Abstract
Background and Objectives:
Dermatophytes possess a wide array of virulence factors and various antifungal susceptibility patterns which influence their pathogenesis in humans and animals. The aim of this study was to evaluate antifungal susceptibility and keratinase and proteinase activity of 49 dermatophyte strains from the genera Microsporum, Trichophyton and Epidermophyton which were isolated from human cases of dermatophytosis.
Materials and Methods:
Forty-nine dermatophyte strains isolated from clinical samples were cultured on general and specific culture media. Keratinase and proteinase activity was screened on solid mineral media and confirmed in liquid cultures. Drug susceptibility toward azoles (fluconazole, ketoconazole and itraconazole), griseofulvin and terbinafine was evaluated using disk diffusion method on Mueller-Hinton agar and minimum inhibitory concentrations (MICs) were determined using microbroth dilution assay according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results:
Our results indicated that clinically isolated dermatophytes from 7 major species produced keratinase and proteinase at different extents. The mean keratinase and proteinase activity was reported as 6.69 ± 0.31 (U/ml) and 2.10 ± 0.22 (U/ml) respectively. Disk diffusion and microbroth dilution (MIC) results of antifungal susceptibility testing showed that ketoconazole was the most effective drug against Epidermophyton floccosum and Trichophyton mentagrophytes, itraconazole against T. rubrum and E. floccosum, and griseofulvin and terbinafine against Trichophyton verrucosum. Our results showed that all dermatophyte isolates were resistant to fluconazole. Overall, ketoconazole and itraconazole were the most effective drugs for all dermatophyte species tested.
Conclusion:
Our results showed that antifungal susceptibility testing is an urgent need to select drugs of choice for treatment of different types of dermatophytosis and further indicated the importance of keratinase and proteinase in pathogenesis of dermatophyte species.
Keywords: Dermatophytes, Antifungal susceptibility testing, Keratinase activity, Proteinase activity, Virulence factors
INTRODUCTION
Dermatophytes are a frequent label for a group of three genera of fungi that result in various types of skin disorders in both animals and humans (1–5). These types of fungi affect millions of people establishing public health problems annually due to prolonged therapeutic approaches and refractivity to treatment (6–8). The global prevalence of dermatomycoses has been estimated to be about 20% (9). However, a sophisticated understanding of dermatophyte pathogenesis has remained uncertain (10). These fungi obtain their nutrients from keratinized material and their colonization in the keratin tissues causes inflammation as the host responds to metabolic by-products (11). Colonies of dermatophytes are frequently restricted to the nonliving cornified layer of the epidermis. In total, invasion of fungi does elicit a host response ranging from mild to severe. Acid proteinases, elastase and keratinases have been reported to involve in dermatophyte pathogenesis (12). The development of cell-mediated immunity correlated with delayed type hypersensitivity and an inflammatory response is associated with clinical cure, whereas the lack of or a defective cell-mediated immunity predisposes the host to chronic or recurrent dermatophyte infection (13). In this regard, the evaluation of the specific types and role of virulence factors in onset of infection and sequence analysis of special conserved genes for identifying of closely-related dermatophytes are of major importance especially for native clinical isolates (14). Moreover, there is evidence that some fungal strains are resistant to certain antifungal drugs which resulting in therapeutic failures (8). Some reasons for failure of dermatophytosis treatment in clinical practice are peripheral vascular disease, resistant structures such as subungual dermatophytom as and the presence of dormant fungal spores (15). Occasionally, in some cases, antifungal therapy is a failure because of resistance to the antifungal drugs by the fungi (16). Thus, determining antifungal susceptibility of clinical dermatophytes is very vital not only for directing the treatment protocols, but also for studying mechanisms of drug resistance in these fungi (17). Overall, it is essential to evaluate the resistant dermatophytes using a standard, simple and reproducible in vitro assay to determine the antifungal activity of drugs against isolates (18).
Evaluation of virulence factors, the assessment of their role in infection, and the evaluation of gene sequences in specific regions to identify and subtraction of dermatophytes as a group of fungi with morphological similarities is very important for differentiating clinical endemic species (19). In addition, determining drug susceptibility of clinical strains of fungi for targeting treatment and studying the mechanisms of drug resistance is very important (20, 21). In the present study, antifungal susceptibility of 49 clinically isolated dermatophytes from 7 different species was evaluated against azoles drugs, griseofulvin and terbinafine by disk diffusion and microbroth dilution assays. Enzymatic activity of the dermatophytes for two important enzymes involved in fungal pathogenesis i.e. keratinase and proteinase was also studied.
MATERIALS AND METHODS
Dermatophyte strains and culture conditions.
In this study, standard strains of M. canis (PFCC 5069), M. gypseum (PFCC 5070), T. mentagrophytes (PFCC 5054), T. verrucosum (PFCC 1069), as well as 49 clinical dermatophyte strains provided from Pathogenic Fungi Culture Collection, Mycology Department of Pasteur Institute of Iran (http://fa.pasteur.ac.ir/Pages.aspx?id=1152) were studied (Table 1). All the dermatophytes were inoculated on Sabouraud Dextrose agar (E. Merck, Germany) supplemented with chloramphenicol (0.005%) and cycloheximide (0.04%). Cultures were maintained at 28 °C for 7 to 10 days. After the incubation time, the spore suspension was prepared by adding sterile distilled water contained 0.1% Tween 80 and the number of spores (mixture of microconidia and macroconidia) was counted using a Neubauer slide and adjusted to 1 × 106 to 5 × 106 spores/ml according to the CLSI M38-A protocol.
Table 1.
Clinico-epidemiological data of dermatophyte species isolated from clinical specimens.
| Dermatophyte | Strain No. | Patient (Sex-Age) | Site of isolation |
|---|---|---|---|
| T. interdigitale | 2788 | F-42 | Body |
| 379 | M-28 | Hand | |
| 1839 | M-54 | Sole | |
| 425 | M-50 | Wrist | |
| 500 | M-34 | Foot | |
| 471 | F-56 | Sole | |
| 803 | M-54 | Sole | |
| 1523 | F-48 | Sole | |
| 897 | M-43 | Hand | |
| 833 | F-34 | Trunk | |
| 298 | M-65 | Sole | |
| T. rubrum | 384 | M-57 | Toe lees |
| 1842 | F-42 | Toe lees | |
| 1739 | M-45 | Groin | |
| 391 | M-35 | Hand | |
| 1792 | M-28 | Wrist | |
| 2422 | F-44 | Trunk | |
| M. canis | 2349 | M-7 | Body |
| 1319 | F-47 | Forearm | |
| 1215 | F-55 | Forearm | |
| 5065 | F-22 | Body | |
| 1612 | F-40 | Trunk | |
| 2349 | M-38 | Leg | |
| T. tonsurans | 1734 | F-16 | Leg |
| 1935 | M-64 | Toe lees | |
| 457 | M-15 | Head hair | |
| 2142 | M-27 | Groin | |
| 1922 | M-14 | Face | |
| 2096 | F-9 | Head | |
| 430 | M-16 | Neck | |
| M. gypseum | 1019 | F-60 | Sole |
| 1891 | F-46 | Elbow | |
| 1783 | F-25 | Chest | |
| 1998 | M-40 | Toe lees | |
| 1861 | M-22 | Groin | |
| E. floccosum | 1757 | F-38 | Groin |
| 1889 | F-34 | Forearm | |
| 2070 | M-46 | Body | |
| 1952 | M-37 | Groin | |
| 1934 | M-28 | Groin | |
| 2534 | F-54 | Toe lees | |
| 2286 | M-60 | Toe lees | |
| 225 | M-23 | Groin | |
| 2068 | M-23 | Groin | |
| T. verrucosum | 50 | F-63 | Foot nail |
| 2918 | M-47 | Hand | |
| 921 | F-19 | Forearm | |
| 2319 | F-44 | Face | |
| 320 | M-51 | Toe lees | |
| 50 | F-63 | Foot nail |
Assessment of virulence factors.
For screening keratinase activity, dermatophytes (106 cells/ml) were cultured in solid mineral medium (0.1 g K2HPO4, 0.5 g MgSO4, 2.0 g NaNO3, 0.01 g FeSO4 7H2O, 0.005 g ZnSO4 7H2O, 3.8 g NaH2PO4, 3.97 g Na2HPO4, 0.5 g cycloheximide, 0.05 g chloramphenicol, 15 g agar in 1000 ml distilled water) and incubated at 25 °C for 7 days. Clear zone diameters around the dermatophyte colonies were considered as an index of keratinase activity. The spectrophotometry was used to assess keratinase activity in liquid mineral medium according to Wawarzkiewicz et al. (22). Keratin powder (20 mg), 3.0 ml phosphate buffer (28 mM, pH 7.8) and 2.0 ml fungal culture filtrate prepared from dermatophyte cultures on liquid mineral medium (mentioned above) were incubated in a shaker water bath (150 rpm) for 1 hour at 37 °C. Tricholoroacetic acid (TCA; 10%) was added to the reaction mixture and centrifuged at 10,000 × g for 15 min. The optical absorption of the supernatant was measured at 280 nm wavelengths using a double-beam UV/VIS 1601 Shimadzu spectrophotometer toward the blank. The blank was treated in the same manner except for the time of TCA addition which was carried out before the initiation of enzyme reaction. The increase of 0.1 unit absorption was equal to one unit of enzyme activity. To screen the production of proteinases, 10 ml of the spore suspension of each dermatophyte (106 cells/ml) was inoculated in solid casein-based medium containing casein 0.5%, glucose 2.0%, K2HPO4 0.1%, MgSO4 0.05%, and Agar 2% in 1000 ml distilled water. The plates were kept at 30 °C for 7 days. The cultures were fixed by Tricholoroacetic (TCA) and then stained with amido-black solution. Clouding of the medium was identified by creating an area of proteolysis surrounding the colonies. Proteinase activity was determined by the measuring diameter of uncolored region around the fungal colonies. The spectrophotometry was used to assess proteinase activity in casein-based liquid medium (mentioned above). An amount of 250 μl of casein prepared in 20 mM Tris-HCl buffer was incubated in a shaker water bath for 1 h. Then, 50 μl of 10% TCA was added to the reaction mixture on ice and centrifuged at 13,000 × rpm for 5 min. Two-hundred μl of resulting supernatant was transferred to each well of a flat-bottom 96 micro-plate and read at 405 nm by an ELISA Plate Reader (Bio Tek Instruments, USA). One unit of proteinase activity was defined as 0.01 increases in absorbance at 405 nm wavelength (23). All the experiments were carried out twice in triplicate sets each.
Antifungal susceptibility testing
Disk diffusion assay.
Dermatophyte strains were evaluated for antifungal susceptibility to commonly used antifungal drugs using standard disks (6.0 mm Dia., MAST Diagnostics) of fluconazole (25 μg), itraconazole (10 μg), 30 mg terbinafine (30 μg), griseofulvin (25 μg), and ketoconazole (10 μg) on Mueller Hinton agar supplemented with glucose (2%) and methylene blue (0.5 μg/ml). Spore suspension (1 × 104 to 5×104 spores/ml) of dermatophytes was spread on agar plates and antifungal disks were placed on the plates. The plates were incubated at 28 °C and checked for clear zones of inhibition around fungal colonies daily up to 7 days (24).
MIC determination by microbroth dilution (CLSI method).
The microbroth dilution assay for antifungal susceptibility testing of der matophytes was performed according to the CLSI guidelines-document M38-A of filamentous fungi. Fluconazole (Pfizer International, New York, NY) was dissolved in distilled water while the other drugs including ketoconazole and itraconazole (Jansen Pharmaceuticals, Beerse, Belgium), terbinafine (Novartis Research Institute, Vienna, Austria) and griseofulvin (Sigma Chemical Company, St. Louis, Mo) were dissolved in 100% dimethylsulfoxide (Sigma-Aldrich). Antifungal drugs were prepared as stock solution and serial two-fold dilutions were obtained to provide final concentrations ranged from 0.0313 to 64 μg/ml for fluconazole, 0.03 to 16 μg/ml for ketoconazole, itraconazole, terbinafine, and griseofulvin. Inoculum suspensions of dermatophytes were prepared from the seven days cultures grown on potato dextrose agar supplemented with chloramphenicol (0.005%) and cycloheximide (0.04%) at 28 °C. The fungal colonies were covered with approximately 10 ml of distilled water, and the suspensions were made by scraping the surface with the tip of a sterile loop. The resulting mixture of conidia and hyphae fragments was withdrawn and transferred to sterile tubes and left for 15 to 20 minutes at room temperature to sediment the heavy particles. Suspensions containing micro- and/or macroconidia were diluted with RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo) to obtain the final inoculum size of approximately 1 to 5 × 104 cells/ml. Aliquots of 100 μl of these suspensions were inoculated in well of microtiter plate containing 100 μl of specific antifungal drug concentration and incubated at 28 °C. (25). Each microdilution well containing 100ml of the diluted drug concentrations was inoculated with 100ml of the diluted microconidia or arthroconidia inoculum suspensions (final volume in each well was 200ml) to bring the dilutions of the inoculum from 1×104 to 5×104 cfu/ml. Growth and sterility controls were included for each isolate tested. Microtiter trays were incubated at 28 °C and MICs were recorded after 5 days of incubation (26). Endpoint determination values were determined visually every 24 h until the indication of growth in drug-free control wells. For azole drugs and griseofulvin, the MIC was defined as the lowest concentration that produced prominent inhibition of growth (approximately 80% inhibition) while for terbinafine, it was defined as the lowest concentration showing 100% growth inhibition (27). All the experiments were carried out twice in triplicate sets each.
Statistical analysis.
Statistical analyses were performed using under windows SPSS version 15 (SPSS Inc., Chicago, IL) by one-way ANOVA. P-values less than 0.05 were considered significant.
RESULTS
Keratinase and proteinase activity.
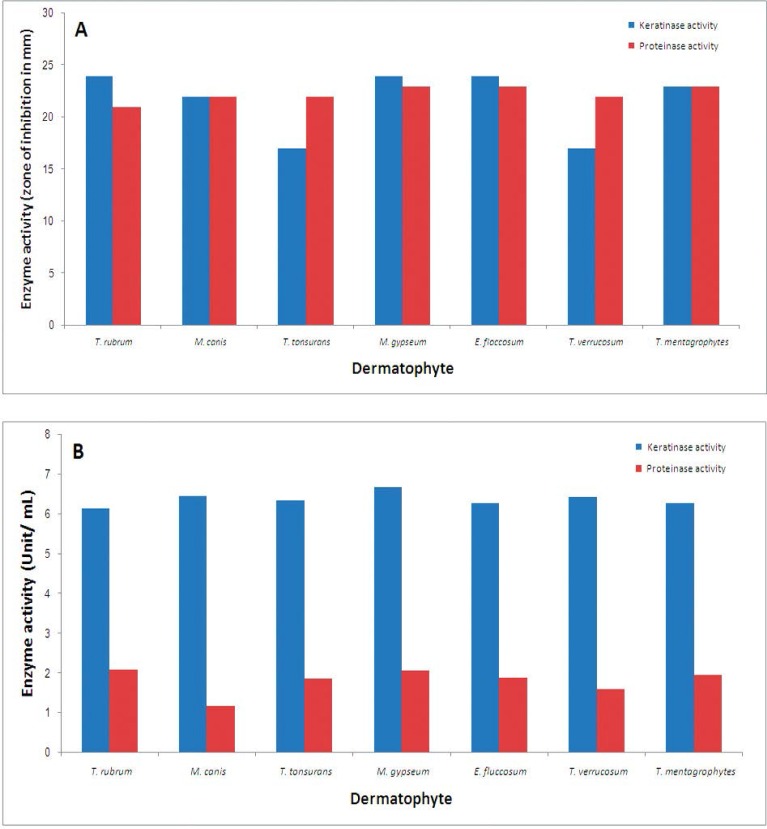
In this study, 7 strains of T. rubrum, 7 strains of M. canis, 12 strains of T. interdigitale, 7 strains of T. tonsurans, 5 strains of M. gypseum, 9 strains of E. floccosum and 6 strains of T. verrucosum were evaluated for keratinase and proteinase activity. No difference was revealed in sex distribution (p = 0.571), however mean age was significantly different in affected samples with the various types of specimens (p = 0.009) (Table 3). Comparative results of the production of keratinase and proteinase in solid and liquid culture media are shown in Fig. 1. In total, the mean keratinase activity in genus of Trichophyton was 6.30 ± 0.75 (U/ml), in the genus of Microsporum was 6.57 ± 0.51 (U/ml), and in the genus of Epidermophyton was 6.29 ± 0.46(U/ml) with no significant difference (p=0.243). Also, the mean proteinase activity in Trichophyton species was 1.90 ± 0.93(U/ml), in Microsporum species was 1.59 ± 0.55 (U/ml), and in Epidermophyton isolates was 1.90 ± 0.69 (U/ml) with no significant difference (p = 0.269). As shown in Table 2, the mean keratinase activity in different strains of dermatophytes determined as 6.16 ± 0.21 (U/ml) in T. rubrum, 6.69 ± 0.54 (U/ml) in M. gypseum and the mean proteinase activity found to be 1.17 ± 0.22 (U/ml) in M. canis and 2.10 ± 0.81 (U/ml) in T. rubrum.
Table 3.
Antifungal drug susceptibility in different dermatophyte species determined by disk diffusion assay
| Dermatophyte species (No.) | Clear zone of fungal growth inhibition (Mean ± SD; mm) | |||
|---|---|---|---|---|
| Ketoconazole | Itraconazole | Griseofulvin | Terbinafine | |
| T. rubrum (7) | 22.0 ± 0.25 | 24.0 ± 0.15 | 16.0 ± 0.15 | 15.0 ± 0.15 |
| M. canis (7) | 22.0 ± 0.25 | 23.0 ± 0.20 | 16.0 ± 0.20 | 16.0 ± 0.12 |
| T. tonsurans (7) | 22.0 ± 0.15 | 22.0 ± 0.25 | 15.0 ± 0.15 | 14.0 ± 0.25 |
| M. gypseum (5) | 22.0 ± 0.15 | 23.0 ± 0.15 | 17.0 ± 0.15 | 17.0 ± 0.14 |
| E. floccosum (9) | 23.0 ± 0.35 | 24.0 ± 0.10 | 16.0 ± 0.10 | 16.0 ± 0.23 |
| T. verrucosum (6) | 22.0 ± 0.20 | 22.0 ± 0.35 | 18.0 ± 0.25 | 18.0 ± 0.15 |
| T. mentagrophytes (12) | 23.0 ± 0.35 | 22.0 ± 0.30 | 17.0 ± 0.30 | 17.0 ± 0.10 |
Fig. 1.
Keratinase and proteinase activity of different strains of dermatophytes in solid (A) and liquid (B) culture media.
Table 2.
Keratinase and proteinase activity (mean ± SD) of different dermatophytes species on solid and liquid culture media
| Dermatophyte species (No.) | Keratinase production in solid medium (mm) | Keratinase activity in liquid culture (Unit/ml) | Proteinase production in solid medium (mm) | Proteinase activity in liquid culture (Unit/ml) |
|---|---|---|---|---|
| T. rubrum (7) | 24.0 ± 0.25 | 6.16 ± 0.31 | 21.0 ± 0.22 | 2.10 ± 0.81 |
| M. canis (7) | 22.0 ± 0.83 | 6.47 ± 0.48 | 22.0 ± 0.23 | 1.17 ± 0.22 |
| T. tonsurans (7) | 17.0 ± 0.45 | 6.34 ± 0.29 | 22.0 ± 0.13 | 1.87 ± 0.50 |
| M. gypseum (5) | 24.0 ± 0.72 | 6.69 ± 0.54 | 23.0 ± 0.22 | 2.08 ± 0.38 |
| E. floccosum (9) | 24.0 ± 0.74 | 6.29 ± 0.46 | 23.0 ± 0.41 | 1.90 ± 0.69 |
| T. verrucosum (6) | 17.0 ± 0.53 | 6.43 ± 0.20 | 22.0 ± 0.24 | 1.60 ± 0.52 |
| T. mentagrophytes (12) | 23.0 ± 0.86 | 6.29 ± 0.61 | 23.0 ± 0.34 | 1.96 ± 0.60 |
Antifungal drug susceptibility
Disk diffusion assay.
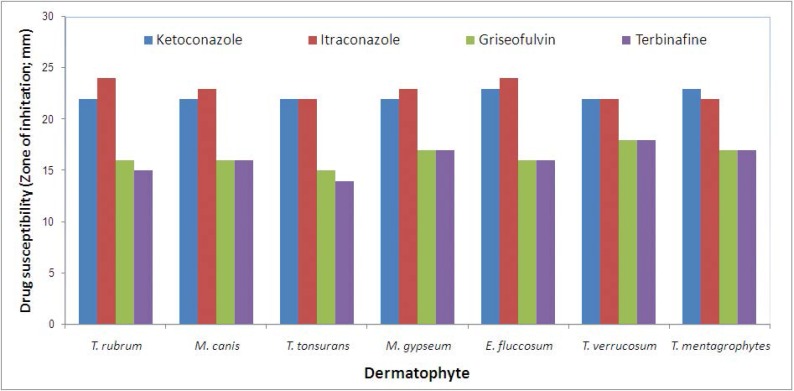
With respect to drug sensitivity of isolates, in genus of Trichophyton, the mean sensitivity to ketoconazole was estimated to be 23 ± 0.35 mm, to itraconazole was 22 ± 0.25 mm, to griseofulvin was 16 ± 0.10 mm, and to terbinafine was 14 ± 0.25 mm (Table 3). In genus of Microsporum, the mean sensitivity to ketoconazole was estimated to be 22 ± 0.15 mm, to itraconazole was 23 ± 0.15 mm, to griseofulvin was 16 ± 0.20 mm, and to terbinafine was 16 ± 0.12 mm and in genus of Epidermophyton, the mean sensitivity to ketoconazole was estimated to be 23 ± 0.35 mm, to itraconazole was 24 ± 0.10 mm, to griseofulvin was 16 ± 0.10 mm, and to terbinafine was 16 ± 0.23 mm (Table 3). Comparative results of drug susceptibility of various dermatophyte species to examined antifungal agents indicated that the highest sensitivity to ketoconazole was related to E. floccosum and T. mentagrophytes, the highest sensitivity to itraconazole was found in T. rubrum and E. floccosum, the highest sensitivity to griseofulvin was found in T. verrucosum, and the highest sensitivity to terbinafine was revealed for T. verrucosum (Fig. 2). In total, the sensitivity to ketoconazole and itraconazole in different strains was higher than to other types of antibiotics including griseofulvin and terbinafine.
Fig 2.
Antifungal drug susceptibility in different strains of dermatophytes by disk diffusion assay
Microbroth dilution assay.
MICs of antifungal agents for 53 dermatophyte isolates were determined after five days when incubated at 28 °C. Table 4 summarizes the MIC range, concentrations inhibiting 50% (MIC50) and 90% (MIC90) of the isolates and geometric mean of the MICs of the five antifungal drugs against 53 strains of dermatophytes. Range of MIC50 for itraconazole was 0.0313 to 0.25 μg/ml and MIC90 was 0.0313 to 1 μg/ml. Range of MIC50 for ketoconazole was 0.0313 to 0.25 μg/ml and MIC90 was 0.25 to 2 μg/ml. For fluconazole, MIC50 range was 12 to 32 μg/ml and MIC90 range was 4 to 64 μg/ ml. These amounts for griseofolvin were 0.5 to 2 μg/ml and 0.5 to 2 μg/ml, respectively. MIC50 range for terbinafine was 0.5 to 2 μg/ml and MIC90 range was 1 to 4 μg/ml. Overall, similar to the results of disk diffusion, MIC data showed that the activity of fluconazole was significantly lower than that of the other antifungal drugs tested.
Table 4.
In-vitro susceptibility testing of 5 antifungal drugs against 7 dermatophytes species by microbroth dilution assay (CLSI method)
| Species (number of strains tested) | Antifungal drug concentration (μg/ml) | |||||
|---|---|---|---|---|---|---|
| ITZ | KTZ | GF | TER | FCZ | ||
| T. interdigitale (n=12) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.0625 | 0.187 | 0.125 | 0.5 | 16 | |
| MIC90 | 0.5 | 2 | 2 | 4 | 32 | |
| GM | 0.1007 | 0.183 | 1 | 1.485 | 16.95 | |
| T. tonsurans (n=7) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.125 | 0.25 | 0.5 | 2 | 16 | |
| MIC90 | 0.25 | 1 | 0.5 | 2 | 32 | |
| GM | 0.09 | 0.182 | 0.82 | 1.811 | 19.50 | |
| T. rubrum (n=7) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.0625 | 0.125 | 0.5 | 2 | 16 | |
| MIC90 | 0.25 | 1 | 2 | 4 | 4 | |
| GM | 0.135 | 0.165 | 0.905 | 1.097 | 23.77 | |
| T. verrucosum (n=6) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.125 | 0.25 | 1 | 2 | 24 | |
| MIC90 | 0.25 | 0.5 | 2 | 2 | 64 | |
| GM | 0.109 | 0.195 | 0.206 | 0.495 | 28.50 | |
| E. floccosum (n=9) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.25 | 0.25 | 0.5 | 0.5 | 16 | |
| MIC90 | 1 | 1 | 2 | 2 | 32 | |
| GM | 0.652 | 0.122 | 0.282 | 0.66 | 18.66 | |
| M. canis (n=7) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.125 | 0.25 | 1 | 2 | 16 | |
| MIC90 | 0.5 | 0.5 | 2 | 4 | 32 | |
| GM | 0.181 | 0.290 | 0.63 | 0.73 | 16 | |
| M. gypseum (n=5) | Range | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 16 | 0.0313 – 64 |
| MIC50 | 0.0313 | 0.0625 | 0.5 | 0.5 | 32 | |
| MIC90 | 0.0313 | 0.25 | 1 | 1 | 64 | |
| GM | 0.154 | 0.165 | 0.49 | 1.22 | 32 | |
MIC50: minimum inhibitory concentration 50%
MIC90: minimum inhibitory concentration 90%
GM: geometric mean MIC
ITZ: itraconazole, KTZ: ketoconazole, GF: griseofulvin, TER: terbinafine, FCZ: fluconazole
DISCUSSION
In our study and with regard to the keratinases and proteinases activity, we found the highest and the lowest keratinases activity in the strains of M. gypseum and T. rubrum, respectively, while, the highest and the lowest proteinases activity was revealed in T. rubrum and M. canis, respectively. On the other hand, although our T. rubrum isolates had the highest proteinases activity, but had the lowest keratinases activity. In overall, the difference in both keratinases and proteinases activity in different genus of dermatophytes was shown to be statistically similar. In a similar study by Sharma and colleagues (28), Trichophyton produced the highest activity of keratinase that was in contrary to our findings. Also, in another study by Muhsin and Salih (29), high keratinase activity was expressed by T. mentagrophytes and M. gypseum that was also in contrary to our result. They also showed that high protease activity that was similar to our result. T. rubrum is the most common dermatophyte of humans and normally colonizes the superficial layers of the epidermis. Several proteinases with a possible role in the metabolism of host proteins have been purified from this fungus (30). The regulation of these enzymes and their role in fungal metabolism were studied at the biochemical level (30). In our previous study, the maximum keratinolytic activity was revealed in the strains of T. mentagrophytes (30). Summarizing the results of our study and previous observations indicates that controversial evidences are in term of keratinases activity of this fungus, but almost all studies have evidenced its high proteinases activity among other strains of dermatophytes. However, because of high prevalence of T. rubrum, followed by E. floccosum, T. mentagrophytes as the most common dermatophytes as pathogenic causes for dermatomycoses (31), the especial importance to assessing keratinases activity of these fungi can result in reducing the trend of these fungal diseases. Overall, we did not find any obvious correlation between enzymatic activities of the dermatophyte species and strains with antifungal susceptibility patterns
Regarding sensitivity to different types of antibiotics, our study could show high sensitivity of different strains of dermatophytes to ketoconazole and itraconazole, while sensitivity to griseofulvin and terbinafine was indicated to be lower. However, the sensitivity of the isolates to all types of studied antibiotics was shown to be high. Goh et al. (32) indicated that of dermatophyte isolates, 82% were sensitive to griseofulvin, 78% to ketoconazole, and 81% to itraconazole. The in vitro antifungal activity of griseofulvin, ketoconazole, and itraconazole were similar against dermatophytes among Singaporean population, however in that population, griseofulvin was given as the first-line drug for treating such infections in Singapore in comparison to our results among Iranian population that ketoconazole and itraconazole seems to be more effective than griseofulvin. In another study by Gupta and Kohli (33), the MIC values for itraconazole was the highest, followed by ketoconazole and terbinafine and thus terbinafine was extremely potent against dermatophytes. We reported that the highest susceptibility of tested dermatophytes to ketoconazole and itraconazole and the lowest susceptibility to fluconazole.
In the present study, T. tonsurans showed the lowest and T. verrucosum showed the highest susceptibility to both griseofulvin and terbinafine. In a study by Ghannoum et al. (34), the MIC values for terbinafine for all dermatophyte isolates were significantly lower than those for itraconazole, ketoconazole, and griseofulvin. Among the antifungal drugs tested, griseofulvin had the highest minimum inhibitory concentration values for T. mentagrophytes isolates. They finally concluded that terbinafine was found to be the most effective antifungal drug against all tested dermatophyte isolates that was not in line with our results. It seems that terbinafine is more effective than griseofulvin to eradicate dermatophytes, while the controversy in effectiveness of this drug in comparison with ketoconazole and itraconazole remains to be further studied.
Agarwa et al. (35) examined a total of 55 strains of dermatophytes for antifungal susceptibility by agar based disk diffusion method and found the susceptibility of the majority of strains to fluconazole, itraconazole, terbinafine and griseofulvin. These results were in contrast to the results of our study for fluconazole and in similar to our results for other antifungal drugs tested. In the present study, the evaluation of in vitro susceptibility showed that the antifungal drugs tested except fluconazole had good activity against the dermatophytes. It is worth mentioning that itraconazole, ketoconazole and terbinafine had the lowest MIC values and geometric mean MICs. The low amounts of MICs found for the three drugs can help to explain the promising results obtained for the treatment of dermatophytosis with these antifungal agents. Although fluconazole showed the highest MIC values among all the antifungal agents tested, we showed that all the strains of T. rubrum, one species that cause a recalcitrant chronic disease, were more susceptible to this drug than T. mentagrophytes and M. canis isolates. The MIC results for itraconazole obtained in our study were similar to those reported by Barros et al. (36) and in contrast to those reported by Fernandez-Torres et al. (37).
CONCLUSION
Taken together, the variation in susceptibility of clinically derived fungal isolates indicates that identification of causative fungi is indispensable for appropriately choosing effective antifungal drugs in the early stages of infection. It seems that using multiple drugs with different antifungal mechanisms against dermatophytes can be effective to treat dermatomycoses. Results of the present study further indicated that in contrast to popular opinion of effectiveness of fluconazole in treatment of dermatophytosis, this drug showed the highest MIC values in comparison with the other tested agents indicating that it is not a choice drug for curing dermatophyte-induced disorders. No obvious correlation was recorded for production of virulence factors and antifungal susceptibility profiles of tested dermatophytes. We found that ketoconazole and itraconazole were the most effective antifungal drugs against dermatophyte isolates from various species and thus, these two antifungals are highly recommended for treatment of clinical cases of dermatophytosis.
Acknowledgments
This work was supported financially by the research deputy of Tarbiat Modares University. Authors wish to thank Hoda Mousa, Akram Moslemi and Fatemeh Sadat Jamzivar from Mycology Department of the Pasteur Institute of Iran for their helpful technical assistance.
REFERENCES
- 1. Ameen M. Epidemiology of superficial fungal infections. J Clin Dermatol 2010;28: 197– 201. [DOI] [PubMed] [Google Scholar]
- 2. Havlickova B, Czaika VA. Epidemiological trends in skin mycoses worldwide. Mycoses 2008; 51: 2– 15. [DOI] [PubMed] [Google Scholar]
- 3. Achterman RR, White TC. Dermatophyte virulence factors: Identifying and analyzing genes that may contribute to chronic or acute skin infections. Int J Microbiol 2012; 358305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jankowska- Konsur A, Dylag M, Hryncewicz- Gwozdz A, Plomer-Niezgoda E, Szepietowski CJ. A 5-year survey of dermatomycoses in southwest Poland, years 2003–2007. Mycoses 2011; 54: 162– 167. [DOI] [PubMed] [Google Scholar]
- 5. Oyeka CA, Eze II. Fungal skin infections among prison inmates in Abakaliki, Nigeria. Mycoses 2007;51: 50– 54. [DOI] [PubMed] [Google Scholar]
- 6. Marques SA, Robles AM, Tortorano AM, Tuculet MA, Negron R, Mendes RP. Mycoses associated with AIDS in the third world. Med Mycol 2000;38: 269– 279. [PubMed] [Google Scholar]
- 7. Ghahfarokhi MS, Goodarzi M, Abyaneh MR, Al-Tiraihi T, Seyedipour G. Morphological evidences for onion-induced growth inhibition of Trichophyton rubrum and Trichophyton mentagrophytes. Fitoterapia 2004; 75: 645– 655. [DOI] [PubMed] [Google Scholar]
- 8. Shams-Ghahfarokhi M, Shokoohamiri MR, Amirrajab N, Moghadasi B, Ghajari A, Zeini F, et al. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia 2006;77: 321– 323. [DOI] [PubMed] [Google Scholar]
- 9. White TC, Oliver BG, Graser Y, Henn MR. Generating and testing molecular hypotheses in the dermatophytes. Eukaryot Cell 2008;7: 1238– 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macura AB. In vitro susceptibility of dermatophytes to antifungal drugs: a comparison of two methods. Int J Dermatol 1993;32: 533– 536. [DOI] [PubMed] [Google Scholar]
- 11. Muhsin T.M, Hadi R.B. Incidence of keratinolytic and dermatophytic fungi in sewage sludge in Basrah, Iraq. J Basrah Res (Sciences) 2011;37: 3– 15. [Google Scholar]
- 12. Cabans FJ. Dermatophytes in domestic animals. In: Kushwaha RKS, Guarro J. (Eds.). Biology of Dermatophytes and other Keratinophilic Fungi. Revista Iberoam Micol 2000; pp. 104– 108. [Google Scholar]
- 13. Sevtap A. Current status of antifungal susceptibility testing methods. Med Mycol 2007;45: 569– 587. [DOI] [PubMed] [Google Scholar]
- 14. Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev 1995;8: 240– 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coelho LM, Aquino-Ferreira R, Maffei CM, Martinez-Rossi NM. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J Antimicrob Chemother 2008;62: 758– 761. [DOI] [PubMed] [Google Scholar]
- 16. Pakshir K, Bahaedinie L, Rezaei Z, Sodaifi M, Zomorodian K. In vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur J Microbiol 2009;2: 158– 163. [Google Scholar]
- 17. Nyilasi I, Kocsube S, Krizsán K, Galgo L, Papp T, Pesti M, Nagy K. Susceptibility of clinically importantdermatophytes against statins and different statin-antifungal combinations. Med Mycol 2014;52: 140– 148. [DOI] [PubMed] [Google Scholar]
- 18. Jessup CJ, Warner J, Isham N, Haasan I, Ghannoum MA. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J Clin Microbiol 2000;38: 341– 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nweze EI, Mukherjee PK, Ghannoum MA. Agar-based disk diffusion assay for susceptibility testing of dermatophytes. J Clin Microbiol 2010;48: 3750– 3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nweze EI, Ogbonna C, Okafor JI. In vitro Susceptibility testing of dermatophytes isolated from pediatric cases in Nigeria against five antifungals. Rev Inst Med Trop S Paulo 2007; 49: 293– 295. [DOI] [PubMed] [Google Scholar]
- 21. Adimi P, Hashemi SJ, Mahmoudi M, Mirhendi H, Shidfar M.R, Emmami M, et al. In-vitro activity of 10 antifungal agents against 320 dermatophyte strains using microdilution method in Tehran. Iranian J Pharmaceut Res 2013; 12: 537– 545. [PMC free article] [PubMed] [Google Scholar]
- 22. Wawarzkiewicz K, Wolski T, Labarzewski J. Screening the keratinolytic activity of derrmatophyte in vitro. Mycopathologia 1991; 114: 1– 8 . [DOI] [PubMed] [Google Scholar]
- 23. Apodaca G, Mckerrow JH. Purification and characterization of a 27,000-M exteracellular proteinase from Trichophyton rubrum. Infect Immun 1989; 57: 3072– 3080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. John HR, Barbara D. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Wayne. A clinical and laboratory standards Institute; 2008; Vol 28: No. 16. [Google Scholar]
- 25. Araujo CR, Miranda KC, Orionald A. In vitro susceptibility testing of dermatophyes isolated in Goiania Brazil, against five antifungal agents by broth microdilution method. Rev Inst Med Trop S Paulo 2009;51: 9– 12. [DOI] [PubMed] [Google Scholar]
- 26. Coelho LM, Aquino-Ferreira R, Maffei CM, Martinez-Rossi NM. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J Antimicrob Chemother 2008;62: 758– 761. [DOI] [PubMed] [Google Scholar]
- 27. Sharma A, Chandra S, Sharma M. Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses 2012; 55: 410– 415. [DOI] [PubMed] [Google Scholar]
- 28. Muhsin TM, Salih TH. Exocellular enzyme activity of dermatophytes and other fungi isolated from ruminants in Southern Iraq. Mycopathologia 2001; 150: 49– 52. [DOI] [PubMed] [Google Scholar]
- 29. Apodaca G, McKerrow JH. Regulation of Trichophyton rubrum proteolytic activity. Infect Immun 1989;57: 3081– 3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shams-Ghahfarokhi M, Razafsha M, Allameh A, Razzaghi-Abyaneh M. Inhibitory effects of aqueous onion and garlic extracts on growth and keratinase activity in Trichophyton mentagrophytes. Iran Biomed J 2003; 7: 113– 118. [Google Scholar]
- 31. Sadeghi G, Abouei M, Alirezaee M, Tolouei R, Shams-Ghahfarokhi M, Mostafavi E, et al. A 4-year survey of dermatomycoses in Tehran from 2006 to 2009. J Med Mycol 2011;21: 260– 265. [Google Scholar]
- 32. Goh CL, Tay YK, Ali KB, Koh MT, Seow CS. In vitro evaluation of griseofulvin, ketoconazole, and itraconazole against various dermatophytes in Singapore. Int J Dermatol 1994;33: 733– 737. [DOI] [PubMed] [Google Scholar]
- 33. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and non-dermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol 2003;149: 296– 305. [DOI] [PubMed] [Google Scholar]
- 34. Ghannoum M, Skaggs B, Sheehan D. Voriconazle susceptibilities of dermatophyte isolates obtained from a worldwide tinea capitis clinical trial. J Clin Microbiol 2006;44: 2579– 2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal RK, Gupta S, Mittal G, Khan F, Roy S, Agarwal A. Antifungal susceptibility testing of dermatophytes by agar-based disk diffusion method. Int J Curr Microbiol Appl Sci 2015;4: 430– 436. [Google Scholar]
- 36. Barros ME, Santos DA, Hamda JS. Evaluation of susceptibility of Trichophyton ment agrophytes and Trichophyton rubrum clinical isolates to antifungal drugs using a modified CLSI microdilution method (M38-A). J Med Microbiol 2007;56: 514– 518. [DOI] [PubMed] [Google Scholar]
- 37. Fernandez-Torres B, Cabanes FJ, Carrillo-Muñoz AJ, Esteban A, Inza I, Abarca L, et al. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microbiol 2002;40: 3999– 4003. [DOI] [PMC free article] [PubMed] [Google Scholar]