Abstract
Background and Objectives:
Unhygienic poultry feedstuffs can lead to nutrient losses and detrimental effect on poultry production and public health. In the present study, mycobiota and colony-forming units per gram in ingredients and finish poultry feed was evaluated with special reference to potentially mycotoxigenic fungi.
Materials and Methods:
Eighty five samples of corn, soybean meal and poultry finished feed were collected from nine poultry feed factories located in three provinces i.e. Tehran, Alborz and Qom in Iran from October 2014 to January 2015. Samples were cultured on Sabouraud dextrose agar (SDA), Aspergillus flavus and parasiticus agar (AFPA) and dichloran rosebengal chloramphenicol agar (DRBC) and incubated at 28 °C for 7–10 days. Purified fungal colonies were identified by a combination of macro- and microscopic morphological criteria. For determining the rate of fungal contamination, samples were cultured on SDA and colony forming units (CFUs) were calculated.
Results:
A total of 384 fungal isolates belonging to 7 genera of filamentous fungi and yeasts were obtained from corn (124 isolates), soybean meal (92 isolates), and feed before (72 isolates), and after pelleting (96 isolates). The most prominent fungal isolate in corn, soybean meal and feed before pelleting (feed as mash form) was Fusarium but in feed after pelleting was Aspergillus. Among 5 Aspergillus species isolated, potentially aflatoxigenic A. flavus isolates was predominant in corn (46.6%), soybean meal (72.7%) and poultry finished feed (75%). CFUs results indicated that 9/22 corn samples (40.9%), none of 22 soybean meal samples, 19/41 finished feed (46.3%) were contaminated higher than the standard limit.
Conclusions:
Our results indicated that corn, soybean meal and finished feed of poultry feed mill are contaminated with various fungal genera by different levels sometimes higher that the standard limits. Contamination with potentially mycotoxigenic fungi especially Aspergillus species may be considered as a human public health hazard.
Keywords: Mycoflora, Aspergillus, Poultry feed, Mycotoxigenic fungi
INTRODUCTION
Contaminated feeds either in the form of ingredients or as a finished product can transmit fungal pathogens to animal farms and sometimes to human food (1). Fungal contamination in raw materials or finished feed not only induces disease in poultry flocks but also causes other infectious diseases in birds (2, 3). It also reduces the use of animal of feed consumption and resulting in a drop in productivity. Among microorganisms, fungi have important effects on quality of food. Fungal growth sometimes leads to non-consumption of feed for poultry (4, 5). These fungi grown on feedstuffs that are kept in poor conditions can widely contaminate feed and agricultural products. Each of these can be infected with one or several fungal toxin that results the final product to be contaminated with various toxins (6–8). Certain strains of filamentous fungi such as Aspergillus, Penicillium and Fusarium often arise as fungi causing toxins. These fungi in the feed mills with unfavorable conditions (temperature and moisture) for storage, grows on finished feeds, and produce fungal toxins (9–12). Agricultural products containing fungal contamination or fungal toxins have not good quality and sold at cheaper prices. The growth of these fungi on raw materials as well as poultry feed, may cause detrimental effects such as suppressing the immune system, reduced performance, increase feed conversion ratio, poultry mortality, and economic losses. According to the World Health Organization (WHO), aflatoxins residue has greater share in contamination comparing with other toxins. According to the International Agency for Research on Cancer (IARC) aflatoxins has been introduced as a human liver carcinogens type A (13). Aflatoxins are produced mainly by Aspergillus flavus and A. parasiticus (10, 14). A. flavus is one of the main types of aflatoxin producing fungi and responsible for the contamination of crops before harvest or during storage is in stock. It should be noted all strains of A. flavus and A. parasiticus are not able to produce aflatoxin. Toxigenic strains cannot produce toxin in all environments (15). The spread of the fungal infections is related to several factors such geographic location, storage conditions, processing of various feeds and moisture. Among the mentioned factors, moisture is the most important factors, so that reducing the moisture of the feed content to less than 12%, fungal growth and aflatoxin production will be stopped (16,17). Aspergillosis is a fungal disease created in poultry farm by Aspergillus species, especially A. flavus and A. fumigatus. Pulmonary aspergillosis is the most common disease in commercial poultry and has been reported in two acute and chronic forms. The disease usually looking for infection, poor nutrition, environmental temperature and relative humidity inappropriate and poisoning caused (3, 18).
Overall, the components of poultry feed prepared in feed mills with fungi contamination is of great importance, so extensive studies by researchers and scientists has been done around the world on this subject (19–23). The production of poultry products in Iran needs to million metric tons of healthy feedstuffs, therefore, study on the poultry feed contamination is necessary in consideration to climatic conditions (temperature and relative humidity), especially in storage and transportation.
The present comprehensive study was designed for the first time in Iran to assess contamination rate and fungal mycoflora of the main components (corn and soybean meal) as well as finished poultry feed (before and after pelleting) with emphasis on isolation and identification of potentially mycotoxigenic Aspergillus species.
MATERIALS AND METHODS
Sample preparation.
Survey was conducted in nine feed poultry mill factories from October 2014 to January 2015 in three provinces in Iran. Samples of feed factories were collected from provinces of Tehran, Alborz and Qom that licensed and permit from the Veterinary Authority. Sampling was carried out based on number code 7570, Institute of Standards and Industrial Research of Iran as well as a role of Iranian Veterinary Organization (IVO 2015). Samples were taken from the main raw material feed ingredients including corn and soybean meal, and the final product before entering the “pelletizer” and then ready to feed “pellet form”. According to the sampling tables, sample size was determined. Samples were corn (22 samples), soybean meal (22 samples), before (22 samples) and after pelleting (19 samples). Each sample was collected from at least three parts of bulks or bags. The sampling was performed randomly to provide an equal chance of selection. Corn samples from silos or power supply line system production, soybean meal samples of soy silos or warehouse. Feed samples before the pellet form of the mixer device and pellet finished feed samples were collected after pelletizer device. The cup or grape method was performed depending on the type of feedstuffs and factories. Pre-samples were thoroughly mixed to obtain a working sample. At this stage, random samples (1kg) of corn, soybean meal, and pelleted feed before and after weight for reduction (500 g) prepared in sterile plastic bags were transferred to Mycology Department of Faculty of Veterinary Medicine, University of Tehran.
Fungal isolation and identification
Samples of corn, soybean meal, and finished feed were mixed by a high-speed mixer. For dilution, a dilution of 10−2 was prepared in 9 ml of sterile distilled water into a test tube. Diluted samples were cultured on Sabouraud dextrose agar (SDA, E. Merck, Germany) plates contained 0.005% chloramphenicol (E. Merck, Germany), Dichloran Rose Bengal chloramphenicol agar (DRBC, Thermo Scientific™ Oxoid™, UK) and Aspergillus flavus and parasiticus agar (AFPA, Thermo Scientific™ Oxoid™, UK) and incubated at 28 °C for 7–10 days. Cultures were checked visually and each suspected fungal colony was sub-cultured on SDA plates for final isolation of fungal isolates. Fungal isolates were identified using a combination of macroscopic and microscopic morphological features at the genus level according to standard methods (17, 24). Aspergillus isolates were identified at species level according to Pitt and Hocking (17). Different colony characters used to identify fungal genera including Aspergillus species were colony size, surface, color (surface and reverse), texture and margins. Microscopic morphology of isolated fungi was studied using slide culture technique.
Determination of colony forming unit (CFU).
For this purpose, 25 g of each sample (corn, soybean meal and finished feed before and after pelleting) was added to 225 ml peptone water and mixed well for 30 seconds at 260 rpm. Dilutions of 10−1 to 10−4 were prepared using tubes containing 9 ml peptone water. Amounts of 10 ml of each sample from each dilution were spread on plates containing SDA and incubated for 3 to 5 days at 25 °C. After this period of time, average colony numbers were counted per gram to determine the quality control of poultry feed. Standard limits were 5 × 104 and 1 × 103 for ingredient and finished feed respectively, according to the rule of Iranian Veterinary Organization (IVO).
Statistical analysis.
Data were analyzed according to One-way ANOVA using SPSS 16.0 software (Illinois, USA). Means were compared using Duncan’s multiple rang test method. Significant was accepted at the level of p<0.05.
RESULTS
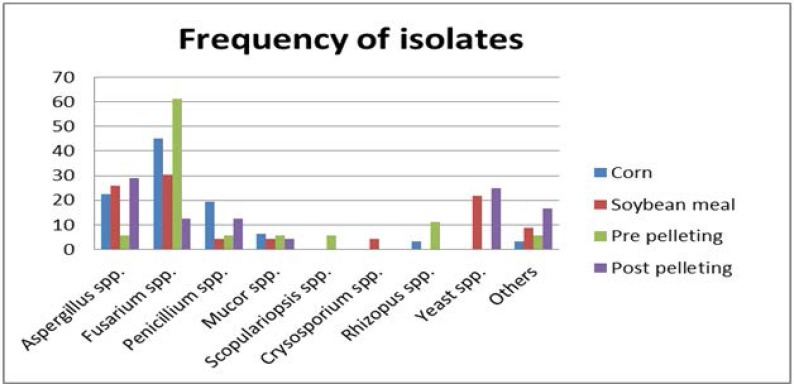
Results of the study fungal flora isolated from ingredients and finished feed in feed mills located in Tehran, Alborz and Qom provinces are presented in Table 1. A total of 384 fungal isolates belonging to 7 genera of filamentous fungi and yeasts were isolated from corn (124 isolates), soybean meal (92 isolates), and feed before (72 isolates), and after pelleting (96 isolates). Fungal genera isolated from corn, soybean meal, and before and after pellets were significantly different (p<0.05). The genus Fusarium was the most prominent fungal group isolated from corn (45.1%), soybean meal (30.5%) and before pelleting (61.2%), whiles the genus Aspergillus was the predominant fungal group in samples of pellets (29.1%). Totally, the most frequent fungus isolated from ingredients and finished feed was Fusarium (36.4%) followed by Aspergillus (21.8%), Penicillium (11.4%) and yeast (11.4%) (Fig. 1).
Table 1.
Determination of fungal mycoflora on ingredients and finished feed in poultry feed mills in Tehran, Alborz and Qom provinces, Iran.
| Fungi | Samples | Ingredients | Finished feed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn | Soybean meal | Pre-pelleting | Post-pelleting | Total | |||||||
| 22a | Fb | 22a | Fb | 22a | Fb | 19a | Fb | 85 | Fc | ||
| Aspergillus spp. | 28 | 22.5 | 24 | 26 | 4.0 | 5.5 | 28 | 29.1 | 84 | 21.8 | |
| Fusarium spp. | 56 | 45.1 | 28 | 30.5 | 44 | 61.2 | 12 | 12.5 | 140 | 36.4 | |
| Penicillium spp. | 24 | 19.3 | 4.0 | 4.3 | 4.0 | 5.5 | 12 | 12.5 | 44 | 11.4 | |
| Mucor spp. | 8.0 | 6.4 | 4.0 | 4.3 | 4.0 | 5.5 | 4 | 4.1 | 20 | 5.2 | |
| Scopulariopsis spp. | 0 | 0 | 0 | 0 | 4.0 | 5.5 | 0 | 0 | 4.0 | 1.0 | |
| Chrysosporium spp. | 0 | 0 | 4.0 | 4.3 | 0 | 0 | 0 | 0 | 4.0 | 1.0 | |
| Rhizopus spp. | 4.0 | 3.2 | 0 | 0 | 8 | 11.1 | 0 | 0 | 12 | 3.1 | |
| Yeast spp. | 0 | 0 | 20 | 21.7 | 0 | 0 | 24 | 25 | 44 | 11.4 | |
| Othersd | 4.0 | 3.2 | 8.0 | 8.7 | 4.0 | 5.5 | 16 | 16.6 | 32 | 8.3 | |
| Total | 124 | 100 | 92 | 100 | 72 | 100 | 96 | 100 | 384 | 100 | |
Number of samples
Frequency percentage of each fungus to all fungi isolated from each sample
Frequency percentage of each fungus to all fungal isolates
Including sterile hyphae or unknown fungus
Fig. 1.
Distribution and frequency of fungi on ingredients and finished feed in poultry feed mills in Tehran, Alborz and Qom provinces, Iran.
The genus Scopulariopsis was isolated from before pelleting samples (5.5%) and Chrysosporium only from soybean meal samples (4.3%). Rhizopus isolated from only the corn (3.2%) and feed before pelleting (11.1%) and yeasts only on soybean meal (21.7%) and post pellets (25%).
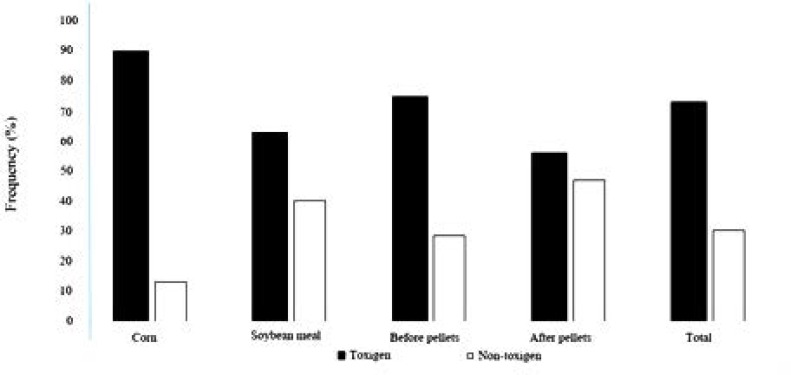
The frequency of the possible mycotoxigenic fungi including Aspergillus, Fusarium and Penicillium and non-toxigenic fungi are shown in Fig. 2 (p<0.05). The ratio of toxigenic/non-toxigenic fungi was shown in corn (87.1/12/9%), soybean meal (60.8/39.2%), before pelleting (72.2/27.8%) and after pelleting (54/1/45.9%). No significant difference was recorded between toxigenic and non-toxigenic fungi in ingredients and final pellet (p>0.05).
Fig. 2.
Frequency of potentially mycotoxigenic fungi on ingredients and finished feed in poultry feed mills in Tehran, Alborz and Qom provinces, Iran.
As shown in Table 2, A. flavus was predominant species in corn (46.6%), soybean meal (72.7%) and feed before and after pelleting (75%). As indicated in Fig. 3, totally, the most frequent isolates from ingredients and finished feed was A. flavus ( 64/3 %) and after that A. niger (16.6%) was the second prevalent species followed by A. fumigatus (10.7%), A. ochraceus and A. glaucus (each 2.4%). There was a significant difference between the frequency of A. flavus species on ingredients and finished feed in poultry (p<0.05).
Table 2.
Aspergillus species isolated from ingredients and finished feed in poultry feed mills in Tehran, Alborz and Qom provinces, Iran.
| Samples | Ingredients | Finished feed | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Aspergillus | Corn | Soybean meal | Pre- pelleting | Post- pelleting | No | ||||
| No | % | No | % | No | % | No | % | ||
| A. flavus | 14 | 46.6 | 16 | 72.7 | 3 | 75 | 21 | 75 | 54 |
| A. fumigatus | 7 | 23.3 | 2 | 9.1 | 0 | 0 | 0 | 0 | 9 |
| A. glaucus | 0 | 0 | 2 | 9.1 | 0 | 0 | 0 | 0 | 2 |
| A. niger | 5 | 16.6 | 1 | 4.5 | 1 | 25 | 7 | 25 | 14 |
| A. ochraceus | 2 | 6.7 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Unidentified | 2 | 6.7 | 1 | 4.5 | 0 | 0 | 0 | 0 | 3 |
| Total | 30 | 100 | 22 | 100 | 4 | 100 | 28 | 100 | 84 |
Fig. 3.
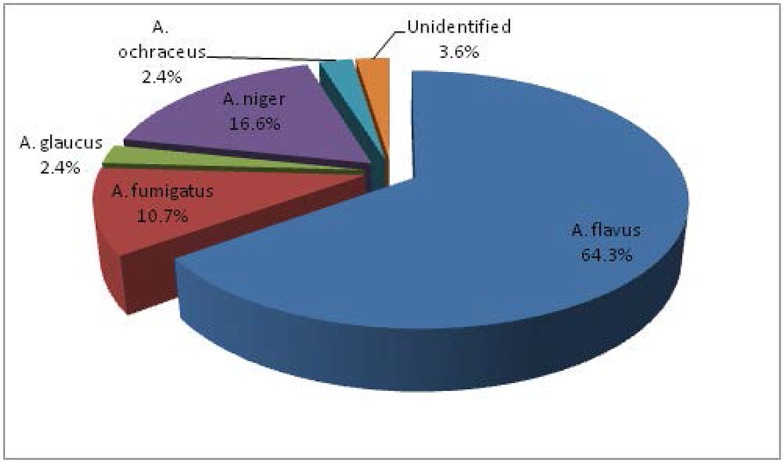
The frequency of total Aspergillus species on ingredients and finished feed in poultry feed mills in Tehran, Alborz and Qom provinces, Iran.
As shown in Table 3, colony forming unit (CFU/g) range of isolated fungi was determined in corn (3×102 to 1.2×105), soybean meal (1×102 to 4×103), before pelleting (1×101 to 5×104) and after pelleting (1×101 to 1.2×103).
Table 3.
Colony forming unit per gram of ingredients and finished feed in poultry feed mills inTehran, Alborz and Qom provinces, Iran.
| Sample | No. | Total CFU | Means of CFU | Minimum of CFU | Maximum of CFU | CFU (higher than Standard limit) | CFU (lower than Standard limit) | ||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||||
| Corn1 | 22 | 332400 | 1.510×104 | 3×102 | 1.2×105 | 9 | 40.9 | 13 | 59.1 |
| Soybean meal1 | 22 | 13320 | 0.060×104 | 1×102 | 4×103 | 0 | 0 | 22 | 100 |
| Pre-pelleting2 | 22 | 105310 | 4.786×103 | 1×101 | 5×104 | 14 | 63.6 | 8 | 36.4 |
| Post-pelleting2 | 19 | 5750 | 0.302×103 | 1×101 | 1.2×103 | 5 | 26.3 | 14 | 73.7 |
Limit of standard for ingredients (5 × 104)
Limit of standard for finished-feed (1 × 103)
Among 22 corn samples fungal CFUs were higher than the standard limits of Iran in 9 samples (40.9%), while in none of 22 samples of soybean meal, contamination was not higher than the standard limit (Table 3). As indicated in Table 3, there was a significant difference between fungal CFUs in samples of after pellets (p<0.05). Fungal contamination was higher than the standard limits in 14 (63.6%) samples of feed before pelleting (feed as mash form) and 5 (26.3%) samples of feed in the form of pellets. The mean percentage of fungal CFUs in ingredients and finished feed are shown in Fig. 4. There was a significant difference (p<0.05) in fungal CFUs between corn and soybean meal and finished feed.
DISCUSSION
In the present study, mycoflora and fungal contamination rate of feed ingredients and finish poultry feed were evaluated. The results of various studies in determining the natural mycobiota of ingredients and finish poultry feed have shown that different fungal genera and species could be isolated (18, 21, 25–27). Orsi et al. (28) studied the mycoflora of corn and showed that Fusarium, Aspergillus and Penicillium were the main fungi isolated. These results are in accordance with our results indicating that these fungal genera are abundance in corn, soybean meal as ingredients of poultry feed. We showed some potentially mycotoxigenic fungi were decrease in processing especially Fusarium and Penicillium, but Aspergillus was increasing after steam pelleting in poultry feed. Many researchers determined that Aspergillus was the main mycoflora of pellets of poultry feed (5, 15, 19, 20, 28–30). In the present study, the most dominant fungus in the feed before pellet was Fusarium followed by Aspergillus and Penicillium and in pellet feed, Aspergillus followed by Penicillium and Fusarium. We found Aspergillus frequency was the most prevalent in mycoflora pellet form in processing in feed mill. The toxigenic fungal contamination of the raw materials occurs during the pre-harvest and/or the postharvest periods, they are exposed during production, processing, transportation, and storage (31). Our result are in accordance with Dalcero et al. (21) and Accensi et al (20) confirmed Aspergillus flavus over than in poultry feed and A. niger was the second prevalent species followed by A. fumigatus. A. flavus predominates in all kinds of poultry feed in the tropical climate (17, 32–34). The thermo-resistant of A. flavus causes of abundantly in poultry feed and the presence in pellet feed may be an indicative of their predominance potentially to produce of aflatoxins. Azarakhsh et al. (35) showed that A. flavus was the most prevalent species followed by A. niger and A. fumigatus in the broiler feeds in Kermanshah province in West of Iran. This difference may be due to the fact that processing stage (temperature, time, etc.) at pelletizer significantly affects the viability of fungal conidia and variable degree of resistance of fungal conidia of various fungi because conditions determines the predominance of mycobiota in feed poultry. According to quality control of samples and standard limits of feed ingredients (5×104) and the finished feed (1×103) in Iran, hazardous contamination higher than standard limits were observed in poultry feed examined in the present study. This contamination may arise from the lack of proper drying of corn in farm operations, transportation and inappropriate storage of silage. Since Iran is a major importer of corn from other countries, the risks of fungal infection and toxin formation must be considered. The low level of fungal CFUs on soybean meal may be account for sounds conditions at harvest, heat treatment, addition of antifungal compounds in soybean meal and providing good storage conditions. In pellets, fungal CFU was relatively low because processing operations in the process pellet which can greatly reduce the chance of contamination. In Iran only a few studies have been conducted on the CFUs contamination of poultry feed commodities and finished feed. Mayahi et al. (36) reported that contaminations of all samples of fish meal, soybean meal, wheat and maize were lower than standards (5× 105) level at that time. Astoreca et al. (26) determined the CFU/g in poultry feed ingredients and showed that corn was the most contaminated feed ingredient (4×104 to 8×104 CFUs/g), while soybean had the least contamination. Also grounded feed (before pelleting) was contaminated higher than pellet poultry feed which is in agreement with the results of our study. These authors showed low level of fungal contamination in raw materials for poultry feed that was good quality indicator of consumption of such materials for humans or animals. Dalcero et al. (21) reported that feed preparing in the form of pellets or crumbles at a high temperature in the poultry feed factories significantly affected the growth of fungal colonies showed that poultry feed pelleting process significantly reduced the fungal counts in the final product which is in agreement with our results.
CONCLUSION
Our results indicated that corn, soybean meal and finished poultry feed were contaminated by various degrees with different fungal genera especially potentially mycotoxigenic fungi belonging to Aspergillus, Fusarium and Penicillium. According to widely distributed Aspergillus spores in the environment, the risk of feeds pellet contamination by this fungus is very high as evidenced in the present study. We showed that some feed ingredients and finished feeds were contaminated with hazardous amounts of fungi higher than the standard limits. These results indicate the need for continuous assessment of the mycological status of finished feed production. The present study showed that untreated such ingredients feed are important vehicles into contaminant poultry feed in Iran. It is therefore, advised to routinely treat ingredients and finished feed with fungal growth inhibitors and necessary of authorizing the code of Hazard Analysis and Critical Control Points (HACCP) for production in poultry factories.
Acknowledgments
This work was supported financially by the Research Council of University of Tehran, Iran. The authors like to thank Dr. Aghil Sharifzadeh for his technical assistance.
REFERENCES
- 1. Khosravi AR, Dakhili M, Shokri H. A mycological survey on feed ingredients and mixed animal feeds in Ghom province, Iran. Pakistan J Nutr 2008; 7: 31– 34. [Google Scholar]
- 2. Kpodo K, Thrane U, Hald B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int J Food Microbiol 2000; 61: 147– 157. [DOI] [PubMed] [Google Scholar]
- 3. Labuda R, Tanvinova D. Fungi recovered from Slovakian poultry feed mixtures and their toxinogenicity. Ann Agric Environ Med 2006; 13: 193– 200. [PubMed] [Google Scholar]
- 4. Magnoli C, Astoreca A, Chiacchiera SM, Dalcero A. Occurrence of ochratoxin A and ochratoxigenic mycoflora in corn and corn based foods and feeds in some South American countries. Mycopathologia 2007; 163: 249– 260. [DOI] [PubMed] [Google Scholar]
- 5. Magnoli C, Hallak C, Astoreca A, Ponsone L, Chiacchiera SM, Palacio G, et al. Surveillance of toxigenic fungi and ochratoxin A in feedstuffs from Córdoba province. Vet Res Commun 2005; 29: 431– 445. [DOI] [PubMed] [Google Scholar]
- 6. Alam MS, Islam MR, Banu MS. Aboundance of fungal flora in relation to moisture content and storage period in different types of poultry feed ingredient. Pakistan J Biol Sci 2001; 41: 194– 198. [Google Scholar]
- 7. Ezekiel CN, Atehnkeng J, Odebode AC, Bandyopadhyay R. Distribution of aflatoxigenic Aspergillus section Flavi in commercial poultry feed in Nigeria. Int J Food Microbiol 2014; 189: 18– 25. [DOI] [PubMed] [Google Scholar]
- 8. Yiannikouris A, Jouan JP. Mycotoxins in feeds and their fate in animals: A review. Anim Res 2002; 51: 81– 99. [Google Scholar]
- 9. Berry CL. The pathology of mycotoxins. J Pathol 1998; 154: 301– 311. [DOI] [PubMed] [Google Scholar]
- 10. Khanafari A, Soudi H, Miraboulfathi M. Biocontrol of Aspergillus flavus and aflatoxin B1 production in corn. Iranian J Environ Health Sci Eng 2007; 4: 163– 168. [Google Scholar]
- 11. Magnoli C, Dalcero A, Chiacchiera SM, Miazzo R, Sáenz M. Enumeration and identification of Aspergillus group and Penicillium species in poultry feeds in Argentina. Mycopathologia 1998; 142: 27 32 [DOI] [PubMed] [Google Scholar]
- 12. Smith JE, Solomons G, Lewis C, Anderson JG. The role of mycotoxins in human and animal nutrition and health. Nat Toxins 1995; 3: 187– 192. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization publication Aflatoxins. International Agency for Research on Cancer (IARC) Summaries & Evaluations 2002; 82: 171– 179. [Google Scholar]
- 14. Alinezhad S, Tolouee M, Kamalzadeh A, Motalebi AA, Nazeri M, Yasemi M, et al. Mycobiota and aflatoxin B1 contamination of rainbow trout (Oncorhinchus mykiss) feed with emphasis to Aspergillus section Flavi. Iranian J Fish Sci 2011; 10: 363– 374. [Google Scholar]
- 15. Fraga ME, Curvello F, Gatti MJ, Cavaglieri LR, Dalcero AM, Rocha-Rasa CA. Potential aflatoxin and ochratoxin A production by Aspergillus species in poultry feed processing. Vet Res Commun 2007; 31: 343– 353. [DOI] [PubMed] [Google Scholar]
- 16. Gibson AM, Baranyi J, Pitt MJ, Eyles MJ, Robert TA. Predicting fungal growth: The effect of water activity on Aspergillus flavus and related species. Int J Food Microbiol 1994; 23: 419– 431. [DOI] [PubMed] [Google Scholar]
- 17. Pitt J, Hocking A. Fungi and Food Spoilage, 3rd edition, Springer, Berlin-Germany, 2009. [Google Scholar]
- 18. Ariyo LA, Anthony MH, Lami ML. Survey of mycotoxigenic fungi in concentrated poultry feed in Niger State. Nigeria J Food Res 2013; 2: 128– 138. [Google Scholar]
- 19. Oliveira GR, Ribeiro JM, Fraga ME, Cavaglieri LR, Direito GM, Keller KM, et al. Mycobiota in poultry feeds and natural occurrence of aflatoxins, fumonisins and zearalenone in the Rio de Janeiro State, Brazil. Mycopathologia 2006; 162: 355– 362. [DOI] [PubMed] [Google Scholar]
- 20. Accensi F, Abarca ML, Cabanes FJ. Occurrence of Aspergillus species in mixed feeds and component raw materials and their ability to produce ochratoxin A. Food Microbiol 2004; 21: 623– 627. [Google Scholar]
- 21. Dalcero A, Magnoli C, Luna M, Ancasi G, Reynoso MM, Chiacchiera S, et al. Mycoflora and naturally occurring mycotoxins in poultry feeds in Argentina. Mycopathologia 1998; 141: 37– 43. [DOI] [PubMed] [Google Scholar]
- 22. González HHL, Resnik SL, Boca RT, Marasas WFO. Mycoflora of Argentinian corn harvested in the main production area in 1990. Mycopathologia 1995; 130: 29– 36. [DOI] [PubMed] [Google Scholar]
- 23. Rosa CAR, Riberio JMM, Fraga MJ, Gatti M, Cavaglieri LR, Magnoli CE, et al. Mycoflora of poultry feed and ochratoxin-producing ability of isolated Aspergillus and Penicillium species. Vet Microbiol 2006; 113: 89– 96. [DOI] [PubMed] [Google Scholar]
- 24. Klich MA, Pitt JI. Differentiation of Aspergillus flavus from A. parasiticus and other closely related species. Trans Brit Mycol Soc 1988; 91: 99– 108 [Google Scholar]
- 25. Ariyo LA, Anthony MH, Lami ML. Survey of mycotoxigenic fungi in concentrated poultry feed in Niger State. Nigeria J Food Res 2013; 2: 128– 138. [Google Scholar]
- 26. Astoreca AL, Dalcero AM, Fernandez Pinto V, Vaamonde G. A survey on distribution and toxigenicity of Aspergillus section Flavi in poultry feeds. Int J Food Microbiol 2011; 146: 38– 43 [DOI] [PubMed] [Google Scholar]
- 27. Atehnkeng J, Ojiambo PS, Donner M, Ikotun T, Sikora RA, Cotty PJ. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int J Food Microbiol 2008; 122: 74– 84. [DOI] [PubMed] [Google Scholar]
- 28. Orsi RB, Corrêa B, Possi CR, Schammass EA, Nogueira JR, Dias SMC, Malozzi MAB. Mycoflora and occurrence of fumonisins in freshly harvested and stored hybrid maize. J Stor Prod Res 2000; 36: 75– 87. [Google Scholar]
- 29. Bragulat MR, Abarca ML, Castella O, Cabañes J. Mycological survey on mixed poultry feeds and mixed rabbit feeds. J Sci Food Agric 1995; 67: 215– 220. [Google Scholar]
- 30. Dalcero A, Magnoli C, Hallak C, Chiacchiera SM, Palacio G, Rosa CDR. Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin by Aspergillus section Nigri in Argentina. Food Addit Contam 2002; 19: 1065– 1072. [DOI] [PubMed] [Google Scholar]
- 31. Khosravi AR, Shokri H, Zaboli F. Grain-Borne Mycoflora and Fumonisin B1 From Fresh-Harvested and Stored Rice in Northern Iran. Jundishapur J Microbiol 2013; 6 (5): 1– 6. [Google Scholar]
- 32. Figueroa S, Centeno S, Calvo MA, Rengel A, Adelantado C. Mycobiota and concentration of ochratoxin A in concentrated poultry feed from Venezuela. Pak J Biol Sci 2009; 12: 589– 594. [DOI] [PubMed] [Google Scholar]
- 33. Okoli IC, Ogbuewu PI, Uchegbu MC, Opara MN., Okorie JO, Omede AA, Okoli GC. Assessment of the mycoflora of commercial poultry feeds sold in the humid tropical environment of Imo State, Nigeria. J Am Sci 2007; 3: 5– 9. [Google Scholar]
- 34. Baliukoniene V, Bakutis B, Stankevicius H. Mycological and mycotoxicological evaluation of grain. Ann Agric Environ Med 2003; 10: 223– 227. [PubMed] [Google Scholar]
- 35. Azarakhsh Y, Sabokbar A, Bayat M. Incidence of the most common toxigenic Aspergillus species in broiler feeds in Kermanshah province, West of Iran. Global Vet 2011; 6: 73– 77. [Google Scholar]
- 36. Mayahi M, RaziJalali M, Slamat N. Isolation of Aspergillus and aflatoxin determination in fish meal, corn and soybean meal. Shahidchamran Uni J Sci 2007; 17: 95– 105. [Google Scholar]