Abstract
Although schizophrenia is defined by waking phenomena, abnormal sleep is a common feature. In particular, there is accumulating evidence of a sleep spindle deficit. Sleep spindles, a defining thalamocortical oscillation of non-rapid eye movement Stage 2 sleep, correlate with IQ and are thought to promote long-term potentiation and enhance memory consolidation. Here we review evidence that reduced spindle activity in schizophrenia is an endophenotype that impairs sleep-dependent memory consolidation, contributes to symptoms and is a novel treatment biomarker. Studies showing that spindles can be pharmacologically enhanced in schizophrenia and that increasing spindles improves memory in healthy individuals suggest that treating spindle deficits in schizophrenia may improve cognition. Spindle activity is highly heritable and recent large-scale genome-wide association studies have identified schizophrenia risk genes that may contribute to spindle deficits and illuminate their mechanisms. For example, the schizophrenia risk gene CACNA1I encodes a calcium channel that is abundantly expressed in the thalamic spindle generator and plays a critical role in spindle activity in a mouse knockout. Future genetic studies of animals and humans can delineate the role of this and other genes in spindles. Such cross-disciplinary research, by forging empirical links in causal chains from risk genes to proteins and cellular functions, through to endophenotypes, cognitive impairments, symptoms and diagnosis, has the potential to advance the mechanistic understanding, treatment and prevention of schizophrenia. This review highlights the importance of deficient sleep-dependent memory consolidation among the cognitive deficits of schizophrenia and implicates reduced sleep spindles as a potentially treatable mechanism.
Keywords: schizophrenia, sleep, spindles, memory, cognition, genetics, endophenotype
Introduction
Neuropsychiatric disorders are primarily defined by waking phenomena, but sleep disturbances are often a prominent feature. While usually viewed as secondary, sleep deprivation can precipitate psychosis (1) and trigger or aggravate a range of neuropsychiatric conditions (2-6). Moreover, as has been shown in depression (7) and attention-deficit/hyperactivity disorder (4), treating sleep can improve both symptoms and cognitive function. This suggests that abnormal sleep is not merely epiphenomenal, but can directly contribute to the defining features of neuropsychiatric disorders. In schizophrenia (SZ), there is a specific deficit in sleep spindles, a defining thalamocortical oscillation of stage 2 non-rapid eye movement sleep (N2). In this review, we describe the nature, correlates and implications of this spindle deficit, and place it in a hypothetical causal chain that links SZ risk genes to cognitive deficits and positive symptoms. We conclude that understanding the neural and genetic bases of spindle deficits can advance the mechanistic understanding and treatment of SZ.
Abnormal sleep is a key feature of SZ and potential target for treatment
In SZ, sleep disturbances have been described since Kraepelin (8) and are associated with poorer coping skills and diminished quality of life (9, 10). They are common throughout the course of SZ (11), in individuals with prodromal symptoms (12, 13), and in young relatives (14). Sleep disturbances are associated with the initial onset of psychosis and predict relapse in remitted patients (15, 16). Findings of sleep disturbances in unmedicated and antipsychotic-naïve SZ patients (17) indicates that disturbed sleep is not merely a medication side-effect. In fact, antipsychotic drugs (APDs) often normalize sleep (18), and withdrawal is associated with a progressive deterioration of sleep quality (19) which, in turn, is associated with relapse (16) and increased positive symptoms (20). Despite the clear association of disturbed sleep with SZ, the exact nature of the disturbance and its relations to pathophysiology, cognitive deficits and symptoms is unclear. If specific sleep abnormalities that contribute to the onset, relapse and manifestations of SZ can be identified, they may serve as targets for intervention to prevent the emergence of SZ, remediate its course and ameliorate core features.
Here, we review evidence that a specific sleep abnormality – reduced sleep spindle activity –predates the onset of SZ, is present throughout its course and contributes to cognitive deficits and symptoms. This evidence indicates that (i) individuals with SZ and their first-degree relatives have reduced sleep spindle activity, (ii) spindle deficits are associated with impaired memory consolidation and positive symptoms, (iii) SZ risk genes are associated with spindle deficits and implicate specific pathophysiologic mechanisms, (iv) spindles can be enhanced and (iv) may serve as a novel treatment biomarker associated with cognition.
Sleep abnormalities in SZ
The most common subjective sleep disturbances in SZ are difficulty initiating and maintaining sleep (i.e., insomnia 15, 17). Polysomnography (PSG) studies variably show reduced sleep efficiency (the fraction of time in bed spent asleep), increased sleep onset latencies, and increased wake time after sleep onset (WASO), in SZ patients compared with healthy individuals (see meta-analyses, 17, 21). Studies also report altered circadian rhythms (22) and increased rates of sleep disorders including obstructive sleep apnea, movement disorders, parasomnias and hypersomnolence (reviewed in, 6, 15).
PSG studies document diverse abnormalities of sleep architecture (i.e., the distribution of time spent in different sleep stages) in SZ. In humans, sleep is divided into rapid eye movement (REM) and non-REM (NREM) sleep and NREM sleep is subdivided into three stages: N1-3 (Figure 1, 23). N3, or slow wave sleep (SWS), is characterized by large delta (.5– 4Hz) waves. Medicated and APD-naïve SZ patients as well as first-degree relatives show N3 abnormalities including reduced duration and delta power (24-27). REM sleep abnormalities, usually decreased REM latency or increased REM density (rapid eye movements per minute) are also reported (25, 26, 28) but neither N3 nor REM abnormalities are consistently observed (28, 29) and meta-analyses have not revealed systematic differences in SZ compared with healthy or psychiatric controls (17, 21).
Figure 1.
Normal sleep architecture. A normal night’s sleep consists of five 90 min cycles that include rapid-eye-movement (REM) sleep (thicker black line). Most of the deep slow-wave sleep (N3) occurs early in the night and most of the REM sleep occurs later in the night. N1 is a transitional state from wake to sleep, characterized by the disappearance of 8-12HZ (alpha) waves from the EEG and appearance of slow (>0.5s) oscillating eye movements (23). N2 is defined by the presence of isolated sharp negative waves followed by a positive component, lasting >0.5s, and sleep spindles. N3 is defined by the presence of large (>75μV peak-to-peak, slow (0.5-2Hz) waves occupying at least 20% of each 30s epoch.
Relatively few studies of SZ venture beyond architecture to examine the spectral characteristics of the sleep electroencephalogram (EEG). Recently, a fairly consistent literature has emerged showing a specific deficit in sleep spindle activity. Sleep spindles, a defining EEG oscillation of N2, are brief (~1s) powerful bursts of 12-15Hz activity organized in a waxing/waning envelope. While spindles also occur during N3, they are less dense (30). Most studies reviewed used N2 spindle density (number of spindles per minute) as the primary measurement, but the amplitude, duration, peak frequency and sigma power (usually 12-15 Hz) of spindles are often reported, as are more general measures of NREM EEG sigma power, which correlate with spindle density (e.g., 31). When summarizing the findings of multiple papers using different measures of spindles, we use the generic term “spindle activity.”
Spindle mechanisms
There is considerable cross-species knowledge about sleep spindle mechanisms. Spindles are generated in the thalamic reticular nucleus (TRN, 32), a thin net-like structure around the thalamus comprised entirely of y-aminobutyric acid (GABA)-ergic neurons (33). TRN neurons project to glutamatergic thalamic neurons that project to the cortex. Cortical neurons, in turn, send glutamatergic inputs back to N-methyl-D-aspartate (NMDA) receptors on TRN neurons (Figure 2). Thus, spindles are the product of a thalamocortical feedback loop that is regulated by GABA-ergic and NMDA-receptor mediated glutamatergic neurotransmission (34). While the TRN can generate spindles in isolation (35, 36), feedback from the cortex is necessary to synchronize spindles across cortical regions (30, 37).
Figure 2.
Thalamic reticular nucleus (TRN) circuitry for generating and synchronizing sleep spindles (adapted from 103). The TRN, a netlike nucleus that sits between the rest of the thalamus and the neocortex, modulates thalmocortical activity. The TRN receives projections from both thalmocortical and corticothalamic neurons. GABAergic TRN neurons project to thalamocortical relay neurons. Glutamatergic corticothalamic neurons, in turn, send projections back to the TRN and other thalamic nuclei.
The voltage-dependent firing properties of TRN neurons are well-described (38). Like most neurons, TRN cells fire in “tonic” mode at resting membrane potential, when most low threshold Ca2+ channels are inactivated. However, when TRN neurons are relatively hyperpolarized to approximately −70mV, these channels are de-inactivated and the neurons fire in “burst” mode. During burst mode, a depolarizing input opens T-type Ca2+ channels, leading to low threshold Ca2+ spikes and rhythmic bursts of action potentials. Rhythmic bursting in TRN neurons produces a powerful and prolonged inhibition followed by rebound spike-bursts in thalamocortical relay neurons that entrain cortical neurons to oscillate at spindle frequency (39).
Dysfunction in spindle-generating circuitry is consistent with current models of SZ that implicate thalamocortical circuitry and both the GABAergic and NMDA receptor-mediated glutamatergic neurotransmission (40) and with evidence of TRN abnormalities in SZ (41).
Sleep spindles mediate memory consolidation
After encoding, memories undergo ‘consolidation’ processes that stabilize, enhance, integrate and reorganize memory traces in the brain. These processes operate outside of conscious awareness during wake and sleep. A wealth of molecular, cellular, neural network, brain activation, and behavioral data from birds (42), rodents (43), cats (44) and humans (45) suggest an evolutionarily conserved function for sleep in the consolidation of multiple forms of memory.
Animal studies suggest that spindles are a key facilitator of the synaptic plasticity involved in memory. Experimental models suggest that spindles induce massive influxes of calcium ions into cortical pyramidal cells (46), where they would be expected to trigger known intracellular calcium-dependent mechanisms that produce synaptic plasticity (47). Trains of stimuli applied to rat cortical pyramidal cells that mimic the neuronal firing patterns that accompany spindles have been shown to induce an NMDA receptor-dependent short-term potentiation and L-type Ca2+ channel-dependent long-term potentiation (48).
In humans, spindles correlate with the sleep-dependent consolidation of both procedural (49-54) and declarative (55-57) memory. In addition, EEG (50, 55), magnetoencephalography (58) and subdural electrode grid (59) studies show increased spindle activity in specific circuits that were involved in pre-sleep learning and that these learning-induced spindles predict sleep-dependent memory consolidation (50, 55, 58-60). Together, these findings suggest that spindles strengthen synapses to consolidate memory during sleep. There is also mounting evidence of a more general role for spindles in cognition based on their correlations with learning ability and IQ (61-63), relationships that may be mediated by memory enhancement.
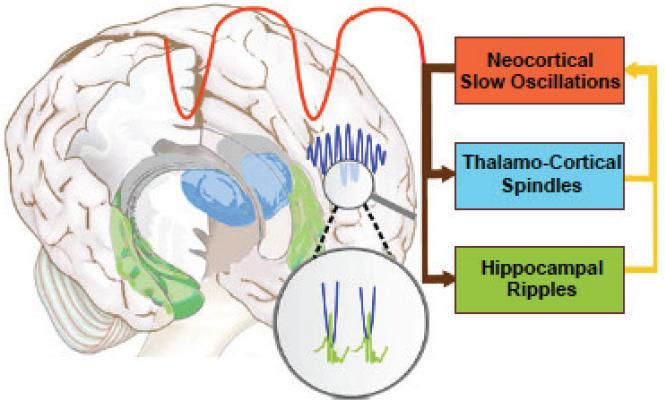
Spindles act in concert with other NREM oscillations. They are temporally correlated with neocortical slow oscillations (.5-1Hz) and hippocampal ripples (~200Hz transient bursts of CA1 pyramidal cell activity), an orchestration that is thought to redistribute recently encoded memories from temporary dependence on the hippocampus to longer-term representation in the cortex (Figure 3, 43, 64, 65). In humans, hippocampal ripples are difficult to measure non-invasively, but simultaneous EEG and fMRI during sleep show that spindles are associated with increased functional connectivity between the hippocampus and neocortex (66). Evidence of a breakdown of this coordination is seen in a rat model of SZ (67). In SZ, there is reduced spindle coherence across the cortex (31). This may reflect reduced modulation by slow oscillations, which are thought to synchronize spindles across the cortex in the service of memory consolidation (64, 68),
Figure 3.
The coordination of sleep spindles with hippocampal ripples and neocortical slow oscillations in the service of consolidating new memories during sleep (adapted from 150). During NREM sleep, neocortical slow oscillations drive the reactivation of hippocampal memory representations during sharp wave ripples (green) in the hippocampus together with spindles (blue) in the TRN. Hippocampal ripples nest in the troughs of spindles, which occur during the up states of slow oscillations. This dialogue between slow oscillations, spindles and hippocampal ripples is thought to mediate the transfer of selected new memories from temporary dependence on the hippocampus to longer-term representation in the neocortex (43).
Sleep spindle deficit in SZ
Three early studies of small samples (n:511) of APD-naïve first-episode (25, 69) and unmedicated (70, 71) SZ patients did not find a spindle deficit (Table 1). A growing literature, however, reports marked reductions of spindle activity In chronic medicated SZ (31, 72-76) and medicated adolescents with early onset SZ spectrum disorder (77). Importantly, with the exception of increased sleep onset latency in two studies (74, 75), the spindle deficit occurred in the context of normal sleep architecture and quality (e.g., efficiency, WASO), indicating that it is not secondary to sleep disruption. This contrast with reports of disrupted sleep in SZ (reviewed above) may reflect that sleep disruption primarily characterizes more acute phases of SZ and that APDs are sedating and tend to normalize sleep architecture (18). The effects of chronic APD treatment on spindles are unknown but a single dose of olanzapine in SZ reduced spindle density (78), and acute administration of haloperidol to healthy participants did not affect spindle density (79).
Table 1.
Studies of spindle density in schizophrenia.
| Study | Medication status |
Patient n |
Healthy Control n |
Sleep stage |
Spindle detection method |
Finding |
|---|---|---|---|---|---|---|
| Hiatt et al., 1985 (71) |
Unmedicated | 5 | 5 | Sampled from midpoints of NREM periods |
Visual | Increased in NREM period 1 |
| Van Cauter et al.,1991 (70) |
Unmedicated | 6 | 6 | First NREM period |
Visual | No difference |
| Poulin et al., 2003 (25); Forest et al., 20071 (69) |
APD-naïve | 11 | 11 | N2 | Visual | No difference |
| Ferrarelli et al., 20072 (74) |
Medicated | 18 | 17 | NREM during first sleep episode |
Algorithm | Reduced |
| Ferrarelli et al., 20102 (75) |
Medicated | 49 | 44 | NREM | Algorithm | Reduced |
| Manoach et al., 2010 (73) |
Medicated | 14 | 15 | N2 | Algorithm | Reduced |
| Seeck- Hirschner et al., 2011 (76) |
Medicated | 20 | 22 | N2 during a nap |
Visual | Reduced |
| Wamsley et al., 2012 (31) |
Medicated | 21 | 17 | N2 | Algorithm | Reduced |
| Manoach et al., 20143 (80) |
APD-naïve | 15 | 25 | N2 | Algorithm | Reduced |
| Goder et al., 2015 (72) |
Medicated | 16 | 16 | N2 | Algorithm and visual |
Reduced |
| Tesler et al., 20154 (77) |
Medicated | 9 | 15 | First hour of NREM |
Algorithm | Reduced |
The eight patients reported in Forest et al., 2007 are a subset of the 11 reported in Poulin et al., 2003.
Measures included spindle number and integrated spindle activity (calculated by integrating the absolute amplitude values of each spindle detected at every electrode, divided by the non-REM sleep duration) rather than density. Included psychiatric control groups whose integrated spindle activity was greater than SZ patients but did not differ from healthy controls.
Included an APD-naïve non-SZ psychotic control group (n=11) whose spindle density was greater than SZ patients at a trend level, and did not differ from healthy controls.
Adolescents with early onset SZ spectrum disorder.
A recent report extended finding of reduced spindle density to early course APD-naïve SZ patients (but not to APD-naive non-SZ psychotic patients). A trend to reduced density and significantly reduced spindle amplitude was also seen in young (mean age=14) nonpsychotic first-degree relatives of SZ patients (80). These findings make it unlikely that the spindle deficit in SZ is due to APDs and suggest the possibility of diagnostic specificity, though replication in larger samples is necessary. Two other studies reported spindle deficits in SZ but not in a mixed psychiatric control group taking APDs (75) or in individuals with a history of depression (74), consistent with another report of normal spindles in depression (81). There are reports, however, of a variety of spindle abnormalities in other neurodevelopmental and neurodegenerative disorders characterized by cognitive impairment including mental retardation (82), phenylketonuria (83), Williams syndrome (84), autism (85, 86) and Parkinson’s disease with dementia (87). Whether the spindle deficits of SZ have unique characteristics and consequences remains to be determined.
Findings of spindle deficits throughout the course of SZ and in first-degree relatives implicate abnormal function of thalamocortical circuitry that may begin before the onset of SZ. This is consistent with the finding of reduced thalamic volume in ultra high-risk adolescents that correlates with sleep disturbance (12). This literature indicates that reduced spindle activity is unlikely to be secondary to APDs or chronicity and instead may be an endophenotype (a trait indicating genetic vulnerability, 88) of SZ.
Reduced spindles are associated with impaired cognition and positive symptoms in SZ
Although sleep is critical for memory, disrupted sleep impairs memory (89-91) and SZ is characterized by both abnormal sleep and impaired memory, few studies have examined the connection. Emerging evidence suggests that reduced sleep spindles contribute to both procedural and declarative memory impairments in SZ.
Using a well-validated probe of sleep-dependent motor procedural memory, the finger-tapping motor sequence task (MST, Figure 4, 49, 92), several studies have demonstrated deficient sleep-dependent enhancement of motor learning in SZ. In healthy individuals, significant performance improvements occur after sleep but not after wake (49, 93-95) and correlate with N2 duration (49) and spindle density (50, 96, 97). In contrast, chronic medicated SZ patients fail to show significant sleep-dependent improvement despite normal learning during training (31, 73, 98-100) and this failure correlated with sleep spindle density in one study (31), but not in two others (73, 100). SZ patients also show reduced sleep-dependent consolidation on a mirror tracing procedural motor task despite intact initial learning. This occurred in the context of reduced sleep spindles, but these deficits were not correlated (76). Sleep-dependent consolidation of declarative memory, tested with a picture recognition task, is also impaired and correlates with reduced sleep spindles (72). Spindle deficits also correlate with worse executive function and lower IQ in APD-naïve early course patients with both SZ and non-SZ psychotic disorders as well as in in nonpsychotic first-degree relatives of SZ patients (80).
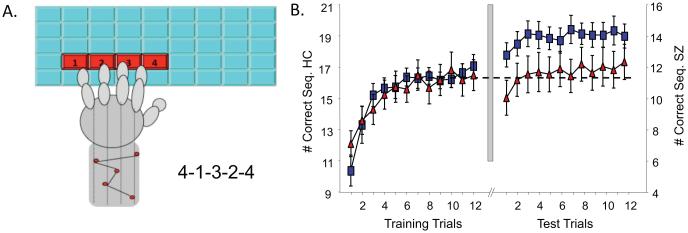
Figure 4.
Finger tapping Motor Sequence Task (MST). A. The MST requires participants to repeatedly type a 5-digit sequence (e.g., 4-1-3-2-4) on a keyboard with the left hand, “as quickly and accurately as possible” for twelve 30 s trials separated by 30 s rest periods. Participants train before sleep and test on and additional 12 trials after sleep. The primary outcome measure is overnight improvement calculated as the percent increase in correctly typed sequences from the last three training trials to the first three test trials (49). B. Sleep-dependent MST performance (data from 98). Left: At training, SZ patients (red triangles) and healthy controls (blue squares) show a similar time course of improvement, although SZ patients are slower overall (see y-axis on right). Right: Following a night of sleep, only the healthy controls show sleep-dependent improvement
A limitation of these studies is that the small sample sizes may leave them underpowered to detect meaningful effects and contribute to inconsistent findings. For example, in one MST study the correlation between spindle density and overnight improvement in SZ was significant (r21=.45, p=.04, 31) but in a smaller study with a similar effect size it was not (r14=.46, p=.10, 73). In summary, the evidence suggests that spindle deficits contribute to cognitive dysfunction in genetically high-risk individuals and in early course and chronic SZ, regardless of medications. These findings are congruent with the large basic literature showing robust correlations between spindle density and a range of cognitive measures including IQ (61).
If TRN dysfunction gives rise to spindle deficits in SZ, there may be other cognitive manifestations. The TRN is strategically positioned between other thalamic nuclei and the cortex to modulate thalamocortical interactions (Figure 2). Consequently, it plays an important role in waking cognition acting as an “attentional searchlight” (101). Different sectors of the TRN receive distinct inputs from the thalamus and neocortex and have distinct projections to thalamic nuclei (102, 103). It is the TRN neurons that project to sensory rather than limbic thalamic nuclei that participate in spindle generation, in the inhibition of sensory processing during sleep and in the augmentation of sensory processing during tasks requiring attention during wake (104). SZ is characterized by deficits in sensory gating (105), attentional modulation, and cortical gamma band oscillations, all of which may depend on the modulation of the flow of information from the thalamus to the neocortex by the TRN (103, 106, 107). Abnormal cortical gamma oscillations in SZ are associated with cognitive deficits and are thought to reflect dysfunction of cortical parvalbumin (PV)-containing GABAergic interneurons (108), which are abnormal in SZ (109). While there is a selective reduction of GABA PV interneurons in anteroventral thalamus (110), to our knowledge, the TRN GABA PV neurons that generate spindles have not been studied. In summary, TRN dysfunction in SZ may contribute to impairments in sensory gating, attention and cortical gamma oscillations during wake and to spindle deficits during sleep.
Reduced spindle activity also correlates with increased positive symptom severity in medicated early onset SZ spectrum disorder (77) and in some (31, 75) but not all (72) studies of chronic medicated SZ. In APD-naïve early course SZ, however, reduced spindle density correlated with decreased positive symptoms (80). The opposite direction of these correlations may reflect differences in the pathophysiological underpinnings of positive symptoms. In chronic medicated SZ, residual positive symptoms have not responded to dopaminergic medications and may arise from GABA or NMDA hypofunction (111), while in early untreated SZ, positive symptoms generally respond to APDs and may reflect dopamine hyperactivity (112). In healthy individuals, spindle density inversely correlates with magical ideation, an index of liability to delusional beliefs, and with glutamine and glutamate levels in the thalamus (113). Schizotypal traits, such as magical ideation, exist on a continuum in the general population and may share neural substrates with the psychotic symptoms of SZ. While the mechanistic link of spindles to positive symptoms is less clear than that for memory, both may reflect abnormal thalamocortical circuit function (114).
Sleep spindles as a novel treatment biomarker for improving cognition in SZ
Cognitive deficits are the strongest predictor of functional outcome in SZ (115). Even after the florid psychotic symptoms are controlled with APDs, debilitating cognitive deficits persist and only ~20% of individuals with SZ work (116). Although ameliorating cognitive deficits is a priority of the SZ research community, effective treatments are lacking (e.g., 117). A better understanding of the pathophysiology of cognitive deficits is needed to guide the development of new treatments.
Although most data linking sleep spindles to cognitive impairments are correlational, recent work supports a causal role for spindles in memory consolidation. In preliminary reports, optogenetic excitation of TRN neurons in mice increased sleep spindles and improved memory, while inhibiting TRN neurons decreased spindles and worsened memory (118, 119). In healthy humans, increasing spindles with zolpidem (120, 121), increasing sigma activity with transcranial stimulation (122, 123) and enhancing the synchronization of sigma activity with slow oscillations using auditory closed-loop stimulation (68) all improve memory, while transcranial stimulation that decreases sigma activity impairs memory (124).
Only a few studies have attempted to improve cognition in SZ by manipulating spindles. In a small sample of patients, transcranial stimulation during N2 did not significantly alter sleep parameters but improved word list recall (125). In a small preliminary study, eszopiclone, which acts on GABA neurons in the TRN (126), significantly increased spindles in SZ but not sleep-dependent memory (127). In another study that did not include PSG measures, eszopiclone improved working memory in SZ, but not symptoms (128). This body of work provides an impetus to develop and test novel therapies for spindle deficits to improve cognition.
Genetic mechanisms of sleep spindles
EEG sigma power is highly heritable in twin studies (heritability estimate: 96%, 129) and shows both high inter-individual variability and within-individual stability, leading to its description as an “electrophysiological fingerprint” (129-131). Despite this, little is known about the genetic underpinnings of sleep spindles. Genome-wide association studies (GWAS) have been conducted for sleep disorders (132), sleep duration (133) and insomnia (134), but genetic studies of the sleep EEG used relatively small samples and only a few candidate genes (135). To understand genetic contributions to spindle deficits in SZ, it is important to conduct well-powered genetic studies of spindles in humans.
Like most human traits, sleep spindles likely have a complex genetic architecture, with allelic variants in many genes combining to influence spindle expression. GWAS with large sample sizes can capture the genetic variation due to common alleles. Alleles identified with statistical confidence can then help to establish the broader gene networks that underlie variation in spindles. GWAS data can also be used to estimate genetic correlations between pairs of traits or diseases: the extent to which genetic influences on one trait are shared by a second trait. If spindle deficits are an endophenotype of SZ, one would expect a significant degree of shared genetic influences (as genes that influence spindles will indirectly influence SZ risk). Contrasting the genetic association profiles across spindles, SZ, related disorders and sleep phenotypes may illuminate causal relations between these traits. It is now also feasible to sequence the entire exome or genome in large numbers of individuals – an approach that can identify rare variants that may have larger effects on spindles, since rare variants are likely to have arisen recently (and might even be de novo in the proband) and are less subject than common variants to natural selection (136).
Recent genetic studies provide clues to potential mechanistic links between spindles and SZ. For example, the largest SZ GWAS to date (137) implicated common variants in CACNA1I in SZ risk. In addition, two missense de novo mutations of CACNA1I were identified in individuals with SZ in a trio study, though not at rates statistically above chance (138).
CACNA1I encodes a T-type calcium channel (Cav3.3) expressed only in the brain and particularly in the TRN (139) (Allen Mouse Brain Atlas, GTex, mouse.brain-map.org, www.gtexportal.org) where it interacts with sarco/endoplasmic reticulum Ca2+-ATPases and small-conductance (SK)-type potassium channels, to shape delta and sigma frequency oscillations (140). Analysis of the two de novo mutations found in SZ revealed that the R1346H variant produces a channel with defective protein maturation and channel trafficking, leading to reduced whole cell currents in a heterologous expression system (manuscript in preparation). This would be expected to reduce the overall expression of Cav3.3 and consequently, the burst firing necessary for spindles. Consistent with this, knocking out CACNA1I in mice causes a spindle deficit (141). Studies now underway are examining the effects of CACNA1I on sleep spindles in humans and whether Cav3.3 channels are viable therapeutic targets. This evidence places reduced sleep spindle activity, a heritable component of the sleep EEG, and a putative endophenotype of SZ that may contribute to cognitive impairment and symptoms, in a hypothetical causal chain from risk gene to diagnosis (Figure 5).
Figure 5.
Hypothetical causal chain. The spindle deficit, a candidate endophenotype of SZ, may link risk genes to fundamental cognitive deficits, symptoms and diagnosis.
Conclusions
This review expands current models of cognitive deficits in SZ by highlighting the importance of deficient sleep-dependent consolidation of both procedural and declarative memory. It implicates reduced sleep spindles as a mechanism and suggests novel pathophysiological targets for treatment. Going forward, we propose several potentially fruitful avenues of research.
First, it will be important to define the scope and consequences of the sleep-dependent memory deficit in SZ. Findings of dissociations (e.g., reduced motor procedural memory in the context of intact spatial memory, 76), suggest that only certain memory types are affected, perhaps those that rely on spindles. It will also be important to understand how memory deficits affect daily function. We have proposed that the sleep-dependent procedural memory deficit represents a breakdown of task automation (98, 142), which normally renders performance faster, less variable, and less dependent on voluntary attention (143). A failure of automation requires the allocation of attentional resources to task demands that should have been automated by sleep. This leaves fewer resources available for higher-order task demands. The interaction between automatic and effortful processes is what allows a limited capacity brain to carry out complex cognitive tasks. Thus, an impairment in sleep-dependent automation could contribute to the generalized cognitive deficits that are a hallmark of SZ (144) and treating it could improve function.
The work reviewed has implications for the development and testing of novel therapies to improve cognition, including pharmacological and transcranial stimulation approaches. As standard neuropsychological tests assess function in a single session, they miss the critical aspects of learning and memory that depend on sleep. Accordingly, it will be important to include probes of sleep-dependent memory in clinical trials. In addition to increasing spindles in SZ, interventions may have to preserve or correct the temporal coordination of spindles with neocortical slow waves and hippocampal ripples to improve memory. It is unclear whether this orchestration of sleep oscillations is preserved in schizophrenia, and animal models are necessary to simultaneously measure all three oscillations. Understanding the pathophysiology and genetic mechanisms of spindle deficits in relation to memory in SZ can guide treatment development.
To evaluate the spindle deficit as an endophenotype it is important to determine its specificity to SZ and to establish its heritability and genetic architecture in larger studies. An impediment to large-scale genetic studies of spindles is the prohibitive cost and difficulty of conducting sleep studies. For this reason, it would be useful to develop waking assays of TRN function to serve as more accessible surrogate markers of spindle activity. TRN activity has seldom been examined in vivo in humans as its size and location make it difficult to identify with neuroimaging (145). Animal models can illuminate the contribution of the TRN to waking cognition and its role in development. The TRN and spindles are thought to contribute to the development of thalamocortical connectivity and synaptic refinement (146, 147), processes that may go awry in SZ (148, 149).
In summary, cross-disciplinary research can foster a more complete understanding of the relationship of sleep spindles to cognition and SZ and can identify pathophysiological targets for treatment. This line of work, by forging empirical links in causal chains from SZ risk genes to cellular and circuit dysfunction to spindle deficits, impaired memory, symptoms and diagnosis, provides unprecedented opportunities to advance our understanding of the genetics and pathophysiology of SZ, and could lead to improved treatment and possibly even prevention.
Acknowledgments
The authors would like to thank Steven Hyman for his comments on the manuscript and to acknowledge support from: K24MH099421 (DSM); R01 MH092638 (DSM, RS); R01MH048832 (RS); R21MH099448 and Stanley Research Center (JQP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: None of the authors have any biomedical financial interests or potential conflicts of interest to report.
Cited Literature
- 1.Tyler DB. Psychological changes during experimental sleep deprivation. Dis Nerv Syst. 1955;16:293–299. [PubMed] [Google Scholar]
- 2.Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 4.Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8:18–30. doi: 10.1016/j.sleep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135:1370–1379. doi: 10.1378/chest.08-1834. [DOI] [PubMed] [Google Scholar]
- 7.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Kraepelin E. Dementia praecox and paraphrenia. E.S. Livingston; Edinburgh, Scotland: 1919. [Google Scholar]
- 9.Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13. doi: 10.1186/1471-244X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman M, Tandon R, DeQuardo JR, Taylor SF, Goodson J, McGrath M. Biological predictors of 1-year outcome in schizophrenia in males and females. Schizophr Res. 1996;21:65–73. doi: 10.1016/0920-9964(96)00021-7. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 12.Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, Smith Watts AK, et al. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151:148–153. doi: 10.1016/j.schres.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller TJ, Zipursky RB, Perkins D, Addington J, Woods SW, Hawkins KA, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the "prodromal" sample. Schizophr Res. 2003;61:19–30. doi: 10.1016/s0920-9964(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 14.Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- 15.Benson KL. Sleep in Schizophrenia: Pathology and Treatment. Sleep medicine clinics. 2015;10:49–55. doi: 10.1016/j.jsmc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Dencker SJ, Malm U, Lepp M. Schizophrenic relapse after drug withdrawal is predictable. Acta Psychiatr Scand. 1986;73:181–185. doi: 10.1111/j.1600-0447.1986.tb10584.x. [DOI] [PubMed] [Google Scholar]
- 17.Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- 18.Krystal AD, Goforth HW, Roth T. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol. 2008;23:150–160. doi: 10.1097/YIC.0b013e3282f39703. [DOI] [PubMed] [Google Scholar]
- 19.Nofzinger EA, van Kammen DP, Gilbertson MW, Gurklis JA, Peters JL. Electroencephalographic sleep in clinically stable schizophrenic patients: two-weeks versus six-weeks neuroleptic-free. Biol Psychiatry. 1993;33:829–835. doi: 10.1016/0006-3223(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 20.Chemerinski E, Ho BC, Flaum M, Arndt S, Fleming F, Andreasen NC. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Compr Psychiatry. 2002;43:393–396. doi: 10.1053/comp.2002.34627. [DOI] [PubMed] [Google Scholar]
- 21.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669-670. [DOI] [PubMed] [Google Scholar]
- 22.Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35:151–163. doi: 10.1016/0006-3223(94)91147-9. [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine; Westchester, Ill: 2007. [Google Scholar]
- 24.Feinberg I, Braun M, Koresko RL, Gottlieb F. Stage 4 sleep in schizophrenia. Arch Gen Psychiatry. 1969;21:262–266. doi: 10.1001/archpsyc.1969.01740210006002. [DOI] [PubMed] [Google Scholar]
- 25.Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophr Res. 2003;62:147–153. doi: 10.1016/s0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res. 2006;82:251–260. doi: 10.1016/j.schres.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Keshavan MS, Reynolds CF, 3rd, Miewald MJ, Montrose DM, Sweeney JA, Vasko RC, Jr., et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55:443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- 28.Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49:185–194. doi: 10.1001/archpsyc.1992.01820030017003. [DOI] [PubMed] [Google Scholar]
- 29.Lauer CJ, Schreiber W, Pollmacher T, Holsboer F, Krieg JC. Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology. 1997;16:51–60. doi: 10.1016/S0893-133X(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 30.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 31.Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, Ely AV, et al. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 33.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. Journal of Neurophysiology. 2001;86:1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 35.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 37.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 38.Sherman SM, Guillery RW. Exploring the Thalamus and it Role in Cortical Function. 2nd The MIT Press; Cambridge, MA: 2006. [Google Scholar]
- 39.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159–179. doi: 10.1113/jphysiol.1996.sp021133. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–1399. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 42.Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 43.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 44.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 45.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 48.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 50.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2008;12:396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 52.Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13-15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–255. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 54.Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17:23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 55.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 56.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, Nanez JE, Sr., et al. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci. 2013;33:13894–13902. doi: 10.1523/JNEUROSCI.1198-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A. 2012;109:18583–18588. doi: 10.1073/pnas.1207532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bang JW, Khalilzadeh O, Hamalainen M, Watanabe T, Sasaki Y. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vision Res. 2014;99:162–171. doi: 10.1016/j.visres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fogel SM, Smith CT. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. European Journal of Neuroscience. 2006;23:1738–1746. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 63.Lustenberger C, Maric A, Durr R, Achermann P, Huber R. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PLoS One. 2012;7:e49561. doi: 10.1371/journal.pone.0049561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- 65.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 66.Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31:10331–10339. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, et al. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012;76:526–533. doi: 10.1016/j.neuron.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Forest G, Poulin J, Daoust AM, Lussier I, Stip E, Godbout R. Attention and non-REM sleep in neuroleptic-naive persons with schizophrenia and control participants. Psychiatry Res. 2007;149:33–40. doi: 10.1016/j.psychres.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Van Cauter E, Linkowski P, Kerkhofs M, Hubain P, L'Hermite-Baleriaux M, Leclercq R, et al. Circadian and sleep-related endocrine rhythms in schizophrenia. Arch Gen Psychiatry. 1991;48:348–356. doi: 10.1001/archpsyc.1991.01810280064009. [DOI] [PubMed] [Google Scholar]
- 71.Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985;42:797–802. doi: 10.1001/archpsyc.1985.01790310059007. [DOI] [PubMed] [Google Scholar]
- 72.Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, et al. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16:564–569. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44:112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 75.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic Dysfunction in Schizophrenia Suggested by Whole-Night Deficits in Slow and Fast Spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2011;44:42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Tesler N, Gerstenberg M, Franscini M, Jenni OG, Walitza S, Huber R. Reduced sleep spindle density in early onset schizophrenia: A preliminary finding. Schiz Res. doi: 10.1016/j.schres.2015.04.042. in press. [DOI] [PubMed] [Google Scholar]
- 78.Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, Serafin I, et al. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry. 2008;41:92–99. doi: 10.1055/s-2007-1004592. [DOI] [PubMed] [Google Scholar]
- 79.Hirshkowitz M, Thornby JI, Karacan I. Sleep spindles: pharmacological effects in humans. Sleep. 1982;5:85–94. doi: 10.1093/sleep/5.1.85. [DOI] [PubMed] [Google Scholar]
- 80.Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, et al. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Frontiers in Human Neuroscience. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plante DT, Goldstein MR, Landsness EC, Peterson MJ, Riedner BA, Ferrarelli F, et al. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146:120–125. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. Sleep. 1982;5:47–57. doi: 10.1093/sleep/5.1.47. [DOI] [PubMed] [Google Scholar]
- 83.De Giorgis GF, Nonnis E, Crocioni F, Gregori P, Rosini MP, Leuzzi V, et al. Evolution of daytime quiet sleep components in early treated phenylketonuric infants. Brain Dev. 1996;18:201–206. doi: 10.1016/0387-7604(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 84.Bodizs R, Gombos F, Kovacs I. Sleep EEG fingerprints reveal accelerated thalamocortical oscillatory dynamics in Williams syndrome. Research in developmental disabilities. 2012;33:153–164. doi: 10.1016/j.ridd.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 85.Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128:1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- 86.Tessier S, Lambert A, Chicoine M, Scherzer P, Soulieres I, Godbout R. Intelligence measures and stage 2 sleep in typically-developing and autistic children. Int J Psychophysiol. 2015;97:58–65. doi: 10.1016/j.ijpsycho.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Latreille V, Carrier J, Lafortune M, Postuma RB, Bertrand JA, Panisset M, et al. Sleep spindles in Parkinson's disease may predict the development of dementia. Neurobiol Aging. 2015;36:1083–1090. doi: 10.1016/j.neurobiolaging.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 89.Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21:417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- 90.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cipolli C, Mazzetti M, Plazzi G. Sleep-dependent memory consolidation in patients with sleep disorders. Sleep Med Rev. 2013;17:91–103. doi: 10.1016/j.smrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 92.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gregory M, Agam Y, Selvadurai C, Nagy A, Vangel M, Tucker M, et al. Resting state connectivity immediately following learning correlates with subsequent sleep-dependent enhancement of motor task performance. NeuroImage. 2014;102P2:666–673. doi: 10.1016/j.neuroimage.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217:117–121. doi: 10.1016/j.bbr.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 97.Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Genzel L, Ali E, Dresler M, Steiger A, Tesfaye M. Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. J Psychiatr Res. 2011;45:555–560. doi: 10.1016/j.jpsychires.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 100.Genzel L, Dresler M, Cornu M, Jager E, Konrad B, Adamczyk M, et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015;77:177–186. doi: 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zikopoulos B, Barbas H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci. 2012;32:5338–5350. doi: 10.1523/JNEUROSCI.4793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, et al. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–821. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Ahrens S, Jaramillo S, Yu K, Ghosh S, Hwang GR, Paik R, et al. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat Neurosci. 2015;18:104–111. doi: 10.1038/nn.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. J Neurosci. 2000;20:8897–8901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 109.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danos P, Baumann B, Bernstein HG, Franz M, Stauch R, Northoff G, et al. Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res. 1998;82:1–10. doi: 10.1016/s0925-4927(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 111.Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–13. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 112.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 113.Lustenberger C, O'Gorman RL, Pugin F, Tushaus L, Wehrle F, Achermann P, et al. Sleep spindles are related to schizotypal personality traits and thalamic glutamine/glutamate in healthy subjects. Schizophr Bull. 2015;41:522–531. doi: 10.1093/schbul/sbu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vukadinovic Z. Sleep abnormalities in schizophrenia may suggest impaired trans-thalamic cortico-cortical communication: towards a dynamic model of the illness. Eur J Neurosci. 2011;34:1031–1039. doi: 10.1111/j.1460-9568.2011.07822.x. [DOI] [PubMed] [Google Scholar]
- 115.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 116.Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66:128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- 117.Sergi MJ, Green MF, Widmark C, Reist C, Erhart S, Braff DL, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007;164:1585–1592. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]
- 118.Shin H. World Congress on Sleep Medicine. Seoul, Korea: 2015. Optogenetic induced spindles alter sleep architecture in mice. [Google Scholar]
- 119.McCarley R, Thankachan S, McNally J, McKenna J, Strecker R, Brown R. American College of Neuropsychopharmacology. Phoenix, AZ: 2014. Optogenetic Study of the Role of Parvalbumin-containing Thalamic Reticular Nucleus Neurons in Spindle Generation: Implications for Schizophrenia. [Google Scholar]
- 120.Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci. 2013;25:1597–1610. doi: 10.1162/jocn_a_00433. [DOI] [PubMed] [Google Scholar]
- 121.Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 123.Del Felice A, Magalini A, Masiero S. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain stimulation. 2015;8:567–573. doi: 10.1016/j.brs.2015.01.410. [DOI] [PubMed] [Google Scholar]
- 124.Marshall L, Kirov R, Brade J, Molle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One. 2011;6:e16905. doi: 10.1371/journal.pone.0016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, et al. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144:153–154. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 126.Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009;328:1000–1006. doi: 10.1124/jpet.108.146084. [DOI] [PubMed] [Google Scholar]
- 127.Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, et al. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013;36:1369–1376. doi: 10.5665/sleep.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tek C, Palmese LB, Krystal AD, Srihari VH, DeGeorge PC, Reutenauer EL, et al. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res. 2014;160:180–185. doi: 10.1016/j.schres.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 130.Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, et al. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64:344–348. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 131.Hori A. Sleep characteristics in twins. The Japanese journal of psychiatry and neurology. 1986;40:35–46. doi: 10.1111/j.1440-1819.1986.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 132.Raizen DM, Wu MN. Genome-wide association studies of sleep disorders. Chest. 2011;139:446–452. doi: 10.1378/chest.10-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parsons MJ, Lester KJ, Barclay NL, Nolan PM, Eley TC, Gregory AM. Replication of Genome-Wide Association Studies (GWAS) loci for sleep in the British G1219 cohort. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:431–438. doi: 10.1002/ajmg.b.32106. [DOI] [PubMed] [Google Scholar]
- 134.Byrne EM, Gehrman PR, Medland SE, Nyholt DR, Heath AC, Madden PA, et al. A genome-wide association study of sleep habits and insomnia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:439–451. doi: 10.1002/ajmg.b.32168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Landolt HP. Genetic determination of sleep EEG profiles in healthy humans. Prog Brain Res. 2011;193:51–61. doi: 10.1016/B978-0-444-53839-0.00004-1. [DOI] [PubMed] [Google Scholar]
- 136.Veltman JA, Brunner HG. De novo mutations in human genetic disease. Nature reviews Genetics. 2012;13:565–575. doi: 10.1038/nrg3241. [DOI] [PubMed] [Google Scholar]
- 137.Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu XB, Murray KD, Jones EG. Low-threshold calcium channel subunit Ca(v) 3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol. 2011;519:1181–1195. doi: 10.1002/cne.22567. [DOI] [PubMed] [Google Scholar]
- 140.Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 141.Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manoach DS, Stickgold R. Does abnormal sleep impair memory consolidation in schizophrenia? Frontiers in Human Neuroscience. 2009;3:21. doi: 10.3389/neuro.09.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atienza M, Cantero JL, Stickgold R. Posttraining sleep enhances automaticity in perceptual discrimination. J Cogn Neurosci. 2004;16:53–64. doi: 10.1162/089892904322755557. [DOI] [PubMed] [Google Scholar]
- 144.Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- 145.Viviano JD, Schneider KA. Interhemispheric interactions of the human thalamic reticular nucleus. J Neurosci. 2015;35:2026–2032. doi: 10.1523/JNEUROSCI.2623-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Evrard A, Ropert N. Early development of the thalamic inhibitory feedback loop in the primary somatosensory system of the newborn mice. J Neurosci. 2009;29:9930–9940. doi: 10.1523/JNEUROSCI.1671-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pinault D. Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull. 2011;37:238–243. doi: 10.1093/schbul/sbq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 149.Vukadinovic Z. Schizophrenia as a disturbance of cortical sensory maps. Translational Neurosci. 2012;3:388–398. [Google Scholar]
- 150.Born J, Wilhelm I. System consolidation of memory during sleep. Psychological research. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]