Abstract
Background and Objectives:
Due to the importance of finding of new antibacterial agents, the antibacterial properties of Prosopis cineraria aerial parts were investigated using a bioassay guided fractionation scheme.
Materials and Methods:
The organic extract was prepared via maceration in methanol, followed by the fractionation using n-hexane and ethyl acetate. The MICs of fractions were determined against some human pathogenic bacteria using broth micro-dilution assay. The primary characterization and identification of bioactive substance(s) was based on a bio-autographical method using HPTLC and flash chromatography in parallel with agar overlay assays. Finally the exact mass of effective compound(s) was determined by LC-MS.
Results:
The best antibacterial activities were related to the ethyl acetate fraction. The effective antibacterial compound of the plant were 2 substances with molecular weight of 348 and 184 Dalton that inhibited the growth of assessed Gram positive bacteria with MIC values lower than 125 to 62.5 μg/ml synergistically.
Conclusion:
Further analysis using nuclear magnetic resonance could reveal the exact structure of these two antibacterial substances. These 2 effective antibacterial compounds could be applied as lead compound for synthesis of new antibacterial agents.
Keywords: Staphylococcus aureus, Prosopis cineraria, Antibiotic resistant, characterization, bioassay
INTRODUCTION
The high frequency of emergence and distribution of multidrug-resistant human bacterial pathogens is a worldwide problem and has been highlighted the requirement of looking for new antibacterial compounds (1, 2). New antibacterial agents which may be derived from natural sources could be useful itself or as novel lead compounds for development of new and more effective antibacterial agents (3).
Plants are helpful and valuable resource for finding of new pharmaceuticals as well as novel antimicrobial agents (4, 5). They have some proven sophisticated defense systems via production, storage and in some cases secretion of variety of antimicrobial compounds for combating with undesirable conditions caused by diverse plant bacterial pathogens during their whole life-time (4, 6).
Prosopis Spp. (Fabaceae) is a plant genus found in Africa and Asia, including some province of Iran such as Fars, Boushehr, Sistan and Balouchestan, Hormozgan and Kerman. It has been used in traditional medicine to treat various diseases such as skin diseases, piles, worms, vertigo and dyspnoea, protection from miscarriage, Eye diseases, Snake bite, asthma, bronchitis, leucoderma, leprosy, muscle tremors, piles and toothache (7–9).
Prosopis cineraria species is endemic in Iran and in the present study, a bioassay-guided fractionation scheme was used to isolate and determine the effective antibacterial compounds of its aerial parts.
MATERIALS AND METHODS
Microbial strains.
Bacterial strains included in the study were as follow: Staphylococcus aureus ATCC 25923, vancomycin-resistant strain of Enterococcus faecium, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, a clinical isolate of Acinetobacter baumanni and Pseudomonas aeruginosa PTCC 1430.
Plant and chemicals preparation.
The aerial parts of Prosopis cineraria were collected in Fars Province, Iran during August 2012 and a voucher specimen was identified and deposited (MPH-2261) in the Medicinal Plants and Drugs Research Institute Herbarium (MPH). Plant materials were immediately dried in a good ventilated room at dark. The organic solvents were purchased from Caledon Lab (Ontario, Canada). The reagents and the high performance thin layer chromatography (HPTLC) silica plates were provided by Merck Co. (KGaA, Darmstadt, Germany). All bacteriological media were from Merck Co. (KGaA, Darmstadt, Germany).
Plant material extraction.
The plant organic extract was prepared via maceration in methanol for 48h at dark with agitation. The resulted waxy pellet was dissolved in distilled water (1:10 w/v ratio) and then was fractioned sequentially by n-hexane and ethyl acetate. The organic solvent extracted material and final aqueous materials were dried under a reduced pressure using rotary evaporator instrument (Heidolph, co, UK) at 40 °C and dried materials were kept at 4 °C.
Antimicrobial assay.
A stock of all samples were dissolved in dimethyl sulfoxide (DMSO) and stored at 4 °C. Antimicrobial susceptibility test was performed using the broth micro-dilution method for determination of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC), as recommended by the Clinical Laboratory Standard Institute (CLSI) (10), with some modifications. Briefly, an 18-hour culture of each test microorganism was used for the susceptibility tests. A serial dilution of each extract (from 64 mg/mL to 0.006 mg/mL) was prepared using sterile, Muller Hinton broth (MHB), containing 0.5% Tween 80 as co-solvent. Microorganisms were added to each well to final concentrations of 0.5–1 × 106 CFU/mL of bacteria. The inoculated 96-well plates were incubated for 20 hours at 37 °C and the MIC values were recorded as the lowest concentration that could inhibit visible growth of microorganisms. Resazurin reagent was used as a growth indicator, as illustrated by Sarker et al. (11). MBCs were determined by sub-culturing 100 μL of each negative well on nutrient agar plates. The MBCs were recorded after 24 hours of incubation at appropriate temperatures, as the lowest concentrations that could kill 99.99 % of the assessed bacteria, as described by CLSI (10). All experiments were performed in triplicates.
High performance thin layer chromatography.
HPTLC was carried out using CAMAG HPTLC instrument. A 1-mg/mL clear solution of Ethyl acetate fraction was dissolved in a solution of Methanol and Ethyl acetate (1:1, v/v) and was spotted onto aluminum silica gel plates PF254 under N2, using Linomat 5, under 0.2 μL/sec speed. Each 6-mm band contained about 30 μg of the extract. A variety of mobile phases were used for achieving the highest separation resolution. All the plates were scanned in the range of 180–800 nm, using CAMAG TLC scanner III (12).
Bio-autography experiments.
The developed TLC plates with the highest resolutions were assessed against S. aureus strain. In brief, a 50 °C Mueller Hinton agar (MHA) medium, containing 107 CFU/mL of bacterium, was poured into developed silica plates and then incubated for 18 hours at 37 °C (13). The effective and ineffective bands were chosen through comparison of the duplicate scanned plates (under 254 nm, 366 nm and visible light) with the bio-autographic plates. Next, the effective and ineffective bands were cut and subjected for further experiments.
Flash chromatography.
Ethyl acetate derived fraction was further fractioned via passing through an open column of silica under vacuum. 5 different mobile phase including n-hexane, n-hexane: ethyl acetate (2:1), n-hexane: ethyl acetate (1:1), n-hexane: ethyl acetate (1:2) and ethyl acetate were applied and each fraction was dried and MIC values of each sample was determined against S. aureus test strain.
Reverse-phase HPLC.
Ethyl acetate fraction derived from first methanol extract and sample of 100% ethyl acetate from flash chromatography experiments were subjected to RP-HPLC. Also 2 bands TLC plates were selected according to the results of bio-autography experiments and resolved by RP-HPLC. The KNAUER HPLC system (Germany) was equipped with an automatic injector (Smartline 3900). A smartline 2800 photodiode array detector set at 254 nm and 210nm, a solvent degasser, an EZchrom software for chromatography data analysis, a binary pump and a 20-μl injection loop were used.
C18 column (Eruspher, 250×4.6 mm, 5 μm, 100 A°) was used for analytical purpose. Binary mobile phase which was used was as follow:
A: Methanol (pH 3 by formic acid) and B: Milli-Q water containing 0.1% Formic acid. The flow rate was 1 ml/min and the column maintained at room temperature. Peaks were recorded by UV (PDA) at wavelengths 254 nm and 210nm. 20 μl of 1000 ppm stock of each mentioned sample was applied.
LC-ESI-MS experiment.
Here HPLC separation was performed on an Agilent LC 1200 series connected to a LCQ ion trap mass spectrometer. HPLC conditions were as follow: C18 column (250× 4.6 mm, 5μm), mobile phase ACN: H2O in 30: 70 ratio. Then LC effluent passed through a UV detector at both 254 nm while the flow rate reduced to 0.5 ml/min and then to the electrospray mass spectrometer. Mass spectra were acquired by a FinniganTM LC-QTM DECA ion trap instrument. ESI was set in both negative and positive mode in a condition including: sheath gas: 60 mL min−1, auxiliary gas: 20 mL min−1, spray voltage:4.5 kV, capillary temperature: 200 °C, capillary voltage: 46 kV, and tube lens: − 60 kV). The Xcalibur 2.0 SR2 software (copyright Thermo Electron Corporation 1998– 2006) was used.
RESULTS AND DISCUSSION
The antibacterial effects of plant extracts are presented in Table 1. It is apparent that not only the overall antibacterial effects of the Ethyl acetate fraction were better than the others, but also it had better inhibitory effects against all Gram-positives and Gram-negative tested bacterial strains. This extract could inhibit the growth of bacteria in concentrations lower than 1 mg/ml and in the case of S. aureus and E. faecium; MIC values were 0.25 mg/ml. So this sample was chosen for further investigations using HPTLC.
Table 1.
MIC & MBC values (mg/ml) of different fractioned materials of P. cineraria.
| Methanol extract | Ethyl acetate fraction | Ethyl acetate 100%* | Band #5 | Cefexime** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. coli | 4 | 8 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | 2 | 8 |
| S. aureus | 2 | 4 | 0.25 | 0.5 | 0.125 | 0.5 | 0.25 | 0.5 | 0.5 | 2 |
| K. pneumoniae | 2 | 8 | 1 | 2 | 0.25 | 1 | 0.25 | 1 | 16 | 64 |
| E. faecium | 1 | 16 | 0.25 | 4 | 0.25 | 1 | 0.25 | 0.25 | 32 | >64 |
| E. faecalis | 0.25 | 16 | 0.031 | 4 | 0.031 | 1 | 0.062 | 0.125 | 2 | 8 |
| A. baumannii | 1 | 8 | 0.25 | 0.5 | 0.125 | 0.5 | 0.25 | 0.5 | 4 | 32 |
Fraction of flash chromatography (100% ethyl acetate),
μg/ml
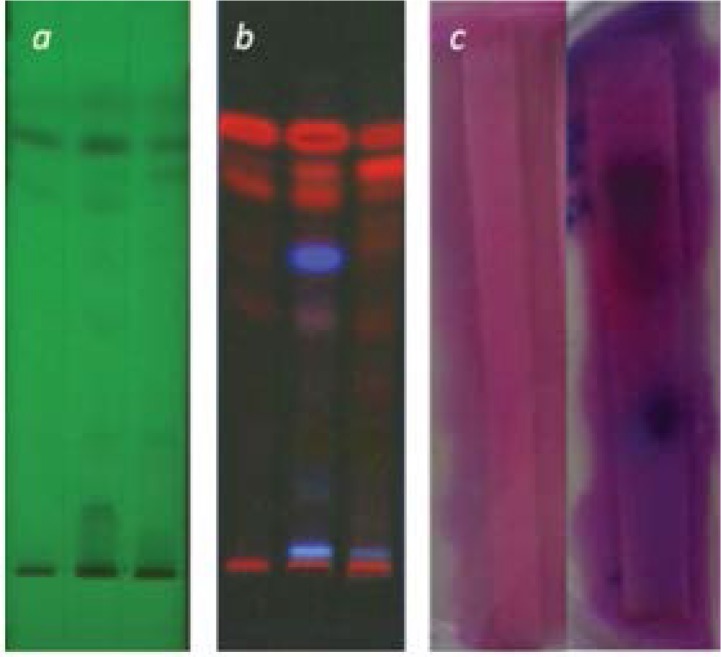
In the HPTLC experiments, the best resolution of bands was obtained by mobile phase of Methanol: n-Hexane: Ethyl acetate (1:2:4) (Fig. 1a & 1b).
Fig. 1.
Silica plates containing developed bands of ethyl acetate fraction (a, under 254 nm and b, under 366 nm). C. indicates control of experiment (left, developed plate without sample) and plate after agar overlay and addition of Resazurin as bacterial growth inhibition (right).
As shown in Fig. 1 (a, b and c), an agar overlay assay was performed in duplicate plates for identification of the bands containing effective antibacterial substances. The results revealed 2 bands without any growth of S. aureus. According to the Bio-autography experiments, there were 2 zones of inhibition around two separated band in Ethyl acetate sample which have been developed by using Resazurin (Fig. 1c). Then a flash chromatography was performed for further separation of substances of ethyl acetate fraction and after determination of MIC values of 5 resulting fraction, it was revealed that 100% ethyl acetate fraction contained effective antibacterial compounds (as shown in the Table 1).
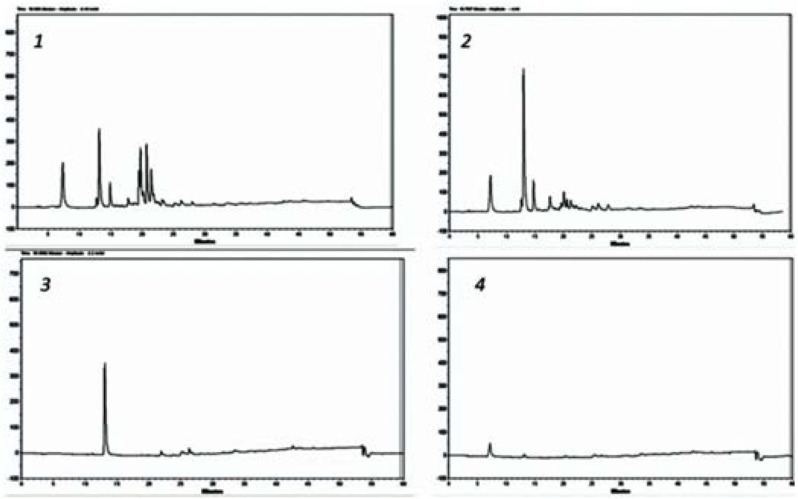
The ethyl acetate fraction and other semi-purified antibacterial substances were subjected to RP-HPLC experiments and the results (Fig. 3) could reveal an increase in the intensity of some peaks during the process (from a to d) which was in accordance with increasing of the antibacterial properties of samples as shown in the Table 1.
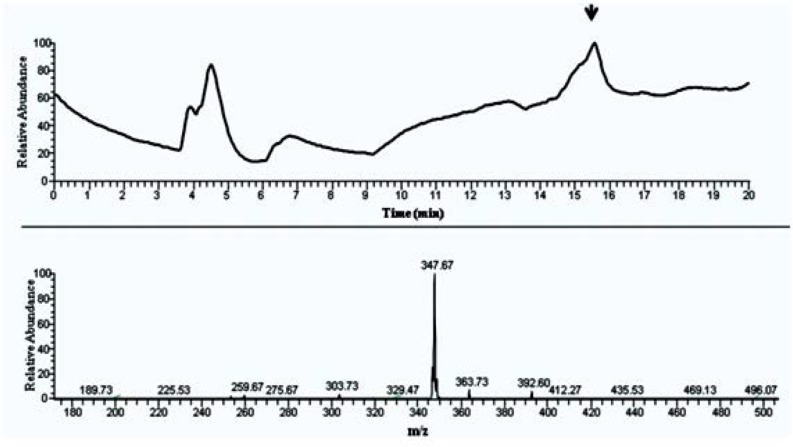
Fig. 3.
MS spectrum and chromatogram of band 5 (from TLC) in LC-ESI-MS analysis. One of the most effective band resolved in TLC, resulted in a sharp peak which corresponds to a 348 Da compound.
Some reports could reveal the antimicrobial activity of P. cineraria against pathogens such as Bacillus subtilis and Staphylococcus aureus (8, 9). But there are no reports on determination of the effective antibacterial substance (s). Although presence of bioactive secondary metabolites like alkaloid, flavonoid, glycosides, saponins, tannins, steroids and terpenoids was reported of this plant (8), it is not clear whether these known components were the actual only contributing components of P. cineraria to the anti-microbial activities against the bacterial pathogen.
The antimicrobial assays were tested against major bacteria with a focus on antibiotics-resistant strains, particularly those of S. aureus. The agar overlay Bioautography method in this study has been used also for preparative separation and purification of P. cineraria bioactive antimicrobials as well as our previous report about determination of antibacterial compounds of Bromus inerrmis (14).
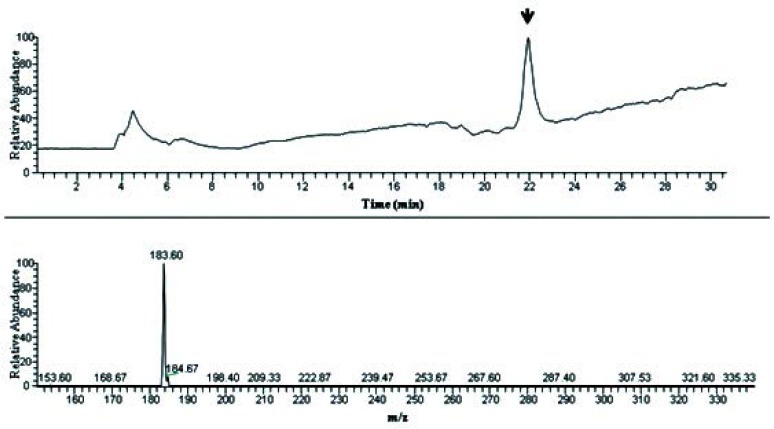
For further analysis, all mentioned sample were analyzed by RP-HPLC (Fig. 2) and all optimized HPLC-DAD separation was extended to LC-ESI-MS equipment. For this both negative and positive mode of ionization were tried. Interestingly we found intensive m/z in only negative mode. Figs 3 and 4 show the presence of 347 m/z and 183 m/z that could be related to compounds with molecular weight of 348 and 184 Dalton. Both UV-Vis and mass spectrum results implying presence of compounds like phenolic or flavonoid structures (data not shown).
Fig. 2.
RP-HPLC chromatograms of fractions: 1. Ethyl acetate fraction, 2. 100% Ethyl acetate after flash chr., 3. Effective band 5 from TLC plate, 4. Effective band 4 from TLC plate.
Fig. 4.
MS spectrum and chromatogram of band 4 (from TLC) in LC-ESI-MS analysis. The most effective band resolved in TLC resulted in a sharp peak which corresponds to a 184 Da compound.
To the best of our knowledge, this is the first report on characterization of antibacterial substances from P. cineraria plant. It can hypothesize that further investigations with nuclear magnetic resonance (NMR) analysis could reveal the identity of the effective substances in our continuous research. Such antibacterial substances can also be considered as the lead compounds for semi-synthesis of new effective antibiotics.
CONCLUSION
According to the result of this study it was concluded that characterized compounds may be applied as lead compound for designing of new and more efficient antibacterial agents. Also bioassay-guided fractionation could be served as an efficient and even cost benefit method for characterization of naturally bioactive compounds.
ACKNOWLEDGEMENTS
This work was jointly supported by Tehran University of Medical Sciences (Project 24753) and Medicinal Plants and Drug Research Institute, Shahid Beheshti University. The authors would like to thank Mr. Mahdi Brazesh for providing plant samples and Dr. Ali Sonboli for kindly identification and recording of the herbarium sample of plant.
REFERENCES
- 1. Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 18: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wenzel RP, Edmond MB. The impact of hospital-acquired bloodstream infections. Emerg Infect Dis 2001; 7: 174– 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klaywong K, Khutrakul G, Choowongkomon K, Lekcharoensuk C, Petcharat N, Leckcharoensuk P, et al. Screening for lead compounds and herbal extracts with potential anti-influenza viral activity. Southeast Asian J Trop Med Public Health 2014; 45: 62– 64. [PubMed] [Google Scholar]
- 4. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12 : 564– 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thevissen K, Kristensen H, Thomma BPH J, Cammue BPA, 4, Francois IEJA. Therapeutic potential of anti-fungal plants and insect defensins. Drug Discov Today 2007; 12 (21/22): 966– 672. [DOI] [PubMed] [Google Scholar]
- 6. Maroti G, Kereszt A, Kondorosi E, Mergaert P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res Microbiol 2011; 162: 363– 374. [DOI] [PubMed] [Google Scholar]
- 7. Sharma N, Garg V, Paul A. Antihyperglycemic, anti-hyperlipidemic and antioxidative potential of Prosopis cineraria bark. Indian J Clin Biochem 2010; 25: 193– 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velmurugan V, Arunachalam G, Ravichandran V. Antibacterial activity of stem bark of Prosopis cineraria (Linn.) Druce. Arch Appl Sci Res 2010; 2: 147– 150. [Google Scholar]
- 9. Mohammad IS, Haji Muhammad Shoaib Khan NA, Rasool F. Biological potential and phytochemical evaluation of Prosopis cineraria. World Appl Sc. 2013; 27: 1489– 1494. [Google Scholar]
- 10. Jorgensen JH, Turnidge JD. (2007). Susceptibility test methods: Dilution and disk diffusion methods. In: Murray PR, Baron EJ, Jorgensen JH, Landry MR, Pfaller MA, editors. Manual of Clinical Microbiology. 9 ed ASM PRESS; Washington DC, pp. 1152– 1172. [Google Scholar]
- 11. Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42: 321– 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waksmundzka-Hajnos M, Sherma J, Kowalska T. (2008). Thin layer chromatography in phytochemistry. CRC Press. [Google Scholar]
- 13. Aliahmadi A, Roghanian R, Emtiazi G, Ghassempour A. A simple method for primary screening of antibacterial peptides in plant seeds. Iran J Microbiol 2011; 3: 104– 108. [PMC free article] [PubMed] [Google Scholar]
- 14. Aliahmadi A , Mirzajani F, Ghassempour A, Sonboli A. Bioassay guided fractionation of an anti-methicillin-resistant Staphylococcus aureus flavonoid from Bromus inermis Leyss Inflorescences. Jundishapur J Microbiol 2014; 7 ( 12). e12739. [DOI] [PMC free article] [PubMed] [Google Scholar]