Abstract
Inhibition of BACE1 is being pursued as a therapeutic target to treat patients suffering from Alzheimer’s disease because BACE1 is the sole β-secretase that generates β-amyloid peptide. Knowledge regarding other cellular functions of BACE1 is therefore critical for the safe use of BACE1 inhibitors in human patients. Neuregulin-1 (Nrg1) is a BACE1 substrate and BACE1 cleavage of Nrg1 is critical for signaling functions in myelination, remyelination, synaptic plasticity, normal psychiatric behaviors and maintenance of muscle spindles. This review summarizes the most recent discoveries associated with BACE1-dependent Nrg1 signaling in these areas. This body of knowledge will help to provide guidance for preventing unwanted Nrg1-based side effects following BACE1 inhibition in humans.
Keywords: BACE1, Alzheimer’s β-secretase, neuregulin-1, myelination, myelin sheath, remyelination, schizophrenia, muscle spindle
Graphical Abstract
To initiate its signaling cascade, membrane anchored Neuregulin (Nrg), mainly type I and III β1 Nrg1 isoforms and Nrg3, requires ectodomain shedding. BACE1 is one of such indispensable sheddases to release functional Nrg signaling fragment. The dependence of Neuregulin on the cleavage by BACE1 is best manifested by disrupting the critical role of Nrg in the control of axonal myelination, schizophrenic behaviors as well as the formation and maintenance of muscle spindles.
INTRODUCTION
BACE, known as β-site amyloid precursor protein (APP) cleaving enzyme (Yan and Vassar 2014), is a membrane-anchored aspartyl protease required for the generation of β–amyloid peptides (Aβ) (Vassar et al. 1999;Yan et al. 1999; Hussain et al. 1999; Sinha et al. 1999; Lin et al. 2000). In brains of patients with Alzheimer’s disease (AD), abnormally accumulated Aβ tends to oligomerize, aggregate and induce synaptic dysfunction and memory loss (Price et al. 2014;Tu et al. 2014; Haass and Selkoe 2007; Walsh et al. 2002; Wang et al. 2013; Gong et al. 2003). Aβ refers to peptide mixtures comprised of variants 38 to 43 amino acids long, but 40 and 42 (Aβ40 and Aβ42) are the most common. Genetic mutations in presenilin-1 and −2 favor the production of Aβ42 (Borchelt et al. 1996; Citron et al. 1997; Duff et al. 1996; Tomita et al. 1997; Li et al. 2014; De et al. 2010), which is more frequently linked to AD pathogenesis. For example, Aβ42, but not Aβ40, has been found to induce tau pathogenesis (Gotz et al. 2001; Hu et al. 2014; Blurton-Jones and LaFerla 2006; Rank et al. 2002; Liraz et al. 2013), which is another major pathological feature in AD brains. Mice completely deficient of BACE1 show abolished Aβ production (Cai et al. 2001; Luo et al. 2001; Roberds et al. 2001), confirming that BACE1 is a critical and indispensable protease for Aβ release. Since BACE1 is critical for Aβ generation, inhibition of BACE1 should reduce levels of Aβ, particularly the more toxic Aβ42, which could benefit AD patients.
However, BACE1 cleaves other cellular substrates in addition to APP. Other BACE1 substrates that have been identified and characterized include neuregulin-1 (Nrg1) (Willem et al. 2006; Fleck et al. 2013; Hu et al. 2006; Hu et al. 2008; Luo et al. 2011), voltage-gated channel proteins such as sodium channel protein β-subunits (Kim et al. 2007; Kim et al. 2011; Wong et al. 2005), the potassium channel proteins KCNE1 and 2 (Sachse et al. 2013; Hitt et al. 2010), neural cell adhesion molecule close homolog of L1 (Zhou et al. 2012; Hitt et al. 2012; Kuhn et al. 2012), the Notch ligands Jagged-1 and Jagged-2 (He et al. 2014; Hu et al. 2013a), and contactin-2 (Gautam et al. 2014). BACE1 is more profoundly expressed in neurons than in non-neuronal cells (Vassar and Zheng 2014) and abolished processing of these substrates in BACE1-null mice is linked to changes in various brain functions.
Among these identified BACE1 substrates, Nrg1 receives considerable attention because Nrg1 is indispensable for neural and cardiac development (Meyer and Birchmeier 1995; Kramer et al. 1996). Additional studies have recently reported a functional link between BACE1 and Nrg1, which form the basis for this review. We will summarize recent findings associated with BACE1-dependent Nrg signaling in neural functions and in various stages of neural development.
Neuregulin signal transduction
I) Neuregulin genes
Neuregulin (Nrg) genes are typically recognized by the presence of exons coding for the epidermal growth factor-like (EGF-like) domain (Holmes et al. 1992; Chang et al. 1997). The Nrg gene family has four members (Nrg1 to Nrg4) in mammals, with more distant orthologues found in the Xenopus laevis and Drosophila melanogaster genomes and even in the emerging vertebrate lineage of the sea lamprey Petromyzon marinus (Libants et al. 2009; Yarnitzky et al. 1997; Yang et al. 1998; Marchionni 2014). Each gene is often expressed by specifically controlled transcription and splicing, which typically produces many mRNA and protein isoforms.
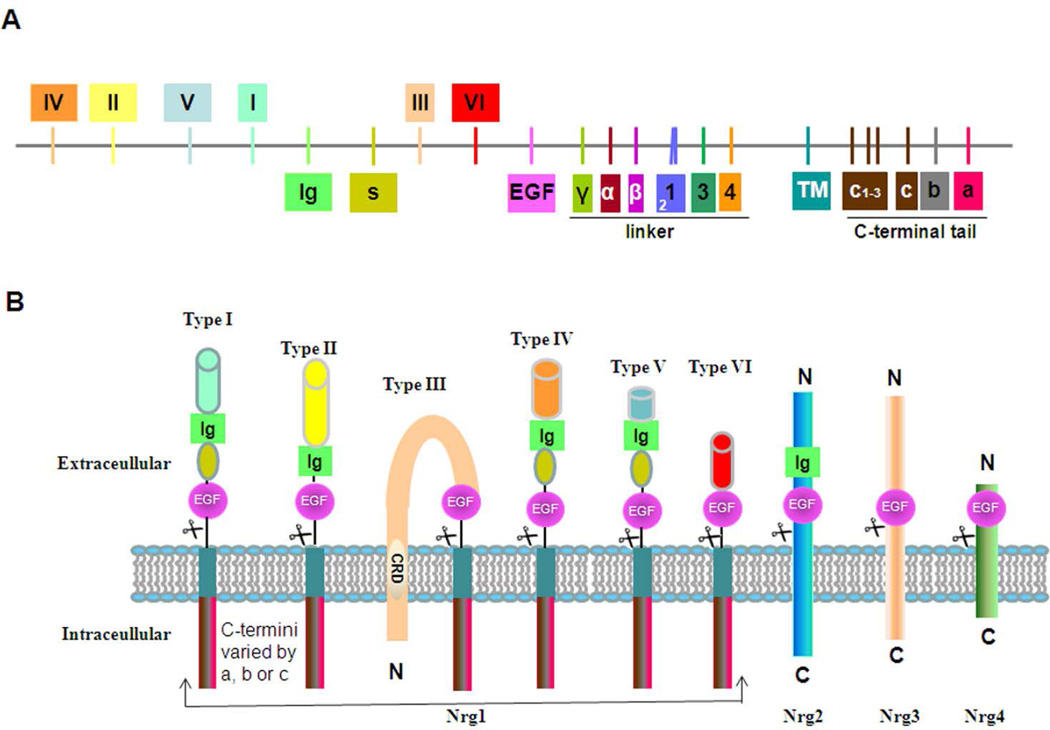
The Nrg1 gene is one of the largest genes in the human genome and its expression is the most complicated in the Nrg family. A single human Nrg1 gene (~1.5 Mb) produces 33 spliced isoforms due to specific uses of six different transcriptional initiation sites as well as multiple splicing isoforms (Brown et al. 2004; Steinthorsdottir et al. 2004; Liu et al. 2011; Tan et al. 2007; Mei and Xiong 2008). As depicted in Figure 1A, six types of Nrg1 are defined by their differences at the N-terminal end. All six isoforms have an EGF-like domain, but only types I, II, IV, and V isoforms have an additional Ig domain. Exons coding for the linker region between the EGF-like domain and the transmembrane domain are often used to distinguish specific Nrg1 isoforms. The Nrg1 γ-isoforms have a stop codon preceding the transmembrane domain and are synthesized as secreted forms. Most Nrg1 α and β isoforms, except for the β3 variant, are synthesized as membrane-anchored molecules (Mei and Xiong 2008). Type III Nrg1 has a cycteine-rich domain (CRD), which is also intercalated into the lipid bilayer (Figure 1B).
Figure 1. Neuregulin-1 isoforms and membrane anchoring.
(A) Schematic illustration of the genetic structure of neuregulin-1 (Nrg1). The Nrg1 gene has 33 exons; each bar represents one or more exons coding for the major structural domain. Exons coding for six different types of Nrg1 are specified as I-VI. Sequences in the space region(s) contain glycosylation sites. Exons coding for the linker region between the EGF-like and transmembrane domains are often alternatively spliced. Each Nrg1 variant can be identified by the presence of exons coding for α, β, or γ-specific sequences. For example, β1 Nrg1 contains both exons for β- and 1-specific sequences. The exon for 2-specific sequence lacks the sequence of KHLGIEFME. The c1, c2 and c3 exons are shared by all membrane-anchored Nrg1 in the C-terminal tail, but vary by either stopping in c or being spliced to include either exon a or b. The γ- and c-exon have an in-frame stop codon; the Nrg1 γ-isoforms are naturally secreted due to this organization. (B) Nrg1 is classified as having six different forms, which differ mainly in their N-terminal part. All six isoforms have an EGF-like domain, but only types I, II, IV, and V contain the Ig domain. The unique N-terminal region of type III Nrg1 has a cysteine rich domain (CRD), which is expected to embed this region in the lipid bilayer. Hence, ectodomain shedding of Nrg1 will only have an EGF-domain containing the type III Nrg1 N-terminal side tethered on the membrane. Although not depicted, Nrg3 has a hydrophobic stretch in the N-terminal domain (residue 61 to 91), and is potentially adopt a membrane topology similar to type III β1 Nrg1.
While Nrg1 isoforms are pleiotropically expressed in various mammalian tissues, including the brain and heart, certain Nrg1 isoforms are expressed under the control of lineage-or development-specific signaling factors (Meyer et al. 1997; Canoll et al. 1996; Kerber et al. 2003). In human brains, all six types of Nrg1 are detectable, but the abundance of each form varies significantly (Liu et al. 2011). By comparing levels of unique mRNA, it has been shown that type III Nrg1 is predominant among all Nrg1 isoforms (accounting for 59–73%, dependent on the age group), while type I Nrg1 is only about 4% in all age groups. Although weakly expressed (less than 1%), type IV is predominantly brain-specific. Within the brain, types I, II, and III are mainly expressed by neurons; astrocytes also express type I, but not types IV, V, or VI Nrg1.
Only in recent years have the discriminative roles of other Nrg family members begun to receive attention. Nrg2 gene produces at least ten transcripts due to alternative splicing and has two different translational initiation sites. It is expressed in the developing nervous system, with the highest levels in granule cells and Purkinje cells of the cerebellum (Carraway, III et al. 1997; Chang et al. 1997), and it is often targeted to dendrites (Longart et al. 2004). Nrg2 is also expressed by motor neurons and Schwann cells (Rimer et al. 2004). Although less complicated compared to Nrg1, the Nrg3 gene still has two alternative ATG start sites and at least four isoforms (Zhang et al. 1997; Howard et al. 2005). Nrg3 is specifically expressed in the human embryonic central nervous system and is required for the survival of oligodendrocytes (Carteron et al. 2006). Nrg4 is the smallest Nrg member with only 115 amino acids, but it has five spliced isoforms (Harari et al. 1999; Hayes and Gullick 2008). Only two Nrg4 isoforms have the transmembrane domain encoded by exon 6. Expression of Nrg4 appears to be more restricted, mainly to pancreas and muscle, and is required for epithelial cell survival (Bernard et al. 2012; McElroy et al. 2014; Hayes et al. 2011). If not synthesized as secreted isoforms, Nrg2, Nrg3, and Nrg4 have a single pass type I membrane domain and all contain EGF-like domains (Figure 1B).
II) ErbB receptors
The discovery of Nrg genes originated from the search for specific ligands that bind to and activate ErbB2 receptors (Wen et al. 1992; Holmes et al. 1992; Falls et al. 1993; Marchionni et al. 1993). The ErbB family comprises four transmembrane tyrosine kinase receptors: ErbB1 (also called Epidermal Growth Factor Receptor, EGFR), ErbB2, ErbB3, and ErbB4. All ErbB proteins have a type I transmembrane topology flanked by a unique N-terminal ligand-binding domain and a C-terminal intracellular domain, which are variable in length and contain either a functional or pseudo tyrosine kinase. Nrg and its cognate receptor have a specific binding preference by forming a functional binding partnership. Nrg1 and Nrg2 bind to ErbB3 and ErbB4, while Nrg3 and Nrg4 preferentially bind to ErbB4 only (Mei and Nave 2014; Hynes and Lane 2005; Yarden and Sliwkowski 2001). Nrg molecules usually do not bind ErbB1, which preferentially binds EGF and is also known as EGFR (Schlessinger 2002). Ligand binding induces dimerization of its cognate receptor. Interestingly, ErbB2 lacks the ligand binding pocket, while ErbB3 has only a pseudo-kinase domain; both require heterodimerization to be a functional unit. ErbB2 often forms a dimer with ErbB3 in many cell types.
III) Nrg-ErbB signal transduction
Upon ligand binding, ErbB intracellular receptor tyrosine kinase is intrinsically activated in response to dimerized structural changes (Weiss and Schlessinger 1998; Roskoski, Jr. 2014). The main activated signaling downstream molecules are the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)–AKT pathways; PLCγ and Stat proteins are recent additions (Britsch 2007; Roskoski, Jr. 2014; Carpenter 2003; Hynes and Lane 2005; Lai and Feng 2004; Yarden and Sliwkowski 2001). Each receptor has multiple intracellular phosphorylated sites, which dictate characteristic preferences for interacting with adaptor proteins. Multiple autophosphorylation sites have been identified in ErbB2 and mediate binding of adaptor molecules such as Grb2, Shc, Doc-R, and CRK for their unique signaling pathways, i.e., the MAPK pathway (Dankort et al. 2001b; Dankort et al. 2001a; Suenaga et al. 2003). Six phosphorylation sites in ErbB3 are normally transactivated, i.e., by the ErbB2 intrinsic kinase, and can be coupled to the p85 adaptor subunit of PI3K to activate the PI3K-AKT pathway. System-wide searches identified 19 potential Tyr sites in ErbB4, which can differentially mediate activation of PI3K-AKT, MAPK, and STAT1 signaling (Kaushansky et al. 2008; Junttila et al. 2000; Kainulainen et al. 2000; Elenius et al. 1999).
Proteolytic cleavage of neuregulin
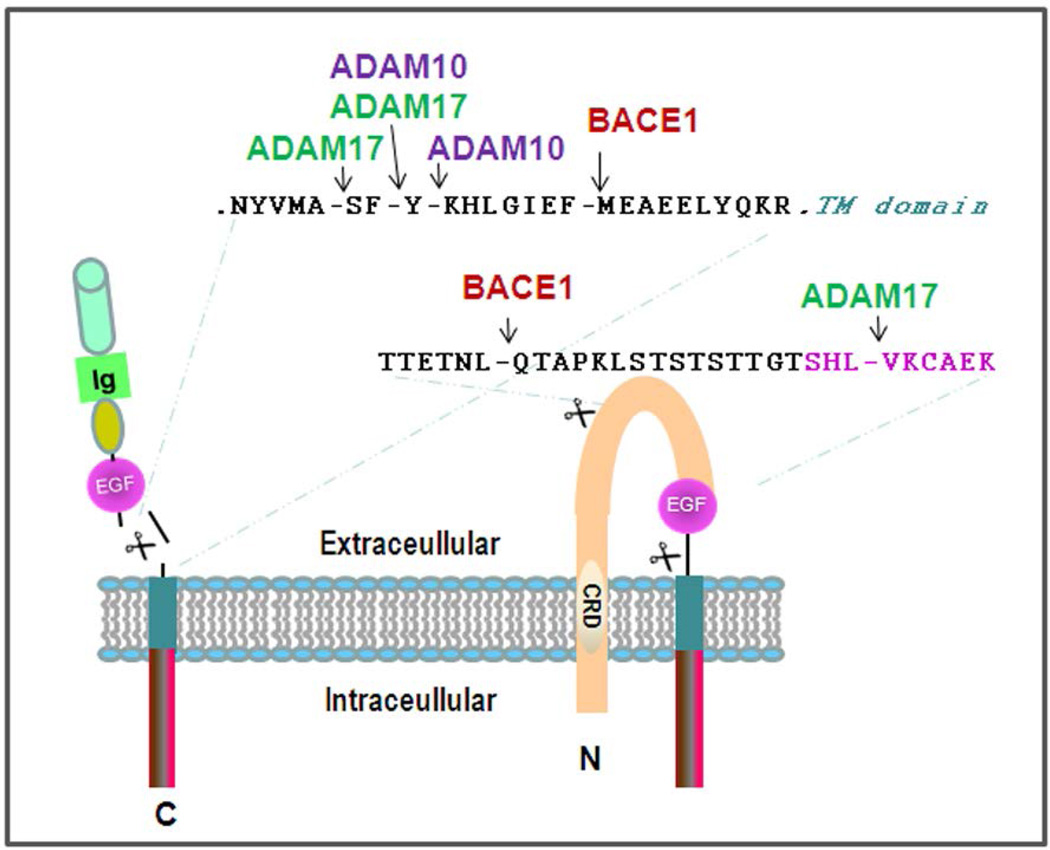
Unless synthesized as functional secreted isoforms, Nrg molecules anchored on the lipid bilayer require proteolytic shedding to release EGF domain-containing fragments for binding to their receptors. Proteolytic cleavages of Nrg1 have been more extensively investigated than other Nrg molecules. Type I Nrg1 can be effectively cleaved at multiple sites by disintegrin and metalloproteinase 10 (ADAM10) and ADAM17 (Figure 2). In vitro MOLDI-TOF mass spectrometric mapping of the cleaved products showed that both ADAM10 and ADAM17 cleave β1 Nrg1 between the F-Y site, 18 residues before the membrane domain (La et al. 2011; Luo et al. 2011; Fleck et al. 2013). ADAM17 can also cleave the neighboring A-S site, while ADAM10 may additionally cleave the Y-K site in cells (Fleck et al. 2013). BACE1 specifically cleaves β1 Nrg1 isoforms at the F-M site (Hu et al. 2008; La et al. 2011; Fleck et al. 2013). Upon cleavage of Nrg1 by either enzyme, the N-terminal of Nrg1 is released for binding to its receptor. The sequence signature (MASFYKHLGIEFMEAEELYQKR) comprising both ADAM and BACE1 cleavage sites is highly conserved among β1 isoforms in different species (human, rodent, dog, and chicken). Although almost all BACE1 substrates are also cleaved by ADAMs (Yan and Vassar 2014), the BACE1 cleavage site is downstream of the ADAM cleavage site, which is unique to Nrg1. This order may competitively favor BACE1 to cleave Nrg1 in early secretory compartments in cells, as ADAM10 and ADAM17 are more enriched at the cell surface (Primakoff and Myles 2000; Deuss et al. 2008).
Figure 2. Cleavage of Nrg1 by BACE1 and ADAMs.
The characterized cleavages of Nrg1 β1 isoforms are illustrated. The β1-containing exons coding for sequences are cleavable by ADAM10, ADAM17, and BACE1. Compared to the β1-isoform, the β2-isoform only lacks the sequence of KHLGIEFME, and is not cleavable by BACE1. The sequences as shown in type III Nrg1 isoforms can also be specifically cleaved by BACE1 and ADAM17, respectively. The EGF-like domain is presumably released after a single enzyme cleavage, such as BACE1 or ADAM17. Letters in pink are residues in the EGF-like domain.
After ectodomain shedding, type I Nrg1 releases its N-terminal fragment (Nrg1-ntf) to the extracellular space, where it binds to ErbB receptors on nearby cells in a paracrine fashion, while type III Nrg1-ntf, which remains tethered on the lipid bilayer due to a hydrophobic CRD in its N-terminus, signals to adjacent cells in a juxtacrine fashion (Warren et al. 2006). A recent study suggested that type III Nrg1 may also mediate autocrine or paracrine signaling due to newly identified cleavage sites preceding the EGF-like domain (Figure 2). It shows that BACE1 cleaves an additional 16 residue site between L-Q, located before the EGF-like domain (Fleck et al. 2013). Enhanced expression of BACE1 facilitates this cleavage. ADAM17 also cleaves type III Nrg1 between L-V, at the N-terminal end of the EGF-like domain. A single cleavage of type III β1 Nrg1 by either BACE1 or ADAM17 may release the EGF-like domain for initiating signaling function (Fleck et al. 2013).
While other ADAMs, including ADAM19, have been suggested to cleave membrane-anchored Nrg1 isoforms, these cleavage sites have not yet been identified (Montero et al. 2000; Kalinowski et al. 2010; Yokozeki et al. 2007; Shirakabe et al. 2001). Type I transmembrane Nrg2, Nrg3, and Nrg4 are also shedded for exerting their signaling activities under specific regulatory conditions. The broad-spectrum matrix metalloproteinase inhibitor, galardin (GM 6001), is capable of inhibiting Nrg4 cleavage (Hayes et al. 2008), although the details remain to be determined. A stretch of eleven residues spanning the BACE1 cleavage site, but not the ADAM cleavage site, is identical between Nrg1 and Nrg3; altered Nrg3 cleavage has been detected in BACE1-null mouse brains (Hu et al. 2008). Since the Nrg3/ErbB4 pathway has been found to regulate the survival of oligodendrocytes in cell cultures (Carteron et al. 2006), this pathway is likely to complement the function of Nrg1 in myelination upon genetic deletion of Nrg1. Amino acid sequences spanning the transmembrane domain of Nrg2 have high homology to those in Nrg1, but lack the canonical BACE1 cleavage site. It is not clear whether Nrg2 and Nrg4 are also physiological BACE1 substrates.
Functional changes via BACE1-dependent Nrg1 signaling
As outlined above, BACE1 mainly cleaves types I and III Nrg1 as well as Nrg3. BACE1 is richly expressed by neurons and its functions in brain development and diseases have recently gained attention (Vassar et al. 2014). Although Nrg1 is known to play roles in both cardiac and neural development, this review will focus on the recent findings related to common functional changes arising from BACE1-dependent Nrg1 signaling in the nervous system.
I) BACE1 and Nrg1 in myelination
In the vertebrate nervous system, most axons are wrapped by myelin membranes, which are specialized, spirally organized plasma membranes synthesized by oligodendrocytes in the central nervous system (CNS) and by Schwann cells in the peripheral nervous system (PNS) (Sherman and Brophy 2005). The protein composition of myelin in the CNS and PNS is significantly different, despite performing the same insulating function. Proteolipid protein (PLP) is the major structural protein of CNS myelin, while P0 protein is the major structural protein of PNS myelin (Tuohy 1994;Lemke and Axel 1985). Myelin basic protein (MBP) and myelin associated glycoprotein (MAG) are present in both CNS and PNS myelin (Boggs 2006; Schachner and Bartsch 2000), but at different levels. A properly myelinated nerve efficiently transmits the electrical impulse by saltatory conduction, i.e. it “jumps” from node to node to ensure rapid axonal conduction.
Two of the well-identified phenotypes in BACE1-null mice are defects in axonal myelination and remyelination in the PNS and CNS. Immunohistochemical staining readily detects a significant reduction of myelin in BACE1-null sciatic nerves. Further electron microscopic analyses confirmed a marked reduction of myelin sheath thickness in BACE1-null mouse sciatic nerves (Hu et al. 2006; Willem et al. 2006). In BACE1-null zebrafish, anterior and posterior lateral line axons, typically ensheathed by Schwann cells, are also hypomyelinated (van et al. 2013). Consequently, abnormal PNS myelination causes impairments of neurological functions in BACE1-null mice, such as reduced grip strength (Hu et al. 2006).
Impaired myelination within the BACE1-null PNS resembles that in mice with type III Nrg1 haploinsufficiency (Nave and Salzer 2006). Mice heterozygous for type III Nrg1 exhibit hypomyelination and reduced nerve conduction velocity in their sciatic nerves (Taveggia et al. 2005; Michailov et al. 2004). This function is evolutionarily conserved, as knocking down type III Nrg1 in zebrafish results in a severe impairment in myelination of anterior and posterior lateral line axons (van et al. 2013). The identification of Nrg1 as a BACE1 substrate has led to molecular insights into BACE1-dependent hypomyelination. In BACE1-null mice, the abrogated cleavage of axonal Nrg1 increases full-length Nrg1 by reducing levels of axonal-anchored type III Nrg1 (Hu et al. 2006; Hu et al. 2008; Willem et al. 2006). This will reduce the transmission of Nrg-ErbB signaling by decreasing the active form of Akt (phosphorylated Akt) and phosphorylated Erk.
Although Nrg1 is also cleaved by ADAM10 and ADAM17, pan-inhibition of ADAM by GM6001 or treatment with ADAM10 siRNA in an in vitro co-culture myelination system shows minimal effects on normal myelination (Luo et al. 2011). Intriguingly, knockdown of ADAM17 in dorsal root ganglia neurons induces hypermyelination in co-cultures and rescues hypomyelination in type III NRG1 heterozygous mice (La et al. 2011), suggesting that ADAM17 is a negative regulator of myelination in the PNS. This effect is likely due to cleavage of Nrg1 within the EGF domain by ADAM17, but this cleavage was not observed with different biochemical mapping (Fleck et al. 2013). Instead, Fleck et al. have argued that either BACE1 or ADAM17 cleaves Nrg1 flanking the EGF-like domain by inducing paracrine Nrg-ErbB signaling (see cleavages in Figure 2). Despite this discrepancy, specific inhibition of BACE1 has been consistently shown to impair axonal myelination (Luo et al. 2011; La et al. 2011), further emphasizing the importance of BACE1-dependent Nrg1 signaling in myelination.
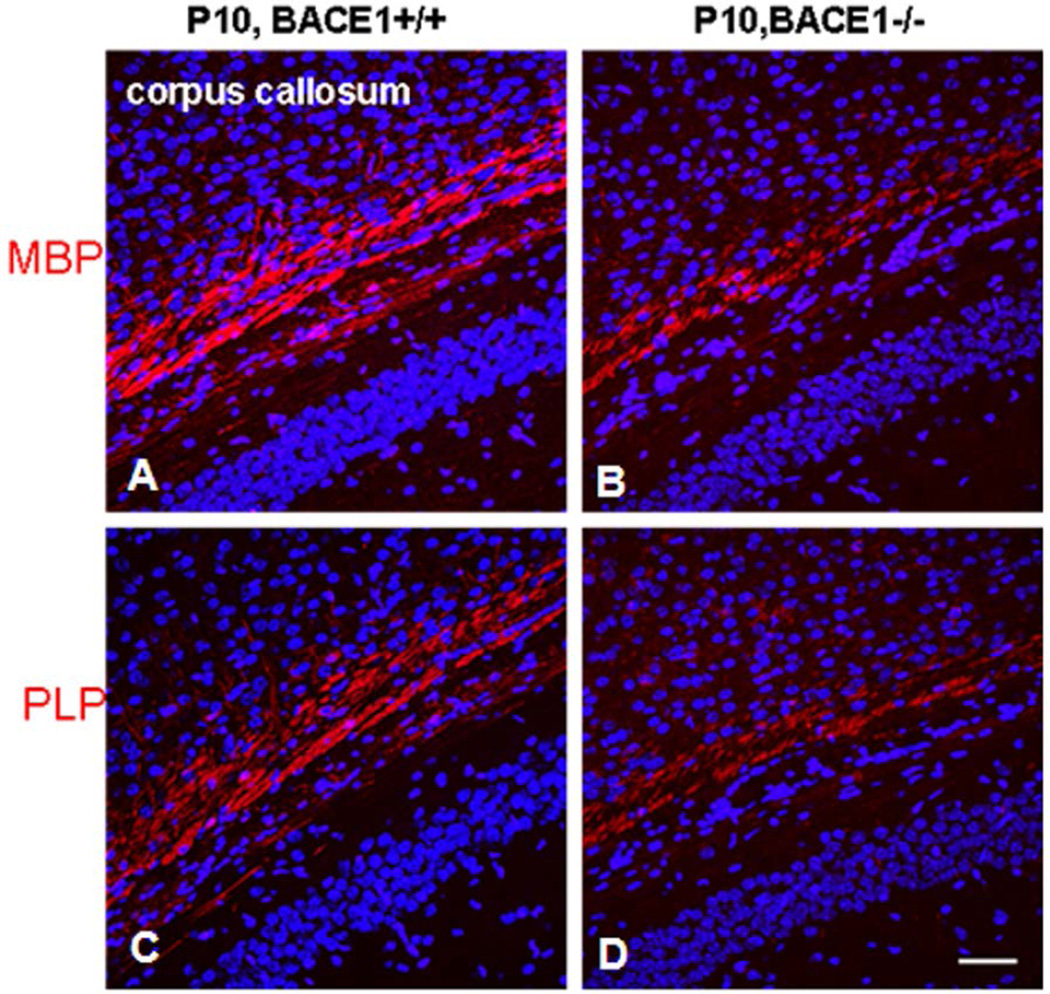
While Nrg1, mainly type III Nrg1, is indispensable for developmental PNS myelination, the effects of BACE1-dependent Nrg1 on developmental CNS myelination is more convoluted. Hypomyelination has been repeatedly observed in BACE1-null optic nerves (Hu et al. 2006), but was not detected in broad brain regions of BACE1-null mice by others (Treiber et al. 2012). In zebrafish, oligodendrocytes and Schwann cells can be highlighted by membrane-bound green fluorescent protein (GFP) under the control of the myelin specific claudin k promoter (Munzel et al. 2012). Mauthner axons are typically myelinated by zebrafish oligodendrocytes and GFP-marked myelin in the Mauthner axons of bace1−/−; claudin k:GFP appears indistinguishable from claudin k:GFP larvae, indicating no obvious effect on CNS myelin in BACE1-null zebrafish (van et al. 2013). Despite this difference in various models, reduced levels of compact MBP, PLP, and MOG were evident in the young BACE1-null mouse hippocampus (Hu et al. 2006). Such a reduction of myelin proteins is visible in hippocampal sections stained by MBP or PLP antibody (Hu et al. 2006) and is more evident during early developmental stages (Figure 3). The eventual normal levels of myelination in this brain region suggest a compensatory mechanism that remains to be determined. Although no obvious changes in myelin sheath thickness in normal adult BACE1-null white matter have been observed, this is remarkably not the case during remyelination. In a cuprizone-induced demyelination model, reduction of myelin sheath thickness was observed in remyelinated axons within the BACE1-null mouse corpus callosum (Treiber et al. 2012).
Figure 3. Delayed myelination of axons in corpus callosum of BACE1-null brains.
Postnatal day 10 (P10) brain sections were fixed for immunohistochemistry with antibody to MBP (A–B) or PLP (C–D). Significantly delayed myelination in the corpus callosum of BACE1-null brain sections was evident with both antibodies, suggesting delayed myelination due to deletion of the BACE1 gene in mice.
One study suggests that type III Nrg1 is a more restricted instructive signal in promoting oligodendrocyte myelination, as mice heterozygous for type III Nrg1 are hypomyelinated only in the forebrain (Taveggia et al. 2008). However, a separate study, using a conditional deletion of the Nrg1 gene in neurons or ErbB3/ErbB4 genes in oligodendrocytes at different time points during development, showed normal myelinated fibers in the grey matter and spinal cord (Brinkmann et al. 2008). Knocking down type III Nrg1 in zebrafish also resulted in normal myelination in the central nerve (van et al. 2013). While the loss-of-Nrg1 function in CNS myelination in animals is controversial, it is no doubt that the enhanced Nrg-ErbB signaling pathway regulates CNS myelination for the following observations. First, in cell cultures, Nrg1 is a mitogen to pro-oligodendrocytes by facilitating lineage development and enhancing survival of oligodendrocytes (Flores et al. 2000; Vartanian et al. 1997; Fernandez et al. 2000; Canoll et al. 1996). In rat optic nerves, injected Nrg1 significantly increases the number of survival oligodendrocytes in the nerve (Fernandez et al. 2000), indicating an important role of Nrg1 in maintaining functional oligodendrocytes. Second, overexpression of either type I or type III Nrg1 has been shown to promote CNS myelination in animals (Taveggia et al. 2008; Brinkmann et al. 2008). Third, disrupting ErbB function by expressing the dominant negative form of ErbB4 induces hypomyelination (Roy et al. 2007). Fourth, Nrg3, another substrate of BACE1 (Hu et al. 2008), is broadly expressed in neurons, is able to activate Akt similar to Nrg1, and acts as a survival factor for oligodendrocytes in cell cultures (Carteron et al. 2006). Nrg3 may potentially compensate for the loss of Nrg1 considering its binding to ErbB receptor like Nrg1. Finally, gain-of-function studies support a role for Akt, a downstream molecule of the Nrg-1/ErbB pathway, in enhancing CNS myelination. Mice overexpressing Akt-DD for gaining Akt function under the control of PLP promoter develop hypermyelination of the central axons (Flores et al. 2008; Barros et al. 2009). Augmenting Akt activity by targeting its downstream mTOR function or inhibiting PTEN by disruption of Pten in Schwann cells also increases hypermyelination (Zou et al. 2014; Narayanan et al. 2009; Lebrun-Julien et al. 2014; Goebbels et al. 2012). Hence, properly enhancing PI3K-Akt signaling function can facilitate myelination in the CNS (Macklin 2010). Consistent with this, BACE1-null mice overexpressing constitutively active Akt (Akt-DD; mutations with D308T and D473S) in oligodendrocytes show reversal of hypomyelination of optic nerves (Hu et al. 2013b). The effect of Akt in oligodendrocytes is also more critical for CNS myelination. Hence, it is likely that BACE1-dependent Nrg signaling can have spatial and temporal effects on CNS myelination.
II) BACE1 and Nrg1 in remyelination
Genetic deletion of BACE1 also affects sciatic nerve remyelination, as demonstrated in the adult sciatic nerve crush model (Hu et al. 2008). When a nerve is severely injured in this manner, the segment proximal to the crush site is subjected to axonal degeneration due to Wallerian degeneration; during recovery, remyelination is initiated by Schwann cells contacting the regenerating axons in the proximal band of Büngner. Myelin sheath thickness remains thinner in BACE1-null regenerated axons and levels of the major myelin proteins P0 and MBP are significantly lower in the distal stump of BACE1-null sciatic nerve (Hu et al. 2008), indicating that BACE1 deficiency also impairs remyelination in peripheral nerves. BACE1 also appears to be required for optimal remyelination of corpus callosum axons, which can be demyelinated by cuprizone treatment (Treiber et al. 2012).
Impaired remyelination can stem from the loss of Nrg1 cleavage by BACE1, as axonal deletion of Nrg1 disrupts remyelination of sciatic nerves in adult mice (Fricker et al. 2011). Peripheral nerve remyelination is also more efficient in transgenic mice overexpressing type III Nrg1. Although it has been suggested that type I Nrg1 has no effect on developmental myelination, transgenic mice overexpressing type I Nrg1 exhibit more efficient remyelination of injured sciatic nerves (Stassart et al. 2013). Moreover, nerve injury triggers expression of type I Nrg1 by Schwann cells, which is normally undetectable in intact nerves (Stassart et al. 2013). Intriguingly, in mice with a conditional deficiency of Nrg1, remyelination is reduced at early stages (up to 2 months post-nerve injury), but not at later recovery stages (Fricker et al. 2013). This late normalization is likely due to compensation by other growth factors. Overall, Schwann cell-derived Nrg1 appears to be dispensable for developmental myelination and myelin maintenance, but exerts an autocrine signaling function for remyelination, as loss of Nrg1 expression in Schwann cells severely impairs remyelination after nerve crush.
We have recently shown that BACE1 in Schwann cells is equally important to axonal BACE1 for remyelination (Hu et al. 2015). In a nerve transplantation experiment, axons in the transplanted sciatic nerve are degenerated, similar to the distal side in the nerve crush model. However, Schwann cells in the transplanted sciatic nerve remain and will form a “Schwann tube”, which can remyelinate the regenerated axons from the recipient proximal segment. We show that in the case of either a BACE1-null nerve segment transplanted into a WT recipient or a WT nerve segment transplanted into a BACE1-null recipient, remyelination is equally impaired, as the increase in g-ratio is almost identical (Hu et al. 2015). Nerve injury not only induces expression of BACE1, but also type I Nrg1 by Schwann cells. Expression of type III Nrg1 in Schwann cells is barely detected, in line with the prior observation that conditional deletion of type I Nrg1, but not type III Nrg1, in Schwann cells is required for optimal remyelination. Hence, axonal and Schwann cell BACE1-dependent Nrg1 signaling differentially contribute to developmental myelination and remyelination of regenerated axons.
III) BACE1 and Nrg1 in schizophrenia
Schizophrenia (SCZ) is a severe chronic neuropsychiatric disorder affecting approximately 1% of the population worldwide, with the average age of onset being between 19 and 25 years (Nasrallah et al. 2011). Clinically, the symptoms include auditory and visual hallucinations, delusions, social withdrawal, and cognitive dysfunction. Although the etiology of SCZ remains contentious, it is commonly accepted that this disease is a multi-factorial neurodevelopmental disorder influenced by both genetic and environmental factors (Shorter and Miller 2015; Purcell et al. 2009; Glessner and Hakonarson 2009; Insel 2010; Lee et al. 2012; Steinberg et al. 2011; Fromer et al. 2014; Purcell et al. 2014; 2014; Ibi and Gonzalez-Maeso 2015). An association study of SCZ families in Iceland initially revealed Nrg1 to be a SCZ-susceptible gene (Stefansson et al. 2002), and this finding was quickly replicated in various ethnic populations (Williams et al. 2003; Li et al. 2004; Stefansson et al. 2003; Yang et al. 2003). Further analyses of Nrg1 polymorphisms support the linkage of altered Nrg1 function as a risk factor for SCZ (Corvin et al. 2004; Tang et al. 2004; Norton et al. 2006). Initial functional studies suggest that mutations in Nrg1 likely lead to loss-of-function, as mice heterozygous for Nrg1 show SCZ-related behavioral deficits such as impaired reciprocal social interaction behaviors as well as impaired short-term and long-term plasticity in the CA3-CA1 pathway (Stefansson et al. 2002; O'Tuathaigh et al. 2008). Biochemical pathway analysis of this risk effect also indicates acting through the receptor level, as mice with haplo-insufficiency of ErbB4 display SCZ-like behaviors, including impaired pre-pulse inhibition (Stefansson et al. 2002). The first clinical evidence of haploinsufficiency of ErbB4 was found in a patient with early myoclonic encephalopathy and profound psychomotor delay with the ErbB4 gene disrupted due to a de novo reciprocal translocation t(2;6)(q34;p25.3) (Backx et al. 2009). An array CGH analysis identified another case of de novo deletion of the ErbB4 gene in a patient with SCZ behaviors (Kasnauskiene et al. 2013). Functional neuroimaging analysis of SCZ patients together with case controls confirmed that individuals carrying risk genotypes for Nrg1 and ErbB4, or these two together with AKT1, are disproportionately less efficient at dorsolateral prefrontal cortex processing (Nicodemus et al. 2010). This epistatic and functional interaction analysis is consistent with prior findings of decreases in AKT1 protein levels and in levels of phosphorylation of GSK3beta at Ser9 in the peripheral lymphocytes and brains of individuals with SCZ (Emamian et al. 2004). Hence, a body of evidence supports the hypothesis that impaired Nrg1-ErbB-AKT1/GSK3 signaling is a risk factor for SCZ (Emamian 2012), although growing evidence also supports association of gain-of-function in Nrg1 with SCZ to be discussed later.
In 2003, BACE1-null mice were reported to display timid behavior and reduced serotonin and dopamine (DA) levels in the hippocampus and striatum, respectively (Harrison et al. 2003); dopamine and serotonin dysfunctions are the basis for many antipsychotic drugs (typical or atypical) developed to ameliorate SCZ positive and negative symptoms (Strange 2008; Amato 2015; Guidotti et al. 2005). However, the link between BACE1 and SCZ pathogenesis has gained recent attention due to the observed SCZ-like endophenotypes in BACE1-null mice and BACE1 cleavage of Nrg1. Specifically, BACE1-null mice display significantly impaired pre-pulse inhibition, hypersensitivity to glutamatergic psychostimulants, reductions in spine density, and hyperactivity (Savonenko et al. 2008). Behavioral studies of BACE1-null mice have also shown poor performance on spatial and temporal hippocampus-dependent memory tasks (Kobayashi et al. 2008; Laird et al. 2005; Eimer and Vassar 2013). All these defects resemble those seen in Nrg1 mutant mice, phenotypically connecting BACE1 and Nrg1 in the same pathway.
Functionally, Nrg1 regulates signaling via inhibitory gamma-aminobutyric acid (GABA)-mediated inhibitory functions and N-methyl-D-aspartate receptor (NMDAR)-mediated glutamatergic excitatory functions (Fazzari et al. 2010; Lu et al. 2014; Okada and Corfas 2004; Woo et al. 2007; Yin et al. 2014; Ting et al. 2011). While the detailed pathways have been well-summarized in several comprehensive reviews (Corfas et al. 2004; Mei and Xiong 2008; Buonanno et al. 2008; Harrison and Law 2006; Mei and Nave 2014; Banerjee et al. 2010), here we mainly discuss Nrg1 function in the hypofunctional NMDAR-mediated glutamatergic system, a hypothesis increasingly gaining attention in SCZ pathogenesis over the past two decades (Coyle 2012; Cohen et al. 2015; Snyder and Gao 2013; Javitt 2012; Stahl 2007; Vukadinovic 2014). Current publications linking BACE1 to SCZ focus mainly in this system (Savonenko et al. 2008). Although altered function of Nrg2-ErbB4 has been shown to regulate NMDA receptor internalization in GABAergic interneurons (Vullhorst et al. 2015), Nrg2 is not a BACE1 substrate and thus will not be included in this discussion. Although Nrg3 is also BACE1 substrate and Nrg3 polymorphisms, perhaps more clustered in the brain-specific exon b, have high ‘delusion factor’ scores and modulate early attentional processes for perceptual sensitivity and vigilance (Kao et al. 2010; Morar et al. 2011; Chen et al. 2009; Wang et al. 2008), its signaling pathway is similar to Nrg1.
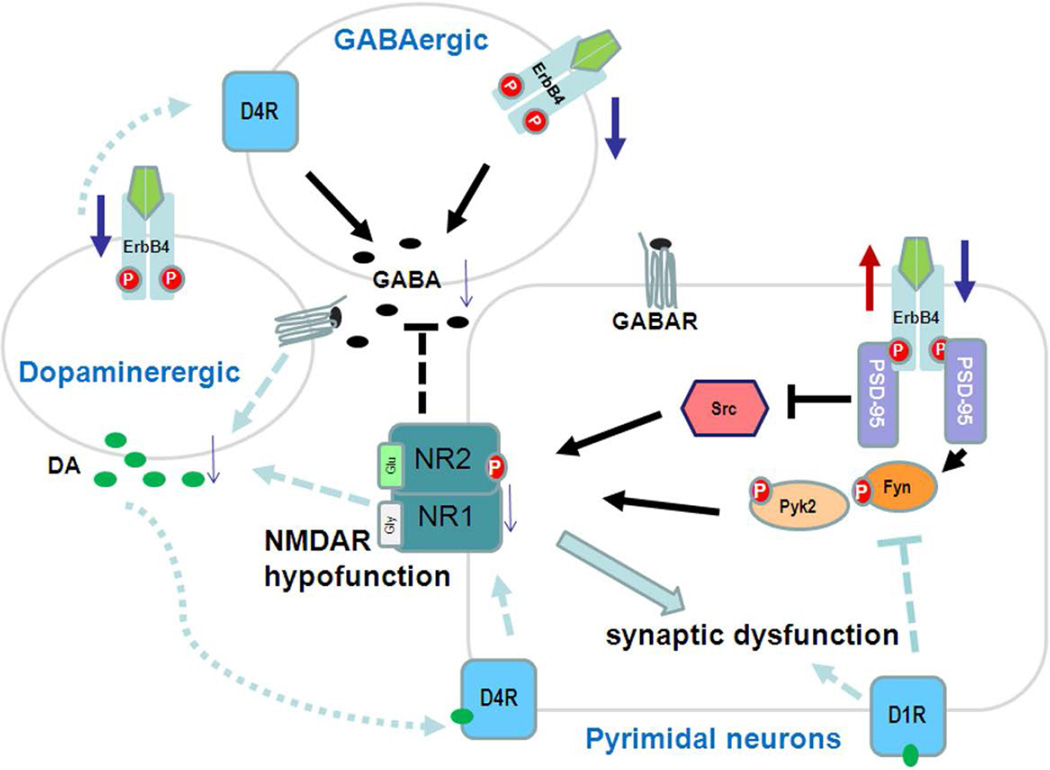
Nrg1 impacts NMDA receptors through multiple mechanisms, such as altered expression and internalization of NMDA receptors (Geddes et al. 2011; Mei and Xiong 2008). (internalization of NMDA or AMPA receptors is artefacts. This cannot be the case, because erbb4 is not expressed in pyramidal neurons as claimed). A binding study using MK-801 in forebrain homogenates from Nrg1 hypomorphs showed reduction of NMDA receptors (Stefansson et al. 2002), supporting an earlier finding that Nrg1β signaling regulates NMDA-mediated excitatory functions by inducing expression of NMDA receptor subunits (Ozaki et al. 1997). Several groups investigated NMDAR expression in postmortem samples from SCZ patients and show reduced NMDA receptor subunit density in the prefrontal cortex, hippocampus, and thalamus (see summary in table 1 by Geddes et al. 2011). Interestingly, both ErbB4 and the NR2 subunit of the NMDA receptor bind to the same adaptor molecule, postsynaptic density protein-95 (PSD-95) (Figure 4), a PDZ domain-containing synaptic scaffold protein (Garcia et al. 2000; Lin et al. 2004; Huang et al. 2000). The interaction between PSD-95 and C-terminal tails of the NR2 subunit increases the number of functional channels at the cell surface and channel opening rate of NMDARs, and this interaction is regulated by phosphorylation of residues within the PDZ-binding domain of NR2 (Chung et al. 2004). The activated ErbB4 receptor, upon Nrg1 binding, is also able to interact with PSD-95 and transduces the downstream signaling cascade. Hence, PSD-95 is clearly the bridging molecule between these two receptors, and impaired binding might contribute to NMDAR hypofunction or altered activities of signaling kinases (Steigerwald et al. 2000; Mori et al. 1998; Rossi et al. 2002). Among various kinases that regulate NMDAR activity, Src/Fyn kinases are particularly important because they are modulated by PKC, Pyk2, and PKA, and thus can serve as a hub of various pathways influencing NMDAR activation (Hahn 2011). ErbB4 has been shown to interact with Fyn, which phosphorylates tyrosine residues on both NR2A and NR2B subunits, affecting channel gating and increasing NMDAR currents (Bjarnadottir et al. 2007; Takasu et al. 2002). Reduced phosphorylation on NR2B Y1472 by Fyn is seen in NRG1+/− mutant mice. Alternatively, overexpressing ErbB4 enhances AMPA synaptic currents and increases dendritic spine size, while reducing Nrg1-ErbB4 activity can destabilize synaptic AMPA receptors, leading to loss of synaptic NMDA currents and dendritic spines (Li et al. 2007). Collectively, these data support the hypothesis that the glutamatergic hypofunction contributing to SCZ is associated with loss-of- function in Nrg1.
Figure 4. The Nrg1-ErbB4 pathway in schizophrenia.
Disrupted functions in dopamine receptor-, GABA receptor-, and NMDAR-mediated neurotransmission are commonly suggested to contribute to schizophrenia etiologies. The ErbB4 receptor is present in pyramidal glutamatergic neurons, GABA-producing interneurons, and dopamine (DA)-producing neurons. Reduced Nrg1 levels decrease ErbB4 signaling in these cells. The consequent reduction in DA transmission will impair synaptic function via the DA-D4R or DA-D1R pathway or decreased stimulation of GABA-producing interneurons. ErbB4 signaling is required for GABA release, which is required for synchronizing synaptic function in glutamergic neurons. Decreased Nrg1-ErbB4 signaling in glutamergic neurons can have a direct impact on NR2 (NR2A and NR2B) phosphorylation via fyn and pyk2 activity, while increased Nrg1 may inhibit src-mediated phosphorylation of NMDARs. PSD-95 has a PDZ domain, which mediates binding of Src/fyn to the ErbB4 receptor.
Conversely, genetic studies also found increased Nrg1 or ErbB4 transcripts and proteins in SCZ patients (Harrison and Law, 2006; Geddes et al., 2011), as well as substantial increases in ErbB4-PSD-95 interactions (Hahn et al. 2006). SCZ-like behaviors are indeed observed in mouse models overexpressing Nrg1 (Yin et al. 2013; Luo et al. 2013; Kato et al. 2010; Agarwal et al. 2014), indicating that balanced Nrg1 function is of critical importance for normal psychiatric behaviors. This notion is strongly supported by studies in two different mouse models utilizing the same tetracycline-inducible promoter system. The Mei lab generated inducible transgenic mice overexpressing full length Nrg1 (Yin et al. 2013), while we developed inducible transgenic mice overexpressing the N-terminal fragment of BACE1-cleaved Nrg1 (Nrg1-ntfβ) (Luo et al. 2013). In both cases, increased transgene expression was found to cause SCZ-like behaviors. After turning off transgene expression, behavioral deficits and synaptic dysfunction in these mice were reversed, indicating that the abnormal functions were due to significant gain-of-function in either full length Nrg1 or Nrg1-ntfβ. In a separate study, transgenic mice with constitutive overexpression of type I full length Nrg1 also exhibited SCZ-like behaviors (Kato et al. 2010). Molecular studies have shown that protein levels of NMDARs are reduced in both transgenic mouse models, which may underlie the observed social and cognitive behavioral impairments. Enhanced Nrg1 reducing NMDARs has been shown to be dependent on functional ErbB receptors (Gu et al. 2005). In an in vitro assay with acutely isolated and cultured prefrontal cortical (PFC) pyramidal neurons, bath perfusion of Nrg1 was found to significantly reduce whole-cell NMDA receptor currents, and this reduction was blocked by application of an ErbB receptor tyrosine kinase inhibitor. The reduced NMDA currents resulting from Nrg1 treatment are attributable to the induced internalization of NR1 subunit of NMRA receptors as demonstrated in cultured PFC neurons, and the membrane-permeable actin stabilizer phalloidin oleate blocks the Nrg1-mediated NR1 internalization (Gu et al. 2005). Nrg1 will not suppress NMDAR currents if NMDAR endocytosis is inhibited by the dynamin inhibitory peptide, suggesting involvement of clathrin/dynamin-dependent endocytosis in Nrg1-induced downregulation of NMDAR currents. Alternatively, others have suggested that Nrg1 is normally required for the physiological upregulation of NMDA receptors in a Src-dependent manner during theta-burst stimulation in the hippocampus and PFC (Pitcher et al. 2011), and enhanced Nrg1 signaling suppresses Src-mediated synaptic NMDAR function (Figure 4). This observation is consistent with the clinical finding of decreases in phosphorylation of Src and Pyk2 in human postmortem brains (Banerjee et al. 2014). Hyperactive ErbB4 is also found in the PFC of patients with SCZ, and reduced tyrosine phosphorylation of NR2A is also observed (Hahn et al. 2006).
Overall, Nrg1-ErbB4 activity can mediate the interaction between synaptic activity and dendritic spines, suggesting that this pathway provides trophic support for glutamatergic functions in development, maturation, and stabilization. Most relevantly, loss of BACE1 activity will reduce Nrg1-ErbB function, which will impair NMDA-mediated synaptic plasticity. Hence, the effect of BACE1 on the pathogenesis of SCZ is likely mainly through impaired Nrg1 signaling. There is no evidence thus far showing that increased BACE1 activity causes SCZ pathogenesis, although abnormally elevated Nrg1 signaling can also contribute to dysfunction of NMDA-mediated pathways.
IV) BACE1 and Nrg1 in muscle spindles
Immuno-electron microscopic analyses in 35 human muscle biopsies, including 5 sporadic inclusion-body myositis (s-IBM), 5 chromosome-9p1-linked quadriceps-sparing inclusion-body myositis (hereditary-IBM), and 25 control muscle biopsies demonstrated that BACE1 is localized in normal adult muscle at the postsynaptic domain of neuromuscular junctions, as well as in cultured human muscle (Vattemi et al. 2003). The role of BACE1 in muscle dystrophy has been suggested due to the finding of elevated expression of BACE1 in s-IMB and h-IMB, which correlated with abnormal Aβ accumulation and oligomerization in necrotizing muscle fibers (Vattemi et al. 2009; Wojcik et al. 2007; Vattemi et al. 2001). Most recently, the important role of BACE1 in muscle spindles has become increasingly clear (Cheret et al. 2013); the functional dependence of Nrg1-ErbB signaling for proper formation of muscle spindles has long been described (Hippenmeyer et al. 2002; Andrechek et al. 2002). The role of BACE1-dependent Nrg1 signaling in this aspect is consistent with observed localization of BACE1, Nrg1, and ErbB4 at the axonal terminus and in synapses (Jaworski and Burden 2006; Kandalepas et al. 2013; Garcia et al. 2000; Rimer 2007; Jo et al. 1995).
Muscle spindles are composed of specialized intrafusal muscle fibers, which are innervated by afferent axons extending from sensory neurons (Hunt 1990). Previous in vitro studies have demonstrated that decreases in Nrg1 in sensory neurons, or its receptor ErbB2 in muscles, reduces the formation of muscle spindles (Andrechek et al. 2002; Hippenmeyer et al. 2002; Leu et al. 2003), which require proper contacts between proprioceptive sensory afferents and developing muscle fibers. This knowledge has driven further examination of coordinated muscle function in BACE1-null mice in the context of Nrg1 cleavage by BACE1 (Cheret et al. 2013). BACE1-null mice show a swaying walking pattern, which is attributable to impared coordination between forelimbs and hindlimbs. Such an ambulatory defect is likely due to dysfunctional proprioception, as governed by muscle spindles. Newborn BACE1-null mice have a more dramatic reduction in the number of muscle spindles, while this reduction is less severe in BACE1-null adult or heterozygous mice (Cheret et al. 2013). In mice treated for 29 days with the compound Ly2811376, which is a specific and nerve-penetrable inhibitor of BACE1 (May et al. 2011), up to 40% of muscle spindles are lost. This role of BACE1 in reduced muscle spindle maintenance is due to abrogated or reduced cleavage of type I Nrg1, which has an Ig domain. The Ig domain-containing Nrg1 isoforms are preferentially expressed by proprioceptive sensory neurons and are sufficient to induce muscle spindle differentiation in animals (Hippenmeyer et al. 2002). Consistently, transgenic mice overexpressing IgNrg1β1 develop supernumerary muscle spindles (Rumsey et al. 2008). Mechanistically, Nrg1 induces expression of members of the early growth response (Egr) family of transcription factors, Egr1, Egr2, and Egr3 (Jacobson et al. 2004); Egr3 expression is regarded as the muscle spindle-specific gene required for the formation of muscle spindle fibers (O'Donovan et al. 1999). Induction of Egr3 expression in intrafusal fibers requires ectodomain-shedded Nrg1 to activate ErbB2 on the muscle fibers and the downstream MAPK pathway; Erg3 promoter contains binding sites for two transcription factors, SRF and CREB, which are inducibly expressed by MAPK signaling activity (Herndon et al. 2013; Herndon et al. 2014). Hence, these data support the concept that BACE1-dependent type I Nrg1 signaling is critical for motor coordination.
Perspective summary
BACE1 inhibitors are currently under clinical trials aimed at treating patients with Alzheimer’s disease (Yan and Vassar 2014). The compound from Merck has shown potent inhibition and safety profiles in phase I trials and is currently in phase II and III combined trials (see reviews by Menting and Claassen 2014; Vassar 2014). Several other companies have also shown great promise with their BACE1 inhibitors in phase I trials. Hence, there is growing optimism that BACE1 inhibitors can reverse Aβ-mediated cognitive failures. Because chemical inhibition of BACE1 in animals has been shown to alter maintenance of muscle spindles and to impair synaptic functions (Filser et al. 2015), monitoring of potential alterations in BACE1-dependent Nrg1 signaling functions in patients who will take BACE1 inhibitor drugs over the long term should receive increasing basic and clinical attention.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Health to R Yan (NS074256, AG025493, AG046929 and NM103942) and a grant from the National Multiple Sclerosis Society to R Yan (RG 4012A1/1).
Abbreviation
- BACE1
β-site APP convertases enzyme
- Nrg1
neuregulin-1
- ErbB, APP
amyloid precursor protein
- Aβ
β–amyloid peptides
- CNS
central nervous system
- PNS
peripheral nervous system
Footnotes
The authors declare no conflicts of interest.
Reference List
- Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Zhang M, Trembak-Duff I, Unterbarnscheidt T, Radyushkin K, Dibaj P, Martins de SD, Boretius S, Brzozka MM, Steffens H, Berning S, Teng Z, Gummert MN, Tantra M, Guest PC, Willig KI, Frahm J, Hell SW, Bahn S, Rossner MJ, Nave KA, Ehrenreich H, Zhang W, Schwab MH. Dysregulated expression of neuregulin-1 by cortical pyramidal neurons disrupts synaptic plasticity. Cell Rep. 2014;8:1130–1145. doi: 10.1016/j.celrep.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Amato D. Serotonin in antipsychotic drugs action. Behav. Brain Res. 2015;277:125–135. doi: 10.1016/j.bbr.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Andrechek ER, Hardy WR, Girgis-Gabardo AA, Perry RL, Butler R, Graham FL, Kahn RC, Rudnicki MA, Muller WJ. ErbB2 is required for muscle spindle and myoblast cell survival. Mol. Cell Biol. 2002;22:4714–4722. doi: 10.1128/MCB.22.13.4714-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx L, Ceulemans B, Vermeesch JR, Devriendt K, Van EH. Early myoclonic encephalopathy caused by a disruption of the neuregulin-1 receptor ErbB4. Eur. J. Hum. Genet. 2009;17:378–382. doi: 10.1038/ejhg.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Macdonald ML, Borgmann-Winter KE, Hahn CG. Neuregulin 1-erbB4 pathway in schizophrenia: From genes to an interactome. Brain Res. Bull. 2010;83:132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Wang HY, Borgmann-Winter KE, Macdonald ML, Kaprielian H, Stucky A, Kvasic J, Egbujo C, Ray R, Talbot K, Hemby SE, Siegel SJ, Arnold SE, Sleiman P, Chang X, Hakonarson H, Gur RE, Hahn CG. Src kinase as a mediator of convergent molecular abnormalities leading to NMDAR hypoactivity in schizophrenia. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JK, McCann SP, Bhardwaj V, Washington MK, Frey MR. Neuregulin-4 is a survival factor for colon epithelial cells both in culture and in vivo. J. Biol. Chem. 2012;287:39850–39858. doi: 10.1074/jbc.M112.400846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J. Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, LaFerla FM. Pathways by which Abeta facilitates tau pathology. Curr. Alzheimer Res. 2006;3:437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol. Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB Signaling Serves Distinct Functions in Myelination of the Peripheral and Central Nervous System. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv. Anat. Embryol. Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Brown DJ, Lin B, Holguin B. Expression of neuregulin 1, a member of the epidermal growth factor family, is expressed as multiple splice variants in the adult human cornea. Invest Ophthalmol. Vis. Sci. 2004;45:3021–3029. doi: 10.1167/iovs.04-0229. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Kwon OB, Yan L, Gonzalez C, Longart M, Hoffman D, Vullhorst D. Neuregulins and neuronal plasticity: possible relevance in schizophrenia. Novartis. Found. Symp. 2008;289:165–177. doi: 10.1002/9780470751251.ch13. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB-4: mechanism of action and biology. Exp. Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Carraway KL, III, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- Carteron C, Ferrer-Montiel A, Cabedo H. Characterization of a neural-specific splicing form of the human neuregulin 3 gene involved in oligodendrocyte survival. J. Cell Sci. 2006;119:898–909. doi: 10.1242/jcs.02799. [DOI] [PubMed] [Google Scholar]
- Chang H, Riese DJ, Gilbert W, Stern DF, McMahan UJ. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature. 1997;387:509–512. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- Chen PL, Avramopoulos D, Lasseter VK, McGrath JA, Fallin MD, Liang KY, Nestadt G, Feng N, Steel G, Cutting AS, Wolyniec P, Pulver AE, Valle D. Fine mapping on chromosome 10q22-q23 implicates Neuregulin 3 in schizophrenia. Am. J. Hum. Genet. 2009;84:21–34. doi: 10.1016/j.ajhg.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret C, Willem M, Fricker FR, Wende H, Wulf-Goldenberg A, Tahirovic S, Nave KA, Saftig P, Haass C, Garratt AN, Bennett DL, Birchmeier C. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013;32:2015–2028. doi: 10.1038/emboj.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George HP, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat. Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 2015 doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Corvin AP, Morris DW, McGhee K, Schwaiger S, Scully P, Quinn J, Meagher D, Clair DS, Waddington JL, Gill M. Confirmation and refinement of an 'at-risk' haplotype for schizophrenia suggests the EST cluster, Hs.97362, as a potential susceptibility gene at the Neuregulin-1 locus. Mol. Psychiatry. 2004;9:208–213. doi: 10.1038/sj.mp.4001412. [DOI] [PubMed] [Google Scholar]
- Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr. Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Jeyabalan N, Jones N, Dumont DJ, Muller WJ. Multiple ErbB-2/Neu Phosphorylation Sites Mediate Transformation through Distinct Effector Proteins. J. Biol. Chem. 2001a;276:38921–38928. doi: 10.1074/jbc.M106239200. [DOI] [PubMed] [Google Scholar]
- Dankort D, Maslikowski B, Warner N, Kanno N, Kim H, Wang Z, Moran MF, Oshima RG, Cardiff RD, Muller WJ. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol. Cell Biol. 2001b;21:1540–1551. doi: 10.1128/MCB.21.5.1540-1551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De SB, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuss M, Reiss K, Hartmann D. Part-Time alpha-Secretases: The Functional Biology of ADAM 9, 10 and 17. Curr. Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Eimer WA, Vassar R. Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol. Neurodegener. 2013;8:2. doi: 10.1186/1750-1326-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol. Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000;28:81–90. doi: 10.1016/s0896-6273(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Filser S, Ovsepian SV, Masana M, Blazquez-Llorca L, Brandt EA, Volbracht C, Muller MB, Jung CK, Herms J. Pharmacological inhibition of BACE1 impairs synaptic plasticity and cognitive functions. Biol. Psychiatry. 2015;77:729–739. doi: 10.1016/j.biopsych.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Fleck D, van BF, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S, Edbauer D, Lichtenthaler SF, Schmid B, Willem M, Haass C. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J. Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J. Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker FR, Antunes-Martins A, Galino J, Paramsothy R, La RF, Perkins J, Goldberg R, Brelstaff J, Zhu N, McMahon SB, Orengo C, Garratt AN, Birchmeier C, Bennett DL. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain. 2013;136:2279–2297. doi: 10.1093/brain/awt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DL. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J. Neurosci. 2011;31:3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O'Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam V, D'Avanzo C, Hebisch M, Kovacs DM, Kim DY. BACE1 activity regulates cell surface contactin-2 levels. Mol. Neurodegener. 2014;9:4. doi: 10.1186/1750-1326-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes AE, Huang XF, Newell KA. Reciprocal signalling between NR2 subunits of the NMDA receptor and neuregulin1 and their role in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:896–904. doi: 10.1016/j.pnpbp.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Hakonarson H. Common variants in polygenic schizophrenia. Genome Biol. 2009;10:236. doi: 10.1186/gb-2009-10-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Wolfer S, Wieser GL, Nientiedt T, Pieper A, Ruhwedel T, Groszer M, Sereda MW, Nave KA. Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy. EMBO Mol. Med. 2012;4:486–499. doi: 10.1002/emmm.201200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J. Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hahn CG. A Src link in schizophrenia. Nat. Med. 2011;17:425–427. doi: 10.1038/nm0411-425. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat. Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, Gonzalez MI, Upton N, Pangalos MN, Dingwall C. BACE1 (beta-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol. Cell Neurosci. 2003;24:646–655. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Hayes NV, Blackburn E, Boyle MM, Russell GA, Frost TM, Morgan BJ, Gullick WJ. Expression of neuregulin 4 splice variants in normal human tissues and prostate cancer and their effects on cell motility. Endocr. Relat Cancer. 2011;18:39–49. doi: 10.1677/ERC-10-0112. [DOI] [PubMed] [Google Scholar]
- Hayes NV, Gullick WJ. The neuregulin family of genes and their multiple splice variants in breast cancer. J. Mammary. Gland. Biol. Neoplasia. 2008;13:205–214. doi: 10.1007/s10911-008-9078-4. [DOI] [PubMed] [Google Scholar]
- Hayes NV, Newsam RJ, Baines AJ, Gullick WJ. Characterization of the cell membrane-associated products of the Neuregulin 4 gene. Oncogene. 2008;27:715–720. doi: 10.1038/sj.onc.1210689. [DOI] [PubMed] [Google Scholar]
- He W, Jinxuan H, Xia Y, Yan R. BACE1 Regulates Notch Signaling by Controlling the Cleavage of Jag1 and Jag2. J. Biol. Chem. 2014 doi: 10.1074/jbc.M114.579862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon CA, Ankenbruck N, Fromm L. The Erk MAP kinase pathway is activated at muscle spindles and is required for induction of the muscle spindle-specific gene Egr3 by neuregulin1. J. Neurosci. Res. 2014;92:174–184. doi: 10.1002/jnr.23293. [DOI] [PubMed] [Google Scholar]
- Herndon CA, Ankenbruck N, Lester B, Bailey J, Fromm L. Neuregulin1 signaling targets SRF and CREB and activates the muscle spindle-specific gene Egr3 through a composite SRF-CREB-binding site. Exp. Cell Res. 2013;319:718–730. doi: 10.1016/j.yexcr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Hitt B, Riordan SM, Kukreja L, Eimer WA, Rajapaksha TW, Vassar R. beta-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J. Biol. Chem. 2012;287:38408–38425. doi: 10.1074/jbc.M112.415505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt BD, Jaramillo TC, Chetkovich DM, Vassar R. BACE1−/− mice exhibit seizure activity that does not correlate with sodium channel level or axonal localization. Mol. Neurodegener. 2010;5:31. doi: 10.1186/1750-1326-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005;19:2078–2090. doi: 10.1101/gad.338505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, He W, Luo X, Tsubota KE, Yan R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep. 2013a;4:40–49. doi: 10.1016/j.celrep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann Cell BACE1 Is Equally Required for Remyelination of Peripheral Nerves. J. Neurosci. 2015;35:3806–3814. doi: 10.1523/JNEUROSCI.5207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li X, Zhao M, Gottesdiener A, Luo W, Paul S. Tau pathogenesis is promoted by Abeta1-42 but not Abeta1-40. Mol. Neurodegener. 2014;9:52. doi: 10.1186/1750-1326-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Schlanger R, He W, Macklin WB, Yan R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. 2013b;27:1868–1873. doi: 10.1096/fj.12-224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Hunt CC. Mammalian muscle spindle: peripheral mechanisms. Physiol Rev. 1990;70:643–663. doi: 10.1152/physrev.1990.70.3.643. [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Ibi D, Gonzalez-Maeso J. Epigenetic signaling in schizophrenia. Cell Signal. 2015 doi: 10.1016/j.cellsig.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12218–12223. doi: 10.1073/pnas.0404240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr. Bull. 2012;38:911–913. doi: 10.1093/schbul/sbs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski A, Burden SJ. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J. Neurosci. 2006;26:655–661. doi: 10.1523/JNEUROSCI.4506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc. Med. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Kainulainen V, Sundvall M, Maatta JA, Santiestevan E, Klagsbrun M, Elenius K. A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J. Biol. Chem. 2000;275:8641–8649. doi: 10.1074/jbc.275.12.8641. [DOI] [PubMed] [Google Scholar]
- Kalinowski A, Plowes NJ, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB J. 2010;24:2567–2575. doi: 10.1096/fj.08-129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer's beta-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–352. doi: 10.1007/s00401-013-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, Law AJ. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasnauskiene J, Ciuladaite Z, Preiksaitiene E, Utkus A, Peciulyte A, Kucinskas V. A new single gene deletion on 2q34: ERBB4 is associated with intellectual disability. Am. J. Med. Genet. A. 2013;161A:1487–1490. doi: 10.1002/ajmg.a.35911. [DOI] [PubMed] [Google Scholar]
- Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS. One. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem. Biol. 2008;15:808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber G, Streif R, Schwaiger FW, Kreutzberg GW, Hager G. Neuregulin-1 isoforms are differentially expressed in the intact and regenerating adult rat nervous system. J. Mol. Neurosci. 2003;21:149–165. doi: 10.1385/JMN:21:2:149. [DOI] [PubMed] [Google Scholar]
- Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, Kovacs DM. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat. Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. Reduced sodium channel Na(v)1.1 levels in BACE1-null mice. J. Biol. Chem. 2011;286:8106–8116. doi: 10.1074/jbc.M110.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS. BACE1 gene deletion: impact on behavioral function in a model of Alzheimer's disease. Neurobiol. Aging. 2008;29:861–873. doi: 10.1016/j.neurobiolaging.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, Haass C, Rossner S, Brase S, Lichtenthaler SF. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La MR, Cerri F, Horiuchi K, Bachi A, Feltri ML, Wrabetz L, Blobel CP, Quattrini A, Salzer JL, Taveggia C. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci. 2011 doi: 10.1038/nn.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Feng L. Neuregulin induces proliferation of neural progenitor cells via PLC/PKC pathway. Biochem. Biophys. Res. Commun. 2004;319:603–611. doi: 10.1016/j.bbrc.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmen C, Trotzmuller M, Kofeler H, Ruegg MA, Hall MN, Suter U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci. 2014;34:8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, DeCandia TR, Ripke S, Yang J, Sullivan PF, Goddard ME, Keller MC, Visscher PM, Wray NR. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat. Genet. 2012;44:247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985;40:501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130:2291–2301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM, Steinthorsdottir V, Januel D, Gudnadottir VG, Petursson H, Ingason A, Gulcher JR, Stefansson K, Collier DA. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol. Psychiatry. 2004;9:698–704. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- Li Y, Bohm C, Dodd R, Chen F, Qamar S, Schmitt-Ulms G, Fraser PE, St George-Hyslop PH. Structural biology of presenilin 1 complexes. Mol. Neurodegener. 2014;9:59. doi: 10.1186/1750-1326-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libants S, Carr K, Wu H, Teeter JH, Chung-Davidson YW, Zhang Z, Wilkerson C, Li W. The sea lamprey Petromyzon marinus genome reveals the early origin of several chemosensory receptor families in the vertebrate lineage. BMC. Evol. Biol. 2009;9:180. doi: 10.1186/1471-2148-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J. Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liraz O, Boehm-Cagan A, Michaelson DM. ApoE4 induces Abeta42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol. Neurodegener. 2013;8:16. doi: 10.1186/1750-1326-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang JZ, Xiong WC, Mei L. Specific regulation of NRG1 isoform expression by neuronal activity. J. Neurosci. 2011;31:8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J. Comp Neurol. 2004;472:156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, Chen YJ, Bean JC, Jiao HF, Liu X, Li BM, Xiong WC, Gao TM, Mei L. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron. 2014;84:835–846. doi: 10.1016/j.neuron.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Luo X, He W, Hu X, Yan R. Reversible Overexpression of Bace1-Cleaved Neuregulin-1 N-Terminal Fragment Induces Schizophrenia-Like Phenotypes in Mice. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Prior M, He W, Hu X, Tang X, Sheng W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD, Yan R. Cleavage of neuregulin-1 by BACE1 or ADAM10 produces differential effects on myelination. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Macklin WB. The myelin brake: when enough is enough. Sci. Signal. 2010;3:e32. doi: 10.1126/scisignal.3140pe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni MA. Neuregulin signaling in pieces--evolution of the gene family. Curr. Pharm. Des. 2014;20:4856–4873. doi: 10.2174/1381612819666131125152146. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, Stout SL, Timm DE, Smith LE, Gonzales CR, Nakano M, Jhee SS, Yen M, Ereshefsky L, Lindstrom TD, Calligaro DO, Cocke PJ, Greg HD, Friedrich S, Citron M, Audia JE. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J. Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SJ, Castle SL, Bernard JK, Almohazey D, Hunter CJ, Bell BA, Al AD, Wang L, Ford HR, Frey MR. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. Am. J. Pathol. 2014;184:2768–2778. doi: 10.1016/j.ajpath.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Nave KA. Neuregulin-ERBB Signaling in the Nervous System and Neuropsychiatric Diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menting KW, Claassen JA. beta-secretase inhibitor; a promising novel therapeutic drug in Alzheimer's disease. Front Aging Neurosci. 2014;6:165. doi: 10.3389/fnagi.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Montero JC, Yuste L, az-Rodriguez E, Esparis-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol. Cell Neurosci. 2000;16:631–648. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- Morar B, Dragovic M, Waters FA, Chandler D, Kalaydjieva L, Jablensky A. Neuregulin 3 (NRG3) as a susceptibility gene in a schizophrenia subtype with florid delusions and relatively spared cognition. Mol. Psychiatry. 2011;16:860–866. doi: 10.1038/mp.2010.70. [DOI] [PubMed] [Google Scholar]
- Mori H, Manabe T, Watanabe M, Satoh Y, Suzuki N, Toki S, Nakamura K, Yagi T, Kushiya E, Takahashi T, Inoue Y, Sakimura K, Mishina M. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron. 1998;21:571–580. doi: 10.1016/s0896-6273(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Munzel EJ, Schaefer K, Obirei B, Kremmer E, Burton EA, Kuscha V, Becker CG, Brosamle C, Williams A, Becker T. Claudin k is specifically expressed in cells that form myelin during development of the nervous system and regeneration of the optic nerve in adult zebrafish. Glia. 2012;60:253–270. doi: 10.1002/glia.21260. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H, Tandon R, Keshavan M. Beyond the facts in schizophrenia: closing the gaps in diagnosis, pathophysiology, and treatment. Epidemiol. Psychiatr. Sci. 2011;20:317–327. doi: 10.1017/s204579601100062x. [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, Rujescu D, Giegling I, Straub RE, McGee K, Gold B, Dean M, Muglia P, Callicott JH, Tan HY, Weinberger DR. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch. Gen. Psychiatry. 2010;67:991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- O'Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, O'Connor AM, O'Sullivan GJ, Lai D, Harvey R, Croke DT, Waddington JL. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous 'knockout' of the schizophrenia risk gene neuregulin-1. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:462–466. doi: 10.1016/j.pnpbp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat. Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KA, Varghese M, Sowa A, Yuk F, Brautigam H, Ehrlich ME, Dickstein DL. Altered synaptic structure in the hippocampus in a mouse model of Alzheimer's disease with soluble amyloid-beta oligomers and no plaque pathology. Mol. Neurodegener. 2014;9:41. doi: 10.1186/1750-1326-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O'Dushlaine C, Chambert K, Bergen SE, Kahler A, Duncan L, Stahl E, Genovese G, Fernandez E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PK, Banks E, Shakir K, Garimella K, Fennell T, DePristo M, Grant SG, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank KB, Pauley AM, Bhattacharya K, Wang Z, Evans DB, Fleck TJ, Johnston JA, Sharma SK. Direct interaction of soluble human recombinant tau protein with Abeta 1–42 results in tau aggregation and hyperphosphorylation by tau protein kinase II. FEBS Lett. 2002;514:263–268. doi: 10.1016/s0014-5793(02)02376-1. [DOI] [PubMed] [Google Scholar]
- Rimer M. Neuregulins at the neuromuscular synapse: past, present, and future. J. Neurosci. Res. 2007;85:1827–1833. doi: 10.1002/jnr.21237. [DOI] [PubMed] [Google Scholar]
- Rimer M, Prieto AL, Weber JL, Colasante C, Ponomareva O, Fromm L, Schwab MH, Lai C, Burden SJ. Neuregulin-2 is synthesized by motor neurons and terminal Schwann cells and activates acetylcholine receptor transcription in muscle cells expressing ErbB4. Mol. Cell Neurosci. 2004;26:271–281. doi: 10.1016/j.mcn.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Rossi P, Sola E, Taglietti V, Borchardt T, Steigerwald F, Utvik JK, Ottersen OP, Kohr G, D'Angelo E. NMDA receptor 2 (NR2) C-terminal control of NR open probability regulates synaptic transmission and plasticity at a cerebellar synapse. J. Neurosci. 2002;22:9687–9697. doi: 10.1523/JNEUROSCI.22-22-09687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, oit-Marand M, Chen C, Moore H, O'Donnell P, Brunner D, Corfas G. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JW, Das M, Kang JF, Wagner R, Molnar P, Hickman JJ. Tissue engineering intrafusal fibers: dose- and time-dependent differentiation of nuclear bag fibers in a defined in vitro system using neuregulin 1-beta-1. Biomaterials. 2008;29:994–1004. doi: 10.1016/j.biomaterials.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse CC, Kim YH, Agsten M, Huth T, Alzheimer C, Kovacs DM, Kim DY. BACE1 and presenilin/gamma-secretase regulate proteolytic processing of KCNE1 and 2, auxiliary subunits of voltage-gated potassium channels. FASEB J. 2013;27:2458–2467. doi: 10.1096/fj.12-214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M, Bartsch U. Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia. 2000;29:154–165. doi: 10.1002/(sici)1098-1136(20000115)29:2<154::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A. Roles of Meltrin beta /ADAM19 in the processing of neuregulin. J. Biol. Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- Shorter KR, Miller BH. Epigenetic mechanisms in schizophrenia. Prog. Biophys. Mol. Biol. 2015;118:1–7. doi: 10.1016/j.pbiomolbio.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Beyond the dopamine hypothesis to the NMDA glutamate receptor hypofunction hypothesis of schizophrenia. CNS. Spectr. 2007;12:265–268. doi: 10.1017/s1092852900021015. [DOI] [PubMed] [Google Scholar]
- Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St CD. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am. J. Hum. Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J. Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, de JS, Andreassen OA, Werge T, Borglum AD, Mors O, Mortensen PB, Gustafsson O, Costas J, Pietilainen OP, Demontis D, Papiol S, Huttenlocher J, Mattheisen M, Breuer R, Vassos E, Giegling I, Fraser G, Walker N, Tuulio-Henriksson A, Suvisaari J, Lonnqvist J, Paunio T, Agartz I, Melle I, Djurovic S, Strengman E, Jurgens G, Glenthoj B, Terenius L, Hougaard DM, Orntoft T, Wiuf C, Didriksen M, Hollegaard MV, Nordentoft M, van WR, Kenis G, Abramova L, Kaleda V, Arrojo M, Sanjuan J, Arango C, Sperling S, Rossner M, Ribolsi M, Magni V, Siracusano A, Christiansen C, Kiemeney LA, Veldink J, van den Berg L, Ingason A, Muglia P, Murray R, Nothen MM, Sigurdsson E, Petursson H, Thorsteinsdottir U, Kong A, Rubino IA, De HM, Rethelyi JM, Bitter I, Jonsson EG, Golimbet V, Carracedo A, Ehrenreich H, Craddock N, Owen MJ, O'Donovan MC, Ruggeri M, Tosato S, Peltonen L, Ophoff RA, Collier DA, St CD, Rietschel M, Cichon S, Stefansson H, Rujescu D, Stefansson K. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum. Mol. Genet. 2011;20:4076–4081. doi: 10.1093/hmg/ddr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Stefansson H, Ghosh S, Birgisdottir B, Bjornsdottir S, Fasquel AC, Olafsson O, Stefansson K, Gulcher JR. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Strange PG. Antipsychotic drug action: antagonism, inverse agonism or partial agonism. Trends Pharmacol. Sci. 2008;29:314–321. doi: 10.1016/j.tips.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Suenaga A, Hatakeyama M, Ichikawa M, Yu X, Futatsugi N, Narumi T, Fukui K, Terada T, Taiji M, Shirouzu M, Yokoyama S, Konagaya A. Molecular dynamics, free energy, and SPR analyses of the interactions between the SH2 domain of Grb2 and ErbB phosphotyrosyl peptides. Biochemistry. 2003;42:5195–5200. doi: 10.1021/bi034113h. [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ, Weinberger DR, Law AJ. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- Tang JX, Chen WY, He G, Zhou J, Gu NF, Feng GY, He L. Polymorphisms within 5' end of the Neuregulin 1 gene are genetically associated with schizophrenia in the Chinese population. Mol. Psychiatry. 2004;9:11–12. doi: 10.1038/sj.mp.4001436. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J. Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Maruyama K, Saido TC, Kume H, Shinozaki K, Tokuhiro S, Capell A, Walter J, Grunberg J, Haass C, Iwatsubo T, Obata K. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber H, Hagemeyer N, Ehrenreich H, Simons M. BACE1 in central nervous system myelination revisited. Mol. Psychiatry. 2012;17:237–239. doi: 10.1038/mp.2011.140. [DOI] [PubMed] [Google Scholar]
- Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer's disease. Mol. Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy VK. Peptide determinants of myelin proteolipid protein (PLP) in autoimmune demyelinating disease: a review. Neurochem. Res. 1994;19:935–944. doi: 10.1007/BF00968703. [DOI] [PubMed] [Google Scholar]
- van BF, Hruscha A, Willem M, Schmid B, Haass C. Loss of Bace2 in zebrafish affects melanocyte migration and is distinct from Bace1 knock out phenotypes. J. Neurochem. 2013 doi: 10.1111/jnc.12198. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Goodearl A, Viehover A, Fischbach G. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. J. Cell Biol. 1997;137:211–220. doi: 10.1083/jcb.137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer's disease. Alzheimers. Res. Ther. 2014;6:89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vassar R, Kuhn PH, Haass C, Kennedy ME, Rajendran L, Wong PC, Lichtenthaler SF. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J. Neurochem. 2014 doi: 10.1111/jnc.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Zheng H. Molecular neurodegeneration: basic biology and disease pathways. Mol. Neurodegener. 2014;9:34. doi: 10.1186/1750-1326-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattemi G, Engel WK, McFerrin J, Buxbaum JD, Pastorino L, Askanas V. Presence of BACE1 and BACE2 in muscle fibres of patients with sporadic inclusion-body myositis. Lancet. 2001;358:1962–1964. doi: 10.1016/S0140-6736(01)06969-0. [DOI] [PubMed] [Google Scholar]
- Vattemi G, Engel WK, McFerrin J, Pastorino L, Buxbaum JD, Askanas V. BACE1 and BACE2 in pathologic and normal human muscle. Exp. Neurol. 2003;179:150–158. doi: 10.1016/s0014-4886(02)00025-0. [DOI] [PubMed] [Google Scholar]
- Vattemi G, Nogalska A, King EW, D'Agostino C, Checler F, Askanas V. Amyloid-beta42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathol. 2009;117:569–574. doi: 10.1007/s00401-009-0511-6. [DOI] [PubMed] [Google Scholar]
- Vukadinovic Z. NMDA receptor hypofunction and the thalamus in schizophrenia. Physiol Behav. 2014;131:156–159. doi: 10.1016/j.physbeh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Vullhorst D, Mitchell RM, Keating C, Roychowdhury S, Karavanova I, Tao-Cheng JH, Buonanno A. A negative feedback loop controls NMDA receptor function in cortical interneurons via neuregulin 2/ErbB4 signalling. Nat. Commun. 2015;6:7222. doi: 10.1038/ncomms8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- Wang H, Ren CH, Gunawardana CG, Schmitt-Ulms G. Overcoming barriers and thresholds - signaling of oligomeric Abeta through the prion protein to Fyn. Mol. Neurodegener. 2013;8:24. doi: 10.1186/1750-1326-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Chen JY, Chen ML, Chen CH, Lai IC, Chen TT, Hong CJ, Tsai SJ, Liou YJ. Neuregulin 3 genetic variations and susceptibility to schizophrenia in a Chinese population. Biol. Psychiatry. 2008;64:1093–1096. doi: 10.1016/j.biopsych.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Warren CM, Kani K, Landgraf R. The N-terminal domains of neuregulin 1 confer signal attenuation. J. Biol. Chem. 2006;281:27306–27316. doi: 10.1074/jbc.M512887200. [DOI] [PubMed] [Google Scholar]
- Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S, O'Donovan MC, Owen MJ. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol. Psychiatry. 2003;8:485–487. doi: 10.1038/sj.mp.4001348. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, Yan R, McFerrin J, Askanas V. NOGO is increased and binds to BACE1 in sporadic inclusion-body myositis and in AbetaPP-overexpressing cultured human muscle fibers. Acta Neuropathol. (Berl) 2007;114:517–526. doi: 10.1007/s00401-007-0281-y. [DOI] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J. Biol. Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol. 2014;13:319–329. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Zhou H, Pun S, Ip NY, Peng HB, Tsim KW. Cloning of cDNAs encoding xenopus neuregulin: expression in myotomal muscle during embryo development. Brain Res. Mol. Brain Res. 1998;58:59–73. doi: 10.1016/s0169-328x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang XL, Zhou M, Zhang HY, Kong QM, Liu C, Zhang DR, Yu YQ, Liu SZ, Ju GZ, Shu L, Ma DL, Zhang D. Association study of neuregulin 1 gene with schizophrenia. Mol. Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Min L, Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 1997;11:2691–2700. doi: 10.1101/gad.11.20.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Liu S, Jiao H, Shen C, Sathyamurthy A, Lin TW, Xiong WC, Li BM, Mei L, Bergson C. Calcyon stimulates neuregulin 1 maturation and signaling. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.131. [DOI] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Lu YS, Bean JC, Sathyamurthy A, Shen C, Liu X, Lin TW, Smith CA, Xiong WC, Mei L. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78:644–657. doi: 10.1016/j.neuron.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki T, Wakatsuki S, Hatsuzawa K, Black RA, Wada I, Sehara-Fujisawa A. Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12:329–343. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski PJ. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Barao S, Laga M, Bockstael K, Borgers M, Gijsen H, Annaert W, Moechars D, Mercken M, Gevaert K, De SB. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J. Biol. Chem. 2012;287:25927–25940. doi: 10.1074/jbc.M112.377465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, Zou J, Zhou L, Yu S, Cui Y, Yang W, Luo L, Lu QR, Liu Y, Chen M, Worley PF, Xiao B. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J. Neurosci. 2014;34:15764–15778. doi: 10.1523/JNEUROSCI.2267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]