Abstract
Considerable work indicates that early cumulative risk exposure is aversive to human development, but very little research has examined neurological underpinnings of these robust findings. We investigated amygdala volume and reactivity to facial stimuli among adults (M = 23.7 years, n = 54) as a function of cumulative risk exposure during childhood (ages 9 and 13). In addition, we tested whether expected, cumulative risk elevations in amygdala volume would mediate functional reactivity of the amygdala during socio-emotional processing. Risks included substandard housing quality, noise, crowding, family turmoil, child separation from family, and violence. Total and left hemisphere adult amygdala volumes, respectively were positively related to cumulative risk exposure during childhood. The links between childhood cumulative risk exposure and elevated amygdala responses to emotionally neutral facial stimuli in adulthood were mediated by the respective amygdala volumes. Cumulative risk exposure in later adolescence (17 years), however, was unrelated to subsequent, adult amygdala volume or function. Physical and socioemotional risk exposures early in life appear to alter amygdala development, rendering adults more reactive to ambiguous stimuli such as neutral faces. These stress-related differences in childhood amygdala development might contribute to well-documented psychological distress as a function of early risk exposure.
Keywords: Children, cumulative risk, amygdala volume, amygdala function
Brain development is sensitive to chronic stress, but most of this work has focused on the hippocampus (Lupien, McEwen, Gunnar & Heim, 2009; McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010), with much less work examining amygdala volume or function. The amygdala is a key brain structure in responses to emotionally salient situations such as threat or ambiguity. The amygdala rapidly responds to environmental demands by triggering a sequence of physiological stress responses including sympathetic adrenal medullary excitation followed by increased hypothalamic pituitary adrenal cortical axis activity (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010). A small number of animal experiments indicate chronic stress enhances dendritic spine density in basolateral amygdala cells in rodents (Bennur et al., 2007; Vyas, Bernal & Chattarji, 2003; Vyas, Pillai & Chattarji, 2004) and amygdala volume in non-human primates with early life stress and short allelic variant of the serotonin transporter gene (Coplan et al., 2014). In concert, human research reveals that children of depressed mothers have larger amygdala volumes when examined at ten years of age (Lupien et al., 2011). Furthermore, prolonged institutional rearing of orphaned children is associated with larger amygdala volumes measured years after institutionalization (Mehta et al., 2009; Tottenham et al., 2010). Hanson and colleagues (2015) however found an inverse association between retrospectively reported stressful life events and amygdala volume among a sample of 12 year olds. Studies of adult PTSD patients find heterogeneity in amygdala volume magnitude with no effects, reduced, and enlarged amygdala volumes among adult PTSD patients relative to controls (Karl et al., 2006). Complexities of PTSD diagnosis and variation in etiology may account for this heterogeneity in PTSD and amygdala volume. Summarizing, animal work demonstrates amygdala hypertrophy with elevated chronic stress; whereas human investigations reveal mixed brain imaging findings on chronic stress and amygdala volume. There is some evidence that developmental timing of risk exposure is important with earlier rather than later stress exposure more consequential for amygdala development (Cohen et al., 2010; Kim et al., 2013; Shonkoff et al., 2009; Sripada et al., 2014). In the current study, we evaluate the hypothesis that elevated cumulative risk exposure early in life will be prospectively associated with increased adult amygdala volume.
In addition to the research on chronic stress and amygdala structure, animal studies (Roozendall, McEwen & Chattarji, 2009) as well as investigations of human trauma victims (Shinn, Rauch & Pitman, 2006) reveal greater amygdala reactivity among organisms in relation to chronic stress exposure and for adolescents exposed to a family history of depression and stressful life events (Swartz et al., 2015a). Moreover these relations also appear to be sensitive to developmental timing with earlier relative to later in life stress more effective in altering amygdala functioning (Tottenham & Sheridan, 2010; Nelson & Sheridan, 2011). There is also evidence that early institutionalization (i.e. maternal deprivation) alters connectivity between the prefrontal cortex (pfc) and the amygdala, accelerating the normal maturational pattern of pfc – amgydala connectivity (Gee et al., 2013). We therefore examined amygdala reactivity herein using salient faces vs. shapes including various emotional expressions and without any emotion regulation instructions. In this context, a number of studies have found (Sommerville et al., 2004; Whalen, 1998; Zaretsky et al., 2010) that the amygdala is particularly responsive to ambiguous rather than to overtly negative stimuli. Interestingly, parallel arguments have been made regarding the effect of socioeconomic status (SES) on physiologic responses to interpersonal interactions in adolescents. These SES physiologic effects were only evident with neutral interpersonal interactions (Chen, Langer, Ralphaelson & Matthews, 2004; Chen & Matthews, 2001; 2003). If as suggested the amygdala is particularly attuned to ambiguity and uncertainty because of the organism's need to interpret and make sense of its surroundings, presenting the amygdala with neutral rather than overtly angry faces ought to constitute a more sensitive task with respect to cumulative risk exposure. We thus examined our subject's responses to both neutral as well as overtly negative and positive facial expressions.
Summarising, we extend research on trauma and stress-related amygdala hyper-reactivity to human facial stimuli and examine whether hypothesized, stress-related volumetric changes in the amygdala also predict alterations in amygdala functioning. We operationalize chronic exposure to stressors with an index of cumulative risk exposure that encompasses multiple physical (e.g., substandard housing) and psychosocial (e.g., violence) stressors during childhood. A large and robust literature documents that cumulative risk adversely affects child development more than singular risk exposure (Evans, Li & Whipple, 2013; Obradovic, Shaffer & Masten, 2012; Pressman, Klebanov & Brooks-Gunn, 2012; Sameroff, 2006). We also examine developmental timing of cumulative risk exposure and alterations in amygdala structure and function. Given prior work we hypothesized that childhood cumulative risk exposure we would be more consequential than later exposure in early adulthood.
Method
Participants
Fifty four adults (M = 23.7, SD = 1.5 years, 54 % male) were drawn based on convenience and childhood poverty status from a parent sample of 341 individuals who were part of a longitudinal cohort that have been monitored since childhood (Evans, 2003). Approximately half of the sample grew up in low-income households and half were from middle-income backgrounds. The mean income-to-needs ratio of the sample was 3.1. All participants had no MRI contraindications (e.g., metallic/ferrous materials in their body), no prior or current diagnosis for any psychiatric disorder (clinician-conducted psychiatric evaluation based on the Structured Clinical Interview for DSM-IV), and no current neurological condition. The neuroimaging subsample closely resembles the parent subject cohort in age, M = 23.5 years, gender = 51% and income = 2.9 income-to-needs ratio. One of the 54 participants could not tolerate the scanner, and one additional participant had excessive movement during the fMRI protocol.
Procedures
At ages 9, 13, and 17, each participant and their mother were independently interviewed in their home as part of a protocol assessing cumulative risk exposure, poverty, and socioemotional development in a sample of low- and middle-income children in rural areas of the Northeast United States.
Cumulative risk exposure
Exposure to six (three psychosocial and three physical) environmental risk factors was assessed at each wave of data collection. Crowding was measured as people/room in the residence. Noise was assessed by a 2 hour monitoring of decibel levels (Leq) in the primary social space in the home. Housing quality was measured by trained raters conducting a walk-through with a standardized checklist that includes structural defects, poor maintenance, clutter and cleanliness, physical hazards, and climatic deficiencies (Evans, Wells, Chan & Saltzman, 2000). At the first wave (age 9), exposure to three psychosocial risk factors (violence, family turmoil, child separation from parents) was assessed from the mother by reports on the Life Events and Circumstances (LEC) checklist (Work, Cowen, Parker & Wyman, 1990; Wyman, Cowen, Work & Parker, 1991). The LEC consists of multiple dichotomous (yes/no) item subscales for each of these risk domains. Example items from each domain include “Our neighborhood has been unsafe”; “Our child has been involved in serious family arguments”; “A close family member was away from home a lot”. At waves 2 and 3 (ages 13 and 17, respectively) the mother again completed the LEC, and the youth also indicated the occurrence of exposure to risks based upon multiple items with the Adolescent Perceived Events Scale (Compas, 1997). Sample items from each domain include “Other kids picked on you, made fun of you, bullied you”; “Pretty serious arguments or fights between parents”; “Parents got divorced/separated”. If either the mother or the youth indicated exposure to a particular item it was counted, but if both indicated exposure, it was only counted once.
For each of these six risk domains, risk exposure was defined dichotomously (0|1) based upon a statistical criterion with 1 equal to a value in the top quartile across the entire sample of children from the original data set at each wave of data collection. A value of 0 was assigned to levels of exposure less than the cutoff. Table 1 shows the mean, range, SD and upper quartile cutoff value for each of the risk components from the original sample used to calculate cumulative risk exposure herein. Thus the cumulative risk index could vary from 0-6 for each wave of data collection. A large body of research indicates that this additive summation of exposure to multiple risk factors is consistently and strongly related to adverse child and adolescent cognitive and socioemotional outcomes (Evans et al., 2013; Obradovic et al., 2012; Pressman et al., 2012; Sameroff, 2006). The additive model is also consistently superior to use of summary metrics that maintain the continuous values of each risk factor. See Evans et al. (2013) for a detailed discussion of the reasons for the use of such an additive model to represent cumulative risk exposure.
Table 1.
Descriptive Information on Cumulative Risk Factors
| Variable | Mean | SD | Range | Upper Quartile Cutpoint |
|---|---|---|---|---|
| Crowding | .61 | .21 | .20-1.75 | .70 |
| Noise | 62.90 | 6.71 | 46.60-80.00 | 67.70 |
| Housing | .60 | .30 | .08-1.68 | .78 |
| Family turmoil | 1.65 | 1.39 | 0-5 | 2 |
| Child separation | 1.88 | 1.38 | 0-6 | 2 |
| Violence | .84 | 1.11 | 0-5 | 1 |
We used the mean of cumulative risk from the two earliest assessments (9 and 13 years) because models with measures of chronic compared to point estimates of childhood stressor exposure predict health better (Miller, Chen & Parker, 2011). We also reasoned that childhood risk exposure assessments would be more salient to brain development than later risk exposure estimates (McEwen & Gianaros, 2010; Lupien et al., 2009; Shonkoff, Boyce & McEwen, 2009). The 9 and 13 year assessments were also more similar and highly correlated (r = .81 for Waves 1 and 2) to each other than to later time points. Wave 3 cumulative risk (age 17) was correlated .39 and .49 with Wave 1 and 2, respectively. The mean and (standard deviation) cumulative risk exposures for each of Waves 1, 2 and 3 were 1.50 (1.39), 1.43 (1.41) and 1.66 (1.35), respectively.
MR Imaging acquisition and data analysis
Structural MR imaging (sMRI) scans were collected on a 3.0 T Philips scanner (VA Ann Arbor fMRI Center). After the participant was positioned in the scanner, a high- quality T1-weighted structural image (sMRI) was obtained using a 3D T1 Turbo field -echo (T1-TFE) pulse sequence (TR: 9.8ms, TE: 4.60 ms., TI: 3000 ms., FA: 8°, FOV: 256 mm, matrix = 256×256, resolution 1 mm; slice thickness = 1 mm3, 180 sagittal slices whole brain). An 8-channel SENSE head coil was used. Similar protocols have been used in previous structural neuroimaging studies (Wang et al., 2010a, b). Functional data were acquired (T2* - weighted EPI, TR: 2000 ms., TE: 30 ms., FA: 90°, FOV: 220 mm, matrix size 64 × 64 × 42 axial slices, voxels: 3.44 × 3.44 × 2.80 mm).
Automated brain structural measurements were made on sMRI images with Freesurfer programs (version 5.1) (http://www.surfer.nmr.mgh.harvard.edu/fswiki) on a Linux workstation (Fischl, 2012). The automated procedure for volumetric measures of subcortical brain regions has been described in detail in previous studies (Fischl et al., 2002; Han & Fischl, 2007). Structural analyses involved preprocessing T1- weighted MRI data, including motion correction, transformation to Talairach space, intensity normalization, and skull stripping. The whole brain segmentation is based on the intensity gradients of signals to distinguish grey matter and white matter and cerebrospinal fluid. Segmentation of subcortical structures is based on a probabilistic atlas provided by Freesurfer. The atlas was created by the Center for Morphometric Analysis http://www.cma.mgh.harvard.edu/) from 20 unrelated, randomly selected healthy people. The automated measures of amygdala volume by Freesurfer are highly correlated with the results of hand tracing of these brain structures, and are sensitive to changes (Frischl et al., 2002; Han & Fischl, 2007). For example, to detect 10% difference in amygdala volume requires only 23 participants per group (Morey et al., 2009). This approach is increasingly used in psychological studies (Inano et al., 2013, Morey et al., 2012; Levy-Gigi, Szabo, Kelemen & Keri, 2013). In this study, we used Freesurfer software to automatically segment bilateral amygdala and measured volume of each respective bilateral structure as well as the total gray matter volume. The amygdala segmentation was visually inspected slice-by-slice, and all segmentation passed manual inspection without manual correction. The manual inspection was done blind to group status, i.e., the inspector had no knowledge of any other aspects of the study. Research has evaluated variation of Freesurfer automated measures (Wang et al., 2010b) and manual inspection with correction enhances accuracy (Dewey et al., 2010).
To examine the relations between amygdala volume and function, we used a well validated and reliable protocol that robustly engages the amygdala (Hariri et al., 2002). The complete account of the functional activation experiment is reported elsewhere (Javanbakht, King, Evans, Swain, Angstadt, Phan, & Liberzon, 2015). Briefly, the Emotional Faces Assessment Task (EFAT) depicts trios of equal numbers of male and female faces expressing angry, fearful, happy, or neutral emotions selected from a validated emotion stimulus set (Gur et al., 2002). The participant's task is to match one of two target faces on the bottom row with the target face on the top row by pressing the left or right response button with their dominant hand. Three, 20 sec blocks of each target facial expression are presented with no target stimulus repeated within or between a set of 24 blocks. Each block contains four sequential matching trials, at 5 sec per trial. In order to maintain attention and allow amygdala activity to return to baseline, the emotional faces blocks are interspersed with baseline stimuli consisting of trios of geometric shapes.
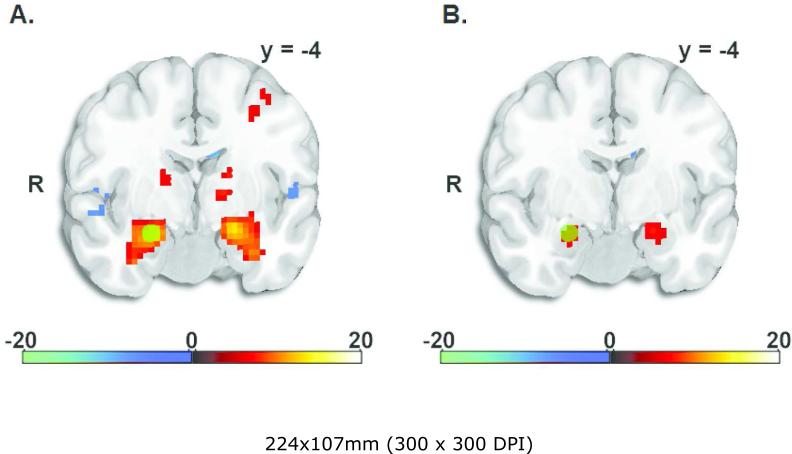
Functional MRI data were analyzed using the statistical parametric mapping software package, SPM8 (2013). Functional volumes were realigned to correct for head motion, spatially normalized to a standard template based upon the Montreal Neurological Institute (MNI) reference brain, and spatially smoothed using an 8-mm FWHM Gaussian kernel. To control for noise due to head motion, six motion parameters and first derivatives of motion parameters were used as nuisance covariates. One participant had movement parameters greater than 3mm and was excluded. In order to conduct a planned analysis examining the connection between the structural change and BOLD reactivity in the amygdala, we examined contrasts of neutral faces and faces bearing emotional expressions to the shapes condition. As we have previously reported (Javanbakht et al., 2015), all of the face probes generated robust bilateral amygdala responses; the neutral faces had bilateral amygdala responses with whole-brain FWE corrected p < .001. For assessment of functional activity of amygdala in the mediation analyses, we extracted the neutral > shapes contrasts using functionally-defined 5 mm spherical ROIs. As recommended by Friston and colleagues (2006), to reduce bias the functional ROIs were derived from a separate contrast of all face types > shapes, which produced peaks of left amygdala (-21, - 7, -17) and right amygdala (24, -4, -17). Amygdala activations in the all faces > shapes and neutral faces > shapes contrasts are shown in Figure 1, with position of the 5 mm ROI shown as a yellow circle. The functional ROIs were extracted for mediational analyses as described in the Results below.
Figure 1.
T-maps showing activations of bilateral amygdala in the contrast of all faces > shapes used to define function ROI peaks (panel A), and the contrast neutral faces > shapes (panel B). The position of the 5 mm sphere right amygdala ROI is shown as yellow circles.
Results
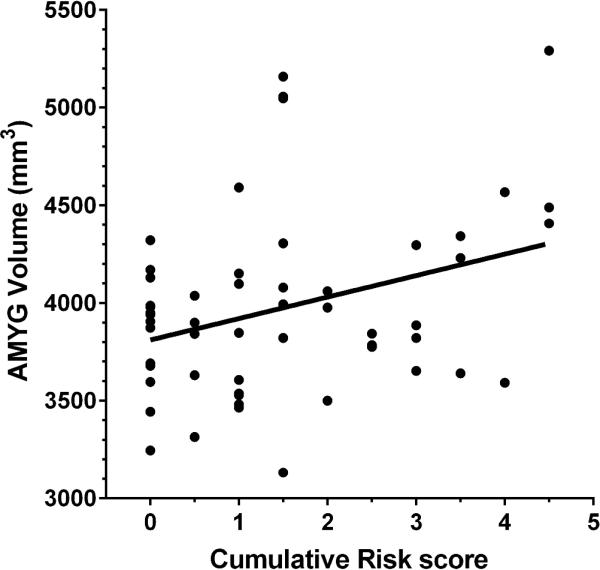
Adults with greater cumulative risk exposure during childhood had larger amygdala volumes. Adults’ total, left, and right amygdala volumes, respectively were regressed onto cumulative risk exposure. Figure 2 depicts the data for total amygdala volume, R2 = .11, p < .01. The results were similar for the left, R2 = .11, p < .01 and right, R2 = .07, p < .05 amygdala volumes, respectively. To gain some sense of the magnitude of the effect, individuals in the highest quartile of cumulative risk exposure had a total amygdala volume 7% larger than those in the lowest quartile of cumualtive risk exposure. Inclusion of statistical covariates for total gray matter volume, gender, age, age of puberty, or income at any wave of data collection, did not alter the results for the total amygdala or left hemisphere volume. However, right amygdala volume was no longer significant when total gray matter volume was included as a covariate. None of the other covariates affected the amygdala results.
Figure 2.
Childhood cumulative risk exposure (M ages 9 and 13) and total amygdala volume.
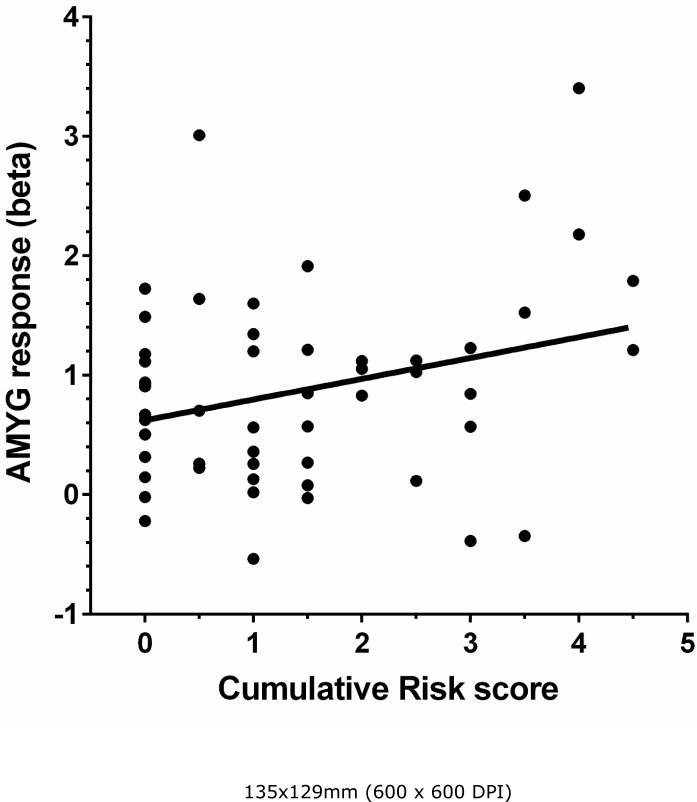
We also analyzed the relation between cumulative risk and amygdala functional responses to emotional stimuli using the EFAT protocol. As we have previously reported, all of the face conditions robustly activated the amygdala (cluster FWE error corrected p < .001). Accuracy on the face matching task was high (>70% face all face types) and was not related to cumulative risk or other income-related variables. For complete presentation of the functional data from the emotional faces protocol, readers are referred to Javanbakht et al. (2015). As can be seen in Figure 3, cumulative risk is related to total amygdala reactivity only for neutral emotional stimuli, R2 = .08, p < 05. The results for the left, R2 = .09, p < .05 and right, R2 = .07, p < .05 hemispheres were similar. There was no relation between amygdala reactivity to emotional faces and cumulative risk exposure.
Figure 3.
Childhood cumulative risk exposure (M ages 9 and 13) and total amygdala function in response to emotionally neutral facial stimuli.
In order to explore the developmental course of cumulative risk exposure, we examined amygdala volume and function with cumulative risk exposures at ages 9, 13, and 17 separately. Only exposures at ages 9 and 13 plus the combined effect of the two earliest cumulative risk assessments were significant. Cumulative risk exposure during early childhood but not by age 17 predicted subsequent enlargement of the adult amygdala as well as reactivity to emotionally neutral stimuli. The mean of the two earlier cumulative risk exposures was more strongly tied to amygdala function likely because it better represents chronic, childhood exposure to cumulative risk than either point estimate.
Finally, we tested our hypothesis that the significant associations uncovered between childhood cumulative risk exposure and amygdala functioning could be explained, at least in part, by increased amygdala volume. Bootstrapping techniques within ordinary least squares (OLS) regression with robust standard errors terms were used to test the mediational hypothesis: cumulative risk → amygdala volume → amygdala reactivity function (Preacher & Hayes, 2008). For total amygdala and left amygdala functioning, we found significant indirect effects, suggesting that one reason why childhood cumulative risk exposure is associated with elevated amygdala activity to emotionally neutral stimuli is because of increased amygdala volume. The significant relation between childhood cumulative risk exposure and total amygdala functioning, b = .17 (SE = .08), was reduced by a statistically significant 41% with the inclusion of the indirect effect of amygdala Volume in the regression equation (b = .01 - .17, 95% confidence interval (CI)). For the left amygdala the same pattern was uncovered, b = .10 (SE = .04), 44% reduction (b = .01 - .10, 95% CI). For the right amygdala the indirect pathway was marginally significant, b = .08 (.05) with a 25% reduction with the inclusion of amygdala Volume in the regression equation, (b = -.00 - .07, 95% CI).
As a partial assessment of the sensitivity and robustness of our mediational findings, we reversed the order of inclusion of the hypothesized medatior (amygdala volume) and the outcome variable (amygdala function) in the regression equations. If one or more unobserved variables were driving the mediational results, then reversing the order of inclusion of these two terms in the regression equation would not change the results. However when the reverse mediational tests were conducted, cumulative risk exposure remained a significant predictor of amygdala volume when amygdala function was tested as the putative mediator. As an additional test of the sensitivity of our findings, we also examined the additivity assumption in the cumulative risk metric. The typical cumulative risk metric, as used herein, is an additive function, thus precluding interactive effects among the risk factors. However considerable empirical research shows that this additive, parsimonious model performs better than various alternatives metrics for multiple stressor exposure (Evans et al., 2013; Obradovic et al., 2012; Pressman et al., 2012; Sameroff, 2006). Combination of multiple continuous variables rarely perform as well as the additive model because the latter does not depend upon the degree of intercorrelation of the specific, singular risk factors. For example in the present case, the correlation between the additive cumulative risk index and total amygdala volume is r = .33. The correlation for the summated continuous variables and total amygdala volume is r = .19.
There are two lines of evidence on the additivity assumption inherent in the typical cumulative risk construction that can be tested. Using the parent data set from which this subsample was derived, we examined with OLS regression whether there was any evidence of a nonlinear function between cumulative risk and several behavioral (e.g., internalizing, externalizing symptoms) and physiological (e.g., allostatic load) outcomes. There was none. Second, for the present subsample data, in addition to regressing amygdala volume and function on to cumulative risk, we examined the functions for each singular risk factor. None of the singular risk factors constituting the cumulative risk metric (crowding, noise, housing quality, family turmoil, violence, separation from family) were significantly related to amygdala volume or function. Both of these results fit well with the more general cumulative risk literature, demonstrating that the cumulative risk metric tends to be additive in structure and outperforms singular risk exposure measurements (Evans et al., 2013; Obradovic et al., 2012; Pressman et al., 2012; Sameroff, 2006).
Discussion
Cumulative risk exposure during childhood was associated prospectively with bilateral amygdala volume in young adults. This is in accord with experimental animal studies indicating that elevated chronic stress causes amygdala enlargement. Both volumetric assessments as well as histological investigations of neuronal development show these trends in animal models (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010). Although the MRI volumetric data are less definitive in the human literature, retrospective examinations of trauma victims report a positive relation between chronic stress and amygdala volume (Lupien et al., 2009; McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010). The present study makes four contributions to research on stress and the human amygdala. One, to our knowledge this is the first prospective examination of chronic stressor exposure during childhood in relation to both volumetric and functional assessments of the amygdala. The prospective assessment is important for analytic reasons and because it enables us to begin to examine the developmental course of childhood risk exposure and human brain development among a non- clinical sample. Two, we shed some light on the developmental timing of cumulative risk exposure and brain development. Our analyses suggest that cumulative risk exposure during childhood but not subsequent cumulative risk exposure in early adulthood is related to amygdala volume and function. This pattern of developmental timing of risk exposure and amygdala volume matches animal work indicating heightened sensitivity of the amygdala during early relative to later stress exposure (Nelson & Sheridan, 2011; Tottenham & Sheridan, 2010). Furthermore it also fits with numerous studies showing that risk exposure during early childhood is related to behavioral conduct disorders, mood disorders (depression and anxiety), and greater physiological stress reactivity (Repetti, Taylor & Seeman, 2012). Three, the data supplement the voluminous behavioral literature on cumulative risk and human behavior (Evans et al., 2013; Obradovic et al., 2012; Pressman et al., 2012; Sameroff, 2006) with brain physiology. Little work to date has explored cumulative risk exposure and neuronal development. Four, we provide unique evidence that the relation between childhood cumulative risk exposure and adult amygdala reactivity to facial stimuli may be accounted for, in part, by elevated amygdala volume.
The finding that amygdala reactivity to neutral but not emotionally expressive faces was sensitive to childhood risk exposure is consistent with some recent research on SES and emotion. Although the present study focuses on cumulative risk exposure and amygdala structure and function, work on SES and the amygdala is potentially illuminating given the overlap between poverty and cumulative risk exposure (Evans, 2004; Evans & Kim, 2010). In a series of studies examining cardiovascular responses to neutral and clearly hostile, threatening interpersonal interactions, Chen found that lower SES adolescents manifested elevated physiological stress responses but only to neutral interpersonal interactions (Chen, Langer, Ralphaelson & Matthews, 2004; Chen & Matthews, 2001; 2003). Negatively charged encounters were uniformly more stressful irrespective of socioeconomic status. On the other hand, adults who retrospectively reported growing up either in households of lower, relative social status (Gianaros et al., 2008) or who retrospectively recalled higher risk exposures during childhood (Taylor et al., 2008) had greater amygdala reactivity to emotionally expressive but not to neutral faces. Some work suggests that rather than emotional salience per se, the amygdala is particularly attuned to ambiguity and uncertainty because of the organism's need to interpret and make sense of its surroundings. Recent experiments using computer morphed faces ranging from highly fearful to neutral emotional expression reveal that more ambiguous facial stimuli consistently evoke greater amygdala responses (Zaretsky, Mendelsohn, Mint & Hendler, 2010). Consistent with this idea, hyper-reactivity to neutral facial stimuli was reported in two groups of children with different psychiatric disorders. Amygdala hyperactivity was found among children with ADHD (Brotman et al. 2010) and among child with Bipolar disorder (Rich et al. 2006) wherein neutral faces were also perceived with more hostility and fear by children with psychiatric diagnoses than control subjects.
One possible explanation for our findings herein on cumulative risk exposure and hyper-reactivity to neutral faces could be the chaotic and unpredictable conditions accompanying risky environments. Perhaps children who grow up in situations with greater risk exposure experience more chaos and uncertainty and consequently become particularly vigilant to ambiguous stimuli - like the neutral faces shown herein. They may also be more likely to attribute threat or harm to emotionally ambiguous stimuli and situations. Future research on childhood risk exposure and brain development should incorporate subjective evaluations of stimulus meaning in conjunction with confidence ratings and then apply signal detection theory to tease apart the influence of risk on sensitivity and response biases to reactions to emotional information. It would also be of value to examine how generalizable emotional reactions are beyond facial stimuli to actual interpersonal encounters or other representations (e.g., semantic) of information varying in emotional tone.
With respect to enlargement of the amygdala in relation to elevated childhood cumulative risk exposure, excess stress hormones might play a role. A few human studies indicate elevated stress hormones with greater cumulative risk exposure (Evans et al., 2013) and as reviewed by McEwen and Gianaros (2010) chronic stress as well as corticosteroid treatment cause dendritic growth in basal amygdala neurons in rodents. Furthermore the basolateral nucleus of the amygdala is networked with multiple cortical areas involved with stressor processing such as the anterior cingulate cortex, ventromedial prefrontal cortex and the orbital prefrontal cortex. Stress hormones also appear to influence the degree of connectivity between the amygdala and the PFC (Gee et al., 2013).
Ideally in future work on childhood risk exposure, brain development, and human wellbeing, neuroimaging would occur in prospective, longitudinal research designs with repeated measures of cumulative risk exposure during childhood and adolescence. Such a design would afford a more sensitive and rigorous examination of the developmental timing of risk exposures and brain development over the life course. Given the particular salience of early risk exposure for brain development (Lupien et al., 2009; McEwen & Gianaros, 2010; Shonkoff et al., 2009), it would have been better to collect earlier in life assessments of cumulative risk exposure. It is worth noting that the findings herein of apparently more salient, early risk exposure for amygdala structure and function comport with a large body of literature on the developmental timing of childhood risk and mental health (Repetti et al., 2002). Exposure to higher levels of risk early in life contribute to psychopathology. Some of this could be due to the effects on brain development as shown herein. For instance, greater amygdala reactivity to threat in college students predicted greater vulnerability to subsequent stressful life events as evidence by more internalizing symptoms (Swartz et al., 2015b). Perhaps for an organism developing within an ecological context of high risk, a more well developed and sensitive system for threat detection and environmental monitoring might be adaptive and, on balance, provide some degree of protection, perhaps at a cost of greater vigilance and anxiety. We also examined whether amygdala structure and function were linked to internalizing and externalizing symptomatology but uncovered no significant associations. However the relatively modest sample size herein may have provided insufficient statistical power for such an investigation. Clearly more work on the inter-relations between early childhood risk, brain development, and mental health is an important priority for future research.
Although the mediational data herein are interesting and provocative, caution is warranted given that amygdala volume and function were assessed at the same age in young adulthood. Another important advantage of a prospective, longitudinal design is that we could more rigorously test the cumulative risk → amygdala volume → amygdala function mediational pathway model. Such a design would enable us to assess amygdala volume subsequent to cumulative risk exposure, as accomplished herein, but prior to amygdala function, which we were unable to do. Another valuable extension of the present paper would be a broader assessment of physical, social, and individual (e.g., genetic) risk factors for consideration in the cumulative risk index. Although we used three major physical (e.g., housing quality) and three major psychosocial risk factors (e.g., exposure to violence), there are many other important risk factors the might also influence brain development. The use of an additive model of summated risk categories (1 = upper quartile of exposure; 0 = lower three quartiles of risk) although a sensitive and robust approach to the operationalization of cumulative risk exposure (Evans et al., 2013) nonetheless equates risk with rarity of exposure rather than conceptually based or normatively derived data. The cumulative risk index as typically used in the literature and herein also equates each of the risk factors in terms of importance to the outcome of interest. Although unweighted linear models tend to be robust, in some cases certain risk factors (e.g., violence) are likely more important than others (e.g., density) in thinking about the structure and function of the brain in general, and the amygdala specifically.
Adults who grew up with higher levels of childhood, cumulative risk exposure have larger amygdala and elevated amygdala reactivity to facial stimuli, respectively relative to those reared in less risky circumstances. The cumulative risk exposures assessed herein included both physical (i.e., noise, crowding, housing quality) and psychosocial (i.e, family turmoil, exposure to violence, separation of child from family) risk factors throughout childhood. Furthermore, mediational analyses suggest that the elevated adulthood amygdala reactivity associated with prior, childhood chronic risk exposure may be explained, in part, by larger amygdala volumes among those from riskier backgrounds. Additionally, cumulative risk exposure later in life appears less important for amygdala volume or function, highlighting the importance of the developmental timing of risk exposure and brain development over the life course.
Significance Statement.
In a prospective analysis, young adults with greater, childhood cumulative risk exposure have larger amygdalae that are more reactive to emotionally neutral, facial stimuli. Moreover, risk related volume enlargement mediates the link between cumulative risk exposure and a more reactive amygdala. Altered amygdala structure and function could help explain well-documented mental health sequelae of risk exposure during childhood.
Acknowledgments
Funding sources: This study was supported by the National Institute of Minority Health and Health Disparities RC2MD004767, the W.T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health, the Robert Wood Johnson Foundation, and the Centers for Disease Control and Prevention U49/CE002099 via the University of Michigan.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors were involved in the development of the study, data acquisition, analysis and interpretation, and in the development of the manuscript.
Literature Cited
- Bennur S, Shankaranarayana RBS, Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial is mediated by tissue-plasminogen activator. Neurosci. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: The role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals Behav Med. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Development of the Cognitive Appraisal and Understanding of Social Events (CAUSE) videos. Health Psych. 2003;22:106–110. doi: 10.1037//0278-6133.22.1.106. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Compas BE. Responses to stress questionnaire. University of Vermont; Burlington, VT.: 1997. [Google Scholar]
- Coplan JD, Fathy HM, Jackowski AP, Tang CY, Perera TD, Mathew SJ, Martinez J, Abdallah CG, Dwork AJ, Pantol G, Carpenter D, Gorman JM, Nemeroff CB, Owens MJ, Kaffman A, Kaufman J. Early life stress and macaque amygdala hypertrophy: preliminary evidence for a role for the serotonin transporter gene. Front Behav Neurosci. 2014;8:342. doi: 10.3389/fnbeh.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezla K, Sem E, Anyanwu JC, Guttmann CR, Navia B, Cohen R, Tate DF, the HIV Neuroimaging Consortium Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2011;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Amer Psych. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the SES-health gradient. NY Acad Sci. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psych Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Evans GW, Wells NM, Chan E, Saltzman H. Housing and mental health. J Consult Clin Psych. 2000;68:526–530. doi: 10.1037//0022-006x.68.3.526. [DOI] [PubMed] [Google Scholar]
- Fischl B. Freesurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. Neuroimage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff G, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Nat Acad Sci. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. SCAN. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendorn M, Marom C, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Han X, Fischl B. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Trans Med Imaging. 2007;26:479–486. doi: 10.1109/TMI.2007.893282. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry. 77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Yoshioka N, Mori H, Kunimatsu A, Abe O, Ohtomo K. Effects of age and gender on neuroanatomical volumes. J Mag Resonance Imagery. 2010;37:1072–1076. doi: 10.1002/jmri.23910. [DOI] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Front Behav Neurosci. 2015;12(9):154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. 2013:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Gigi E, Szabo C, Kelemen O, Keri S. Association among clinical response, hippocampal volume, and FKBPS gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Bio Psychiat. 2013;74:793–800. doi: 10.1016/j.biopsych.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nat Rev Neurosci. 2009;10:434–450. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Seguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Nat Acad Sci. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals NY Acad Sci. 2010;1186:190–220. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study. J Child Psych Psychiat. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psych Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez M. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown V, Haswell CC, Nasser JD, Wagner HR, McCarthy G. Mid-Atlantic MIRECC Workshop. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiat. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR II, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, III, Sheridan MA. Lessons from neuroscience research for understanding causal links between family and neighborhood characteristics and educational outcomes. In: Duncan BJ, Murname RJ, editors. Whither opportunity. Russell Sage Foundatin; New York: 2011. pp. 27–46. [Google Scholar]
- Obradovic J, Shaffer A, Masten AS. Risk and adversity in developmental psychopathology: Progress and future directions. In: Mayes LC, Lewis M, editors. The environment of human development: A handbook of theory and measurement. Cambridge University Press; New York: 2012. pp. 35–57. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Beh Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pressman AW, Klebanov PK, Brooks-Gunn J. New approaches to the notion of environmental risk. In: Mayes LC, Lewis M, editors. The environment of human development: A handbook of theory and measurement. Cambridge University Press; New York: 2012. pp. 152–172. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TW. Risky families: Family social environments and the mental and physical health of offspring. Psych Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. P Natl Acad Sci USA. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendall B, McEwen BS, Chattarji S. Stress, memory, and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Clarke-Stewart A, Dunn J, editors. Identifying risk and protective factors for healthy child development. Families count. New York: Cambridge University Press. 2006:53–76. [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals NY Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sommerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Bio Psychiat. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39:2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical Parametric Mapping 8. Wellcome Trust Center for Neuroimaging, University College; London, UK: [April 16, 2015]. http://www.fil.ion.ucl.ac.uk/spm. [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry. 2015a;172:276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015b;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hiomert CJ, Lieberman MD. Neural bases of moderation of cortisol responses by psychosocial resources. J Personal Soc Psych. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan M. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Fron Neurosci. 2010;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvin A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaladaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neurosci. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang X, Garfinkel SN, King AP, Angstadt M, Dennis MJ, Xie H, Welsh RC, Tamburrino MB, Liberzon I. A multiple-plane approach to measure the structural properties of functionally active regions in the human cortex. Neuroimage. 2010;49:3075–3085. doi: 10.1016/j.neuroimage.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gerken M, Dennis M, Mooney R, Kane J, Khuder S, Xie H, Bauer W, Apkarian AV, Wall J. Profiles of precentral and postcentral cortical mean thicknesses in individual subjects over acute and subacute time-scales. Cerebral Cort. 2010;20:1513–1522. doi: 10.1093/cercor/bhp226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work W, Cowen E, Parker G, Wyman P. Stress resilient children in an urban setting. J Primary Prev. 1990;11:3–17. doi: 10.1007/BF01324858. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psych Sci. 1998;7:177–188. [Google Scholar]
- Wyman P, Cowen E, Work W, Parker G. Developmental and family milieu correlates of resilience in urban children who have experienced major life stress. Am J Comm Psych. 1991;19:405–26. doi: 10.1007/BF00938033. [DOI] [PubMed] [Google Scholar]
- Zaretsky M, Mendelsohn A, Mint M, Hendler T. In the eye of the beholder: Internally driven uncertainty of danger recruits the amygdala and the dorsomedial prefrontal cortex. J Cog Neurosci. 2010;22:2263–2275. doi: 10.1162/jocn.2009.21402. [DOI] [PubMed] [Google Scholar]