Abstract
Background
Electrical stimulation (NMES) is a highly sought after but poorly studied treatment for dysphagia among head and neck cancer (HNC) patients with dysphagia. This study investigated the efficacy of NMES in this patient population.
Methods
In this double-blinded, randomized controlled trial, 170 HNC patients experiencing post-treatment dysphagia were randomized into active NMES + swallow exercise versus sham NMES + swallow exercise groups. Outcomes after a 12-week program included changes in fluoroscopy measures, diet, and quality of life.
Results
After the 12-week program, the active NMES group had significantly worse Penetration Aspiration Scale scores than the sham group. Both groups reported significantly better diet and quality of life. No other measures were significant.
Conclusions
NMES did not add benefit to traditional swallow exercises. Unfortunately swallow exercises were not effective by themselves either. For HNC patients with moderate-severe dysphagia caused by radiation therapy, current behavioral therapies are of limited help in reversing long-term dysphagia.
Keywords: Dysphagia, Head and neck cancer, radiation therapy, electrical stimulation, exercise
INTRODUCTION
In the 21st century, head and neck cancer (HNC) patients experience improved local tumor control and lower mortality rates because of advancements in radiation therapy (RT), chemoradiotherapy (CRT), and surgery.1 Unfortunately, elimination of the cancer can leave devastating side effects, including the inability to eat and swallow normally. Organ preservation strategies are often the preferred treatment, but can leave functional deficits that can produce and magnify a dysphagia. As many as two thirds of HNC patients are left with permanent swallowing problems, and dysphagia symptoms can deteriorate for several years post treatment.2–4 The dysphagia impacts nutrition, hydration, and pulmonary health, and leaves patients with significantly diminished quality of life.
Speech pathologists use behavioral therapies (compensatory and rehabilitative) to alleviate such swallowing problems, with limited benefit.5,6 Compensatory strategies such as liquid washes have been found to be partially effective, especially in patients with mild dysphagia. However, they do not restore a patient’s swallowing, and may be ineffective in patients with more severe dysphagia.7 Alternatively, rehabilitative strategies are meant to have a permanent effect by making the swallow stronger or faster. These strategies include “non-swallow exercises” which aim to strengthen isolated muscles used in swallowing (such as tongue strengthening) and “swallowing exercises” that aim to strengthen all the muscles used in swallowing while executing a hard, effortful, or prolonged swallow. To date, no randomized clinical trials have shown that rehabilitative strategies are efficacious in HNC patients who are post-RT.8
Neuromuscular electrical stimulation (NMES), often referred to as electrical stimulation (estim), was introduced as a novel therapy for dysphagia in the late 1980s. The principles of NMES in the limb rehabilitation literature are well established.9,10 However published protocols applying NMES to swallowing function have shown mixed results.11–13 Two recent clinical trials tested the effect of NMES on swallowing with HNC patients.14,15 Both claimed that estim was more successful than an alternate therapy in improving swallowing. Unfortunately, both studies had very small sample sizes and lacked adequate controls, making their results questionable. Despite limited evidence supporting estim for dysphagia in HNC patients, the popularity of this therapy has remained high.
This clinical trial aimed to determine whether NMES was an effective therapeutic intervention for dysphagia in patients who had completed RT or CRT for HNC. Our rationale was that facilitating muscle contraction would lead to a stronger swallow and perhaps push through the fibrotic tissue caused by radiation damage. The stimulation was paired with “swallowing exercises” in keeping with the neuroplasticity principle of specificity of training.
METHODS
Study Structure
This was a phase III, double blinded, randomized clinical trial (patients and research staff blinded) with two arms: Active NMES vs Sham NMES. From 2009 to 2012, 170 patients were enrolled at 16 institutions throughout the U.S. Modified Barium Swallow (MBS) studies were sent to an independent Central Laboratory for analysis, and all data was sent to an independent Data Coordinating Center (DCC). The DCC also generated the randomization scheme (2:1), served as the data repository center, managed data cleaning, and performed statistical analyses. A Data Safety Monitoring Board (DSMB) met bi-annually to review safety and study progress.
Subjects
Eligible patients were over 21 years old, cancer free, had completed a full dose (≥50Gy) of C/RT at least 3 months prior to enrollment, and demonstrated moderate-severe dysphagia on a MBS study defined as Penetration-Aspiration Score (PAS)16 ≥4 on at least on one bolus. Key exclusion criteria included history of dysphagia unrelated to HNC, prior use of electrical stimulation, neurologic disease, presence of pacemaker/defibrillator, floor of mouth resection, and/or inability to follow the study protocol. All patients gave written informed consent and this study was approved by each participating site’s IRB.
Enrollment and Randomization
Patients who met initial eligibility criteria, determined by the clinician-researchers, received an MBS study that was recorded and sent to the central laboratory. If they confirmed a PAS score of ≥4, the patient was randomized using a 2:1 experimental to control treatment arm scheme. Of note, the initial criteria required a PAS ≥ 6, but low enrollment prompted an easing in this requirement.
The MBS study consisted of 15 swallows of thin liquid, thick liquid, pudding , banana, and cracker. If the patient aspirated on 2 consecutive boluses of thin liquid of the same volume, the clinician did not administer the larger volumes of thin liquid. Similarly, if the clinician judged that boluses of more solid food would result in severe aspiration, these boluses were skipped. All boluses judged to be unsafe were given a PAS of 8 by the Central Lab.
Treatment Plan
The estim device was the BMR NeuroTech (NT) 2000 (Galway, Republic of Ireland). Most patients received the default settings with minor alterations for some patients to help them attain maximum performance. See Table 1 for the default parameters. The sham device looked and performed identically to the real device, but the internal current carrying wires were disabled. A visual bar and audio tone were activated when the stimulation was supposedly being transmitted, identical to the active device.
Table 1.
Parameters of the BMR NT2000
| Parameter | Default setting/ Possible Range |
|---|---|
| Program | 0 or 1 |
| Frequency | 70 Hertz |
| Pulse Width | 300 microseconds (ranged from 130 – 300) |
| Contraction | 4 seconds (ranged from 4 – 8) |
| Relaxation | 12 seconds (ranged from 12 – 16) |
| Ramp Up | 2 seconds (ranged from 2 – 4) |
| Ramp Down | 0 seconds |
| Amplitude Limit | 0–99 (real) 0–25 (sham) |
| Options | 5 (sound) is “on”, all others are “off” |
| Treatment Time | 20 minutes or longer if needed |
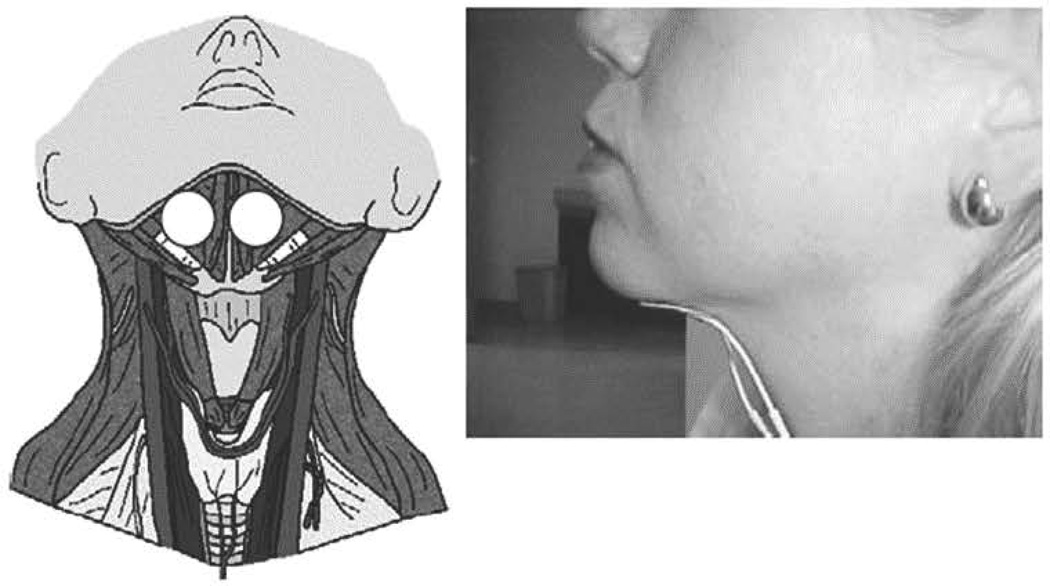
As shown in Figure 1, electrodes were placed in a bipolar fashion, above the hyoid, beneath the mandible. Electrical stimulation was delivered to the submental region to stimulate the supra-hyoid muscles. Patients in the Active estim group were able to set the amplitude to a level where they felt a comfortable contraction.
Figure 1.
Electrode Placement
Subjects were taught to swallow as the electric current (and/or audio/visual cues) came on, and to rest between cycles. The subjects were taught three standard swallow maneuvers for their exercises: the Mendelsohn, the Super Supraglottic, and the Effortful Swallow.17–22 They were taught to “work their muscles hard” so that the swallow maneuver would become an exercise. Each session consisted of a 5-minute warm-up stretching protocol followed by swallowing 60 times in synchrony with the stimulation, alternating regular swallows with the swallow maneuvers as shown in Table 2 Compliance was documented on daily logs kept by patients and in the “time log” recorded on the devices.
Table 2.
Sequence of Swallow Maneuvers for Each Therapy Session
| Patients performed 60 sequential swallows, where they were given 4 seconds to initiate and execute a swallow, and then 12 seconds to rest. This protocol was typically performed in 16–20 minutes. | |||||
|---|---|---|---|---|---|
| 10 | 10 | 10 | 10 | 10 | 10 |
| Super- supraglottic Swallows |
Regular Swallows |
Mendelsohn Swallows |
Regular Swallows |
Effortful Swallows |
Regular Swallows |
After enrollment, three training sessions ensured that the subject could competently execute the home-based protocol, performed two times a day, six days a week, for 12 weeks. The patient returned to clinic every 3 weeks to assess competence and compliance. Repeat MBS studies, diet assessments [Performance Status Scale (PSS)]23 and quality of life assessments [Head Neck Cancer Inventory (HNCI)]24 were performed mid-way through the treatment (week 7) and at completion of treatment (week 13).
Masking/Blinding
Clinicians of sham group patients “programmed” the device to specific parameters in front of the patient suggesting the preparation of an active device, although possibly with a low current. Patients were also informed that they may not be able to feel the stimulation because of RT-induced fibrosis and reduced sensation. All aspects of the protocol were otherwise identical to the experimental group.
Aims and Outcomes of Interest
The first aim of this study was to determine whether NMES added benefit to a therapy program comprised of aggressive swallowing exercises and stretching. Performance between the groups was compared at completion of treatment, after adjustment for baseline differences. The primary outcome measure was swallowing function as measured by the Penetration-Aspiration Score (PAS)16. Two other swallow measures, Oropharyngeal Swallow Efficiency (OPSE)25, and hyoid excursion (in mm), were secondary outcome measures. Diet, measured by the PSS23 and Quality of Life, measured by the HNCI24 were other secondary outcomes.
The second aim of this study was to determine whether either or both of the groups improved over the trial. The two groups were assessed independently and when pooled together to measure changes from baseline to treatment completion. The same outcome measures were used.
The total, or composite PAS score, was the average of 14 PAS scores (one for each bolus swallowed minus the swallow in the AP projection). Mean subscale PAS scores were computed for each consistency; thin liquid, thick liquid, pudding, banana, and cracker. OPSE scores and hyoid excursion were calculated similarly. PSS scores were calculated for each domain (diet, public, speech) as a total score (mean of domain scores). HNCI scores were similarly calculated by domain (speech, eating, aesthetics, social disruption) and as a total (mean domain) score.
Central Laboratory Fidelity
Two research technicians shared the analysis of the swallow studies for this project, and were initially trained in their accuracy until their inter- and intra-observer reliability was at least 0.85. Once the study commenced, ten percent of the analyzed swallows were chosen at random for re-evaluation by the lab coordinator. Those selected swallows were reanalyzed by both research technicians to determine inter- and intra-judge reliability for 7 fluoroscopic variables including oral residue, pharyngeal residue, aspiration before the swallow, aspiration during the swallow, oral transit time, pharyngeal response time, and pharyngeal delay time. Lin’s Concordance Coefficient was used to quantify reliability.26
Statistical Design and Analysis
Baseline characteristics and comparability of the two treatment groups were assessed by the two-sample t-test for continuous variables and by chi-square or Fisher’s exact test for categorical variables. To evaluate attrition, the dropout patterns were compared between the treatment groups using Fisher’s exact test. Then the permutation test was used to assess deviation from the assumption of missingness completely at random (MCAR) for the data.27
To address the first aim of the study, we used analysis of covariance (ANCOVA) comparing changes in study outcomes from baseline to week 13 adjusting for differences in baseline measures of swallow performance, diet and quality of life across the two treatment arms. These analyses only included subjects with both baseline and week 13 outcome data. Using similar ANCOVA methodology, analyses of compliance were performed.
To address the second aim, we performed longitudinal analyses using general linear models for repeated measures of outcome data collected at baseline, 7, and 13 weeks. In these analyses the outcome was expressed as change from baseline to 7 and 13 weeks, and the baseline measure was included as a covariate along with time, treatment group and time-by-treatment interaction term. These analyses included all subjects with available outcome data at baseline assessment regardless of dropout. An intention to treat analysis was included as part of this model. The effect of time was expressed as change per month. SAS 9.2 statistical software (SAS Institute Inc., Cary, NC) was used for all computations.
RESULTS
Of 488 screened patients, 170 were randomized into the study. Average time since completion of RT was 53.7 months (median=24.5 months; range=3–267 months). An extensive list of patient, disease, and cancer treatment variables can be found in Table 3. Subjects in the two arms were not significantly different for any patient variables of interest at time of entry.
Table 3.
Patient Demographics at Baseline
| Characteristic | Overall (N=168) |
NMES (N=116) |
Sham (N=52) |
p-value |
|---|---|---|---|---|
| Site | ||||
| 1 | 28 (16.7%) | 19 (16.4%) | 9 (17.3%) | |
| 3 | 17 (10.1%) | 12 (10.3%) | 5 (9.6%) | |
| 4 | 16 (9.5%) | 11 (9.5%) | 5 (9.6%) | |
| 7 | 11 (6.5%) | 7 (6%) | 4 (7.7%) | |
| 8 | 2 (1.2%) | 1 (0.9%) | 1 (1.9%) | |
| 9 | 9 (5.4%) | 6 (5.2%) | 3 (5.8%) | |
| 11 | 9 (5.4%) | 7 (6%) | 2 (3.8%) | |
| 14 | 29 (17.3%) | 19 (16.4%) | 10 (19.2%) | |
| 15 | 7 (4.2%) | 5 (4.3%) | 2 (3.8%) | |
| 18 | 10 (6%) | 7 (6%) | 3 (5.8%) | |
| 19 | 4 (2.4%) | 3 (2.6%) | 1 (1.9%) | |
| 20 | 4 (2.4%) | 4 (3.4%) | 0 (0.0%) | |
| 21 | 3 (1.8%) | 2 (1.7%) | 1 (1.9%) | |
| 22 | 4 (2.4%) | 3 (2.6%) | 1 (1.9%) | |
| 24 | 1 (0.6%) | 1 (0.9%) | 0 (0.0%) | |
| 91 | 14 (8.3%) | 9 (7.8%) | 5 (9.6%) | |
| Gender | ||||
| Male | 144 (85.7%) | 100 (86.2%) | 44 (84.6%) | 0.814 |
| Female | 24 (14.3%) | 16 (13.8%) | 8 (15.4%) | |
| Age | ||||
| Mean ± SD | 61.9±9.6 | 62.1±9.2 | 61.5±10.6 | 0.722 |
| Median and Range | 62 (33–87) | 62 (33–83) | 62 (33–87) | |
| Ethnicity | ||||
| Hispanic or Latino | 13 (7.9%) | 10 (8.8%) | 3 (5.9%) | 0.756 |
| Not Hispanic or Latino | 152 (92.1%) | 104 (91.2%) | 48 (94.1%) | |
| Race | ||||
| White | 121 (72%) | 82 (70.7%) | 39 (75%) | 0.911 |
| Black | 19 (11.3%) | 14 (12.1%) | 5 (9.6%) | |
| Hispanic | 13 (7.7%) | 10 (8.6%) | 3 (5.8%) | |
| Asian | 14 (8.3%) | 9 (7.8%) | 5 (9.6%) | |
| Multiple | 1 (0.6%) | 1 (0.9%) | 0 (0.0%) | |
| Stage | ||||
| 1 | 7 (5%) | 7 (7.4%) | 0 (0.0%) | 0.082 |
| 2 | 14 (9.9%) | 7 (7.4%) | 7 (15.2%) | |
| 3 | 26 (18.4%) | 20 (21.1%) | 6 (13%) | |
| 4 | 94 (66.7%) | 61 (64.2%) | 33 (71.7%) | |
| Time since completion of radiation therapy | ||||
| Mean ± SD | 53.7±60.9 | 56.5±65.1 | 47.4±50 | 0.395 |
| Median and Range | 24.5 (3–267) | 29 (3–267) | 23 (3–186) | |
| Prior Radiation Therapy | ||||
| Yes | 8 (4.9%) | 5 (4.5%) | 3 (5.9%) | 0.706 |
| No | 155 (95.1%) | 107 (95.5%) | 48 (94.1%) | |
| Chemotherapy | ||||
| Yes | 121 (75.6%) | 83 (74.1%) | 38 (79.2%) | 0.552 |
| No | 39 (24.4%) | 29 (25.9%) | 10 (20.8%) | |
| RT: Intensity Modulated Radiation Therapy (IMRT) | ||||
| Yes | 86 (51.5%) | 56 (48.3%) | 30 (58.8%) | 0.241 |
| No | 81 (48.5%) | 60 (51.7%) | 21 (41.2%) | |
| RT: Brachytherapy | ||||
| Yes | 4 (2.4%) | 3 (2.6%) | 1 (2%) | 0.999 |
| No | 163 (97.6%) | 113 (97.4%) | 50 (98%) | |
| RT: Conventional | ||||
| Yes | 70 (41.9%) | 51 (44%) | 19 (37.3%) | 0.497 |
| No | 97 (58.1%) | 65 (56%) | 32 (62.7%) | |
| RT: Stereotactic | ||||
| Yes | 2 (1.2%) | 0 (0.0%) | 2 (3.9%) | 0.092 |
| No | 165 (98.8%) | 116 (100%) | 49 (96.1%) | |
| RT site: Oral | ||||
| Yes | 14 (8.4%) | 11 (9.5%) | 3 (5.9%) | 0.555 |
| No | 153 (91.6%) | 105 (90.5%) | 48 (94.1%) | |
| RT site: Nasopharynx | ||||
| Yes | 17 (10.2%) | 10 (8.6%) | 7 (13.7%) | 0.404 |
| No | 150 (89.8%) | 106 (91.4%) | 44 (86.3%) | |
| RT site: Oropharynx | ||||
| Yes | 78 (46.7%) | 55 (47.4%) | 23 (45.1%) | 0.867 |
| No | 89 (53.3%) | 61 (52.6%) | 28 (54.9%) | |
| RT site: Hypopharynx | ||||
| Yes | 23 (13.8%) | 14 (12.1%) | 9 (17.6%) | 0.339 |
| No | 144 (86.2%) | 102 (87.9%) | 42 (82.4%) | |
| RT site: Larynx | ||||
| Yes | 22 (13.2%) | 13 (11.2%) | 9 (17.6%) | 0.320 |
| No | 145 (86.8%) | 103 (88.8%) | 42 (82.4%) | |
| RT site: Other | ||||
| Yes | 18 (10.8%) | 14 (12.1%) | 4 (7.8%) | 0.589 |
| No | 149 (89.2%) | 102 (87.9%) | 47 (92.2%) | |
| Surgery | ||||
| Yes | 82 (49.4%) | 58 (50%) | 24 (48%) | 0.866 |
| No | 84 (50.6%) | 58 (50%) | 26 (52%) | |
| RT Modality | ||||
| Intensity Modulated Radiation Therapy (IMRT) | 84 (50.3%) | 55 (47.4%) | 29 (56.9%) | 0.423 |
| Brachytherapy | 1 (0.6%) | 1 (0.9%) | 0 (0.0%) | |
| Conventional | 68 (40.7%) | 50 (43.1%) | 18 (35.3%) | |
| Stereotactic | 1 (0.6%) | 0 (0.0%) | 1 (2%) | |
| Multiple | 13 (7.8%) | 10 (8.6%) | 3 (5.9%) | |
| RT site | ||||
| Oral | 8 (4.8%) | 7 (6%) | 1 (2%) | 0.316 |
| Nasopharynx | 16 (9.6%) | 9 (7.8%) | 7 (13.7%) | |
| Oropharynx | 70 (41.9%) | 51 (44%) | 19 (37.3%) |
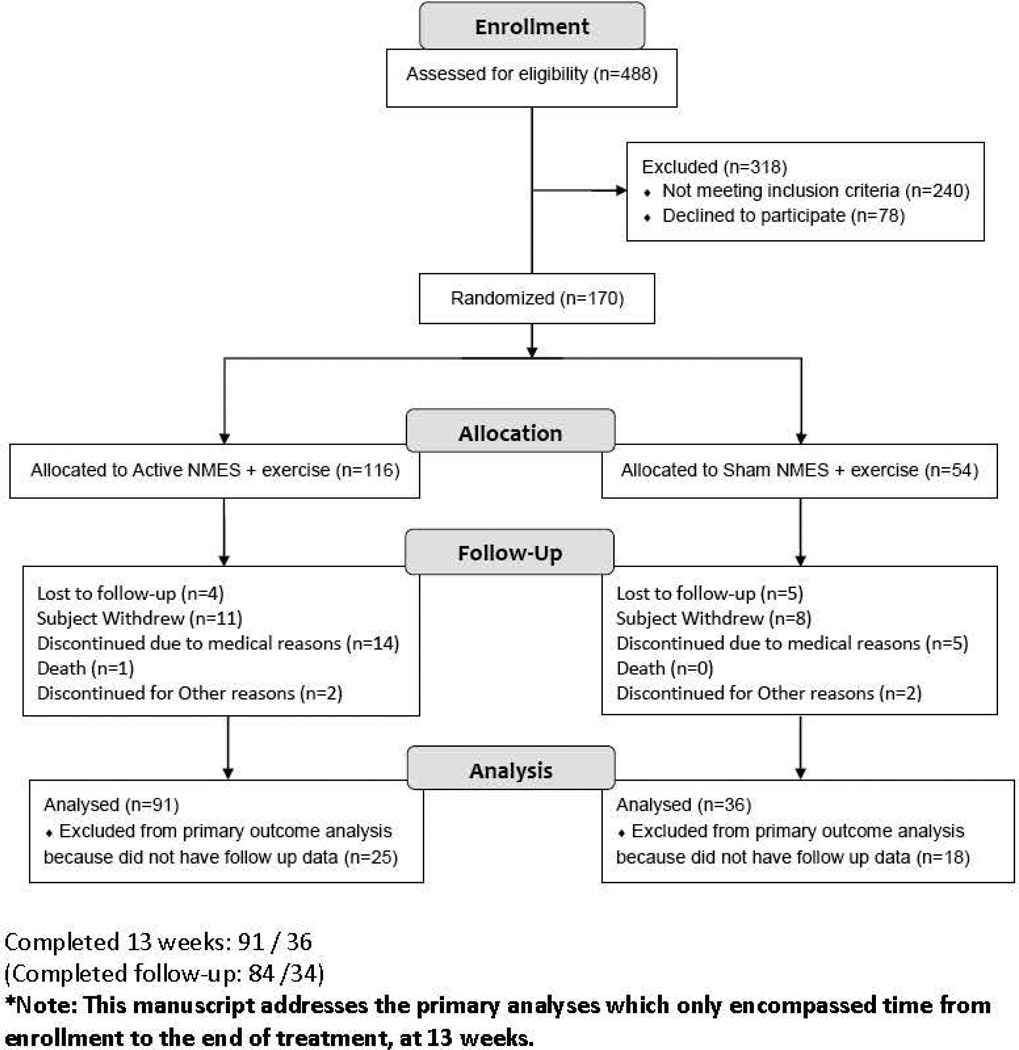
116 patients were randomly assigned to the Active NMES + exercise group while 54 were assigned to the Sham NMES + exercise group. 91 subjects in the Active NMES group and 36 subjects in Sham NMES group were included in the primary analyses. Drop-out was not significantly different between the treatment groups (Fisher’s exact test p=0.394). The permutation test identified no significant deviation from the MCAR and hence the reported results are unlikely to be biased due to attrition. The Consort Flow Diagram is depicted in Figure 2.
Figure 2.
Consort Diagram
Outcomes between the Two Treatment Arms at the End of Treatment
Table 4 summarizes the raw scores at baseline and 13 weeks for both groups for the major outcomes of interest. Table 5 summarizes the analysis done to compare the two groups at end of treatment, adjusting for differences in baseline performance.
Table 4.
Raw Scores at Baseline and 13 week (end of treatment) in selected outcome vari
| Variable | Treatment Group |
N | Baseline | Week 13 | Change |
|---|---|---|---|---|---|
| PASa Total | NMES | 90 | 5.13 (1.88) | 5.14 (1.84) | 0.01 (1.04) |
| Sham | 35 | 5.48 (1.66) | 4.91 (2.11) | −0.57 (1.62) | |
| OPSEb | NMES | 81 | 41.47 (20.98) | 41.81 (20.1) | 0.34 (14.75) |
| Sham | 30 | 35.88 (19.93) | 40.72 (19.74) | 4.84 (17.44) | |
| Hyoid Anterior Total | NMES | 76 | 7.05 (4.07) | 6.42 (4.47) | −0.62 (3.49) |
| Sham | 32 | 6.57 (3.4) | 5.86 (3.24) | −0.71 (2.47) | |
| Hyoid Superior Total | NMES | 76 | 16.81 (9.16) | 15.94 (7.87) | −0.87 (6.72) |
| Sham | 32 | 16.91 (7.82) | 17.31 (8.85) | 0.4 (5.58) | |
| % Residue in Pharynx | Sham | 85 | 30.81 (15.65) | 30.55 (17.24) | −0.26 (10.02) |
| NMES | 34 | 35.84 (19.58) | 31.74 (16.31) | −4.1 (18.26) | |
| PSSc Total | NMES | 91 | 60.73 (22.11) | 66.98 (21.55) | 6.25 (14.45) |
| Sham | 35 | 58.38 (20.36) | 62.9 (21.31) | 4.52 (16.06) | |
| HNCId Eating | NMES | 86 | 32.54 (21.04) | 38.85 (23.97) | 6.31 (17.92) |
| Sham | 34 | 24.18 (18.58) | 30.93 (20.46) | 6.74 (15.59) |
Penetration Aspiration Scale (PAS)
Oralpharyngeal Swallowing Efficiency (OPSE)
Performance Status Scale (PSS)
Head and Neck Cancer Inventory (HNCI)
Table 5.
Difference between the groups at week 13 for primary and secondary outcome measures, adjusted for baseline differences.
| Outcome Measure | Difference between the 2 groups: NMES-Sham (95%CI) N=127 |
p-value |
|---|---|---|
| Primary Outcome | ||
| PASa Total | 0.52 (0.06,0.98) | 0.027* |
| PAS Thin | 0.81 (0.29,1.33) | 0.002* |
| PAS Thick | −0.13 (−0.96,0.71) | 0.767 |
| PAS Pudding | 0.23 (−0.57,1.04) | 0.570 |
| PAS Banana | 0.03 (−0.78,0.84) | 0.943 |
| PAS Saltine | 0.11 (−0.76,0.98) | 0.806 |
| Secondary Outcomes | ||
| OPSEb Total | −2.75 (−8.63,3.14) | 0.361 |
| Hyoid Anterior Total | 0.22 (−1.03,1.47) | 0.732 |
| Hyoid Superior Total | −1.30 (−3.63,1.03) | 0.274 |
| PSSc Total | 2.30 (−3.10,7.69) | 0.404 |
| PSS Diet | 0.37 (−7.48,8.22) | 0.926 |
| PSS Public | 6.20 (−3.40,15.81) | 0.206 |
| PSS Speech | −0.20 (−4.65,4.25) | 0.929 |
| HNCId Speech | −3.37 (−9.81,3.06) | 0.304 |
| HNCI Eating | 1.41 (−5.28,8.10) | 0.679 |
| HNCI Aesthetics | 0.49 (−7.98,8.95) | 0.910 |
| HNCI Social Disruption | −3.11 (−10.28,4.05) | 0.395 |
Penetration Aspiration Scale (PAS)
Oralpharyngeal Swallowing Efficiency (OPSE)
Performance Status Scale (PSS)
Head and Neck Cancer Inventory (HNCI)
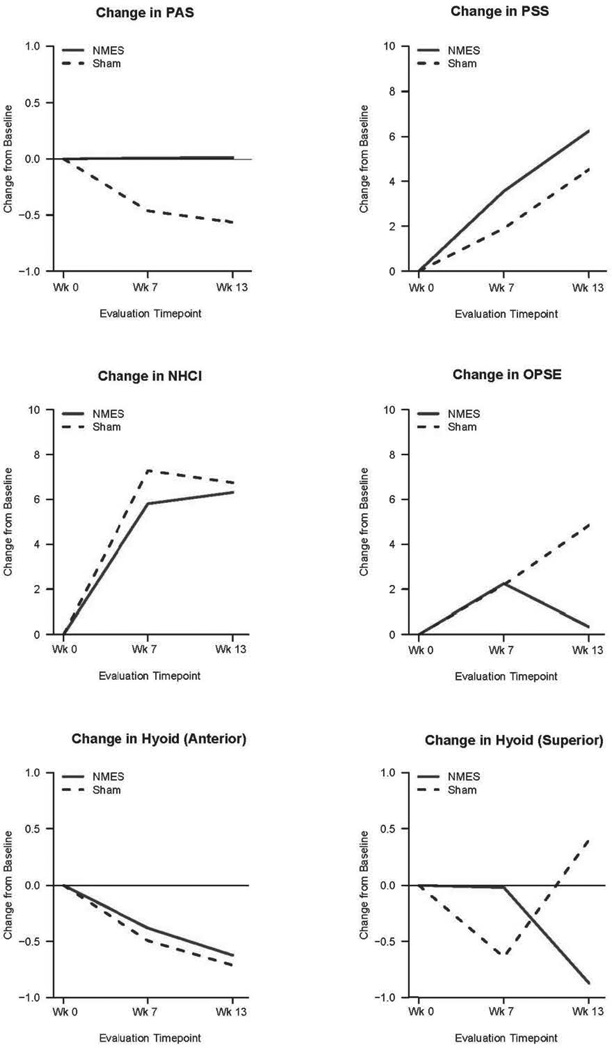
Over the course of treatment, the mean PAS score remained unchanged in the Active NMES + exercise group and decreased (improved) in Sham NMES + exercise group. At end of treatment, the Active NMES Total PAS mean raw score was 5.1 (+/−1.8) compared to the Sham NMES Total PAS of 4.9 (+/− 2.1). Adjusting for differences in baseline, this resulted in a difference of 0.52 on PAS (95% CI: 0.06–0.98, p=0.027) indicating greater improvement in the Sham NMES + exercise group. This difference was driven primarily by the PAS Thin Liquid subscale. None of the other outcome measures showed a significant difference between the two groups. Figure 3 depicts the difference in scores between the groups graphically.
Figure 3.
Comparison of scores for Active and Sham NMES groups at three times, adjusted for baseline differences.
Although statistically significant, the raw score differences between the two groups for our primary outcome, PAS, were not dramatically different. Thus an effect size analysis was conducted comparing this difference. A Cohen’s d of 0.472 was found, suggesting a small effect size. In clinical terms, a difference of less than 1 PAS score is of marginal significance.
Longitudinal Analyses: Change in Outcome Measures over Time
The longitudinal analysis of change from baseline to 13 weeks is shown in Table 6. The Sham NMES group showed significant improvement in Total PAS score over time (p<0.001), while the Active NMES showed no significant change. The significant change in the Sham NMES group was driven primarily by PAS Thin Liquid and PAS Cracker subscales. Overall, combining both groups, the p-value for a change in PAS lost its significance. There was a significant decrease of Hyoid Anterior excursion in the Active NMES group (p=0.038) and when all patients were combined (p=0.014). There were no significant differences in OPSE measures in either group or overall.
Table 6.
Change over time for primary and secondary outcome measures in the two groups.
| Outcome Measure | Active NMES: Change per month (95%CI) N=116 |
p-value | Sham NMES: Change per month (95%CI) N=54 |
p-value | Overall (combined groups) Change per month (95%CI) N=170 |
p-value |
|---|---|---|---|---|---|---|
| Primary Outcome | ||||||
| PASa Total | 0.01 (−0.07,0.08) | 0.879 | −0.20 (−0.31,−0.09) | <0.001* | −0.05 (−0.11,0.01) | 0.084 |
| PAS Thin | 0.02 (−0.07,0.11) | 0.671 | −0.28 (−0.41,−0.14) | <0.001* | −0.06 (−0.14,0.01) | 0.097 |
| PAS Thick | −0.01 (−0.15,0.13) | 0.897 | 0.03 (−0.19,0.25) | 0.780 | 0.00 (−0.12,0.12) | 0.967 |
| PAS Pudding | 0.00 (−0.15,0.15) | 0.973 | −0.14 (−0.37,0.09) | 0.232 | −0.04 (−0.17,0.09) | 0.540 |
| PAS Banana | −0.07 (−0.21,0.08) | 0.376 | −0.13 (−0.35,0.09) | 0.249 | −0.08 (−0.21,0.04) | 0.173 |
| PAS Saltine | −0.04 (−0.20,0.12) | 0.600 | −0.25 (−0.50,−0.01) | 0.043* | −0.10 (−0.24,0.03) | 0.131 |
| Secondary Outcomes | ||||||
| OPSEb Total | 0.27 (−0.75,1.29) | 0.601 | 1.43 (−0.17,3.04) | 0.080 | 0.60 (−0.26,1.46) | 0.168 |
| Hyoid Anterior Total | −0.25 (−0.48,−0.01) | 0.038* | −0.24 (−0.60,0.11) | 0.177 | −0.25 (−0.44,−0.05) | 0.014* |
| Hyoid Superior Total | −0.27 (−0.71,0.17) | 0.230 | 0.01 (−0.66,0.67) | 0.988 | −0.19 (−0.55,0.18) | 0.314 |
| PSSc Total | 1.96 (1.06,2.86) | <0.001* | 1.42 (0.02,2.81) | 0.046* | 1.81 (1.06,2.57) | <0.001* |
| PSS Diet | 1.87 (0.62,3.12) | 0.003* | 2.25 (0.32,4.19) | 0.023* | 1.99 (0.94,3.03) | <0.001* |
| PSS Public | 2.75 (1.06,4.45) | 0.002* | 1.05 (−1.57,3.67) | 0.430 | 2.28 (0.86,3.70) | 0.002* |
| PSS Speech | 1.18 (0.29,2.06) | 0.009* | 1.05 (−0.32,2.43) | 0.132 | 1.15 (0.41,1.89) | 0.003* |
| HNCId Speech | 1.29 (0.24,2.35) | 0.016* | 2.93 (1.28,4.58) | 0.001* | 1.75 (0.86,2.64) | <0.001* |
| HNCI Eating | 2.22 (1.19,3.24) | <0.001* | 2.44 (0.86,4.01) | 0.003* | 2.28 (1.43,3.14) | <0.001* |
| HNCI Aesthetics | 0.40 (−1.09,1.90) | 0.596 | 1.30 (−1.05,3.65) | 0.276 | 0.66 (−0.59,1.92) | 0.300 |
| HNCI Social Disruption | 1.14 (−0.07,2.34) | 0.065 | 2.31 (0.43,4.19) | 0.016* | 1.46 (0.45,2.48) | 0.005* |
Penetration Aspiration Scale (PAS)
Oralpharyngeal Swallowing Efficiency (OPSE)
Performance Status Scale (PSS)
Head and Neck Cancer Inventory (HNCI)
Diet, measured by total PSS score, improved significantly over time in both the Active (p=<0.001) and Sham (p=0.046) groups. Significant improvement was also seen in the HNCI quality of life scores in each treatment group for Speech (p=0.016 and p=0.001) and Eating (p<0.001 and p=0.003) domains. Both diet and quality of life scores showed a significant improvement overall, with both groups combined.
Compliance
Active and Sham NMES groups were similarly “compliant” defined as performing 10 or more sessions per week. 57% of the Active NMES group and 48% of the Sham NMES group were deemed compliant, yielding a non-significant chi square p-value of 0.2958.
Inter- and Intra-Observer Reliability of Videofluoroscopic Data
Lin’s Concordance Coefficient yielded excellent inter- and intra-observer reliability with a mean of 0.989 and 0.992, respectively. Mean IRR coefficients ranged from 0.964 to 1.000 for each of the 7 variables that were tested.
DISCUSSION
This randomized controlled clinical trial is the largest trial to date to investigate the effect of specific behavioral interventions on swallowing ability in post-radiated HNC patients with dysphagia. The sample size was larger and the therapy program more robust than any other similar clinical trial to date. Most subjects had been treated with CRT for advanced stage cancer and were past the acute stages of recovery (mean of 4.5 years post-CRT). The hope that a systematic, aggressive exercise therapy, augmented by electrical stimulation, would improve swallowing in this population was not supported. The fluoroscopic measures of swallowing showed no added benefit for NMES; in fact, hyoid excursion decreased (worsened) in both groups. Conversely, patients in both arms reported significant improvement in diet (PSS) and quality of life (HNCI) domains related to swallowing.
The fact that NMES did not improve swallowing in this group of patients is noteworthy. This modality of swallowing therapy has now been tested in several clinical trials, mainly with post-stroke patients, and most of the results have been negative. This current study adds to the increasing list of negative trials and puts the value of electrical stimulation in doubt. Certainly, it should not be recommended for chronic HNC patients with dysphagia. One might question whether NMES is, in fact, detrimental for swallowing recovery, since there was a significant difference between the two groups in our primary outcome of PAS, with the Active NMES group showing no change but the sham group showing improved PAS scores. The effect analysis tempered this conclusion, showing a small effect that was not impressive clinically. The more reasoned conclusion may be that NMES simply did not help the swallow.
In addition to assessing the efficacy of estim, this clinical trial also tested the effect of three exercises on improving swallow function in post-radiated HNC patients. The three exercises used in this study are considered best practice therapy; they all incorporate swallowing into the exercise and thus are considered “swallow-specific” exercises that favor the principles of neuroplasticity. They have all been shown to have immediate effects on swallowing18,20,28–30 but whether they strengthen the swallow after several weeks of execution has not been adequately determined. One recent study of stroke patients found some improved swallow scores after practicing the Mendelsohn Maneuver, but it was a pilot study with a cross-over design with only two weeks of using this maneuver.31 To date, the Super-supraglottic Swallow (SSGS) and the Effortful Swallow have not been tested for their long-term effects in patients with dysphagia. Waters, et al reported on a controlled trial with 79 long-term HNC survivors engaged in a program of exercises including the SSG, Effortful, and Mendelsohn Swallows lasting 16 weeks.32 The patients did not demonstrate significant improvement in swallow function after completing the exercise program. These disappointing results are consistent with the current study.
One might hypothesize that perhaps a different outcome would have occurred if non-swallow exercises had been tested. One small clinical trial has tested the efficacy of tongue strengthening exercises (a common non-swallow exercise) on post-radiated HNC patients. Unfortunately, neither swallowing nor measures of tongue strength improved.8 No other specific non-swallow exercises have been tested for their long term effect in patients with HNC.
Compliance is often a factor that influences outcomes, and poor compliance can impact the validity of clinical trial results. This is especially true for patients undergoing CRT who are often non-compliant with prescribed swallow exercises.32,33 Unlike patients who are in the throes of cancer diagnosis and treatment, the patients in this study were mostly far removed from that time. Because of their long-standing dysphagia, they were eager to participate in the trial and some even exercised more than was asked of them. Thus, compliance was a minor problem.
Given the lack of objective improvement in swallow physiology, it is unclear why the patients reported better diet and quality of life outcomes at the end of this trial. The quality of life improvement may have reflected a “placebo” effect that is commonly found after subjects have participated in clinical trials. They want to improve, they anticipate that they will improve, they are actively doing something to improve, and they feel they have improved.35
The improvement in diet is not so easily explained by the placebo effect. Many patients said they could eat faster and more easily at the end of the trial. They were not as afraid of aspirating, and they were able to eat with others more comfortably. Perhaps this suggests that the simple act of practicing swallowing over and over for each session improved their skill, ease, and rate of eating, helping them to more safely and efficiently try more challenging foods.
CONCLUSIONS
This large, randomized controlled clinical trial determined that NMES did not add any benefit to traditional dysphagia therapy in post-radiated HNC patients. It also suggested that traditional swallowing-specific exercises and stretching may not help rehabilitate dysphagia in this group of patients with chronic dysphagia. Interestingly, all patients reported significant improvements in diet and quality of life. For the majority of patients, it appears that once post-radiation dysphagia is well-established, current interventions are limited in reversing the decline in swallow function.
Acknowledgements
This clinical trial was supported fully by the following granting agency: NIH/NCI RO1 CA 120950-05 A1 ClinicalTrials.gov Identifier: NCT062965
The authors wish to recognize the contributions of Dr. Jerilyn Logemann who was one of the original investigators of this study. Her guidance throughout the clinical trial and her vast knowledge of dysphagia benefitted everyone involved in the trial.
Clinicians at 16 sites participated in this clinical trial by enrolling subjects, completing the examinations and training sessions, working with the subjects, completing all forms, and participating in regular conference calls. Their experiences and advice helped to make the study a success. We especially wish to thank the Principal Investigators at each site: Danielle Lodewyck, PhD (New York University Hospitals), Gary Gramigna, M.S. (VA Boston Healthcare), Eva Michalakis, M.S. (Lahey Clinic), Michelle Graham M.S. (North Shore LIJ Lenox Hill Hospital), Cathy Lazarus, PhD (Beth Israel Medical Center, NY), Tim McCulloch, M.D. (University of Wisconsin), Barbara Messing, M.S. (Greater Baltimore Medical Center), Tanya Meyer, M.D. (University of Washington), Alice Silbergleit PhD (Henry Ford Hospitals), Darlene Graner, M.S. (Mayo Clinic, Rochester), Steve Goldman, M.S. (University of California, San Diego), Kristin Larsen, M.S. (Northwestern University Hospitals), Cynthia Wagner, M.S. (Beth Israel Deaconess Medical Center, Boston), Tamar Kotz, M.S. (Mount Sinai Hospital, New York), and Lisa Crujido, M.S. (Mayo Clinic, Phoenix).
We wish to thank Gad Alon, PhD (University of Maryland) and Arthur Miller, PhD (UCSF) for their expert advice in the early stages of this proposal, and Teresa Biber, M.S. for teaching all the clinicians the principles and techniques of delivering NMES. We also thank the DSMB members for their support and guidance throughout the study.
Finally, we thank Bracco Diagnostics for their contribution of barium sulfate products for the fluoroscopy studies, BMR NeuroTech for their generous contributions and maintenance of the NMES devices, and Electrodes-to-Go to for contributing electrodes for the NMES devices.
Footnotes
Preliminary data from the clinical trial was presented at the Dysphagia Research Society annual meeting on March 14, 2013, in Seattle, WA.
Contributor Information
Susan E Langmore, Department of Otolaryngology, Boston University School of Medicine; Department of Speech Language Hearing, Boston University, langmore@bu.edu.
Timothy M McCulloch, Division of Otolaryngology Head and Neck Surgery, Department of Surgery, University of Wisconsin-Madison, mccull@surgery.wisc.edu.
Gintas P Krisciunas, Department of Otolaryngology, Boston University Medical Center, gintas@bu.edu.
Cathy L. Lazarus, Associate Professor, Department of Otolaryngology, Icahn School of Medicine at Mount Sinai; Research Director, THANC Foundation, Department of Otolaryngology Head & Neck Surgery, Mount Sinai Beth Israel, clazarus@chpnet.org.
Douglas J Van Daele, Department of Otolaryngology-Head and Neck Surgery, University of Iowa Carver College of Medicine, douglas-van-daele@uiowa.edu.
Barbara Roa Pauloski, Communication Sciences and Disorders, Northwestern University, pauloski@northwestern.edu.
Denis Rybin, Data Coordinating Center, Boston University School of Public Health, drybine@bu.edu.
Gheorghe Doros, Associate Professor of Biostatistics, Department of Biostatistics, Boston University, doros@bu.edu.
REFERENCES
- 1.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus CL. Effects of radiation therapy and voluntary maneuvers on swallow functioning in head and neck cancer patients. Clin Commun Disord. 1993;3(4):11–20. [PubMed] [Google Scholar]
- 3.Smith RV, Kotz T, Beitler JJ, Wadler S. Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Arch Otolaryngol Head Neck Surg. 2000;126(3):384–389. doi: 10.1001/archotol.126.3.384. [DOI] [PubMed] [Google Scholar]
- 4.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy--results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(5):1161–1171. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 5.Grobbelaar EJ, Owen S, Torrance AD, Wilson JA. Nutritional challenges in head and neck cancer. Clin Otolaryngol Allied Sci. 2004;29(4):307–313. doi: 10.1111/j.1365-2273.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 6.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118(23):5793–5799. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazarus C, Logemann JA, Gibbons P. Effects of maneuvers on swallowing function in a dysphagic oral cancer patient. Head Neck. 1993;15(5):419–424. doi: 10.1002/hed.2880150509. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus CL, Husaini H, Falciglia D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg. 2014;43(5):523–530. doi: 10.1016/j.ijom.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez M, Lucia A, Rivero JL, et al. Effects of transcutaneous short-term electrical stimulation on M. vastus lateralis characteristics of healthy young men. Pflugers Arch. 2002;443(5–6):866–874. doi: 10.1007/s00424-001-0769-6. [DOI] [PubMed] [Google Scholar]
- 10.Siff M. Applications of electrical electrostimulation in physical conditioning: a review. J Appl Sports Sci Res. 1990;4:20–26. [Google Scholar]
- 11.Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. J Rehab Med. 2009;41(3):174–178. doi: 10.2340/16501977-0317. [DOI] [PubMed] [Google Scholar]
- 12.Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal Dysphagia. Dysphagia. 2007;22(1):1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23(3):302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JS, Kang JY, Park JY, et al. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral Oncol. 2009;45(8):665–668. doi: 10.1016/j.oraloncology.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Lin PH, Hsiao TY, Chang YC, et al. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19(1):91–99. doi: 10.1007/s00520-009-0792-2. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 17.Ding R, Larson CR, Logemann JA, Rademaker AW. Surface electromyographic and electroglottographic studies in normal subjects under two swallow conditions: normal and during the Mendelsohn manuever. Dysphagia. 2002;17(1):1–12. doi: 10.1007/s00455-001-0095-3. [DOI] [PubMed] [Google Scholar]
- 18.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82(12):1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27(3):418–426. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus C, Logemann JA, Shi G, Kahrilas P, Pelzer H, Kleinjan K. Does laryngectomy improve swallowing after chemoradiotherapy? A case study. Arch Otolaryngol Head Neck Surg. 2002;128(1):54–57. doi: 10.1001/archotol.128.1.54. [DOI] [PubMed] [Google Scholar]
- 21.Logemann JA, Kahrilas PJ, Cheng J, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992;262(2 Pt 1):G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 22.Martin BJ, Logemann JA, Shaker R, Dodds WJ. Normal laryngeal valving patterns during three breath-hold maneuvers: a pilot investigation. Dysphagia. 1993;8(1):11–20. doi: 10.1007/BF01351472. [DOI] [PubMed] [Google Scholar]
- 23.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Funk GF, Karnell LH, Christensen AJ, Moran PJ, Ricks J. Comprehensive head and neck oncology health status assessment. Head Neck. 2003;25(7):561–575. doi: 10.1002/hed.10245. [DOI] [PubMed] [Google Scholar]
- 25.Rademaker AW, Vonesh EF, Logemann JA, et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head Neck. 2003;25(12):1034–1041. doi: 10.1002/hed.10317. [DOI] [PubMed] [Google Scholar]
- 26.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. [PubMed] [Google Scholar]
- 27.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 28.Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol. 1991;260(3 Pt 1):G450–G456. doi: 10.1152/ajpgi.1991.260.3.G450. [DOI] [PubMed] [Google Scholar]
- 29.Logemann JA. Swallowing physiology and pathophysiology. Otolaryngol Clin North Am. 1988;21(4):613–623. [PubMed] [Google Scholar]
- 30.Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg. 1997;116(3):335–338. doi: 10.1016/S0194-59989770269-9. [DOI] [PubMed] [Google Scholar]
- 31.McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabil. 2012;19(3):234–243. doi: 10.1310/tsr1903-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters TM, Logemann JA, Pauloski BR, et al. Beyond efficacy and effectiveness: conducting economic analyses during clinical trials. Dysphagia. 2004;19(2):109–119. doi: 10.1007/s00455-003-0507-7. [DOI] [PubMed] [Google Scholar]
- 33.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. "Pharyngocise": randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):210–219. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 34.van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Rasch CR, Hilgers FJ. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26(2):155–170. doi: 10.1007/s00455-010-9288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doering BK, Rief W, Petrie KJ. Lessons to be learned from placebo arms in psychopharmacology trials. Handb Exp Pharmacol. 2014;225:273–290. doi: 10.1007/978-3-662-44519-8_15. [DOI] [PubMed] [Google Scholar]