Abstract
Chemokines play important roles in the central nervous system, including mediating neuroinflammation and guiding the intracortical migration of interneurons during development. Alteration in parvalbumin-positive interneurons is a key neuropathological hallmark of multiple mental conditions. We recently reported a significant reduction in the expression of CXCL12 in olfactory neurons from sporadic cases with schizophrenia compared with matched controls, suggesting a role for CXCR4/CXCL12 signaling in mental conditions. Thus, we depleted the chemokine receptor Cxcr4 from mice using the parvalbumin-2A-Cre line. The conditional knockout mice exhibited a unique behavioral phenotype involving increased stereotypy. Stereotypy is observed in many psychiatric conditions, including schizophrenia, autism, and dementia. Thus, the Cxcr4 conditional knockout mice may serve as a model for this symptomatic feature.
Chemotactic cytokines, or chemokines, play several important roles in the central nervous system (Adler et al., 2005; Mithal et al., 2012). They mediate the infiltration of leukocytes into the central nervous system during neuropathology (Jaerve and Muller 2012) and may also be involved in neurogenesis, neuroprotection, and neurotransmission (Edman et al., 2008; Guyon 2014). During development, the chemokine CXCL12 (also called stromal cell derived factor-1 or SDF-1) and its receptor CXCR4 guide the tangential intracortical migration of GABAergic interneurons to their correct laminar positions (Stumm et al., 2003; Lopez-Bendito et al., 2008). In addition to its role in the pathology of multiple sclerosis, Alzheimer's disease, and HIV-associated dementia, we have previously reported altered expression of the CXCR4/CXCL12 cascade in olfactory neurons from sporadic schizophrenia patients (Toritsuka et al., 2013).
Cxcr4 is constitutively expressed throughout the brain in neurons, astrocytes, and to a lesser extent microglia (Banisadr et al., 2002). Among cortical interneurons, Cxcr4 is especially critical for the migration of PV-positive interneurons (Zhao et al., 2008; Wang et al., 2010; Meechan et al., 2012). However, the postnatal expression of Cxcr4 in PV neurons may be relatively low (Stumm et al., 2007). As far as we are aware, the influence of CXCR4 on behavior has been addressed only with Sox1-Cre;Cxcr4fl/fl mice, in which Cxcr4 is deleted in neural stem and progenitor cells, including populations in the lateral ventricle walls, dentate gyrus, and Purkinje cell layer of the cerebellum. That study focused on the cerebellum and detected abnormal motor behaviors (Huang et al., 2014). In contrast, here we address the influence of Cxcr4 on a variety of behaviors relevant to multiple dimensions of mental disorders (Cuthbert and Insel 2013). We thus aim to examine the specific effect of Cxcr4-mediated PV-positive neuron deficits on behavior.
We intended to genetically deplete Cxcr4 in PV-positive neurons by crossing floxed Cxcr4 mice [B6.129P2-Cxcr4tm2Yzo/J mice (Jax 008767)] (Nie et al., 2004) with B6.Cg-Pvalbtm1.1(cre)Aibs/J mice (Jax 012358) (Madisen et al., 2010). Experiments were performed with homozygous conditional Cxcr4 knockout males and their wild-type littermate controls. All animal experiments were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 823) and were approved by the Johns Hopkins IACUC.
We tested adult males (at least 3 months old) in the following sequence of behavioral tests: open field, Y maze, three-chamber sociability, prepulse inhibition, and forced swim. Tests were performed from less to more stressful and approximately one week apart to minimize inter-trial interference.
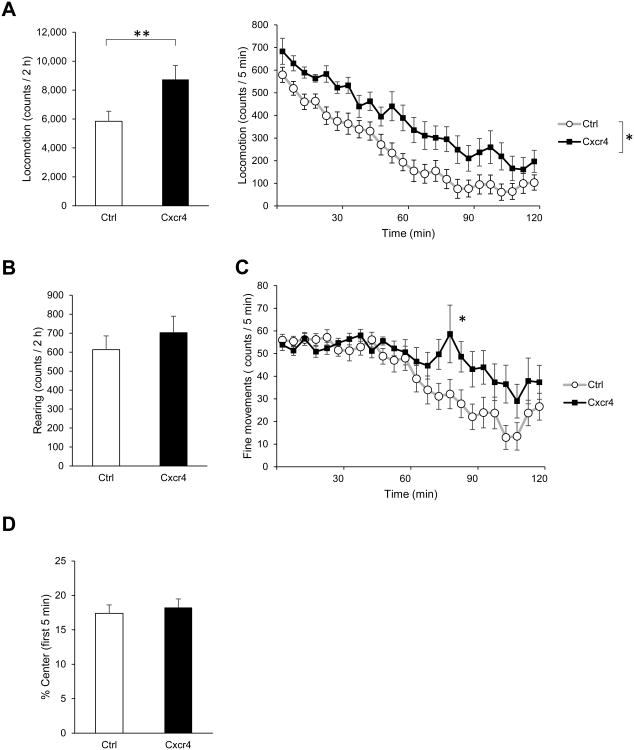
In the open field paradigm, each mouse was allowed to roam freely in a novel open field box (40 cm × 40 cm; San Diego Instruments, San Diego, CA) for 2 h. Horizontal and vertical locomotion and fine (stereotypic) movements were automatically recorded by an infrared activity monitor (San Diego Instruments). Single beam breaks are reported as “counts.” PV-Cxcr4-/- mice showed hyperlocomotion over the whole 2 h (Fig. 1A, left) and their horizontal activity was higher than that of controls at all time points (Fig. 1A, right). Both PV-Cxcr4-/- mice and controls showed normal habituation of horizontal activity throughout the test period. Although vertical movements (rearing) were slightly increased in the PV-Cxcr4-/- mice, there was no significant difference from controls (Fig. 1B). PV-Cxcr4-/- mice showed increased fine/stereotypic movements compared to controls during the second hour, suggesting abnormally slow habituation (Fig. 1C). Both groups spent a similar amount of time in the anxiogenic center of the open field vs. the safer periphery during the first 5 min (Fig. 1D).
Fig. 1.
Behavior of PV-Cxcr4-/- mice in a novel open field. (A) Cxcr4 mice were hyperactive in the open field as measured by horizontal locomotion. Left, total counts over the 2 h, **p<0.01. Right, locomotion over time, two-way repeated measures ANOVA showed a significant effect of the genotype: F(1,26)=6.26, *p<0.05. (B) Cxcr4 mice did not differ significantly from control mice in rearing over the 2 h. (C) Cxcr4 mice made more fine/stereotypic movements during the second h in the open field. Two-way repeated measures ANOVA: significant genotype × time interaction, F(23,598)=2.24, ***p<0.001. Bonferroni post-hoc analysis indicated a significant difference in the fine movements in the 5 min interval 80-85 min, p<0.05. (D) Cxcr4 mice did not differ from control mice in percentage time spent in the center of the open field during the first 5 min, suggesting no difference in anxiety.
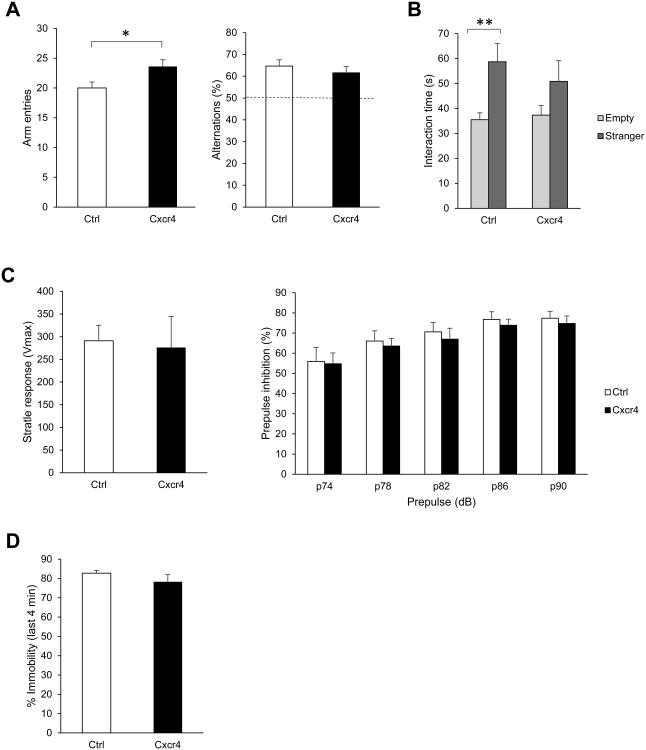
In the Y-maze paradigm, we recorded arm entries for each mouse over a 5 min free exploration period in a Y-shaped maze. Spontaneous alternation was calculated as the percentage of triads of successive arm entries containing entries into all three arms. PV-Cxcr4-/- mice had a higher number of arm entries, supporting our conclusion of hyperactivity seen in the open field test (Fig. 2A, left). However, they did not differ from the controls in the percentage of alternations between the three arms of the maze, suggesting normal short-term spatial memory (Fig. 2A, right).
Fig. 2.
Behavior of PV-Cxcr4-/- mice in additional standard assays. (A) Cxcr4 had more arm entries in the Y-maze (left, *p<0.05), but showed no difference in percentage of alternations, suggesting normal short term spatial memory. (B) In contrast to control mice, which showed a clear preference for interacting with a stranger mouse over an empty enclosure, Cxcr4 mice did not show a significant preference. Bonferroni post-hoc comparison for empty vs. stranger following two way repeated measures ANOVA: Ctrl, p**<0.01; Cxcr4, non-significant. (C) Cxcr4 mice did not differ from control mice in startle response to a 120 dB stimulus or in prepulse inhibition of acoustic startle.
Following the open field and Y-maze, we conducted the three-chamber sociability test. Mice were habituated to the three-chamber apparatus for three consecutive days prior to the experiment by being allowed to freely roam the apparatus for 10 min. The experiment consisted of a 5 min habituation period followed by a 10 min trial to measure sociability. A young, unfamiliar mouse of the same background and sex as the experimental mice was placed in an enclosure in one of the side chambers while the enclosure in the other side chamber was left empty. Mice will normally interact with the enclosure containing the unfamiliar mouse more than the empty enclosure. In contrast to control mice, PV-Cxcr4-/- mice did not significantly prefer to interact with the stranger over the empty enclosure, suggesting reduced sociability (Fig. 2B).
We next examined prepulse inhibition of the startle response. Acoustic startle and prepulse inhibition responses were measured in a startle chamber (San Diego Instruments). Each mouse was subjected to six pseudorandomly-distributed sets of three trial types: pulse-alone trials, prepulse-pulse trials, and no-stimulus trials. The pulse used was 120 dB and the prepulses were 74, 78, 82, 86, and 90 dB emitted over a constant background noise of 70 dB. PV-Cxcr4-/- mice showed normal startle response (Fig. 2C, left) and prepulse inhibition, suggesting normal sensorimotor gating (Fig. 2C, right).
Lastly, we conducted a forced swim test in which each mouse was placed into a large glass beaker containing room-temperature water for 6 min. We recorded the length of time spent swimming vs. immobile. PV-Cxcr4-/- mice did not differ from controls in percentage of time spent immobile, suggesting that they are not more or less prone to developing learned helplessness (Fig. 2D).
We used the Student's t-test for statistical analysis of the open field, Y maze, and forced swim assays, and two-way repeated measures ANOVA for the sociability and prepulse inhibition assays. p<0.05 was considered significant.
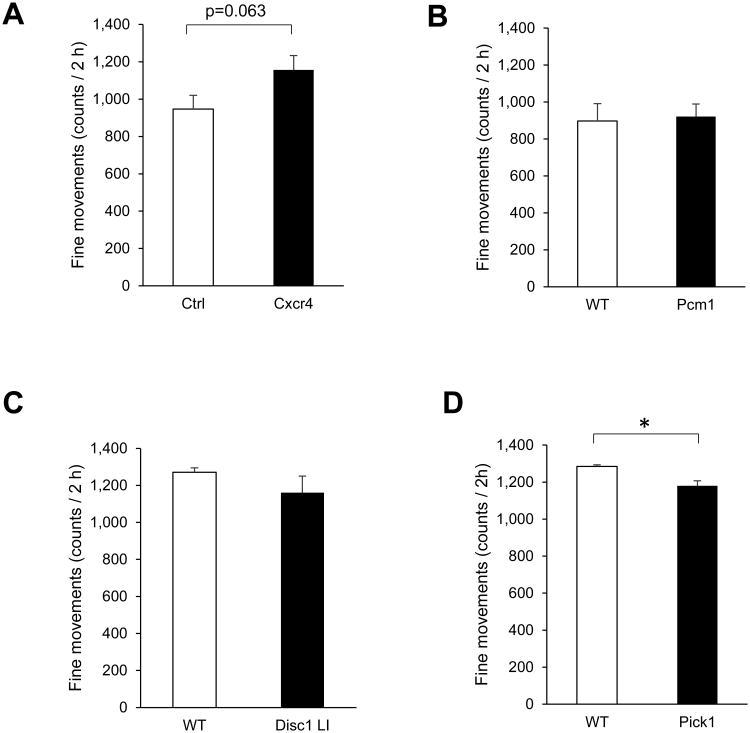
To summarize, PV-Cxcr4-/- mice were hyperactive in the open field and showed impaired sociability. Among these phenotypes, the increased stereotypic movement caught our attention. We therefore compared this feature to other mouse models we have tested similarly in the past (Fig. 3). Over the 2 h in the open field, we found that Pcm1 haploinsufficient mice (Zoubovsky et al., 2015) showed very similar stereotypic movement to wild-type littermate controls (Fig. 3B); Disc1 locus impairment mice (Shahani et al., 2015) showed a non-significant trend towards less stereotypic movement than controls (Fig. 3C); and Pick1 knockout mice (Nomura et al., 2015) showed significantly less stereotypic movement than controls, although they showed hyperlocomotion over part of the time (Fig. 3D).
Fig. 3.
Comparison of fine/stereotypic movements over 2 h in a novel open field among four brain oriented genetically engineered mouse models. (A) PV-Cxcr4-/- mice showed a strong trend of increased fine movements, p=0.063. (B) Pcm1 haploinsufficient mice made fine movements at a level very similar to that of WT littermate controls. (C) Disc1 locus impairment (LI) mice showed a non-significant trend of decreased fine movements. (D) Pick1 knockout mice had significantly lower fine movements that WT littermates, *p<0.05.
In the present study, we aimed to explore the role of Cxcr4 in behaviors relevant for mental conditions. As far as we are aware, there was only one study that addressed the effect of Cxcr4 on behavior, but focused on motor functions by using a Sox1-Cre line (Huang et al. 2014). In contrast, Cxcr4 conditional knockout mice we used here exhibited a unique behavioral phenotype involving increased stereotypy. Stereotypy is observed in many psychiatric conditions, including schizophrenia, autism, and dementia. We propose that the increased stereotypy in the present Cxcr4 model is relatively unique among several mouse models relevant to schizophrenia and psychosis.
Since the primary goal was to study behavioral deficits, we selected the strategy of cross-breeding floxed Cxcr4 mice [B6.129P2-Cxcr4tm2Yzo/J mice (Jax 008767)] with B6.Cg-Pvalbtm1.1(cre)Aibs/J mice (Jax 012358) to maintain C57BL/6J background. However, we need to state potential limitation in the choice of the mouse line. Due to the high efficiency of the 2A sequence in mediating bicistronic translation, it may drive Cre expression not only in interneurons where the levels of PV expression is high, but also in other neuronal populations and germ cells in which the levels of PV expression is lower (Madisen et al. 2010; Kobayashi and Hensch 2013). In order to address the question whether Cxcr4 in PV-positive interneurons is specifically important for stereotypical behavior, another Cre line (more specific to PV interneuron in its expression) (Hippenmeyer et al., 2005) will be useful. At least at present, the line under the C57BL/6J background is not commercially available. Thus, to carry out reliable behavioral assessment, we may need to adjust the genetic background by backcross breeding. Alternatively, we may employ stereotaxic injection of a viral vector that can express Cre under PV promoter into the forebrain. Testing of stereotypy in conventional Cxcr4+/- could also provide a useful comparison to assess the role of Cxcr4 specific to PV neurons.
Comparison of stereotypic movement among several different genetically engineered mouse models revealed that the present PV-Cxcr4-/- mice demonstrate uniquely increased stereotypic movement. Mutations in PV-positive neurons are not always associated with the stereotypic phenotype. For example, mice with PV-specific knockout of the NMDA receptor subunit NR1 showed normal stereotypic behavior (Carlen et al., 2012). We also note that knockout of TrkB in PV-positive neurons showed increased stereotypy (Lucas et al., 2014).
Locomotor activity is thought to be associated with the mesocorticolimbic dopamine pathway, whereas fine movements involved in stereotypy are thought to be associated with the nigrostriatal dopamine pathway (Broderick 2002). Accordingly, dopamine agonists can induce hyperlocomotion and stereotypy. Dopamine transporter knockout mice also show hyperlocomotion and increased stereotypic movements in the open field (Wong et al., 2012). Our primary hypothesis is that the depletion of Cxcr4 from PV-interneurons may be a major driver for stereotypy in the present model. Nonetheless, we do not exclude the possibility that the stereotypy may be driven by depletion of Cxcr4 from the dopaminergic system. Indeed, Cxcr4 is reportedly expressed in the dopaminergic system including the substansia nigra (Banisadr et al. 2002), and Sdf1/Cxcl12 could increase dopamine release (Skrzydelski et al., 2007).
Stereotypy is observed in many psychiatric conditions, including schizophrenia, autism, ADHD, obsessive-compulsive disorder, and Tourette's syndrome (Ghosh et al., 2013). Recent nosology in psychiatry has introduced, instead of classic categorical approaches, dimensional approaches in which mechanisms for each behavioral construct are investigated at the molecular, cellular, and circuitry levels (Cuthbert and Insel 2013). Furthermore, recent genetic studies have indicated that each categorized disease (e.g., schizophrenia, autism, and ADHD) may not be mutually exclusive but overlap with each other in ethological viewpoint (Owen 2014). Thus, the present model that focuses on a unique behavioral construct (e.g., stereotypy) may be useful to address a key mechanism for the behavioral changes underlying more than one psychiatric conditions.
Highlights.
We studied the behavioral effects of deleting Cxcr4 using parvalbumin-2A-Cre
PV-Cxcr4-/- mice showed increased stereotypical movements in an open field
PV-Cxcr4-/- may serve as a model for increased stereotypy
Acknowledgments
Funding statement: A.S. was supported by NIH grants (MH-084018, MH-094268 Silvo O. Conte center, MH-069853, MH-085226, MH-088753, MH-092443) as well as foundation grants from Stanley, S-R, RUSK, NARSAD, MSCRF. Funding from Astellas Pharm. Co. Ltd. was also used for the present study.
Footnotes
Conflict of interest statement: This study was conducted in part by using the fund from Astellas Pharm. Co. Ltd. for an academic research collaboration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. The AAPS journal. 2005;7:E865–870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. The European journal of neuroscience. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Broderick PA. Interleukin 1alpha alters hippocampal serotonin and norepinephrine release during open-field behavior in Sprague-Dawley animals: differences from the Fawn-Hooded animal model of depression. Progress in neuro-psychopharmacology & biological psychiatry. 2002;26:1355–1372. doi: 10.1016/s0278-5846(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman LC, Mira H, Erices A, Malmersjo S, Andersson E, Uhlen P, Arenas E. Alphachemokines regulate proliferation, neurogenesis, and dopaminergic differentiation of ventral midbrain precursors and neurospheres. Stem cells (Dayton, Ohio) 2008;26:1891–1900. doi: 10.1634/stemcells.2007-0753. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Rajan PV, Erenberg G. A comparative study of primary and secondary stereotypies. Journal of child neurology. 2013;28:1562–1568. doi: 10.1177/0883073812464271. [DOI] [PubMed] [Google Scholar]
- Guyon A. CXCL12 chemokine and GABA neurotransmitter systems crosstalk and their putative roles. Frontiers in cellular neuroscience. 2014;5:115. doi: 10.3389/fncel.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS biology. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GJ, Edwards A, Tsai CY, Lee YS, Peng L, Era T, Hirabayashi Y, Tsai CY, Nishikawa SI, Iwakura Y, Chen SJ, Flint J. Ectopic Cerebellar Cell Migration Causes Maldevelopment of Purkinje Cells and Abnormal Motor Behaviour in Cxcr4 Null Mice. PloS one. 2014;9:e86471. doi: 10.1371/journal.pone.0086471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaerve A, Muller HW. Chemokines in CNS injury and repair. Cell and tissue research. 2012;349:229–248. doi: 10.1007/s00441-012-1427-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hensch TK. Germline recombination by conditional gene targeting with Parvalbumin-Cre lines. Frontiers in neural circuits. 2013;7:168. doi: 10.3389/fncir.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Jegarl A, Clem RL. Mice lacking TrkB in parvalbumin-positive cells exhibit sexually dimorphic behavioral phenotypes. Behavioural brain research. 2014;274:219–225. doi: 10.1016/j.bbr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18601–18606. doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithal DS, Banisadr G, Miller RJ. CXCL12 signaling in the development of the nervous system. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:820–834. doi: 10.1007/s11481-011-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. The Journal of experimental medicine. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura J, Jaaro-Peled H, Lewis E, Nunez-Abades P, Huppe-Gourgues F, Cash-Padgett T, Emiliani F, Kondo MA, Furuya A, Landek-Salgado MA, Ayhan Y, Kamiya A, Takumi T, Huganir R, Pletnikov M, O'Donnell P, Sawa A. Role for neonatal D-serine signaling: prevention of physiological and behavioral deficits in adult Pick1 knockout mice. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Shahani N, Seshadri S, Jaaro-Peled H, Ishizuka K, Hirota-Tsuyada Y, Wang Q, Koga M, Sedlak TW, Korth C, Brandon NJ, Kamiya A, Subramaniam S, Tomoda T, Sawa A. DISC1 regulates trafficking and processing of APP and Abeta generation. Molecular psychiatry. 2015;20:874–879. doi: 10.1038/mp.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. Journal of neurochemistry. 2007;102:1175–1183. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Hollt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. The Journal of comparative neurology. 2007;502:382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toritsuka M, Kimoto S, Muraki K, Landek-Salgado MA, Yoshida A, Yamamoto N, Horiuchi Y, Hiyama H, Tajinda K, Keni N, Illingworth E, Iwamoto T, Kishimoto T, Sawa A, Tanigaki K. Deficits in microRNA-mediated Cxcr4/Cxcl12 signaling in neurodevelopmental deficits in a 22q11 deletion syndrome mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17552–17557. doi: 10.1073/pnas.1312661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, Rubenstein JL. Dlx5 and Dlx6 regulate the development of parvalbuminexpressing cortical interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Chang CC, Marx CE, Caron MG, Wetsel WC, Zhang X. Pregnenolone rescues schizophrenia-like behavior in dopamine transporter knockout mice. PloS one. 2012;7:e51455. doi: 10.1371/journal.pone.0051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Flandin P, Long JE, Cuesta MD, Westphal H, Rubenstein JL. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. The Journal of comparative neurology. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubovsky S, Oh EC, Cash-Padgett T, Johnson AW, Hou Z, Mori S, Gallagher M, Katsanis N, Sawa A, Jaaro-Peled H. Neuroanatomical and behavioral deficits in mice haploinsufficient for Pericentriolar material 1 (Pcm1) Neuroscience research. 2015 doi: 10.1016/j.neures.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]