Abstract
From the IGEMS Consortium, data were available from 26,579 individuals aged 23 to 102 years on 3 subjective health items: self-rated health (SRH), health compared to others (COMP), and impact of health on activities (ACT). Marital status was a marker of environmental resources that may moderate genetic and environmental influences on subjective health. Results differed for the 3 subjective health items, indicating that they do not tap the same construct. Although there was little impact of marital status on variance components for women, marital status was a significant modifier of variance in all 3 subjective health measures for men. For both SRH and ACT, single men demonstrated greater shared and nonshared environmental variance than married men. For the COMP variable, genetic variance was greater for single men vs. married men. Results suggest gender differences in the role of marriage as a source of resources that are associated with subjective health.
Keywords: subjective health, marital status, age differences, gender differences, GxE interaction, moderation model
Subjective health is the focus of great research interest because of the role it plays in predicting objective health and mortality. In fact, measures of subjective health predict mortality above and beyond objective health measures (Idler & Benyamini, 1997; Latham & Peek, 2013; McFadden et al., 2009). Many researchers have posited explanations for the paradoxical observation that putatively simple questions about health perceptions can provide information about objective health-related outcomes distinct from multiple objective measures of health (Benyamini, 2011). A recent analysis tested four conceptualizations of subjective health (Franz et al., in revision); tests of age and gender moderation of genetic and environmental variance in subjective health measures supported the idea that subjective health taps personal intuitions about health and that these personal intuitions reflect cultural definitions and personal concepts of health (Bailis, Segall, & Chipperfield, 2003; Jylhä, 2009, 2010). These conceptions of subjective health rely primarily on mechanisms within the individual – intuitions and perceptions about health. In the current analysis, we shifted the focus to an external mechanism, marriage, which may influence subjective health and thus may moderate the genetic and environmental contributions to subjective health.
Research has demonstrated that subjective health is related to external factors such as education, financial status, social support, marital status, and neighborhood characteristics that indicate the extent of resources individuals have to support and maintain their health (Benyamini, 2011; Subramanian, Kubzansky, Berkman, Fay, & Kawachi, 2006). Although marriage has many meanings, at its most basic level marital status can reflect socioeconomic status as well as social and physical support (Benyamini, 2011; Zheng & Thomas, 2013). The beneficial association between marriage and physical health has been amply demonstrated (Carr & Springer, 2010; Robles & Kiecolt-Glaser, 2003) and a recent meta-analysis supported the lower relative risk for mortality among married people compared with non-married groups (Manzolo, Villari, Pirone, & Boccia, 2007). Research suggests that marriage supports maintenance of health behaviors, thus affecting disease prevention rather than treatment or recovery from severe illnesses (Zheng & Thomas, 2013). For example, in a sample of twins discordant for marital status, the unmarried twin was more likely to smoke and less likely to exercise (Osler, McGue, Lund, & Christensen, 2008). Epidemiological studies of this nature cannot determine cause and effect, however; thus there is ongoing discussion about whether the association between marriage and health reflects selection or causation (Silventoinen, Moustgaarid, Peltonen, & Martikainen, 2013).
The association of marital status with subjective health is nearly as well established as the association with physical health (Liu & Umberson, 2008; Waite & Gallagher, 2000). Evidence suggests that the relationship between marital status and subjective health reflects a tendency for married adults to be somewhat overconfident about their health status. In fact, Zheng and Thomas (2013) conclude that adults perceive marriage as a source of resources to support health, which results in both overestimation of health and delay in seeking medical care. Historical trends indicate that as gender roles and the meaning of marriage have changed over the last several decades, the relationship between marital status and subjective health has also changed (Liu & Umberson, 2008).
The association between marital status and physical and subjective health may not be the same for men and women (Liu & Umberson, 2008). Research suggests that men and women have diverse experiences of physical aging. Men tend to have earlier and more compressed histories of major illnesses and disability prior to death, while women live longer, have more health complaints across the life course, and higher prevalence of chronic disabling but not fatal diseases later in life (Sainio et al., 2006). As a result, men may focus more on life-threatening conditions when judging their own health, whereas women may focus on chronic conditions that are a greater part of their experience of aging (Deeg & Kriegsman, 2003). Consistent with this, women tend to report poorer subjective health, and subjective health appears to be a weaker predictor of mortality in women than in men (Benyamini, 2011; Benyamini, Blumstein, Lusky, & Modan, 2003; Deeg & Kriegsman, 2003). Evidence for a gender difference in the association between marital status and subjective health is mixed, with some researchers finding a stronger protective effect of marriage for men than women (Liu & Umberson, 2008; Williams & Umberson, 2004), while others report no gender differences (Zheng & Thomas, 2013).
Whereas previous studies focused primarily on gender differences in means and the predictive power of subjective health measures, we examined how genetic and environmental components of variance in subjective health are moderated by marital status, and whether that moderation effect differs for men and women. Multiple studies have reported heritability estimates for subjective health; however, to our knowledge, no other study has examined marital status moderation of these estimates. Studies of adult twins in Australia, Denmark, Finland, Sweden, and the U.S. have reported heritability estimates for subjective health primarily in the range of 25% to 30% (for a review see (Franz et al., in revision). A recent twin analysis that included 12,900 individuals aged 25 to 102 from the Interplay of Genes and Environment across Multiple Studies consortium (IGEMS; (Pedersen et al., 2013), which is also the basis for the present study, provided a more nuanced understanding of genetic and environmental influences on subjective health. Results indicated that heritability varied significantly by age, gender, and subjective health measure. Here, we expanded those analyses to examine how age, sex, and marital status moderated genetic and environmental influences on subjective health. Although marital status is not purely an environmental measure (Trumbetta, Markowitz, & Gottesman, 2007), we focused on relationship status as a marker for resources to support health by differentiating individuals who were living with partners (married or cohabitating) from those living alone (single, divorced, or widowed). We predict that living with a partner provides a protective or stabilizing influence that to some degree buffers individuals against age differences in genetic and environmental influences on subjective health identified by Franz and colleagues (in revision). Furthermore, given the possibility of gender differences in the role of marital status in subjective health, we predict that living with a partner will modulate the heritability of subjective health differently for men and women. Finally, based on previous results, we also predict that the moderation effect of marriage will vary across different measures of subjective health.
METHOD
Participants
IGEMS is an international consortium of twin studies from the Nordic countries and the U.S. covering the adult lifespan (Pedersen et al., 2013). The sample sizes and age ranges from the IGEMS studies included here are presented in Table 1: a total of 26,579 individuals contributed relevant data to the current study. Age ranged from 23-102 years, with a mean of 55.2 (sd = 16.6). For reporting of sample sizes and means, the sample was divided into four approximately equal age groups: age less than 50, 50-59, 60-69, and greater than 70 years. For the moderator analyses, both members of a twin pair were needed: the same-sex twin pairs available for each subjective health measure in each age group are presented in Table 2. Although sample size is presented separately by age group to indicate coverage across the lifespan, age was included as a continuous moderator in the biometric models.
Table 1.
IGEMS studies
| Study | Label | Reference | N subjects |
Age range |
Vars |
|---|---|---|---|---|---|
| Finnish Twin Cohort | FTC | Kaprio and Koskenvuo (2002) |
7870 | 53-67 | SRH |
| Finntwin16 | FT16 | Kaprio, Pulkkinen, and Rose (2002) |
4246 | 21-29 | SRH |
| Longitudinal Study of Aging Danish Twins |
LSADT | Christensen, Holm, McGue, Corder, and Vaupel (1999) |
3311 | 70-102 | SRH COMP ACT |
| Middle-Age Danish Twins | MADT | Osler et al. (2008) | 4037 | 45-68 | SRH COMP ACT |
| Midlife in the United States | MIDUS | South and Krueger (2012) |
1764 | 25-74 | SRH COMP ACT |
| Minnesota Twin Study of Adult Development and Aging |
MTSADA | Finkel and McGue (1993) |
835 | 25-92 | COMP ACT |
| Origins of Variance in the Oldest-Old |
OCTO- Twin |
McClearn et al. (1997) |
666 | 79-98 | SRH COMP ACT |
| Swedish Adoption Twin Study of Aging |
SATSA | Finkel and Pedersen (2004) |
1711 | 26-93 | SRH COMP ACT |
| Twin and Offspring Study in Sweden |
TOSS | Neiderheiser and Lichtenstein (2008) |
1069 | 32-60 | SRH COMP ACT |
| Vietnam Era Twin Study of Aging |
VETSA | Kremen et al. (2006) |
1070 | 51-60 | SRH COMP ACT |
Note: SRH – self-rated health; COMP = health compared with others; ACT = health influences activities
Table 2.
Number of twin pairs
| Age | SRH | ACT | COMP | |||
|---|---|---|---|---|---|---|
| Group | Men | Women | Men | Women | Men | Women |
| <50 | ||||||
| MZ | 525 | 674 | 303 | 359 | 316 | 361 |
| DZ | 584 | 648 | 329 | 340 | 332 | 350 |
| 50-59 | ||||||
| MZ | 778 | 548 | 590 | 237 | 587 | 234 |
| DZ | 858 | 801 | 517 | 256 | 516 | 258 |
| 60-69 | ||||||
| MZ | 380 | 464 | 202 | 247 | 201 | 248 |
| DZ | 549 | 706 | 209 | 223 | 212 | 222 |
| 70+ | ||||||
| MZ | 248 | 390 | 251 | 394 | 246 | 393 |
| DZ | 315 | 597 | 319 | 593 | 316 | 581 |
| TOTAL | 9065 | 5373 | 5369 | |||
Measures
Marital Status
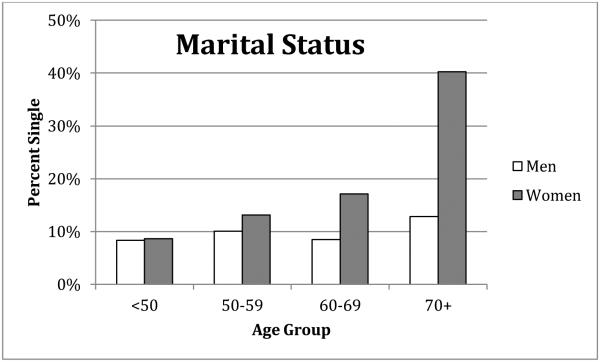
Marital status was recorded in various categories in the IGEMS studies. Because the focus of the current study was on partner presence as a marker of resources to support health, we created a dichotomous variable, combining married and cohabitating in one category, and widowed, divorced, and single in the other category. For simplicity, the two categories were labeled “married” and “single”. The distributions of marital status across the four age groups for men and women are presented in Figure 1. Percent single increased modestly but significantly from 8% to 12% for men across the four age groups (χ2 (df=3, N=12201) = 202.2, p<.01). It increased more dramatically from 9% to 41% for women, following population trends (χ2 (df=3, N=14378) = 2046.9, p<.01).
Figure 1.
Distribution of marital status across age groups and gender
Subjective Health
Three different types of questions were used to assess subjective health in the IGEMS studies (see Table 1). Nine of the studies included the most common question used to assess subjective health: “How would you rate your overall health?” In the literature, the acronym SRH is typically used to identify this question. Eight IGEMS studies asked participants to compare their health with others (COMP) using two slightly different forms: “compared to others your age, how would you rate your overall health?” was used by six studies and “I am as healthy as anyone I know” from the SF-36 version 1 (Ware, Kosinski, & Keller, 1994) used by two. Participants in eight studies also indicated how their health affected their daily activities (ACT); five studies included a single question, “Is your health condition preventing you from doing things you like to do?” Three indicated whether their health affected their physical functioning in a list of multiple behaviors from the SF-36. Responses to activities were averaged to create a single ACT score for these three studies.
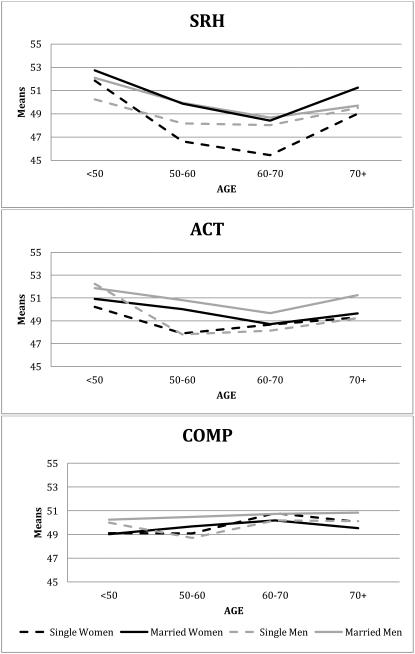
Although the subjective health questions administered across the studies were similar or identical, the response scales varied from dichotomous options to 7-point Likert scales. To examine and reconcile differences among these putatively similar measures, we engaged in a harmonization process, collecting new data on all combinations of questions and answer schemes from an independent international sample of 1065 participants aged 30 to 98 (Gatz et al., in press). The harmonization sample allowed us to verify that similarly worded questions correlated substantially, regardless of exact wording or response scales. Average correlations across response scales were .77 for SRH, .78 for ACT, and .63 for COMP. Average correlations across the three different subjective health questions were rSRH·COMP = .63, rSRH·ACT = .57, and rCOMP·ACT = .46. Comparison of three types of harmonization methods indicated that the optimal approach involved standardizing scores within samples to achieve a common metric, then pooling data across studies. To that end, the three subjective health questions were standardized separately within each sample and converted to T-scores (mean 50, SD 10). For all measures, high scores indicated better subjective health. Means across age groups in the combined IGEMS sample are presented in Figure 2, indicating age, gender, and marital status effects. Different trends are evident for each subjective health item, with the smallest group mean differences seen for the COMP variable. Continuous age trends in mean subjective health estimated by the age moderation model resulted in the same pattern of results.
Figure 2.
Means for subjective health variables across age groups, gender, and marital status
Statistical Methods
To evaluate whether the genetic and environmental influences on subjective health ratings differed as a function of marital status, we utilized a modified version of the univariate twin model in which age and marital status were included as moderating variables (Purcell, 2002; Van der Sluis, Dolan, Neale, & Posthuma, 2008). The standard univariate twin model incorporates monozygotic (MZ) twins and dizygotic (DZ) twins to decompose the variance of any phenotype into the proportion attributed to additive genetic influences (A), common or shared environmental influences (C), and unique environmental influences (E). The model used in the present study allows for differences in the A, C, and E parameters as a function of two continuous moderator variables (age and age-squared) and one categorical moderator variable (marital status). Moderation of the genetic and/or environmental variance components indicates that the contributions of these factors to the variance of subjective health vary by age and marital status. All models were tested using the structural equation-modeling package Classic Mx 1.68 (Neale, Boker, Xie, & Maes, 2003). Evaluation of relative model fit was performed using the likelihood-ratio-test (LRT). Significant LRT values indicate that the reduction in parameters resulted in a significant reduction in model fit.
RESULTS
Previous analyses have focused on age and sex moderation of subjective health (Franz et al., in revision); the focus here was primarily on marital status moderation of subjective health for men and women. Therefore, model comparison focused on testing marital status moderation parameters. The first phase of model testing examined gender differences in these parameters and two models were compared: one in which all 19 model parameters were allowed to vary across gender versus a model in which the 3 marital status moderation parameters (for A, C, and E) were set equal across genders. Comparison of these two models indicated significant gender differences in marital status moderation of subjective health for SRH (LRT = 8.13, df = 3, p < .05) and for ACT (LRT = 24.64, df = 3, p < .01), but not for COMP (LRT = 2.01, df = 3, ns).
In the next phase of model fitting, five models were tested separately for each gender (see Table 3). First the full model estimated all variance components and moderator parameters. In model 2, all marital status moderation parameters were dropped. In models 3 through 5, marital status moderation of each variance component (A, C, and E) was tested independently. For each model, all other parameters were retained: the primary A, C, and E variance components and the age and age-squared moderation of these components.
Table 3.
Model-fit statistics
| Model | SRH | ACT | COMP | |||
|---|---|---|---|---|---|---|
| -2LL | df | -2LL | df | -2LL | df | |
| Women | ||||||
| 1. Full model | 75425 | 10198 | 38845 | 5214 | 38843 | 5205 |
| 2. Drop all MS moderation | 75435* | 10201 | 38849 | 5217 | 38847 | 5208 |
| 3. Drop MS moderation on A | 75425 | 10199 | 38848 | 5215 | 38843 | 5206 |
| 4. Drop MS moderation on C | 75427 | 10199 | 38845 | 5215 | 38843 | 5206 |
| 5. Drop MS moderation on E | 75427 | 10199 | 38845 | 5215 | 38844 | 5206 |
| Men | ||||||
| 1. Full model | 68817 | 9310 | 39369 | 5368 | 39822 | 5377 |
| 2. Drop all MS moderation | 68844** | 9313 | 39399** | 5371 | 39831* | 5380 |
| 3. Drop MS moderation on A | 68817 | 9311 | 39369 | 5369 | 39826* | 5378 |
| 4. Drop MS moderation on C | 68821* | 9311 | 39373* | 5369 | 39824 | 5378 |
| 5. Drop MS moderation on E | 68821* | 9311 | 39526** | 5369 | 39823 | 5378 |
Model fit differs significantly from model 1 at p < .05
Model fit differs significantly from model 1 at p < .01
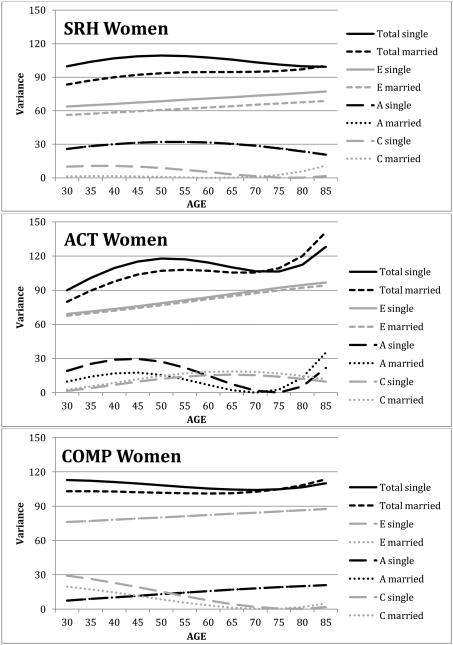
Comparing model 2 to model 1 indicated significant marital status moderation of only one subjective health measure in women: SRH. Testing each marital status moderation parameter separately (models 3 through 5) failed to identify the source of the marital status moderation of variance components of SRH in women. Minimization of Akaike’s Information Criterion (log-likelihood – 2* degrees of freedom) can be used to identify the best-fitting model. In this case, AIC was smallest for model 3 for SRH in women, suggesting modest marital status moderation of C and E components. The estimates from the ACE model with full moderation (model 1) were used to depict marital status moderation of subjective health measures for women across age (see Figure 3). A, C, and E components of variance, along with total variance, are presented for single and married women for the three subjective health measures. For SRH, slightly more C and E variance was evident for single women than for married women, resulting in greater total variance in SRH for single women than married women. The estimates for the A variance component from single and married women were nearly identical, so the lines on the graph overlap. Results for ACT suggest somewhat more A variance for single women than married women; however, the moderation parameter did not achieve significance (model 3 vs. model 1 = 3.20, df = 1, n.s.). Little distinction can be detected in the A, C, and E variance components for COMP in women; the lines for A and E variance components overlap. The general pattern of variance components across age matches the results reported by Franz and colleagues (in revision). Heritability of SRH was estimated at 28% for women across most of the age range, with a somewhat lower heritability estimated in late adulthood (17%). Heritability for ACT showed a curvilinear trend over age, with highest estimates for younger women (17% and 25%) and lower estimates for older women (2%). Heritability for COMP increased across age from 7% for younger women to 19% for older women.
Figure 3.
Genetic and environemental components of variance across age and marital status for women. SRH: self-rated health; ACT: health impacts activites; COMP: health compared with other.
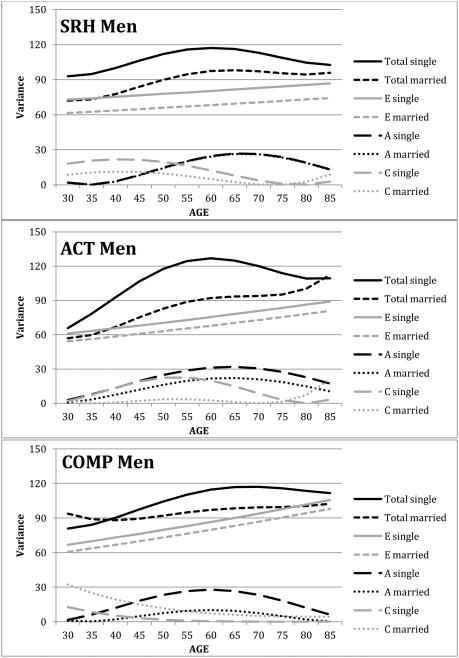
In contrast, evidence for significant marital status moderation of variance was found for all three subjective health measures in men, although the pattern of results differs across measures. For both SRH and ACT, model fitting indicated significant marital status moderation of C and E components of variance; whereas for COMP, model comparison indicated significant marital status moderation of the A variance component. The impact of marital status on variance components of the three subjective health measures in men is presented in Figure 4. For SRH, single men demonstrate significantly more C and E variance than married men, resulting in more total variance. The difference in C variance between single and married men declines with age, as does total C variance, which reaches nearly zero for both groups of men at age 75. Genetic variance in SRH was basically identical for single and married men, so the lines on the graph overlap. Heritability is lower in younger men and the highest estimate is at age 70 (23%).
Figure 4.
Genetic and environemental components of variance across age and marital status for men. SRH: self-rated health; ACT: health impacts activites; COMP: health compared with other.
For the ACT variable, A, C, and E components of variance are higher for single men then for married men, but only the differences in C and E components achieve significance (see Figure 4). Whereas the differences in A and E variances are constant across the age range, marital status differences in C variance peak in midlife, around age 55; group differences are minimized earlier and later in the measured age range (age 30 and 75). Across most of the age range, total variance is about 30% higher for single men compared with married men. Similar to SRH in men, heritability for ACT is highest at age 65 (26%).
A markedly different pattern of marital status moderation of variance was found for the COMP variable. In this instance, marital status significantly moderated A variance, only (see Figure 4). A variance is highest in midlife for both single and married men, but genetic variance estimated for single men is nearly three times higher then the genetic variance estimated for married men. As a result, heritability estimates in midlife for the COMP variable are 24% versus 10% for single and married men, respectively. Note, however, that regardless of the different moderation patterns suggested for COMP for men and women, model comparisons indicated that the pattern of marital status moderation of COMP did not differ significantly between men and women. For all three subjective health measures, total variance was greater for single men than married men.
DISCUSSION
Our examination of marital status moderation of genetic and environmental influences on subjective health across adulthood revealed varied patterns of moderation that differed for men and women and for the three distinct measures of subjective health. In addition, we replicated the pattern of age moderation of genetic and environmental influences on subjective health reported by Franz and colleagues (in revision) in a smaller sample of 12,900 individuals from the IGEMS consortium.
For men, marital status moderated shared and nonshared environmental components of variance for SRH and ACT and the genetic component of variance for COMP. As a result, shared and nonshared environmental components of variance were significantly greater for single men than for married men for the SRH and ACT measures. Whereas the differences in nonshared environmental variance were generally consistent across the age range, differences in shared environmental variance were higher for younger men than for older men. In fact, for ACT, estimates of shared environment were near zero for married men but peaked at 19% at age 50 for single men. Thus, for men, marriage apparently provided a buffer that resulted in more stability in components of variance for subjective health across the age range. The marital status category that differed the most across the age groups for men was widowhood: 10.2% of single men in the 50s, 23.1% of single men in their 60s, and 57.6% of single men over 70 were widowed. Paralleling this pattern, nonshared environmental variance of subjective health was greater in single men across the same age range. The percentage of single men reporting that they were divorced peaked in the 50-60 age range, approximately the same point in the age range that shared environmental variance peaked for SRH and ACT for single men and genetic variance peaked for COMP in single men. It would appear, then, that without marriage as a protective factor, the fluctuations in genetic and environmental components of variance are amplified.
The pattern of results for women was far less complex: marital status played only a modest role in greater environmental variance in single women for SRH. Thus, marriage provided at most a limited buffer against the environmental impact of health perceptions with age. However, across the life course—but especially after age 50—women are less likely to remarry after divorce or bereavement (Waite, Laumann, Das, & Schumm, 2009); thus marital status during this time period may be more stable for women than for men, resulting in less marital status moderation of variation in perceptions of health compared with men. Previous evidence for gender differences in the influence of marital status on mean subjective health has been mixed (Liu & Umberson, 2008; Williams & Umberson, 2004; Zheng & Thomas, 2013). In the current analyses, despite gender differences in (a) the experiences of physical aging (Sainio et al., 2006), (b) the impact of marital status on environmental resources in these cohorts (Weaver, 2010), and (c) the incidence of bereavement, marital status had very little impact on sources of variance in subjective health in women. It may be that women in these cohorts are better able than men to maintain sources of social support independent of marital status, with the result that variance composition of subjective health is fairly consistent for single and partnered women. Some evidence suggests that men have smaller support networks than women and thus marriage constitutes a larger portion of men’s social support networks (Dykstra & Fokkema, 2007). Moreover, especially in these older cohorts, women are often responsible for maintaining and fostering the social interactions of both members of the pair (Dykstra & de Jong Gierveld, 1994; Rosenthal, 1985). Thus bereavement for men can mean loss of emotional and instrumental support for maintaining health (Chipperfield & Havens, 2001). Consequently, although both men and women experience increased mortality rates immediately following bereavement, mortality rates tend to remain elevated for men, only (Kaprio, Koskenvu, & Hell, 1987). Regardless of marital status, then, women are more likely than men to be able to tap their larger support networks for the emotional and instrumental resources that result in stable heritability estimates for subjective health.
Finally, there were striking differences in genetic and environmental components of variance, and the marital status moderation of variance, across measures of subjective health. In fact, gender differences in marital status moderation of the COMP variable did not achieve significance. Different subjective health items tap different frames of reference (Manderbacka, Kåreholt, Martikainen, & Lundberg, 2003; Vuorisalmi, Lintonen, & Jylhä, 2006), reflecting diverse combinations of psychological dispositions, situational factors, shared cultural values, and characteristics such as age, gender, class, or ethnicity (Jylhä, 2009, 2010; Sprangers & Schwartz, 1999). Some subjective health questions trigger more internal frames of reference (e.g., rate your overall health), whereas in others the frame of reference may be more external (e.g., rate your health compared to others your age; does health prevent you from doing things you like to do?) and may trigger more conscious or unconscious consideration of environmental support factors. The current results suggest that the different frames of reference triggered by the subjective health items were differentially affected by marital status (at least for men).
Our conclusions are subject to methodological limitations. First, combining data across studies was both a strength and a weakness of our approach. Combining studies provided sufficient power to examine effects simultaneously across age groups, gender, and marital status, which is impossible with smaller cohorts. However, it also necessitated harmonizing somewhat different measures of subjective health. The independent crosswalk study of our measures (Gatz et al., in press) supported our approach. Moreover, consistent with our data, a cross-national comparison of self-rated health found that relationships among SRH and covariates, including marital status and gender, were homogeneous across countries (Bardage et al., 2005). Second, we interpreted marital status as a measure of environmental resources to support physical and subjective health. Although alternative interpretations of the relationship between marital status and subjective health exist, marital status as a marker for health resources has been supported by the literature (Benyamini, 2011; Zheng & Thomas, 2013). Still, marital status does not tap only environmental variance, but genetic variance as well. However, the heritable influences on marital status appear to decline from 40% in early adulthood to 0% by age 50 and beyond (Trumbetta et al., 2007). Third, the participants were all from the U.S. and the Nordic countries, and in fact the Finnish twin studies contributed nearly half the available data for SRH. Results for ACT, which was not included in the Finnish data, are similar to the results for SRH. Moreover, previous reports of these analyses that did not include the Finnish twin studies produced very similar results (Finkel, Horwitz, & Gatz, 2014): addition of the Finnish twin sample provided more power but did not change the overall conclusions. Finally, as in any study including older adults, the sample was subject to survivor effects, particular in the oldest age groups. The slight reductions in total variance generally evident in late adulthood (particularly for men) likely resulted from absence of individuals in poorest health from the sample.
Overall, we observed that external factors, such as those tapped by marital status, were associated with genetic and environmental contributions to subjective health, indicative of gene by environment interaction. Gender and age differences, combined with marital status differences that may impact access to resources to support health, were associated with fluctuations in genetic and environmental components of variance in health perceptions. This relationship was far more pronounced for men than for women, likely as a result of different roles that marriage and partners play in social networks for men and women in these cohorts. Finally, these results join a growing body of evidence that not all measures of subjective health are equal. The manner in which the question is posed triggers a frame of reference that will impact the interplay of genetic and environmental influences on health perceptions.
Acknowledgements
IGEMS is supported by the National Institutes of Health grant no. R01 AG037985. SATSA was supported by grants R01 AG04563, R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141). OCTO-Twin was supported by grant R01 AG08861. TOSS was supported by grant R01 MH54610 from the National Institute of Health. The Danish Twin Registry is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the US National Institute of Health (P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by NIA grant R01 AG 06886. VETSA was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry. MIDUS twin study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by National Institute on Aging Grant AG20166. Data collection and analyses in the Finnish twin cohort have been supported by ENGAGE – European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement number 201413, National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to R J Rose), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278 and 264146 to J. Kaprio). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA.
References
- Bailis DS, Segall A, Chipperfield JG. Two view of self-rated general health status. Social Science & Medicine. 2003;56:203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Bardage C, Pluijm SMF, Pedersen NL, Deeg DJH, Jylhä M, Noale M, Otero Á. Self-rated health among older adults: A cross-national comparison. European Journal of Ageing. 2005;2:149–158. doi: 10.1007/s10433-005-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamini Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychology and Health. 2011;26:1407–1413. doi: 10.1080/08870446.2011.621703. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health--mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? The Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Carr D, Springer KW. Advances in families and health research in the 21st century. Journal of Marriage and Family. 2010;72:743–761. [Google Scholar]
- Chipperfield JG, Havens B. Gender differences in the relationship between marital status transitions and life satisfaction in later life. Journal of Gerontology: Psychological Sciences. 2001;56B:P176–P186. doi: 10.1093/geronb/56.3.p176. [DOI] [PubMed] [Google Scholar]
- Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. Journal of Aging and Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- Deeg DJH, Kriegsman DMW. Concepts of self-rated health: Specifying the gender difference in mortality risk. The Gerontologist. 2003;43:376–386. doi: 10.1093/geront/43.3.376. [DOI] [PubMed] [Google Scholar]
- Dykstra PA, de Jong Gierveld J. The theory of mental incongruity, with a specific application to loneliness among widowed men and women. In: Erber R, Gilmour R, editors. Theoretical frameworks in personal relationships. Erlbaum Associates, Inc.; Hillsdale, NJ: 1994. pp. 235–259. [Google Scholar]
- Dykstra PA, Fokkema T. Social and emotional loneliness among divorced and married men and women: Comparing deficit and cognitive perspectives. Basic and Applied Social Psychology. 2007;29:1–12. [Google Scholar]
- Finkel D, Horwitz BN, Gatz M. Marital status moderates gender differences in genetic and environmental influences on subjective health [Abstract] The Gerontologist. 2014;54 doi: 10.1007/s10519-015-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, McGue M. The origins of individual differences in memory among the elderly: A behavior genetic analysis. Psychology and Aging. 1993;8:527–537. doi: 10.1037//0882-7974.8.4.527. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. [Google Scholar]
- Franz CE, Finkel D, Panizzon MS, Spoon K, Christensen K, Gatz M, Pedersen NL. Genetic and Environmental Influences on The Many Facets of Subjective Health from Early Adulthood to Old Age. Journal of Aging and Health. doi: 10.1177/0898264315625488. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Finkel D, Hahn C, Zhou Y, Zavala C. Data harmonization in aging research: Not so fast. Experimental Aging Research. doi: 10.1080/0361073X.2015.1085748. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Jylhä M. Self-rated health between psychology and biology. A response to Huisman and Deeg. Social Science & Medicine. 2010;70:655–657. [Google Scholar]
- Kaprio J, Koskenvu M, Hell R. Mortality after bereavement: A prospective study of 95, 647 widowed persons. American Journal of Public Health. 1987;77:283–287. doi: 10.2105/ajph.77.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Research. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Research. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, Lyons MJ. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Latham K, Peek CW. Self-rated health and morbidity onset among late midlife U.S. adults. Journals of Gerontology Series B: Psychological Sciences & Social Sciences. 2013;68:107–116. doi: 10.1093/geronb/gbs104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Umberson D. The times they are a changin': Marital status and health differentials from 1972 to 2003. Journal of Health and Social Behavior. 2008;49:239–253. doi: 10.1177/002214650804900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderbacka K, Kåreholt I, Martikainen P, Lundberg O. The effect of point of reference on the association between self-rated health and mortality. Social Science & Medicine. 2003;56:1447–1452. doi: 10.1016/s0277-9536(02)00141-7. [DOI] [PubMed] [Google Scholar]
- Manzolo L, Villari P, Pirone GM, Boccia A. Marital status and moralit in the elderly: A systematic review and meta-analysis. Social Science & Medicine. 2007;6:77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- McFadden E, Luben R, Bingham S, Wareham N, Kinmonth A-L, Khaw K-T. Does the association between self-rated health and mortality vary by social class? Social Science & Medicine. 2009;68:275–280. doi: 10.1016/j.socscimed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry; VCU Box 900126, Richmond, VA 23298: 2003. [Google Scholar]
- Neiderheiser JM, Lichtenstein P. The Twin and Offspring Study in Sweden: Advancing our understanding of genotype-environment interplay by studying twins and their families. Acta Psychologica Sinica. 2008;40:1116–1123. [Google Scholar]
- Osler M, McGue M, Lund R, Christensen K. Marital status and twins' health and behavior: an analysis of middle-aged Danish twins. Psychosom Med. 2008;70:482–487. doi: 10.1097/PSY.0b013e31816f857b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Christensen K, Dahl A, Finkel D, Franz CE, Gatz M, Reynolds CA. IGEMS: The Consortium on Interplay of Genes and Environment across Multiple Studies. Twin Research and Human Genetics. 2013;16:481–489. doi: 10.1017/thg.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research and Human Genetics. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiol. Behav. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. Kinkeeping in the familial division of labor. Journal of Marriage and the Family. 1985;47:965–974. [Google Scholar]
- Sainio P, Koskinen S, Heliovaara M, Martelin T, Harkanen T, Hurri H, Aromaa A. Self-reported and test-based mobility limitations in a representative sample of Finns aged 30. Scandinavian Journal of Public Health. 2006;34:378–286. doi: 10.1080/14034940500489859. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Moustgaarid H, Peltonen R, Martikainen P. Changing associations between partnership history and risk of accidents, violence and suicides. Journal of Epidemiology and Community Health. 2013;67:265–270. doi: 10.1136/jech-2012-201311. [DOI] [PubMed] [Google Scholar]
- South SC, Krueger RF. Genetic strategies for probing conscientiousness and its relationship to aging. Developmental Psychology. 2012;50:1362–1376. doi: 10.1037/a0030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers MAG, Schwartz CE. Integrating response shift into health-related qualty of life research: A theoretical model. Social Science & Medicine. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Kubzansky L, Berkman L, Fay M, Kawachi I. Neighborhood effects on the self-rated health of elders: Uncovering the relative importance of structural and service-related neighborhood environments. Journal of Gerontology: Social Sciences. 2006;61B:S153–S160. doi: 10.1093/geronb/61.3.s153. [DOI] [PubMed] [Google Scholar]
- Trumbetta SL, Markowitz EM, Gottesman II. Marriage and genetic variation across the lifespan: Not a steady relationship? Behavior Genetics. 2007;37:362–375. doi: 10.1007/s10519-006-9132-1. [DOI] [PubMed] [Google Scholar]
- Van der Sluis S, Dolan CV, Neale MC, Posthuma D. A General Test for Gene– Environment Interaction in Sib Pair-based Association Analysis of Quantitative Traits. Behavior Genetics. 2008;38:372–389. doi: 10.1007/s10519-008-9201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorisalmi M, Lintonen T, Jylhä M. Comparative vs global self-rated health: associations with age and functional ability. Aging Clinical and Experimental Research. 2006;18:211–217. doi: 10.1007/BF03324651. [DOI] [PubMed] [Google Scholar]
- Waite L, Gallagher M. The Case for Marriage: Why Married People Are Happier, Healthier, and Better Off Financially. Doubleday; New York: 2000. [Google Scholar]
- Waite L, Laumann EO, Das A, Schumm LP. Sexuality: measures of partnerships, practices, attitudes, and problems in the National Social Life, Health, and Aging Study. Journal of Gerontology: Social Sciences. 2009;64:56–66. doi: 10.1093/geronb/gbp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A Users' Manual. The Health Institute; Boston: 1994. [Google Scholar]
- Weaver DA. Widows and social security. Social Security Bulletin. 2010;70:89–109. [PubMed] [Google Scholar]
- Williams K, Umberson D. Marital status, marital transitions, and health: A gendered life course perspective. Journal of Health and Social Behavior. 2004;45:81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Thomas PA. Marital status, self-rated health, and mortality: Overestimation of health or diminishing protection of marriage? Journal of Health and Social Behavior. 2013;54:128–143. doi: 10.1177/0022146512470564. [DOI] [PMC free article] [PubMed] [Google Scholar]