Abstract
Budding yeast cells have a finite replicative life span; that is, a mother cell produces only a limited number of daughter cells before it slows division and dies. Despite the gradual aging of the mother cell, all daughters are born rejuvenated and enjoy a full replicative lifespan. It has been proposed that entry of mother cells into senescence is driven by the progressive accumulation and retention of damaged material, including protein aggregates. This additionally allows the daughter cells to be born damage free. However, the mechanism underlying such asymmetrical segregation of protein aggregates by mother and daughter cells remains controversial, in part because of the difficulties inherent in tracking the dynamics and fate of protein aggregates in vivo. To overcome such limitations, we have developed single-cell real-time imaging methodology to track the formation of heat-induced protein aggregates in otherwise unperturbed dividing cells. By combining the imaging data with a simple computational model of protein aggregation, we show that the establishment of asymmetrical partitioning of protein aggregates upon division is driven by the large bud-specific dilution rate associated with polarized growth and the absence of significant mother/bud exchange of protein aggregates during the budded phase of the cell cycle. To our knowledge, this study sheds new light on the mechanism of establishment of a segregation bias, which can be accounted for by simple physical arguments.
Introduction
The accumulation of misfolded proteins into large aggregates is thought to impair normal cellular physiology and is a hallmark of many age-related degenerative diseases (1). Protein aggregation is also thought to play an important role in the normal aging process of unicellular organisms (2, 3, 4). In budding yeast, which divides asymmetrically, mother cells generate buds that become daughter cells after division. A mother cell can produce only a limited number of daughter cells, ∼20–30, before it enters replicative senescence and ultimately dies (5); however, daughters of aging mothers are born with full replicative potential (6) and display normal physiology (7), implying the existence of an unknown rejuvenation process. The main hypothesis is that senescence is a consequence of the progressive accumulation in mothers of deleterious factor(s) that are not transmitted to their progeny (7). More recently, aging cells were shown to undergo a sharp transition into a slow replicative state, termed the senescence entry point, which suggests a threshold effect of the cellular response to the accumulated damage (8).
Over the last 15 years, several potential aging factors have been identified, including extrachromosomal rDNA circles (9) and dysfunctional mitochondria (10, 11, 12). In addition, carbonylated proteins have a tendency to accumulate and form amorphous aggregates within mother cells (2, 13). The asymmetrical mother/daughter partitioning of such aggregates is directly controlled by the expression of protein chaperones and the pleiotropic longevity regulatory gene SIR2 (13). More recent work providing important mechanistic insights into how aggregates are partitioned has been extensively debated and experimentally refined. Heat shock-induced protein aggregates (PAs) have been monitored and characterized indirectly using the green fluorescent protein (GFP)-tagged disaggregase Hsp104, which binds amorphous protein clusters. Previous studies suggested that PAs in the bud may undergo retrograde transport to the mother cell through tethering to polarized actin cables (14, 15). These results led to the proposal that an active spatial protein quality control mechanism helps to maintain daughters aggregate free upon mitosis and might be involved in the rejuvenation process observed in the progeny of aging mothers. This hypothesis was later challenged by Zhou et al., who did not observe biased transport of aggregates through the bud neck (16). Using quantitative measurements of aggregate diffusion, these authors also showed that PA retention within mother cells can be explained by physical arguments; that is, the slow and anomalous diffusive properties of these large structures within a confined environment makes their transport through the bud neck very unlikely over cell cycle timescales. This conclusion was further supported by a recent theoretical study investigating the transport properties of cellular materials in various cell biology contexts (17) and by experiments in bacteria that led to similar explanations (4, 18). However, previous work has shown that amorphous PAs do not freely diffuse within the cytosol but, rather, are targeted to perinuclear or perivacuolar compartments called JUNQ (which is also referred to as INQ (19)) and IPOD (20, 21), respectively. Therefore, tethering of PAs to these structures could explain the limited diffusive properties of the PAs and, importantly, drive their asymmetrical inheritance in yeast (21). A subsequent study also supported the idea that PA retention is mediated by their localization to subcellular organelles (22). In that study, PAs resulting from various proteotoxic stresses were shown to associate with the endoplasmic reticulum or mitochondria or at the interface of the two structures. In this model, asymmetrical segregation of mitochondria-bound PAs would result from the biased transport of mitochondria to the bud, a process that remains to be understood (22).
Therefore, our understanding of the mechanism of PA segregation clearly remains incomplete. Several independent factors may have contributed to this situation. For example, even when colocalization assays of the Hsp104-GFP-bound PAs with specific organelles are performed with high-resolution imaging techniques, the complex aggregation kinetics of misfolded proteins, which involves the recruitment of multiple chaperone proteins, is superposed on the dynamic behavior of cellular organelles (such as mitochondria). Similarly, it is still unclear whether PAs are formed directly on their target organelles, as proposed recently (22), or whether small PAs are formed and then fuse before their recruitment to specific compartments (21, 23). This distinction may have important consequences for their ability to segregate between mother and bud. Previous studies have used one or more proteotoxic stresses to destabilize protein conformation or the protein quality control machinery, thereby triggering PA formation. The dynamics or localization of the PAs was then assessed following a recovery period (typically 30 min). Although it is essential to distinguish between the establishment and the maintenance of unequal PA localization, this distinction is generally not possible (except in (15, 24)), and therefore most previous studies did not mark the budding state at the time the stressor is applied. Notably, the model of cytoskeleton-driven aggregate transport proposes an active mechanism of segregation between the mother and daughter cell (i.e., it addresses asymmetry establishment) (14), whereas all other studies have focused on the retention of preexisting PAs by mothers (i.e., they address asymmetry maintenance) (16). In addition, the question of resolution/clearance versus segregation of PAs cannot be distinguished by static snapshots of the aggregation pattern determined after cell recovery. There is thus a clear need for a specific methodology allowing cell fate to be monitored during and after the application of stress to untangle the contributions of the establishment, maintenance, and clearance of PAs to their biased segregation.
To overcome the technical limitations mentioned previously and to further understand the establishment of an unequal PA repartition in mother versus bud following temperature stress, we have developed an assay to monitor the kinetics of PA formation triggered by a rapid increase in temperature from 30°C to 38°C (hereafter referred to as temperature shift, TS) in real time with single-cell resolution under the microscope, as described previously (23). In combination with a computational model of protein aggregation, our data clearly reveal that the establishment of biased accumulation of PAs in mother/daughter cells arises from the polarized growth of the bud during the budded period of the cell cycle.
Materials and Methods
Strains and plasmids
All strains were congenic to S288C. The strain carrying Hsp104-GFP was purchased from Invitrogen (Carlsbad, CA) and the bni1 deletion mutant was from Euroscarf (Bad Homburg, Germany). The strain carrying the Cdc10-mCherry fusion was a gift from Jeremy Thorner (University of California, Berkeley, CA), and the preCox4-mCherry marker was generously provided by Dan Gottschling (Fred Hutchinson Cancer Research Center, Seattle, WA). The 2μ pESC-GAL1p:Ubc9ts:mCherry -ura3 plasmid was a kind gift from Michel Toledano and was transformed into the Hsp104-GFP strain. Other strains were generated by crossing and were scored by tetrad dissection according to standard techniques.
Microfabrication
The microfluidic master for heat shock studies was made using reactive ion etching (Bosch process). Prototypic molds were replicated in epoxy. The microchannels were cast by curing polydimethylsiloxane (Sylgard 184,10:1 mixing ratio) and then covalently bound to a 24 × 50 mm coverslip using plasma surface activation (Diener, Jettingen, Germany).
Time-lapse microscopy
Freshly thawed cells were grown overnight at various final cell densities. In the morning, log phase cells (OD ∼0.2–0.5) were transferred into the microfluidic device, and heat shock experiments were started 4–6 h later. The device was perfused with medium (synthetic complete with 2% glucose; SCD) throughout the experiment using a peristaltic pump (Ismatec, Wertheim, Germany; flow rate 25 μl/min). Cells were imaged using an inverted Zeiss Axio Observer Z1 microscope (Zeiss, Oberkochen, Germany). Focus was maintained using a custom focus search algorithm and commercial focus stabilization hardware (Definite Focus; Zeiss). Fluorescence illumination was achieved using LED light (precisExcite, CoolLed, Andover, UK), and light was collected using a 100× N.A. 1.4 objective and an EM-CCD Luca-R camera (Andor, Belfast, UK). The camera was triggered on the precisExcite module using a transistor-transistor logic signal. We used an automated stage to follow up to five positions in parallel over the course of the experiment. Images were acquired every 3 min, and three z-stacks (with 1 μm spacing) were collected to image most of the foci within the cells while maintaining phototoxicity at a minimal level. Cells were followed for up to 12 h. For the experiment combining Hsp-104 foci and Ubc9ts-mCherry observation, a similar protocol was applied as with Cdc10-mCherry, except that cells were perfused with synthetic complete medium –ura + raffinose 2% + galactose 2%, to induce Ubc9ts-mCherry aggregate formation.
To control the temperature in the chip and to perform the temperature shift, we built a custom sample holder with thermoelectric modules and an objective heater with heating resistors (see Text S1 in the Supporting Material). Temperature control was achieved using a proportional-integral-derivative controller (5C7-195, Oven Industries, Mechanicsburg, PA).
Image analysis
Raw images were processed using custom software, called phyloCell, based on MATLAB (The MathWorks, Natick, MA) and the image-processing toolbox (8). This software features a comprehensive graphical user interface to perform segmentation/tracking and to introduce manual error corrections. The software is available for download on GitHub (https://github.com/gcharvin/phyloCell). In this study, the software was used to segment cell contours based on phase contrast images, to detect aggregates from fluorescence images, to track cells over time, and to measure the fluorescence within the cells. Relative fluorescence concentrations were obtained by normalizing total fluorescence to cell volume. We checked that cell volume scaled with projected area3/2 using cytoplasmic Hsp104-GFP fluorescence measurements (under stress-free conditions) in mother cells and in buds of varying sizes (see Fig. S8). In addition, we checked that an Hsp104-GFP foci scales linearly with the aggregates size by using an independent Ubc9ts-mCherry marker (Fig. S9), which has been extensively used to ectopically generate protein aggregates (22, 23). Custom background subtraction was performed by measuring background within the cell cluster.
Drug treatment
During the course of a standard time lapse experiment, cycloheximide (C4859; Sigma Aldrich, St. Louis, MO) was perfused in the medium at various concentrations (2 μg/ml and 100 ng/ml), concomitantly with the temperature shift, to inhibit cell growth.
Mathematical model for establishment of asymmetry by cell growth
See Text S1 in the Supporting Material for the detailed model used to explain the establishment of PA asymmetry.
Results
Unequal accumulation of PAs in mothers and buds following temperature shift
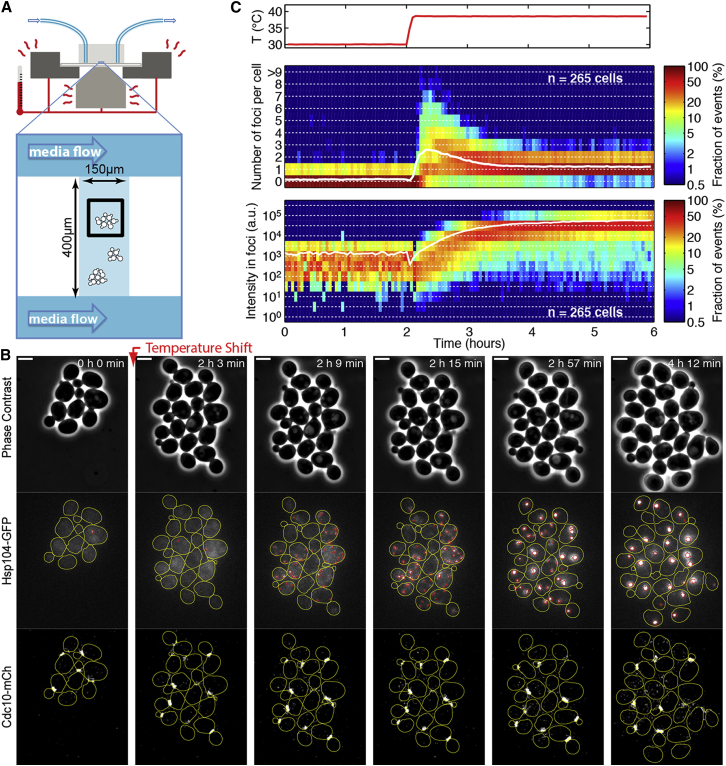
To overcome the technical limitations associated with the assessment of PA segregation bias in mothers versus buds, we developed, to our knowledge, a new strategy to track the kinetics of PA formation in real time at single-cell resolution. We expressed GFP fused with Hsp104, a heat stress-induced chaperone that associates with PAs, to visualize the formation of PA foci in cells growing in a microfluidic device (Figs. 1 A and S1). Whereas severe (42°C) heat shock induces a complete growth stall and requires a recovery period before cells are observed ((14, 16)), a TS from 30°C to 38°C (within minutes, see Fig. S1, C and D) induced a brief and transient cell growth arrest but was sufficient to induce massive formation of PAs (Figs. 1 B and S2 B; Movie S1). Because the cell division process was not significantly affected during this procedure, we were able to investigate the establishment of PA segregation bias in budded cells by continuous observation of cell proliferation.
Figure 1.
Monitoring formation of protein aggregates in real time following temperature shift. (A) Sketch of the experimental setup. Cells are grown in chambers on a microfluidic chip and fed by diffusion using media perfusion channels. Temperature shift is performed in situ with a custom heating stage and objective heater. (B) Sequence of phase contrast and fluorescence (Hsp104-GFP and Cdc10-mCherry) images obtained at the indicated times after initiation of heat shock by TS from 30°C to 38°C. On the fluorescence images, yellow and red lines represent the cell contours and the position of protein aggregates, respectively. Scale bar, 4 μm. (C) Quantification of the kinetics of protein aggregate induction. Upper panel: actual stage temperature over 6 h. Middle panel: two-dimensional histogram of the distribution of foci number per cell as a function of time. White line indicates the mean number of foci per cell. Lower panel: two-dimensional histogram of the distribution of total fluorescence intensity in foci as a function of time. White line indicates the mean fluorescence intensity. To see this figure in color, go online.
We quantified both the number of PA foci and their total fluorescence intensity over time using a custom MATLAB-based image-processing pipeline (Figs. 1 C and S3; Text S1 in the Supporting Material). The number of foci peaked at an average of 2.6 ± 0.1 foci/cell (n = 265 cells) at 10 min after TS and declined to 1.2 ± 0.05 foci/cell after 30 min, thus revealing the kinetics of fusion of small PAs into larger ones. The increase in foci number was concomitant with the increase in total foci fluorescence after subtraction of cytoplasmic fluorescence (Fig. 1 C).
To assess the respective accumulation of PAs in mother and bud in budded cells following TS, we used a Cdc10-mCherry fusion as a marker of budding (Fig. 1 B) and we measured the cellular concentration of PAs ([Fluo.], defined as the total fluorescence within foci divided by the computed cell volume, see Materials and Methods) in every mother/bud (M/B) pair present at TS and at several time points post-TS, including at division (Fig. 2, A and B). Immediately after TS, the PA concentration in buds ([B. Fluo.]) was slightly higher than in mothers ([M. Fluo.]) (Fig. 2 B, left panel), giving a [B. Fluo.]/[M. Fluo.] ratio of 1.6 (Fig. 2 C). Of importance, this finding allowed us to rule out the hypothesis that foci are preferentially formed in mothers. Although PA concentration increased in both compartments upon division (Fig. 2 B; p < 3 × 10−9 for both compartments), the [B. Fluo.]/[M. Fluo.] median ratio dropped significantly to 0.6 (Fig. 2 C; p = 4 × 10−5). This result clearly indicated that unequal concentrations of PAs in mother and bud arose during the budded phase of the cell cycle following TS.
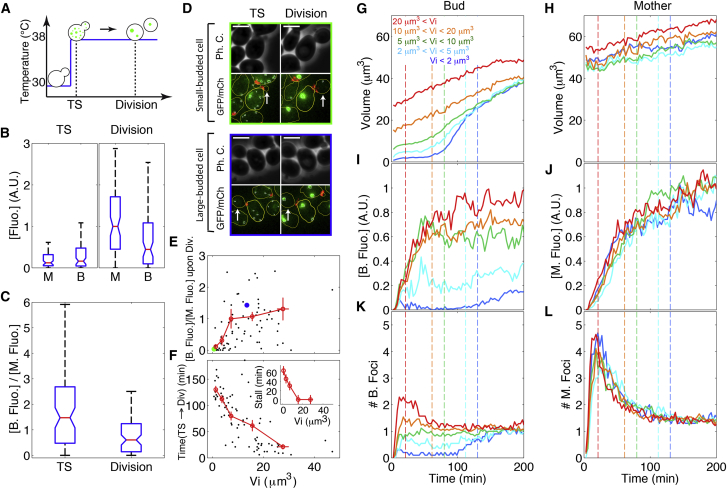
Figure 2.
Establishment of unequal distribution of protein aggregates following temperature shift. (A) Sketch illustrating the TS assay and the specific time points at which PA concentration ([Fluo.]) was analyzed (dotted lines): 12 min after temperature shift (indicated by TS) and upon division. (B) Boxplots of PA concentration (measured as [Fluo.]; total fluorescence in foci/cell volume) in mothers (M) and buds (B) at the time points described in (A). The central red line indicates the median; the lower and upper box edges indicate the 25th and 75th percentiles, respectively; the notches indicate 5% confidence intervals; and the whiskers indicate the most extreme data points not considered outliers. (C) Boxplots of the ratio of [Fluo.] in bud versus mother at the same time points (see B for explanation). (D) Typical phase contrast and fluorescence images (green: Hsp104-GFP; red: Cdc10-mCherry) of small-budded (upper) and large-budded (lower) cells at the time points indicated in (A). Scale bar, 4 μm. (E) Median [B. Fluo.]/[M. Fluo.] ratio at division as a function of initial bud size at the time of TS. Black dots represent individual cells and red circles with error bars (statistical error on mean) display the same data after binning data by size. Green and blue dots correspond to the individual cells in the upper and lower panels shown in (D), respectively. (F) Time from TS to cell division for individual cells (black dots) and after binning (red symbols). Inset: growth arrest as a function of bud size upon TS. (G–L) Evolution with time of cell size (G and H), fluorescence concentration (I and J), and number of aggregates (foci) per cell (K and L) for the buds (left column) and the corresponding mothers (right column). Each colored line represents a group of individual time traces of mother-bud pairs grouped according to the initial volume of the bud Vi as indicated in the figure. Total number of cells analyzed = 78. Vertical dashed lines indicate the average time of division for each group of cells. To see this figure in color, go online.
Because these experiments were performed with unsynchronized cells, we reasoned that the degree of asymmetrical PA distribution might be strongly dependent on the phase of the cell cycle at the time of heat shock. Indeed, plotting the [B. Fluo.]/[M. Fluo.] ratio as a function of bud size at the time of the TS revealed that the segregation bias was much stronger for small-budded cells ([B. Fluo.]/[M. Fluo.] = 0.16) than for large-budded ones, in which the average [B. Fluo.]/[M. Fluo.] ratio was close to 1 (Fig. 2, D and E; Movie S2). We noticed that the time between TS and division displayed a strong negative correlation with initial bud size, most likely because large-budded cells at the TS were close to division (Fig. 2 F). Regardless of the mechanism for establishing unequal PA concentration in mother and bud, the absence of asymmetrical segregation upon division of initially large-budded cells could reflect the shorter time during which segregation could occur.
To account for the cell-to-cell variability in PA repartition, we grouped single-cell data according to the initial bud size Vi (Fig. 2, G–L). We found that the time between TS and division of initially small-budded cells was longer than that of the large-budded cells, not only because of the growth requirements for completion of the cell cycle but also because, unexpectedly, the cells experienced a cell size-dependent transient growth arrest following TS (Figs. 2 F, inset, and G). Most importantly, the concentration of PAs was much lower in initially small buds (i.e., Vi < 5 μm3) than in larger ones (i.e., Vi > 10 μm3) (Fig. 2 I). This contrasted sharply with their mothers, which uniformly accumulated PAs (Fig. 2 J). Similarly, the average number of PAs in buds following TS was strongly dependent on the initial bud size (Fig. 2 K), but this was not observed with their mothers (Fig. 2 L). These data suggested that bud growth during the budded period of the cell cycle could determine the PA asymmetry upon division.
PA dilution induced by the polarized growth of the bud drives asymmetrical PA distribution upon division
To test this hypothesis, we turned to a computational model that integrated both the formation and the fusion of PAs and the dynamics of cell growth following TS. Whereas physical arguments based on limited diffusion in a crowded environment (4, 17, 18) or tethering to specific organelles (20, 22) can successfully describe the maintenance of unequal PA concentration across the bud neck, it is important to note that none of them can explain how such a difference can emerge during the budding process. Therefore, we simulated the formation and fusion of PAs within a cell using a previously developed coalescence framework (25, 26) that was recently used to model the distribution of PAs in fission yeast (25). The aggregation of misfolded proteins was represented by an aggregation kernel that describes the frequency of fusion of particles of different sizes (maximal radius of particles amax = 0.6 μm (Fig. S4), constant rate k = 0.0035 min−1; see Figs. S4 and S5; see Text S1 in the Supporting Material for details about the model and the determination of parameters value) in a cellular compartment of volume V. One major advantage of this strategy is that it bypasses the spatial description of aggregate diffusion, which is cumbersome and computationally intensive. Using the Gillespie algorithm, we computed the stochastic kinetics of PA accumulation in cellular compartments, assuming that the M/B size evolved according to Fig. 2, G and H.
We purposely neglected the diffusion of particles through the bud neck, in agreement with experimental observations as well as previous theoretical work, as described in the following: the equilibration of PA in mothers and buds was shown to be slow enough to not interfere with the kinetics of aggregation following heat shock (according to (16, 17), only ∼10% of PAs originally present in the mother cell are found in the bud after 90 min). In line with this, by scoring the number of foci transports from a bud to its mother over the course of the experiment (Fig. S6, A and B), we found that the frequency of these events was very low (11%, n = 78, Fig. S6 C). This hypothesis was also motivated by the observation that bni1 mutants, which are defective in actin cable assembly, have an increased bud neck aperture (Fig. S7, A and B), and therefore a larger probability of PA escape, compared to wild-type yeast, but they display a comparable asymmetrical PA repartition ratio upon division (Fig. S7 C) and similar PA accumulation kinetics in mothers and buds as the wild-type strain (Fig. S7, D–I). Therefore, in this model, mother and bud were treated as independent cellular compartments, even though we could not exclude the possibility that small invisible particles may exchange across the bud neck.
For consistency with our experiments, we introduced a particle visibility threshold such that small PAs would be considered invisible until their size reached a fraction T of the size of the largest observed PAs (T ∼0.01). We found that the formation of individual misfolded proteins should be modeled as a zeroth order process, the rate of which declines exponentially with time following TS (decay rate α = 0.011 min−1). This assumption was necessary to fit the observation that the kinetics of the fluorescence increase in PAs was considerably slower than would be expected from the aggregation dynamics of particles if generated all at once following the TS. Finally, cell growth was modeled to reproduce the dynamics of the volume increase observed in buds of various initial sizes (Fig. 2 G). For the sake of simplicity, we assumed that the initial post-TS cell cycle stall was followed by exponential growth until the bud reached a fraction of the size of its mother, after which the growth rate was greatly reduced (see Text S1 in the Supporting Material for more details related to the model description).
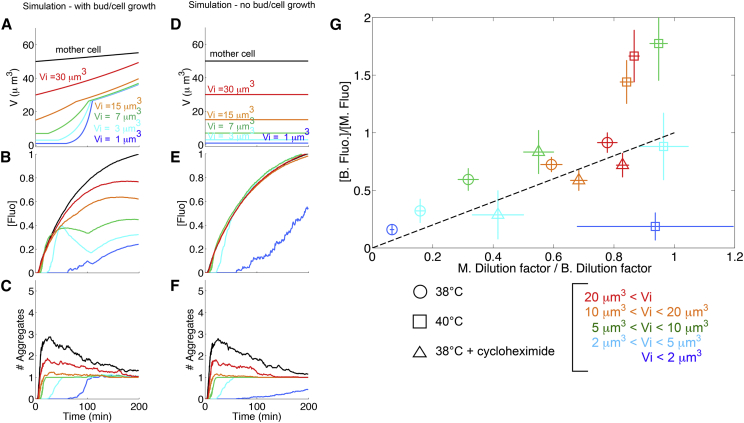
Remarkably, we observed that such a simple framework (with only four experimentally constrained parameters related to PA formation: amax, k, α, and T) was sufficient to quantitatively describe the evolution of the number and concentration of PAs as a function of time, both in buds of various initial sizes and in mother cells (Fig. 3, A–C). In particular, the model successfully captured the size dependence and lower concentration of PA fluorescence in bud versus mother (colored versus black lines, respectively, in Fig. 3 B), thus illustrating the asymmetry in PA distribution across the bud neck.
Figure 3.
Effect of growth-induced dilution on mother/bud asymmetry in PA concentration: numerical model and experimental evidence. (A–C) Stochastic simulation of protein aggregation (all curves represent averages of 30 runs). Parameter values: amax = 0.6 μm; α = 0.02; T = 0.01; and k = 0.035 min−1. (A) Cell volume as a function of time (cell growth is deterministic, and parameters for cell growth are set by the corresponding experimental curves in Fig. 2). Each colored line corresponds to a specific initial bud volume (Vi = 1,3,7,15, and 30 μm3). The black line represents a mother cell of initial size 50 μm3. See Supporting Material text for other parameter values. (B) Concentration of misfolded proteins in PAs as a function of time. Each colored line corresponds to a specific initial bud volume (1, 3, 7, 15, and 30 μm3). The black line represents a mother cell of initial size 50 μm3. (C) Evolution of number of aggregates as a function of time. Each colored line corresponds to a specific initial bud volume (1, 3, 7, 15, and 30 μm3). The black line represents a mother cell of initial size 50 μm3. (D–F) same as (A)–(C), but with growth rate set to 0. (G) Bud/mother ratio in PA concentration (measured at t = 200 min following the temperature shift) as a function of the M/B ratio in dilution factor (i.e., cell volume increase factor between t = 0 min and t = 200 min) for the indicated conditions: 38°C (circles), 40°C (squares), and 38°C + 130 ng/ml cycloheximide (triangles). Each colored point corresponds to a group of cells with initial volume Vi, as indicated on the figure legend. Error bars represent standard error of the mean. The dashed line represents the diagonal. To see this figure in color, go online.
This nonintuitive behavior can be understood as the net result of several counteracting forces. First, the visibility threshold tends to make PAs invisible in tiny buds, which explains why fluorescence concentration is close to 0 when growth is stalled. Second, as PAs become increasingly visible, there is competition between the coalescence of newly unfolded proteins (which tend to increase the PA concentration) and the dilution due to cell growth (which decreases PA concentration). This latter effect seems to dominate the kinetics of PA accumulation and leads to a decrease in (or at least stabilization of) the PA concentration during the polarized growth phase of the cell cycle in a bud size-dependent manner (Fig. 3 B). Consistent with this, setting the growth rate to 0 in the simulation (Fig. 3 D), showed that the difference in PA concentration between mother and bud was fully abolished, except in tiny buds (Fig. 3 E).
Therefore, this computational simulation suggested that the dilution induced by bud growth may drive the unequal distribution of PAs in mother and bud. Consistently, by plotting the bud/mother ratio of PA concentration (at t = 200 min following TS) as a function of the mother/bud ratio of volume fold change between t = 0 and t = 200 min (referred to as the dilution factor), we observed that single-cell data grouped by initial bud volume were close to the diagonal (see colored open circles on Fig. 3 G). This suggested that concentration ratios could simply be set by the ratio of dilution factors.
To further support this conclusion, we used two independent strategies to perturb the polarized growth of the cell and therefore affect the dilution factor in the bud. First, we performed a TS experiment at 40°C. Unlike at 38°C, bud growth was almost completely arrested (Fig. S10) and, therefore, the mother/bud ratio of dilution factor measured for both small-budded cells and large-budded cells was close to 1 (see colored open squares on Fig. 3 G). However, we noticed that bud-to-mother transport events were much more frequent under these conditions (27% at 40°C vs. 11% at 38°C, p = 0.018, Fig. S6 C), with a strong dependency on the initial bud size (Fig. S6 D), due to bud growth arrest over a long time period. Whereas these transport events played a negligible role on the kinetics of PA accumulation at 38°C (Fig. S6, E and G), they lead to a significant underestimate of PA concentration at 40°C (Fig. S6, F and H). By filtering out the cells in which transport of foci occurred at 40°C, we found that the bud/mother ratio of PA concentration was close to 1 for most cell groups (except for tiny buds), providing a satisfying agreement with the dilution model. (Noteworthy, some ratio measurements are close to 1.5 on Fig. 3 G, which is quite consistent with the overall ratio measured right after TS, i.e., when dilution plays no role, see Fig. 2 C.)
Second, we used cycloheximide (CHX) to inhibit cell growth. A high dose of CHX (100 μg/ml) was reported to prevent the formation of heat shock-induced Hsp104-bound aggregates (22), a phenomenon that we could reproduce with a concentration as low as 2 μg/ml (middle panel in Fig. S10). However, lowering further the concentration of CHX to 100 ng/ml significantly impaired cell growth, yet did not prevent the formation of PAs (although at a lower level, see bottom panel Fig. S10). Here again, we found an overall good scaling between bud/mother concentration ratios and M/B dilution ratios. Of importance, Fig. 3 G revealed that the different data sets could all be accounted for by a simple dilution mechanism, even though the data were obtained in several conditions in which the kinetic parameters of aggregates formation may vary.
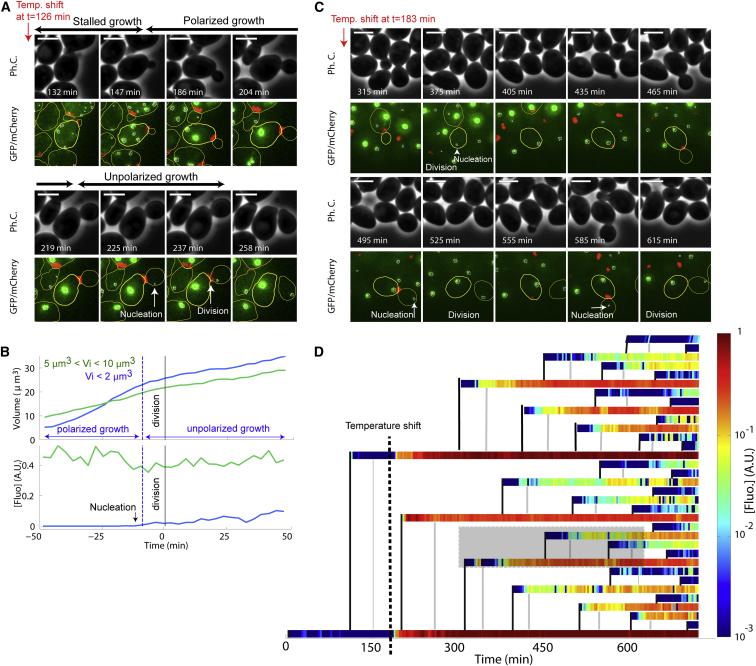
To confirm that bud growth plays an essential role in determining the unequal PA concentration across the bud neck, we postulated that the sharp switch from strongly polarized to unpolarized bud growth by the end of the S/G2 phase should markedly affect the dynamics of PA accumulation. To test this hypothesis, we performed an in silico synchronization of temporal traces with respect to cell division, to make the cell polarization switch more obvious. As in Fig. 2, cells were grouped by initial bud size. By restraining our analysis to a specific window around cell division (between 50 min before and 50 min after division), we found that the emergence of small foci (referred to as “nucleation” in the following) in initially small buds was concomitant with the switch to unpolarized growth (see blue curves in Fig. 4, A and B). In contrast, initially larger buds, which displayed no pronounced change in growth rate, also showed no significant change in the rate of PA accumulation (see green curve in Fig. 4 B). Interestingly, the nucleation of PAs in the bud before division was also consistently observed in dividing cells that were born long after the initial TS (Fig. 4 C; Movie S3), indicating that unfolded proteins were still being generated during maintenance at 38°C. By quantifying the level of PA across a microcolony of growing cells, we found that successive buds of the founder cells were systematically free of PAs during their growth period, and nucleation of a small Hsp104-GFP focus occurred immediately before division (Fig. 4 D). Noteworthy, although PA concentration in founder cells converged to a very high level, cells that were born after the temperature switch reached a much lower plateau. These observations suggest that the rate of formation of misfolded proteins decreases over time, i.e., cells progressively adapt to elevated temperature.
Figure 4.
Nucleation of protein aggregates in growing buds. (A) Nucleation of small PAs during unpolarized bud growth. Sequence of images (phase contrast, overlaid Hsp104-GFP, and Cdc10-mCherry fluorescence) acquired at the indicated time points after TS (126 min). Scale bar, 4 μm. (B) Evolution with time of cell volume and [PA] after in silico synchronization with respect to cell division (indicated by the solid black line). Blue and green curves correspond to initially small and larger buds, respectively, as indicated. The dashed lines indicate the switch from polarized to nonpolarized growth for the initially small buds (blue curve). The onset of [Fluo] increase (nucleation) is indicated. (C) Successive nucleation of PAs in dividing cells following TS. Images are displayed as described in (A). (D) Pedigree of dividing cells before and following the TS (black dashed line). Each horizontal colored bar indicates the evolution of [PA] ([fluo] on a log scale; right vertical bar) as a function of time. The vertical solid black lines indicate the mother/daughter parentage and the onset of budding. The gray lines indicate the times of division. The gray box corresponds to the cell and its progeny displayed in (C). To see this figure in color, go online.
Collectively, these results demonstrate that mother/daughter bias in PA accumulation is established by the strong dilution of PAs in the bud due to the polarized growth during the budded period of the cell cycle, as summarized in Fig. 5. The fact that none of the observed daughter cells (0 of 78 cells analyzed; Fig. 4 D) inherited a large aggregate from their mothers also confirmed that any preestablished bias was maintained over a multigeneration timescale.
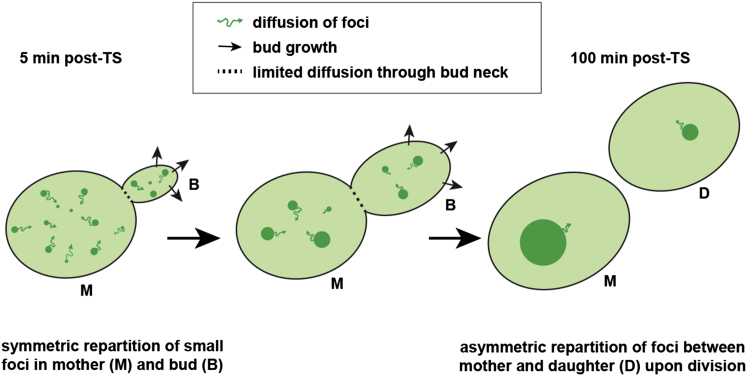
Figure 5.
Schematic model describing the establishment of asymmetric protein aggregates repartition between mother and bud during cell cycle progression. Following an initial temperature shift (left panel), mother cell and bud share similar concentrations of small protein aggregates (PAs), depicted as green dots. These small PAs undergo random movements (curly arrows) and fuse together into larger structures (middle panel). Because of its polarized growth (arrows), PAs get more diluted in the bud than in the mother cell. This—as well as the limited diffusion of PAs through the bud neck (symbolized by the dashed line)—leads to unequal concentrations of PAs across the bud neck upon cell division. To see this figure in color, go online.
Discussion
In this work, we combined quantitative live cell imaging, microfluidics, and a numerical simulation to investigate the establishment of asymmetrical segregation of heat shock-induced PAs between mother and daughter yeast cells. Unlike previous studies based either on static or on very short timescale analyses of the spatial partitioning of PAs and their interactions with subcellular organelles, we focused specifically on the real-time kinetics of PA accumulation in the cell cycle timescale. This approach, in which protein aggregation was observed concomitantly with the cell division process, allowed us to directly investigate contradicting models for the establishment and maintenance of the segregation bias.
Previous work has clearly demonstrated that slow and anomalous diffusion of PAs within cellular compartments prevents their passive transport to the bud on cell cycle timescales (16, 17). Although the maintenance of asymmetrical segregation was proposed to result from the limited diffusion of these large inclusion bodies and their geometric constraints, the question of how biased PA accumulation is established has not been investigated using a limited set of physical arguments. We directly addressed this issue by monitoring the formation and fusion of PAs in unperturbed dividing cells. Both experimental and computational data clearly demonstrated that the Bud/Mother ratio in PA concentration depends on the initial bud size. Any quantitative description of the dynamics of PA concentration must necessarily take the dilution due to cell growth in account. Our analysis revealed that this sole physical argument is sufficient to explain the unequal partitioning of PAs across the bud neck, because the Bud/Mother PA ratio equals the Mother/Bud ratio in dilution factor (Fig. 3 G). Any additional mechanism generating an asymmetry (e.g., that would limit the level of PA concentration in the bud) should have further decreased the Bud/Mother PA ratio upon division below the level expected by the dilution model, contrasting with our observations. Therefore, although we cannot formally exclude the existence of such a bud-specific program, we anticipate that it would only have a minor contribution to the Bud/Mother PA ratio upon division. Rather, we conclude that a combination of bud growth and PA retention in the mother may be the main mechanisms leading to the establishment of unequal PA concentration across the bud neck (Fig. 5). This mechanism may also explain the unequal partitioning of aggregates in the mother versus daughter lineage on a multigeneration timescale (Fig. 4, C and D), which is consistent with the previous observation that the fraction of buds with aggregates decreases as a function of time following a temperature shift (14). Yet, our long-term single-cell imaging assay revealed that low PA concentration in daughters was the consequence of the absence of PA nucleation during most of the budding period (and cellular adaptation to high temperature), rather than resulting from an active retrograde transport (14).
In fission yeast, the fusion of aggregates into one unique structure promotes its asymmetrical segregation into one of the two sibling cells, leaving the other cell aggregate free (25). In our study, although PA fusion is responsible for a sharp slowdown in PA diffusion (see Fig. S4) and therefore is likely to limit transport through the bud neck, the main mechanism responsible for the differential accumulation of PAs is the polarized growth of the cell. We speculate that this mechanism, in combination with the large kinetic barrier associated with transport across the bud neck, may account for the bias observed in aggregates distribution between mother and daughters in an aging context.
Following initial studies proposing that PAs are recruited to various subcellular compartments (20), a growing body of evidence suggests that PAs generated by various proteotoxic stresses may be associated with mitochondria (22) and the endoplasmic reticulum (23, 27). Our data indicate that the aggregation process can be accurately described using a coalescence model based on free particle diffusion. These two a priori conflicting descriptions of aggregation can be reconciled by noting that mitochondria undergo very dynamic fusion/fission events, such that the apparent dynamics of PAs resembles that of freely diffusing particles (with low diffusion coefficients). To refine this analysis, further quantitative studies will be necessary to precisely determine whether the kinetics of PA fusion is limited by the diffusion of particles in a crowded environment or by the intrinsic dynamics of the mitochondrial network. Regardless, it is striking that the differential accumulation of PAs between mother and daughter can be simply described using a limited set of physical arguments and experimentally constrained parameters. Our work highlights the power of microfluidics-based single-cell assays to test and refine existing models of asymmetrical segregation of protein aggregates.
Author Contributions
C.P. and G.C. designed the project. C.P. performed most experiments; S.Q. and A.M. contributed additional experiments requested by reviewers. C.P. and G.C. analyzed the data. G.C. developed the numerical model. G.C. and C.P. wrote the article and S.Q. edited the manuscript.
Acknowledgments
We thank Youlian Goulev for help with statistical analyses and the whole G.C. laboratory for careful reading of the manuscript. We thank Michel Toledano for reagents and useful discussions.
G.C. was supported by the ATIP-AVENIR program and C.P. was funded by a fellowship from the French Ministry of Higher Education and Research.
Editor: Rong Li.
Footnotes
Supporting Materials and methods, ten figures, and three movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)300035-2.
Supporting Material
Overlay of phase contrast and Hsp104-GFP (green) images. Scale bar, 4 μm.
The fluorescence signal corresponds to Hsp104-GFP. Lines represent cell and foci contours. Scale bar, 4 μm.
The fluorescence signal corresponds to Hsp104-GFP. Lines represent cell and foci contours. Scale bar, 4 μm.
References
- 1.Lindner A.B., Demarez A. Protein aggregation as a paradigm of aging. Biochim. Biophys. Acta. 2009;1790:980–996. doi: 10.1016/j.bbagen.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Aguilaniu H., Gustafsson L., Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 3.Lindner A.B., Madden R., Taddei F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. USA. 2008;105:3076–3081. doi: 10.1073/pnas.0708931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler J., Seybert A., Bukau B. Quantitative and spatio-temporal features of protein aggregation in Escherichia coli and consequences on protein quality control and cellular ageing. EMBO J. 2010;29:910–923. doi: 10.1038/emboj.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortimer R.K., Johnston J.R. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy B.K., Austriaco N.R., Jr., Guarente L. Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J. Cell Biol. 1994;127:1985–1993. doi: 10.1083/jcb.127.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egilmez N.K., Jazwinski S.M. Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1989;171:37–42. doi: 10.1128/jb.171.1.37-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehrmann S., Paoletti C., Charvin G. Aging yeast cells undergo a sharp entry into senescence unrelated to the loss of mitochondrial membrane potential. Cell Reports. 2013;5:1589–1599. doi: 10.1016/j.celrep.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair D.A., Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 10.Hughes A.L., Gottschling D.E. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFaline-Figueroa J.R., Vevea J., Pon L.A. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veatch J.R., McMurray M.A., Gottschling D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009;137:1247–1258. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erjavec N., Larsson L., Nyström T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev. 2007;21:2410–2421. doi: 10.1101/gad.439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B., Larsson L., Nyström T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Liu B., Larsson L., Nyström T. Segregation of protein aggregates involves actin and the polarity machinery. Cell. 2011;147:959–961. doi: 10.1016/j.cell.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C., Slaughter B.D., Li R. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinkhabwala A., Khmelinskii A., Knop M. Analytical model for macromolecular partitioning during yeast cell division. BMC Biophys. 2014;7:1–10. doi: 10.1186/s13628-014-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coquel A.-S., Jacob J.-P., Berry H. Localization of protein aggregation in Escherichia coli is governed by diffusion and nucleoid macromolecular crowding effect. PLOS Comput. Biol. 2013;9:e1003038. doi: 10.1371/journal.pcbi.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller S.B.M., Mogk A., Bukau B. Spatially organized aggregation of misfolded proteins as cellular stress defense strategy. J. Mol. Biol. 2015;427:1564–1574. doi: 10.1016/j.jmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Kaganovich D., Kopito R., Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spokoini R., Moldavski O., Kaganovich D. Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Reports. 2012;2:738–747. doi: 10.1016/j.celrep.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C., Slaughter B.D., Li R. Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells. Cell. 2014;159:530–542. doi: 10.1016/j.cell.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escusa-Toret S., Vonk W.I.M., Frydman J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat. Cell Biol. 2013;15:1231–1243. doi: 10.1038/ncb2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill S.M., Hao X., Nyström T. Life-span extension by a metacaspase in the yeast Saccharomyces cerevisiae. Science. 2014;344:1389–1392. doi: 10.1126/science.1252634. [DOI] [PubMed] [Google Scholar]
- 25.Coelho M., Lade S.J., Tolić I.M. Fusion of protein aggregates facilitates asymmetric damage segregation. PLoS Biol. 2014;12:e1001886. doi: 10.1371/journal.pbio.1001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurenzi I.J., Bartels J.D., Diamond S.L. A general algorithm for exact simulation of multicomponent aggregation processes. J. Comput. Phys. 2002;177:418–449. [Google Scholar]
- 27.Song J., Yang Q., Nyström T. Essential genetic interactors of SIR2 required for spatial sequestration and asymmetrical inheritance of protein aggregates. PLoS Genet. 2014;10:e1004539. doi: 10.1371/journal.pgen.1004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overlay of phase contrast and Hsp104-GFP (green) images. Scale bar, 4 μm.
The fluorescence signal corresponds to Hsp104-GFP. Lines represent cell and foci contours. Scale bar, 4 μm.
The fluorescence signal corresponds to Hsp104-GFP. Lines represent cell and foci contours. Scale bar, 4 μm.