 2010;13:373–379. [PubMed] [Google Scholar]
- 9.Fraipont A, Van Erck E, Ramery E, Richard E, Denoix JM, Lekeux P, Art T. Subclinical diseases underlying poor performance in endurance horses: diagnostic methods and predictive tests. Vet. Rec. 2011;169:154. doi: 10.1136/vr.d4142. [DOI] [PubMed] [Google Scholar]
- 10.Valentine BA, McDonough SP, Chang YF, Vonderchek AJ. Polysaccharide storage myopathy in Morgan, Arabian, and Standardbred related horses and Welsh-cross ponies. Vet. Pathol. 2000;37:193–196. doi: 10.1354/vp.37-2-193. [DOI] [PubMed] [Google Scholar]
- 11.Estill CT, Valentine BA. Severe rhabdomyolysis due to polysaccharide storage myopathy in an Arabian mare. Equine Vet. Educ. 2007;19:139–142. [Google Scholar]
- 12.McCue ME, Armien AG, Lucio M, Mickelson JR, Valberg SJ. Comparative skeletal muscle histopathologic and ultrastructural features in two forms of polysaccharide storage myopathy in horses. Vet. Pathol. 2009;46:1281–1291. doi: 10.1354/vp.08-VP-0177-M-FL. [DOI] [PubMed] [Google Scholar]
- 13.Lindholm A, Piehl K. Fibre composition, enzyme activity and concentrations of metabolites and electrolytes in muscles of standardbred horses. Acta Vet. Scand. 1974;15:287–309. doi: 10.1186/BF03547460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry OH, Passonneau JV. A Flexible System for Enzymatic Analysis. Academic Press; New York: 1972. A kinetic primer for tissue analysts; pp. 21–25. [Google Scholar]
- 15.Cumming WJK, Fulthorpe JJ, Hudgson P, Mahon M. Histological and histochemical procedures. In: Cumming JK, Fulthorpe J, Hudgson P, Mahon M, editors. Color Atlas of Muscle Pathology. Mosby-Wolfe; London: 1994. pp. 183–188. [Google Scholar]
- 16.Ribeiro WP, Valberg SJ, Pagan JD, Gustavsson BE. The effect of varying dietary starch and fat content on serum creatine kinase activity and substrate availability in equine polysaccharide storage myopathy. J. Vet. Intern. Med. 2004;18:887–894. doi: 10.1892/0891-6640(2004)18<887:teovds>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc. Res. 2008;77:667–675. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- 18.Selcen D. Myofibrillar myopathies. Neuromuscul. Disord. 2011;21:161–171. doi: 10.1016/j.nmd.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel AG. Myofibrillar myopathy. Ann. Neurol. 1999;46:681–683. doi: 10.1002/1531-8249(199911)46:5<681::aid-ana1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Olive M, Odgerel Z, Martinez A, Poza JJ, Bragado FG, Zabalza RJ, Jerico I, Gonzalez-Mera L, Shatunov A, Lee HS, Armstrong J, Maravi E, Arroyo MR, Pascual-Calvet J, Navarro C, Paradas C, Huerta M, Marquez F, Rivas EG, Pou A, Ferrer I, Goldfarb LG. Clinical and myopathological evaluation of early- and late-onset subtypes of myofibrillar myopathy. Neuromuscul. Disord. 2011;21:533–542. doi: 10.1016/j.nmd.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selcen D, Ohno K, Engel AG. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain. 2004;127:439–451. doi: 10.1093/brain/awh052. [DOI] [PubMed] [Google Scholar]
- 22.Claeys KG, van der Ven PF, Behin A, Stojkovic T, Eymard B, Dubourg O, Laforet P, Faulkner G, Richard P, Vicart P, Romero NB, Stoltenburg G, Udd B, Fardeau M, Voit T, Fürst DO. Differential involvement of sarcomeric proteins in myofibrillar myopathies: a morphological and immunohistochemical study. Acta Neuropathol. 2009;117:293–307. doi: 10.1007/s00401-008-0479-7. [DOI] [PubMed] [Google Scholar]
- 23.McCue ME, Ribeiro WP, Valberg SJ. Prevalence of polysaccharide storage myopathy in horses with neuromuscular disorders. Equine Vet. J. 2006;38(Suppl. 36):340–344. doi: 10.1111/j.2042-3306.2006.tb05565.x. [DOI] [PubMed] [Google Scholar]
- 24.Shelton GD, Sammut V, Homma S, Takayama S, Mizisin AP. Myofibrillar myopathy with desmin accumulation in a young Australian Shepherd dog. Neuromuscul. Disord. 2004;14:399–404. doi: 10.1016/j.nmd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Barash IA, Peters D, Friden J, Lutz GJ, Lieber RL. Desmin cytoskeletal modifications after a bout of eccentric exercise in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R958–R963. doi: 10.1152/ajpregu.00185.2002. [DOI] [PubMed] [Google Scholar]
- 26.Goebel HH, Bornemann AB. Desmin pathology in neuromuscular diseases. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;64:127–135. doi: 10.1007/BF02915105. [DOI] [PubMed] [Google Scholar]
- 27.Quist EM, Dougherty JJ, Chaffin MK, Porter BF. Equine rhabdomyolysis. Vet. Pathol. 2011;48:E52–E58. doi: 10.1177/0300985811414034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.