Abstract
Acetyl-CoA carboxylases (ACCs) catalyse the committed step in fatty-acid biosynthesis: the ATP-dependent carboxylation of acetyl-CoA to malonyl-CoA. They are important regulatory hubs for metabolic control and relevant drug targets for the treatment of the metabolic syndrome and cancer. Eukaryotic ACCs are single-chain multienzymes characterized by a large, non-catalytic central domain (CD), whose role in ACC regulation remains poorly characterized. Here we report the crystal structure of the yeast ACC CD, revealing a unique four-domain organization. A regulatory loop, which is phosphorylated at the key functional phosphorylation site of fungal ACC, wedges into a crevice between two domains of CD. Combining the yeast CD structure with intermediate and low-resolution data of larger fragments up to intact ACCs provides a comprehensive characterization of the dynamic fungal ACC architecture. In contrast to related carboxylases, large-scale conformational changes are required for substrate turnover, and are mediated by the CD under phosphorylation control.
 Acetyl-CoA carboxylases are central regulatory hubs of fatty acid metabolism and are important targets for drug development in obesity and cancer. Here, the authors demonstrate that the regulation of these highly dynamic enzymes in fungi is governed by a mechanism based on phosphorylation-dependent conformational variability.
Acetyl-CoA carboxylases are central regulatory hubs of fatty acid metabolism and are important targets for drug development in obesity and cancer. Here, the authors demonstrate that the regulation of these highly dynamic enzymes in fungi is governed by a mechanism based on phosphorylation-dependent conformational variability.
Biotin-dependent acetyl-CoA carboxylases (ACCs) are essential enzymes that catalyse the ATP-dependent carboxylation of acetyl-CoA to malonyl-CoA. This reaction provides the committed activated substrate for the biosynthesis of fatty acids via fatty-acid synthase1,2. By catalysing this rate-limiting step in fatty-acid biosynthesis, ACC plays a key role in anabolic metabolism. ACC inhibition and knock-out studies show the potential of targeting ACC for treatment of the metabolic syndrome3,4,5. Furthermore, elevated ACC activity is observed in malignant tumours6,7. A direct link between ACC and cancer is provided by cancer-associated mutations in the breast cancer susceptibility gene 1 (BRCA1), which relieve inhibitory interactions of BRCA1 with ACC8,9. Thus, ACC is a relevant drug target for type 2 diabetes and cancer10,11. Microbial ACCs are also the principal target of antifungal and antibiotic compounds, such as Soraphen A12,13,14.
The principal functional protein components of ACCs have been described already in the late 1960s for Escherichia coli (E. coli) ACC15,16: Biotin carboxylase (BC) catalyses the ATP-dependent carboxylation of a biotin moiety, which is covalently linked to the biotin carboxyl carrier protein (BCCP). Carboxyltransferase (CT) transfers the activated carboxyl group from carboxybiotin to acetyl-CoA to yield malonyl-CoA. Prokaryotic ACCs are transient assemblies of individual BC, CT and BCCP subunits17. Eukaryotic ACCs, instead, are multienzymes, which integrate all functional components into a single polypeptide chain of ∼2,300 amino acids2. Human ACC occurs in two closely related isoforms, ACC1 and 2, located in the cytosol and at the outer mitochondrial membrane, respectively18,19. In addition to the canonical ACC components, eukaryotic ACCs contain two non-catalytic regions, the large central domain (CD) and the BC–CT interaction domain (BT). The CD comprises one-third of the protein and is a unique feature of eukaryotic ACCs without homologues in other proteins. The function of this domain remains poorly characterized, although phosphorylation of several serine residues in the CD regulates ACC activity20,21,22. The BT domain has been visualized in bacterial carboxylases, where it mediates contacts between α- and β-subunits23,24.
Structural studies on the functional architecture of intact ACCs have been hindered by their huge size and pronounced dynamics, as well as the transient assembly mode of bacterial ACCs. However, crystal structures of individual components or domains from prokaryotic and eukaryotic ACCs, respectively, have been solved25,26,27,28,29. The structure determination of the holoenzymes of bacterial biotin-dependent carboxylases, which lack the characteristic CD, such as the pyruvate carboxylase (PC)30, propionyl-CoA carboxylase23, 3-methyl-crotonyl-CoA carboxylase24 and a long-chain acyl-CoA carboxylase31 revealed strikingly divergent architectures despite a general conservation of all functional components. In these structures, the BC and CT active sites are at distances between 40 and 80 Å, such that substrate transfer could be mediated solely by the mobility of the flexibly tethered BCCP.
Human ACC1 is regulated allosterically, via specific protein–protein interactions, and by reversible phosphorylation. Dynamic polymerization of human ACC1 is linked to increased activity and is regulated allosterically by the activator citrate and the inhibitor palmitate20,21, or by binding of the small protein MIG-12 (ref. 32). Human ACC1 is further regulated by specific phosphorylation-dependent binding of BRCA1 to Ser1263 in the CD. BRCA1 binds only to the phosphorylated form of ACC1 and prevents ACC activation by phosphatase-mediated dephosphorylation9,33. Furthermore, phosphorylation by AMP-activated protein kinase (AMPK) and cAMP-dependent protein kinase (PKA) leads to a decrease in ACC1 activity. AMPK phosphorylates ACC1 in vitro at Ser80, Ser1201 and Ser1216 and PKA at Ser78 and Ser1201. However, regulatory effects on ACC1 activity are mainly mediated by phosphorylation of Ser80 and Ser1201 (refs 34, 35). Phosphorylated Ser80, which is highly conserved only in higher eukaryotes, presumably binds into the Soraphen A-binding pocket36. The regulatory Ser1201 shows only moderate conservation across higher eukaryotes, while the phosphorylated Ser1216 is highly conserved across all eukaryotes. However, no effect of Ser1216 phosphorylation on ACC activity has been reported in higher eukaryotes.
For fungal ACC, neither spontaneous nor inducible polymerization has been detected despite considerable sequence conservation to human ACC1. The BRCA1-interacting phosphoserine position is not conserved in fungal ACC, and no other phospho-dependent protein–protein interactions of fungal ACC have been described. In yeast ACC, phosphorylation sites have been identified at Ser2, Ser735, Ser1148, Ser1157 and Ser1162 (ref. 37). Of these, only Ser1157 is highly conserved in fungal ACC and aligns to Ser1216 in human ACC1. Its phosphorylation by the AMPK homologue SNF1 results in strongly reduced ACC activity22,38.
Despite the outstanding relevance of ACC in primary metabolism and disease, the dynamic organization and regulation of the giant eukaryotic, and in particular fungal ACC, remain poorly characterized. Here we provide the structure of Saccharomyces cerevisiae (Sce) ACC CD, intermediate- and low-resolution structures of human (Hsa) ACC CD and larger fragments of fungal ACC from Chaetomium thermophilum (Cth; Fig. 1a). Integrating these data with small-angle X-ray scattering (SAXS) and electron microscopy (EM) observations yield a comprehensive representation of the dynamic structure and regulation of fungal ACC.
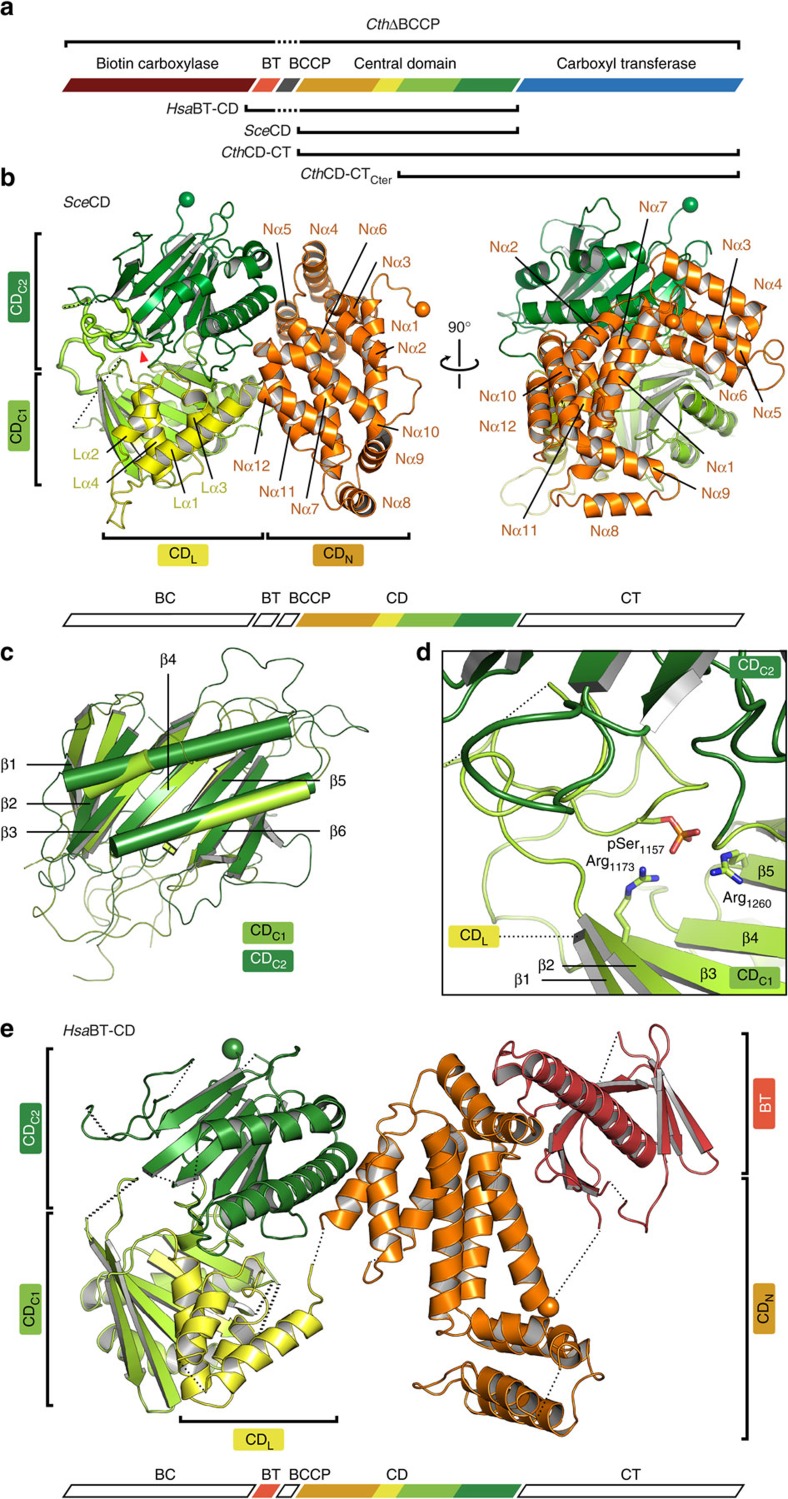
Figure 1. The phosphorylated central domain of yeast ACC.
(a) Schematic overview of the domain organization of eukaryotic ACCs. Crystallized constructs are indicated. (b) Cartoon representation of the SceCD crystal structure. CDN is linked by a four-helix bundle (CDL) to two α–β-fold domains (CDC1 and CDC2). The regulatory loop is shown as bold cartoon, and the phosphorylated Ser1157 is marked by a red triangle. The N- and C termini are indicated by spheres. (c) Superposition of CDC1 and CDC2 reveals highly conserved folds. (d) The regulatory loop with the phosphorylated Ser1157 is bound into a crevice between CDC1 and CDC2, the conserved residues Arg1173 and Arg1260 coordinate the phosphoryl-group. (e) Structural overview of HsaBT-CD. The attachment points to the N-terminal BCCP domain and the C-terminal CT domain are indicated with spheres. All colourings are according to scheme a.
Results
The organization of the yeast ACC CD
First, we focused on structure determination of the 82-kDa CD. The crystal structure of the CD of SceACC (SceCD) was determined at 3.0 Å resolution by experimental phasing and refined to Rwork/Rfree=0.20/0.24 (Table 1). The overall extent of the SceCD is 70 by 75 Å (Fig. 1b and Supplementary Fig. 1a,b), and the attachment points of the N-terminal 26-residue linker to the BCCP domain and the C-terminal CT domain are separated by 46 Å (the N- and C termini are indicated with spheres in Fig. 1b). SceCD comprises four distinct domains, an N-terminal α-helical domain (CDN), and a central four-helix bundle linker domain (CDL), followed by two α–β-fold C-terminal domains (CDC1/CDC2). CDN adopts a letter C shape, where one of the ends is a regular four-helix bundle (Nα3-6), the other end is a helical hairpin (Nα8,9) and the bridging region comprises six helices (Nα1,2,7,10–12). CDL is composed of a small, irregular four-helix bundle (Lα1–4) and tightly interacts with the open face of CDC1 via an interface of 1,300 Å2 involving helices Lα3 and Lα4. CDL does not interact with CDN apart from the covalent linkage and forms only a small contact to CDC2 via a loop between Lα2/α3 and the N-terminal end of Lα1, with an interface area of 400 Å2. CDC1/CDC2 share a common fold; they are composed of six-stranded β-sheets flanked on one side by two long, bent helices inserted between strands β3/β4 and β4/β5. CDC2 is extended at its C terminus by an additional β-strand and an irregular β-hairpin. On the basis of a root mean square deviation of main chain atom positions of 2.2 Å, CDC1/CDC2 are structurally more closely related to each other than to any other protein (Fig. 1c); they may thus have evolved by duplication. Close structural homologues could not be found for the CDN or the CDC domains.
Table 1. Crystallographic data collection and refinement statistics.
| SceCD | SceCD Thimerosal | SceCD Eu | HsaBT-CD | HsaBT-CD Cd2+ | CthCD-CTCter1 | CthCD-CTCter2 | CthCD-CT | CthΔBCCP | |
|---|---|---|---|---|---|---|---|---|---|
| Data collection | |||||||||
| Space group | P43212 | P43212 | P43212 | I4122 | I4122 | P212121 | P212121 | P31212 | P6422 |
| Cell dimensions | |||||||||
| a, b, c (Å) | 110.86, 110.86, 131.12 | 111.22, 111.22, 131.49 | 108.65, 108.65, 127.36 | 267.27, 267.27, 210.61 | 267.67, 267.67, 210.46 | 97.66, 165.34, 219.23 | 100.17, 153.45, 249,24 | 295.02, 295.02, 189.52 | 462.20, 462.20, 204.64 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 120 | 90, 90, 120 |
| Resolution* (Å) | 3.0 | 3.4 | 4.0 | 3.7 | 4.1 | 3.6 | 4.5 | 7.2 | 8.4 |
| RMerge† | 18.2 (389.6) | 20.5 (306.1) | 40.6 (327.0) | 7.5 (400.9) | 15 (730.5) | 14.5 (384.5) | 27.4 (225.6) | 5.6 (302.6) | 29.4 (381.7) |
| CC ½*,† | 100 (58.3) | 99.9 (42.6) | 99.9 (48.5) | 100 (59.4) | 99.8 (73.2) | 99.9 (50.9) | 99.5 (46.7) | 100 (33.3) | 99.7 (35) |
| I/σI† | 24.68 (1.46) | 7.99 (0.89) | 17.92 (1.85) | 21.24 (1.07) | 16.53 (1.41) | 10.61 (0.97) | 6.35 (1.00) | 18.95 (0.92) | 9.05 (0.9) |
| Completeness† | 99.9 (99.9) | 99.6 (100) | 99.7 (96.8) | 99.8 (99.1) | 99.8 (99.7) | 99.7 (99.9) | 99.4 (98.6) | 99.6 (100) | 99.1 (99.9) |
| Redundancy† | 39.1 (39.8) | 12.1 (14.3) | 81.6 (65.2) | 13.7 (13.7) | 20.9 (19.1) | 12.7 (13.5) | 6.1 (6.5) | 9.9 (10.4) | 18.5 (18.2) |
| Refinement | |||||||||
| Resolution* (Å) | 46.4–3.0 | 84.5–3.7 | 49.2–3.6 | 49.1–4.5 | 49.9–7.2 | 50.0–8.4 | |||
| Reflections | 16,928 | — | — | 40,647 | — | 41,799 | 23,340 | 14,046 | 12,111 |
| Rwork/Rfree | 0.20/0.24 | — | — | 0.35/0.38‡ | — | 0.20/0.24 | 0.24/0.24 | 0.23/0.25 | 0.30/0.32 |
| Number of atoms | |||||||||
| Protein | 5,465 | 6,925 | 16,592 | 16,405 | 22,543 | 22,445 | |||
| Waters | 43 | — | — | — | — | — | — | — | — |
| Ligand/ion | 7 | — | — | 5 | — | — | — | — | — |
| B-factors | |||||||||
| Protein | 130 | 158 | 226 | 275 | 272 | 250 | |||
| Waters | 84 | — | — | — | — | — | — | — | — |
| Ligand/ion | 90 | — | — | 189 | — | — | — | — | — |
| R.m.s.d. | |||||||||
| RMS (angles, °) | 0.97 | — | — | 0.83 | — | 1.07 | 1.11 | 1.15 | 1.01 |
| RMS (bonds, Å) | 0.01 | — | — | 0.01 | — | 0.01 | 0.01 | 0.01 | 0.01 |
*Resolution cutoffs determined based on internal correlation significant at the 0.1% level as calculated by XDS.
†Highest-resolution shell is shown in parentheses.
‡Modelled only as poly-alanine.
A regulatory loop mediates interdomain interactions
To define the functional state of insect-cell-expressed ACC variants, we employed mass spectrometry (MS) for phosphorylation site detection. In insect-cell-expressed full-length SceACC, the highly conserved Ser1157 is the only fully occupied phosphorylation site with functional relevance in S. cerevisiae. Additional phosphorylation was detected for Ser2101 and Tyr2179; however, these sites are neither conserved across fungal ACC nor natively phosphorylated in yeast. MS analysis of dissolved crystals confirmed the phosphorylated state of Ser1157 also in SceCD crystals. The SceCD structure thus authentically represents the state of SceACC, where the enzyme is inhibited by SNF1-dependent phosphorylation.
In the SceCD crystal structure, the phosphorylated Ser1157 resides in a regulatory 36-amino-acid loop between strands β2 and β3 of CDC1 (Fig. 1b,d), which contains two additional less-conserved phosphorylation sites (Ser1148 and Ser1162) confirmed in yeast39, but not occupied here. This regulatory loop wedges between the CDC1 and CDC2 domains and provides the largest contribution to the interdomain interface. The N-terminal region of the regulatory loop also directly contacts the C-terminal region of CDC2 leading into CT. Phosphoserine 1157 is tightly bound by two highly conserved arginines (Arg1173 and Arg1260) of CDC1 (Fig. 1d). Already the binding of phosphorylated Ser1157 apparently stabilizes the regulatory loop conformation; the accessory phosphorylation sites Ser1148 and Ser1162 in the same loop may further modulate the strength of interaction between the regulatory loop and the CDC1 and CDC2 domains. Phosphorylation of the regulatory loop thus determines interdomain interactions of CDC1 and CDC2, suggesting that it may exert its regulatory function by modifying the overall structure and dynamics of the CD.
The functional role of Ser1157 was confirmed by an activity assay based on the incorporation of radioactive carbonate into acid non-volatile material40. Phosphorylated SceACC shows only residual activity (kcat=0.4±0.2 s−1, s.d. based on five replicate measurements), which increases 16-fold (kcat=6.5±0.3 s−1) after dephosphorylation with λ protein phosphatase. The values obtained for dephosphorylated SceACC are comparable to earlier measurements of non-phosphorylated yeast ACC expressed in E. coli41.
The variable CD is conserved between yeast and human
To compare the organization of fungal and human ACC CD, we determined the structure of a human ACC1 fragment that comprises the BT and CD domains (HsaBT-CD), but lacks the mobile BCCP in between (Fig. 1a). An experimentally phased map was obtained at 3.7 Å resolution for a cadmium-derivatized crystal and was interpreted by a poly-alanine model (Fig. 1e and Table 1). Each of the four CD domains in HsaBT-CD individually resembles the corresponding SceCD domain; however, human and yeast CDs exhibit distinct overall structures. In agreement with their tight interaction in SceCD, the relative spatial arrangement of CDL and CDC1 is preserved in HsaBT-CD, but the human CDL/CDC1 didomain is tilted by 30° based on a superposition of human and yeast CDC2 (Supplementary Fig. 1c). As a result, the N terminus of CDL at helix Lα1, which connects to CDN, is shifted by 12 Å. Remarkably, CDN of HsaBT-CD adopts a completely different orientation compared with SceCD. With CDL/CDC1 superposed, CDN in HsaBT-CD is rotated by 160° around a hinge at the connection of CDN/CDL (Supplementary Fig. 1d). This rotation displaces the N terminus of CDN in HsaBT-CD by 51 Å compared with SceCD, resulting in a separation of the attachment points of the N-terminal linker to the BCCP domain and the C-terminal CT domain by 67 Å (the attachment points are indicated with spheres in Fig. 1e). The BT domain of HsaBT-CD consists of a helix that is surrounded at its N terminus by an antiparallel eight-stranded β-barrel. It resembles the BT of propionyl-CoA carboxylase23; only the four C-terminal strands of the β-barrel are slightly tilted.
On the basis of MS analysis of insect-cell-expressed human full-length ACC, Ser80 shows the highest degree of phosphorylation (90%). Ser29 and Ser1263, implicated in insulin-dependent phosphorylation and BRCA1 binding, respectively, are phosphorylated at intermediate levels (40%). The highly conserved Ser1216 (corresponding to S. cerevisiae Ser1157), as well as Ser1201, both in the regulatory loop discussed above, are not phosphorylated. However, residual phosphorylation levels were detected for Ser1204 (7%) and Ser1218 (7%) in the same loop. MS analysis of the HsaBT-CD crystallization sample reveals partial proteolytic digestion of the regulatory loop. Accordingly, most of this loop is not represented in the HsaBT-CD crystal structure. The absence of the regulatory loop might be linked to the less-restrained interface of CDL/CDC1 and CDC2 and altered relative orientations of these domains. Besides the regulatory loop, also the phosphopeptide target region for BRCA1 interaction is not resolved presumably because of pronounced flexibility.
At the level of isolated yeast and human CD, the structural analysis indicates the presence of at least two hinges, one with large-scale flexibility at the CDN/CDL connection, and one with tunable plasticity between CDL/CDC1 and CDC2, plausibly affected by phosphorylation in the regulatory loop region.
The integration of CD into the fungal ACC multienzyme
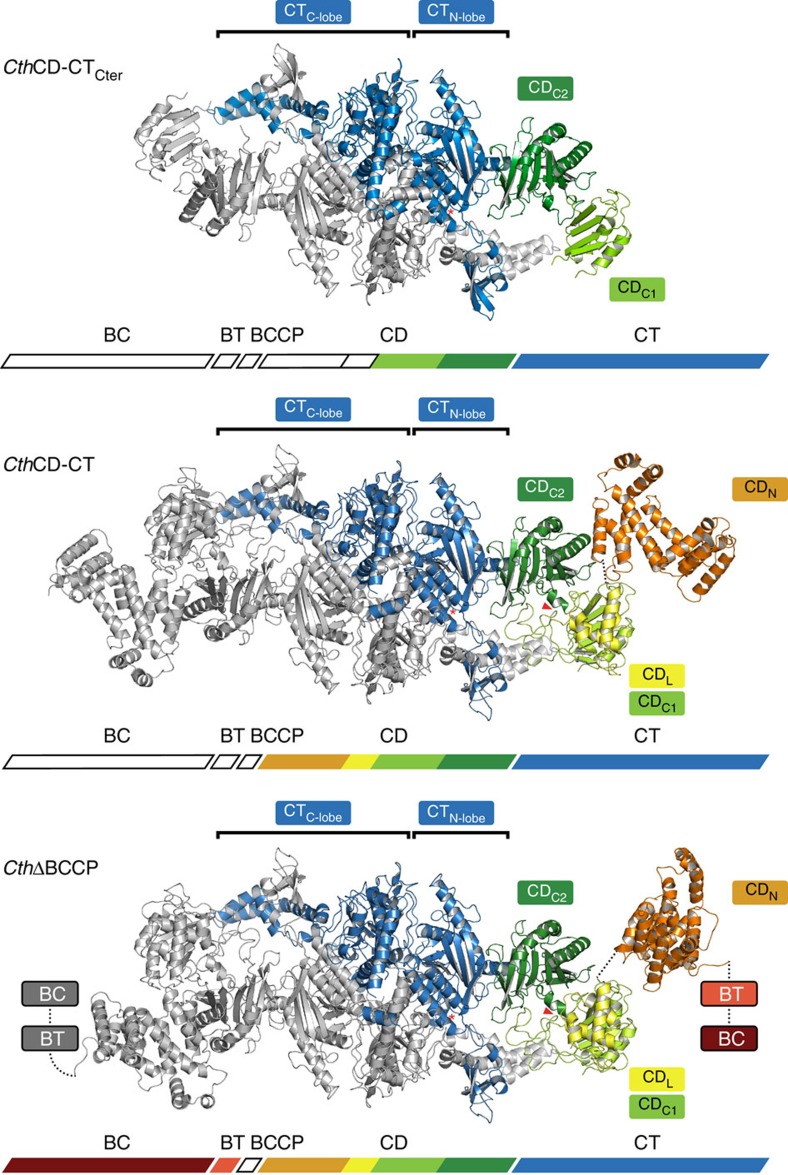
To further obtain insights into the functional architecture of fungal ACC, we characterized larger multidomain fragments up to the intact enzymes. Using molecular replacement based on fungal ACC CD and CT models, we obtained structures of a variant comprising CthCT and CDC1/CDC2 in two crystal forms at resolutions of 3.6 and 4.5 Å (CthCD-CTCter1/2), respectively, as well as of a CthCT linked to the entire CD at 7.2 Å resolution (CthCD-CT; Figs 1a and 2, Table 1). No crystals diffracting to sufficient resolution were obtained for larger BC-containing fragments, or for full-length Cth or SceACC. To improve crystallizability, we generated ΔBCCP variants of full-length ACC, which, based on SAXS analysis, preserve properties of intact ACC (Supplementary Table 1 and Supplementary Fig. 2a–c). For CthΔBCCP, crystals diffracting to 8.4 Å resolution were obtained. However, molecular replacement did not reveal a unique positioning of the BC domain. Owing to the limited resolution the discussion of structures of CthCD-CT and CthΔBCCP is restricted to the analysis of domain localization. Still, these structures contribute considerably to the visualization of an intrinsically dynamic fungal ACC.
Figure 2. Architecture of the CD–CT core of fungal ACC.
Cartoon representation of crystal structures of multidomain constructs of CthACC. One protomer is shown in colour and one in grey. Individual domains are labelled; the active site of CT and the position of the conserved regulatory phosphoserine site based on SceCD are indicated by an asterisk and a triangle, respectively.
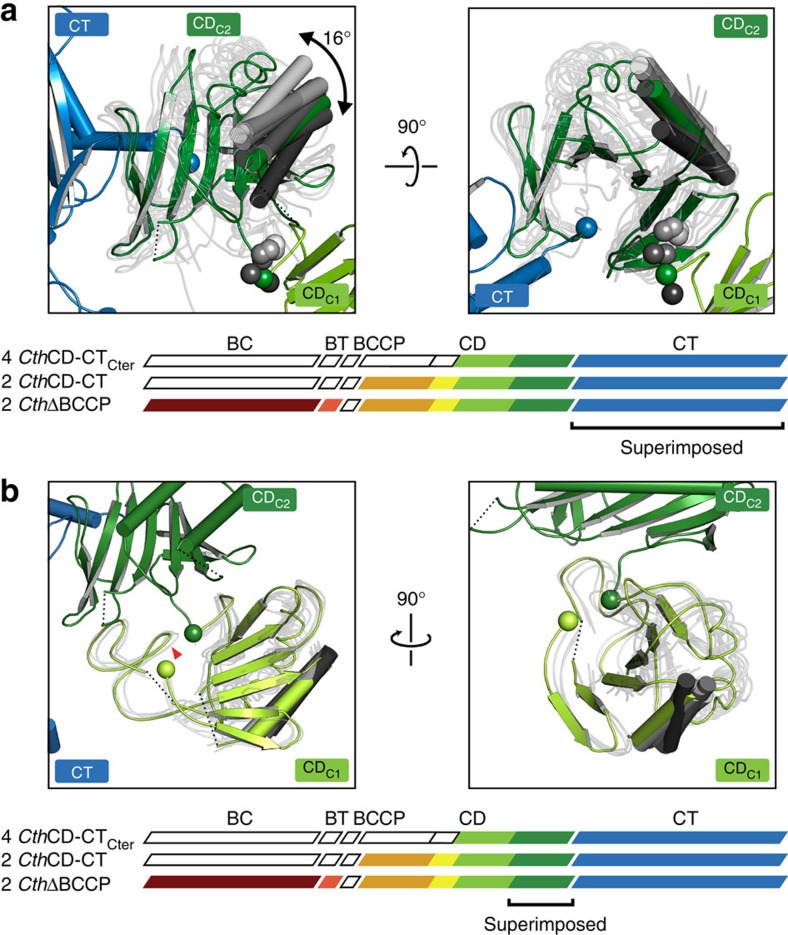
In all these crystal structures, the CT domains build a canonical head-to-tail dimer29, with active sites formed by contributions from both protomers (Fig. 2 and Supplementary Fig. 3a). The connection of CD and CT is provided by a 10-residue peptide stretch, which links the N terminus of CT to the irregular β-hairpin/β-strand extension of CDC2 (Supplementary Fig. 3b). The connecting region is remarkably similar in isolated CD and CthCD-CTCter structures, indicating inherent conformational stability. CD/CT contacts are only formed in direct vicinity of the covalent linkage and involve the β-hairpin extension of CDC2 as well as the loop between strands β2/β3 of the CT N-lobe, which contains a conserved RxxGxN motif. The neighbouring loop on the CT side (between CT β1/β2) is displaced by 2.5 Å compared to isolated CT structures (Supplementary Fig. 3c). On the basis of an interface area of ∼600 Å2 and its edge-to-edge connection characteristics, the interface between CT and CD might be classified as conformationally variable. Indeed, the comparison of the positioning of eight instances of the C-terminal part of CD relative to CT in crystal structures determined here, reveals flexible interdomain linking (Fig. 3a). The CDC2/CT interface acts as a true hinge with observed rotation up to 16°, which results in a translocation of the distal end of CDC2 by 8 Å.
Figure 3. Variability of the connections of CDC2 to CT and CDC1 in fungal ACC.
(a) Hinge properties of the CDC2–CT connection analysed by a CT-based superposition of eight instances of the CDC2-CT segment. For clarity, only one protomer of CthCD-CTCter1 is shown in full colour as reference. For other instances, CDC2 domains are shown in transparent tube representation with only one helix each highlighted. The range of hinge bending is indicated and the connection points between CDC2 and CT (blue) as well as between CDC1 and CDC2 (green and grey) are marked as spheres. (b) The interdomain interface of CDC1 and CDC2 exhibits only limited plasticity. Representation as in a, but the CDC1 and CDC2 are superposed based on CDC2. One protomer of CthΔBCCP is shown in colour, the CDL domains are omitted for clarity and the position of the phosphorylated serine based on SceCD is indicated with a red triangle. The connection points from CDC1 to CDC2 and to CDL are represented by green spheres.
The interface between CDC2 and CDL/CDC1, which is mediated by the phosphorylated regulatory loop in the SceCD structure, is less variable than the CD–CT junction, and permits only limited rotation and tilting (Fig. 3b). Analysis of the impact of phosphorylation on the interface between CDC2 and CDL/CDC1 in CthACC variant structures is precluded by the limited crystallographic resolution. However, MS analysis of CthCD-CT and CthΔBCCP constructs revealed between 60 and 70% phosphorylation of Ser1170 (corresponding to SceACC Ser1157).
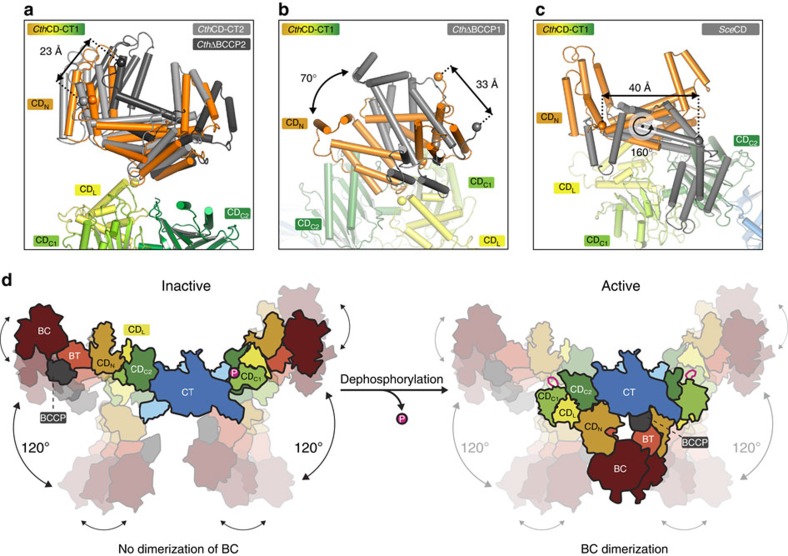
The CDN domain positioning relative to CDL/CDC1 is highly variable with three main orientations observed in the structures of SceCD and the larger CthACC fragments: CDN tilts, resulting in a displacement of its N terminus by 23 Å (Fig. 4a, observed in both protomers of CthCD-CT and one protomer of CthΔBCCP, denoted as CthCD-CT1/2 and CthΔBCCP1, respectively). In addition, CDN can rotate around hinges in the connection between CDN/CDL by 70° (Fig. 4b, observed in the second protomer of CthΔBCCP, denoted as CthΔBCCP2) and 160° (Fig. 4c, observed in SceCD) leading to displacement of the anchor site for the BCCP linker by up to 33 and 40 Å, respectively.
Figure 4. The conformational dynamics of fungal ACC.
(a–c) Large-scale conformational variability of the CDN domain relative to the CDL/CDC1 domain. CthCD-CT1 (in colour) serves as reference, the compared structures (as indicated, numbers after construct name differentiate between individual protomers) are shown in grey. Domains other than CDN and CDL/CDC1 are omitted for clarity. The domains are labelled and the distances between the N termini of CDN (spheres) in the compared structures are indicated. (d) Schematic model of fungal ACC showing the intrinsic, regulated flexibility of CD in the phosphorylated inhibited or the non-phosphorylated activated state. Flexibility of the CDC2/CT and CDN/CDL hinges is illustrated by arrows. The Ser1157 phosphorylation site and the regulatory loop are schematically indicated in magenta.
Conformational variability in the CD thus contributes considerably to variations in the spacing between the BC and CT domains, and may extend to distance variations beyond the mobility range of the flexibly tethered BCCP. On the basis of the occurrence of related conformational changes between fungal and human ACC fragments, the observed set of conformations may well represent general states present in all eukaryotic ACCs.
Large-scale conformational variability of fungal ACC
To obtain a comprehensive view of fungal ACC dynamics in solution, we employed SAXS and EM. SAXS analysis of CthACC agrees with a dimeric state and an elongated shape with a maximum extent of 350 Å (Supplementary Table 1). The smooth appearance of scattering curves and derived distance distributions might indicate substantial interdomain flexibility42 (Supplementary Fig. 2a–c). Direct observation of individual full-length CthACC particles, according to MS results predominantly in a phosphorylated low-activity state, in negative stain EM reveals a large set of conformations from rod-like extended to U-shaped particles. Class averages, obtained by maximum-likelihood-based two-dimensional (2D) classification, are focused on the dimeric CT domain and the full BC–BCCP–CD domain of only one protomer, due to the non-coordinated motions of the lateral BC/CD regions relative to the CT dimer. They identify the connections between CDN/CDL and between CDC2/CT as major contributors to conformational heterogeneity (Supplementary Fig. 4a,b). The flexibility in the CDC2/CT hinge appears substantially larger than the variations observed in the set of crystal structures. The BC domain is not completely disordered, but laterally attached to BT/CDN in a generally conserved position, albeit with increased flexibility. Surprisingly, in both the linear and U-shaped conformations, the approximate distances between the BC and CT active sites would remain larger than 110 Å. These observed distances are considerably larger than in static structures of any other related biotin-dependent carboxylase. Furthermore, based on an average length of the BCCP–CD linker in fungal ACC of 26 amino acids, mobility of the BCCP alone would not be sufficient to bridge the active sites of BC and CT. Consequently, increased flexibility or additional modes of conformational changes may be required for productive catalysis. The most relevant candidate site for mediating such additional flexibility and permitting an extended set of conformations is the CDC1/CDC2 interface, which is rigidified by the Ser1157-phosphorylated regulatory loop, as depicted in the SceCD crystal structure.
Discussion
Altogether, the architecture of fungal ACC is based on the central dimeric CT domain (Fig. 4d). The CD consists of four distinct subdomains and acts as a tether from the CT to the mobile BCCP and an oriented BC domain. The CD has no direct role in substrate recognition or catalysis but contributes to the regulation of all eukaryotic ACCs. In higher eukaryotic ACCs, regulation via phosphorylation is achieved by combining the effects of phosphorylation at Ser80, Ser1201 and Ser1263. In fungal ACC, however, Ser1157 in the regulatory loop of the CD is the only phosphorylation site that has been demonstrated to be both phosphorylated in vivo and involved in the regulation of ACC activity. In its phosphorylated state, the regulatory loop containing Ser1157 wedges between CDC1/CDC2 and presumably limits the conformational freedom at this interdomain interface. However, flexibility at this hinge may be required for full ACC activity, as the distances between the BCCP anchor points and the active sites of BC and CT observed here are such large that mobility of the BCCP alone is not sufficient for substrate transfer. The current data thus suggest that regulation of fungal ACC is mediated by controlling the dynamics of the unique CD, rather than directly affecting catalytic turnover at the active sites of BC and CT. A comparison between fungal and human ACC will help to further discriminate mechanistic differences that contribute to the extended control and polymerization of human ACC.
Most recently, a crystal structure of near full-length non-phosphorylated ACC from S. cerevisae (lacking only 21 N-terminal amino acids, here denoted as flACC) was published by Wei and Tong41. In flACC, the ACC dimer obeys twofold symmetry and assembles in a triangular architecture with dimeric BC domains (Supplementary Fig. 5a). In their study, mutational data indicate a requirement for BC dimerization for catalytic activity. The transition from the elongated open shape, observed in our experiments, towards a compact triangular shape is based on an intricate interplay of several hinge-bending motions in the CD (Fig. 4d). Comparison of flACC with our CthΔBCCP structure reveals the CDC2/CT hinge as a major contributor to conformational flexibility (Supplementary Fig. 5b,c). In flACC, CDC2 rotates ∼120° with respect to the CT domain. A second hinge can be identified between CDC1/CDC2. On the basis of a superposition of CDC2, CDC1 of the phosphorylated SceCD is rotated by 30° relative to CDC1 of the non-phosphorylated flACC (Supplementary Fig. 5d), similar to what we have observed for the non-phosphorylated HsaBT-CD (Supplementary Fig. 1d). When inspecting all individual protomer and fragment structures in their study, Wei and Tong also identify the CDN/CDC1 connection as a highly flexible hinge, in agreement with our observations.
The only bona fide regulatory phophorylation site of fungal ACC in the regulatory loop is directly participating in CDC1/CDC2 domain interactions and thus stabilizes the hinge conformation. In flACC, the regulatory loop is mostly disordered, illustrating the increased flexibility due to the absence of the phosphoryl group. Only in three out of eight observed protomers a short peptide stretch (including Ser1157) was modelled. In those instances the Ser1157 residue is located at a distance of 14–20 Å away from the location of the phosphorylated serine observed here, based on superposition of either CDC1 or CDC2. Applying the conformation of the CDC1/CDC2 hinge observed in SceCD on flACC leads to CDN sterically clashing with CDC2 and BT/CDN clashing with CT (Supplementary Fig. 6a,b). Thus, in accordance with the results presented here, phosphorylation of Ser1157 in SceACC most likely limits flexibility in the CDC1/CDC2 hinge such that activation through BC dimerization is not possible (Fig. 4d), which however does not exclude intermolecular dimerization. In addition, EM micrographs of phosphorylated and dephosphorylated SceACC display for both samples mainly elongated and U-shaped conformations and reveal no apparent differences in particle shape distributions (Supplementary Fig. 7). This implicates that the triangular shape with dimeric BC domains has a low population also in the active form, even though a biasing influence of grid preparation cannot be excluded completely.
Large-scale conformational variability has also been observed in most other carrier protein-based multienzymes, including polyketide43 and fatty-acid synthases (with the exception of fungal-type fatty-acid synthases)44,45, non-ribosomal peptide synthetases46 and the pyruvate dehydrogenase complexes47, although based on completely different architectures. Together, this structural information suggests that variable carrier protein tethering is not sufficient for efficient substrate transfer and catalysis in any of these systems. The determination of a set of crystal structures of SceACC in two states, unphosphorylated41 and phosphorylated at the major regulatory site Ser1157, provides a unique depiction of multienzyme regulation by post-translational modification (Fig. 4d). The phosphorylated regulatory loop binds to an allosteric site at the interface of two non-catalytic domains and restricts conformational freedom at several hinges in the dynamic ACC. It disfavours the adoption of a rare, compact conformation, in which intramolecular dimerization of the BC domains41 results in catalytic turnover. The regulation of activity thus results from restrained large-scale conformational dynamics rather than a direct or indirect influence on active site structure. To our best knowledge, ACC is the first multienzyme for which such a phosphorylation-dependent mechanical control mechanism has been visualized. However, the example of ACC now demonstrates the possibility of regulating activity by controlled dynamics of non-enzymatic linker regions also in other families of carrier-dependent multienzymes. Understanding such structural and dynamic constraints imposed by scaffolding and linking in carrier protein-based multienzyme systems is a critical prerequisite for engineering of efficient biosynthetic assembly lines.
Methods
Protein expression and purification
All proteins were expressed in the Baculovirus Expression Vector System. The MultiBac insect cell expression plasmid pACEBACI (Geneva Biotech) was modified to host a GATEWAY (LifeTechnologies) cassette with an N-terminal 10xHis-tag, named pAB1GW-NH10 hereafter. Full-length HsaACC (Genebank accession #Q13085), SceACC (#Q00955) and CthACC (#G0S3L5) were cloned into pAB1GW-NH10 using GATEWAY according to the manufacturer's manual. Truncated variants were constructed by PCR amplification, digestion of the template DNA with DpnI, phosphorylation of the PCR product and religation of the linear fragment to a circular plasmid. The following constructs were used for this study: SceACC (1–2,233), CthACC (1–2,297), CthΔBCCP (1–2,297, Δ700–765), CthCD-CT (788–2,297), CthCD-CTCter (1,114–2,297), SceCD (768–1,494) and HsaBT-CD (622–1,584, Δ753–818). Bacmid and virus production was carried out according to MultiBac instructions48. Baculovirus generation and amplification as well as protein expression were performed in Sf21 cells (Expression Systems) in Insect-Xpress medium (Lonza). The cells were harvested 68–96 h post infection by centrifugation and stored at −80 °C until being processed.
Cells were lysed by sonication and the lysate was cleared by ultracentrifugation. Soluble protein was purified using Ni-NTA (Genscript) and size exclusion chromatography (Superose 6, GE Healthcare). The affinity tag was removed by tobacco etch virus (TEV) protease cleavage overnight at 4 °C. TEV protease and uncleaved protein were removed by orthogonal Ni-NTA purification before size exclusion chromatography. SceACC, CthACC and CthΔBCCP were further purified by high-resolution anion exchange chromatography before size exclusion chromatography. Purified SceCD, CthCD-CTCter, CthCD-CT, CthΔBCCP, CthACC and SceACC were concentrated to 10 mg ml−1 in 30 mM 3-(N-morpholino) propanesulfonic acid (MOPS) pH 7, 200 mM ammonium sulfate, 5% glycerol and 10 mM dithiothreitol. Purified HsaBT-CD was concentrated to 20 mg ml−1 in 20 mM bicine pH 8.0, 200 mM NaCl, 5% glycerol and 5 mM tris(2-carboxyethyl) phosphine (TCEP). Proteins were used directly or were stored at −80 °C after flash-freezing in liquid nitrogen.
Protein crystallization
All crystallization experiments were conducted using sitting drop vapour diffusion. SceCD crystals were grown at 19 °C by mixing protein and reservoir solution (0.1 M BisTrisPropane pH 6.5, 0.05–0.2 M di-sodium malonate, 20–30% polyethylene glycol (PEG) 3350, 10 mM trimethylamine or 2% benzamidine) in a 1:1 or 2:1 ratio. Crystals appeared after several days and continued to grow for 20–200 days. Crystals were cryoprotected by short incubation in mother liquor supplemented with 22% ethylene glycol and flash-cooled in liquid nitrogen. For heavy metal derivatization the crystals were incubated in stabilization solution supplemented with 1 mM Thimerosal or 10 mM EuCl2, and then backsoaked for 15 s in stabilization solution without heavy metal.
Initial crystals of HsaBT-CD grew in 0.1 M Tris pH 8.5, 0.35 M tri-potassium citrate and 2–3.5% PEG10000 at 19 °C. After several rounds of optimization, good-quality diffraction crystals were obtained at 19 °C in 0.1 M MES pH 6, 0.25–0.35 M tri-potassium citrate, 2–5% PEG10000 and 0.01–0.04 M cadmium chloride. The protein drop contained a 1:1 ratio of protein and reservoir solution. Crystals grew immediately and stopped growing after 3 days. They were dehydrated and cryoprotected in several steps in artificial mother liquor containing incrementally increasing concentrations of tri-potassium citrate, PEG10000 and ethylene glycol and then flash-cooled in liquid nitrogen. The final solution was composed of 0.1 M MES pH 6, 0.5 M tri-potassium citrate, 6.75% PEG10000, 0.01 M cadmium chloride and 22% ethylene glycol.
CthCD-CTCter crystals were grown at 19 °C by mixing protein and reservoir solution (0.1 M HEPES pH 7.5, 2–7% Tacsimate pH 7, 7.5–15% PEG monomethyl ether 5000) in a 1:1 ratio. Crystals appeared after several days and continued to grow for up to 2 weeks. Crystals were cryoprotected by short incubation in mother liquor supplemented with 22% ethylene glycol.
CthCD-CT ACC crystals were grown at 19 °C by mixing protein and reservoir solution (0.1 M Bicine pH 8.5–9.5, 4–8% PEG8000) in a 1:1 or 1:2 ratio. Crystals grew 8– 10 days and were cryoprotected by short incubation in mother liquor supplemented with 22% ethylene glycol before flash-cooling in liquid nitrogen.
CthΔBCCP ACC crystals were grown at 19 °C by mixing protein and reservoir solution (0.1 M Morpheus buffer 3 (Molecular Dimensions, MD2-100-102), 7–12% Morpheus ethylene glycols mix (MD2-100-74), 8–12% PEG4000, 17–23% glycerol) in a 1:1 or 1:2 ratio. Crystals grew up to 3 weeks and were cryoprotected in reservoir solution before flash-cooling in liquid nitrogen.
Structure determination and analysis of phosphorylation
All X-ray diffraction data were collected at beamlines X06SA (PXI) or X06DA (PXIII) at the Swiss Light Source (SLS, Paul Scherrer Institute, Villigen, Switzerland) equipped with PILATUS detectors. The wavelength of data collection was 1.000 Å for native crystals, and 1.527 and 1.907 Å for crystals derivatized with europium and cadmium, respectively. Raw data were processed using XDS49. Molecular replacement was carried out using Phaser 2.5.7 and 2.6.0, density modification was performed using Parrot50,51 and resolve, multicrystal averaging52 was carried out using phenix. All model building procedures were conducted using Coot53 and figures were prepared using PyMOL (Schrödinger LLC).
Diffraction of initial SceCD crystals in space group P43212 with unit cell dimensions of a=b=110.3 Å and c=131.7 Å was limited to 3.5 Å. The resolution was improved to 3 Å by addition of trimethylamine or benzamidine to the reservoir solution without significant changes in unit cell dimensions. Crystals derivatized with thimerosal and europium were used for initial SAD phase determination using the SHELXC/D package54. Two mercury and four europium sites were located, and an initial model was placed in the resulting maps. Since crystals derivatized with europium were slightly non-isomorphous with a c axis length of 127 Å, multicrystal averaging was used for density modification and provided directly interpretable maps. Iterative cycles of model building and refinement in Buster (version 2.10.2; Global Phasing Ltd) converged at Rwork/Rfree of 0.20/0.24. The final model lacks the disordered N terminus (amino acids 768–789), an extended loop in the CDC1 domain (1,203–1,215), a short stretch (1,147–1,149) preceding the regulatory loop and the two very C-terminal residues (1,493–1,494). On the basis of temperature factor analysis, the start and end of the regulatory loop show higher disorder than the region around the interacting phosphoserine 1157. MS analysis of dissolved crystals detected quantitative phosphorylation of the regulatory Ser1157, as also found for full-length SceACC, and additionally albeit with much lower occurrence, phosphorylation of Ser790, Ser1137, Ser1148 and Ser1159. A modelled phosphoryl position for Ser1159 could overlap with the one of Ser1157, and might be represented in the crystal. For all other phosphorylation sites no difference density could be observed, probably because of very low occupancy. PDBeFold55 was used to search for structural homologues. The thresholds for lowest acceptable percentage of matched secondary structure elements were 70% for the search query and 20% for the result.
Initial HsaBT-CD crystals were obtained in space group I4122 with a=b=240.1 Å and c=768.9 Å and diffracted to 7.5 Å. Optimized and dehydrated crystals also belonged to space group I4122 but with unit cell parameters a=b=267.3 Å and c=210.6 Å and diffracted to a resolution of 3.7 Å. Phase information was obtained from SAD based on bound cadmium ions from the crystallization condition. Six cadmium positions were located in a 4.0-Å resolution data set at 1.9 Å wavelength using SHELXC/D54 via the HKL2MAP interface56. Density modification and phasing based on this anomalous data set, a 3.7-Å resolution data set at 1.0 Å wavelength and additional non-isomorphous lower-resolution data sets led to a high-quality electron density map. At the intermediate resolution obtained, the map was interpreted by a poly-alanine model, which was guided by predicted secondary structure as well as sequence and structural alignment with SceCD. The final model contains five cadmium ions and refines using phenix52 against experimental data with Rwork/Rfree of 0.35/0.38, as expected for a poly-alanine model. Two HsaBT-CD monomers are packed in the asymmetric unit via the CDN and BT domains. Density on top of the β-barrel of one BT most likely representing parts of the BT–CD linker guided the assignment of this BT to its linked CD partner domain. This BT-to-CD assignment was further supported by the analysis of an additional lower-resolution crystal form. Cadmium ions were found to participate in crystal packing.
In HsaACC, phosphorylation at regulatory sites was detected as provided in the main text. No phosphorylation was detected for other phosphosites previously identified in large-scale phosphoproteomics studies, namely serines 5, 23, 25, 48, 53, 78, 488, 786, 1273 (refs 57, 58, 59).
Two different crystal forms were obtained for CthCD-CTCter (denoted as CthCD-CTCter1 and CthCD-CTCter2), diffracting to 3.6 and 4.5 Å. Both forms packed in space group P212121 with unit cell constants of a=97.7 Å, b=165.3 Å and c=219.2 Å or a=100.2 Å, b=153.5 Å and c=249.2 Å, respectively. Phases were determined by molecular replacement using a homology model based on SceCT (pdb 1od2) as search model in Phaser51,60,61; multicrystal averaging was applied in density modification. The CT domain was rebuilt and an initial homology model based on the SceCD structure was fitted into difference density for CthCD-CTCter1. Iterative cycles of rebuilding and refinement in Buster converged at Rwork/Rfree of 0.20/0.24. The refined CD fragment served as a starting model for rebuilding CthCD-CTCter2 at lower resolution. Coordinate refinement in Buster was additionally guided by reference model restraints and converged at Rwork/Rfree of 0.24/0.24. Residues 1,114–1,185, 1,213–1,252, 1,380–1,385, 1,465–1,468 and 2,188–2,195 were disordered in both crystal forms and are not included in the models. Helical regions C terminal to Glu2264 of both protomers of CthCD-CTCter1 and C terminal to Leu2259 and Arg2261 of the two protomers of CthCD-CTCter2, respectively, could not be built unambiguously and were therefore interpreted by placing poly-alanine stretches. Conservation was mapped on the CthCD-CTCter1 crystal structure using al2co62 based on a sequence alignment of 367 fungal ACC sequences calculated by Clustal Omega63. MS analysis of purified protein detected 7% phosphorylation at Ser1170 (corresponding to Ser1157 in SceCD).
CthCD-CT crystallized in space group P31212 with unit cell constants of a=b=195.0 Å and c=189.5 Å and crystals diffracted to a resolution of 7.2 Å. The structure was solved by molecular replacement using a model composed of CthCT and CDC2 as search model in Phaser. CDC1 and CDN were placed manually into the resulting maps, and the model was refined using rigid-body, domain-wise TLS and B-factor refinement and NCS- and reference model-restrained coordinate refinement in Buster to Rwork/Rfree of 0.23/0.25. Owing to the low resolution, the maximum allowed B-factor in Buster refinement was increased from the default value of 300–500 Å2, minimizing B-factor clipping to 5% of all atoms. Residues 1,033–1,035, 1,134–1,152, 1,213–1,252, 1,380–1,385, 1,465–1,468 and 2,188–2,195 were not included in the models. Helical regions C terminal to Leu2259 and Arg2261 on the two protomers, respectively, were interpreted as described for CthCD-CTCter. Loop conformations, including the regulatory loop, were modelled as observed in SceCD. MS analysis of purified protein detected 60% phosphorylation at Ser1170 (corresponding to Ser1157 in SceCD). Conservation was mapped on the CthCD-CT crystal structure as for CthCD-CTCter.
CthΔBCCP ACC crystallized in space group P6422 with unit cell constants of a=b=462.2 Å and c=204.6 Å, resolution was limited to 8.4 Å. Structure determination and refinement was performed as for CthCD-CT, with a maximum allowed B-factor of 500 Å2, minimizing B-factor clipping to 3% of all atoms. Although substantial difference density is observed, no defined positions of the BT and BC domains could be derived because of disorder or partial in situ proteolysis or combinations thereof. In addition, residues 1,032–1,039, 1,134–1,152, 1,213–1,252, 1,380–1,385, 1,465–1,468 and 2,188–2,195 were not included in the model. The MissingAtom macro implemented in Buster was employed to account for missing atoms, the final Rwork/Rfree were 0.30/0.32. A region C terminal to Leu2259 on one protomer was interpreted as poly-alanine. Loop conformations, including the regulatory loop, were modelled as observed in SceCD. MS analysis of purified protein detected 70% phosphorylation at Ser1170 (corresponding to Ser1157 in SceCD).
Small-angle X-ray scattering
Proteins were thawed on ice and dialysed overnight against 30 mM MOPS pH 7, 200 mM ammonium sulfate, 5% glycerol and 10 mM dithiothreitol. Raw scattering data were measured at SAXS beamline B21 at Diamond Light Source. The samples were measured at concentrations of 2.5, 5 and 10 mg ml−1. Data were processed using the ATSAS package64 according to standard procedures65,66. A slight increase in scattering in the very low-resolution range was observed with increasing protein concentrations, which may be because of interparticle attraction or minor aggregation. Scattering intensities were thus extrapolated to zero concentration using point-wise extrapolation implemented in Primus67. Direct comparison of raw scattering curves demonstrates the similarity of CthACC and CthΔBCCP, and the derived values such as Rg and Porod Volume match within expected error margins. Molecular mass estimations based on the SAXS–MOW method68 derive values of 534.7 and 534.0 kDa for CthACC and CthΔBCCP, respectively. The relative discrepancies to the theoretical weights of 516.8 kDa (CthACC) and 503.0 kDa (CthΔBCCP) are 3.5% and 6.2%, respectively, which is in a typical range for this method68.
Electron microscopy
Full-length CthACC was diluted to 0.01 mg ml−1 in 30 mM MOPS pH 7.0, 200 mM ammonium sulfate, 5% glycerol and 10 mM dithiothreitol. Protein sample was adsorbed to a 200-μm copper grid and stained with 2% uranyl acetate. Grids of CthACC were imaged on a CM-200 microscope (Philips) equipped with a TVIPS F416 4k CMOS camera (Tietz Video and Image Processing Systems). The voltage used was 200 kV, and a magnification of × 50,000 results in a pixel size of 2.14 Å. Initial image processing and particle picking was carried out using Xmipp69,70. Overall, 22,309 particles were picked semi-automatically from 236 micrographs with a box size of 300 × 300 pixels. After extraction, particles with a z-score of more than three were discarded and 22,257 particles were aligned and classified into 48 2D class averages using the maximum-likelihood target function in Fourier space (MLF2D). After 72 iterations, 4,226 additional particles were discarded and the remaining 18,031 particles were re-aligned and classified into 36 classes using MLF2D with a high-resolution cutoff of 30 Å. After 44 iterations the alignment converged and class averages were extracted.
In vitro biotinylation and activity assay
To ensure full functionality, SceACC was biotinylated in vitro using the E. coli biotin ligase BirA. The reaction mixture contained 10 μM ACC, 3.7 μM BirA, 50 mM Tris-HCl, pH 8, 5.5 mM MgCl2, 0.5 mM biotin, 60 mM NaCl, 3 mM ATP and 10% glycerol, and the reaction was allowed to proceed for 7 h at 30 °C.
The catalytic activity of phosphorylated and dephosphorylated SceACC was measured by following the incorporation of radioactive 14C into acid-stable non-volatile material40. Dephosphorylated ACC was prepared by overnight treatment with λ protein phosphatase (New England Biolabs) of partially purified ACC before the final gel filtration step. The removal of the phosphoryl group from Ser1157 was confirmed by MS. The reaction mixture contained 0.5 μg recombinant ACC in 100 mM potassium phosphate, pH 8, 3 mM ATP, 5 mM MgCl2, 50 mM NaH14CO3 (specific activity 7.4 MBq mmol−1) and 1 mM acetyl-CoA in a total reaction volume of 100 μl. The reaction mixture was incubated for 15 min at 30 °C, stopped by addition of 200 μl 6 M HCl and subsequently evaporated to dryness at 85 °C. The non-volatile residue was redissolved in 100 μl of water, 1 ml Ultima Gold XR scintillation medium (Perkin Elmer) was added and the 14C radioactivity was measured in a Packard Tricarb 2000CA liquid scintillation analyser. Measurements were carried out in five replicates and catalytic activities were calculated using a standard curve derived from measurements of varying concentrations of NaH14CO3 in reaction buffer.
Additional information
Accession codes: Atomic coordinates and structure factors have been deposited in the Protein Data Bank with accession codes 5I6E (SceCD), 5I87 (HsaBT-CD), 5I6F/5I6G (CthCD-CTCter1/2), 5I6H (CthCD-CT) and 5I6I (CthΔBCCP).
How to cite this article: Hunkeler, M. et al. The dynamic organization of fungal acetyl-CoA carboxylase. Nat. Commun. 7:11196 doi: 10.1038/ncomms11196 (2016).
Supplementary Material
Supplementary Figures 1-7 and Supplementary Table 1
Acknowledgments
We thank the staff of beamlines X06DA and X06SA at Paul Scherrer Institute, Villigen, Switzerland, for support with crystallographic data collection and the beamline scientists of B21 at Diamond Light Source, Oxfordshire, UK, for support with SAXS measurements. We thank K. Goldie and H. Stahlberg (C-CINA, Basel, Switzerland) for their support with electron microscopy, the Biophysics facility of the Biozentrum for protein characterization and the Proteomics core facility for mass spectrometry and identification of post-translational modifications. M.H. acknowledges generous support by a Novartis Excellence Fellowship. A.H. is supported by a ‘Fellowship for Excellence' of the Biozentrum Basel International PhD programme. This work was supported by Swiss National Science Foundation grants 138262, 159696 and 145023.
Footnotes
Author contributions M.H. cloned, expressed, purified and crystallized fungal ACC constructs, determined their structure and carried out SAXS analysis. E.S. cloned, expressed and crystallized human ACC CD and determined its structure. EM analysis was carried out by E.S., M.H. and A.H. S.I. contributed to structural analysis and figure preparation. T.M. designed and supervised work and analysed crystallographic data; all authors contributed to manuscript preparation.
References
- Wakil S. J., Titchener E. B. & Gibson D. M. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim. Biophys. Acta 29, 225–226 (1958). [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K. & Joshi V. C. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 52, 537–579 (1983). [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L., Matzuk M. M., Abo-Hashema K. A. & Wakil S. J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291, 2613–2616 (2001). [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L., Oh W., Kordari P. & Wakil S. J. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc. Natl Acad. Sci. USA 100, 10207–10212 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood H. J. J. Acetyl-CoA carboxylase inhibition for the treatment of metabolic syndrome. Curr. Opin. Invest. Drugs 5, 283–289 (2004). [PubMed] [Google Scholar]
- Milgraum L. Z., Witters L. A., Pasternack G. R. & Kuhajda F. P. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin. Cancer Res. 3, 2115–2120 (1997). [PubMed] [Google Scholar]
- Swinnen J. V., Brusselmans K. & Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 9, 358–365 (2006). [DOI] [PubMed] [Google Scholar]
- Williams R. S., Lee M. S., Hau D. D. & Glover J. N. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat. Struct. Mol. Biol. 11, 519–525 (2004). [DOI] [PubMed] [Google Scholar]
- Shen Y. & Tong L. Structural evidence for direct interactions between the BRCT domains of human BRCA1 and a phospho-peptide from human ACC1. Biochemistry 47, 5767–5773 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol. Life Sci. 62, 1784–1803 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S. J. & Abu-Elheiga L. A. Fatty acid metabolism: target for metabolic syndrome. J. Lipid Res. 50, (Suppl): S138–S143 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Volrath S. L., Weatherly S. C., Elich T. D. & Tong L. A mechanism for the potent inhibition of eukaryotic acetyl-coenzyme A carboxylase by soraphen A, a macrocyclic polyketide natural product. Mol. Cell 16, 881–891 (2004). [DOI] [PubMed] [Google Scholar]
- Campbell J. W. & Cronan J. E. Jr. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu. Rev. Microbiol. 55, 305–332 (2001). [DOI] [PubMed] [Google Scholar]
- Weatherly S. C., Volrath S. L. & Elich T. D. Expression and characterization of recombinant fungal acetyl-CoA carboxylase and isolation of a soraphen-binding domain. Biochem. J. 380, 105–110 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W. & Vagelos P. R. Acetyl CoA carboxylase. I. Requirement for two protein fractions. Proc. Natl Acad. Sci. USA 59, 561–568 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Nervi A. M. & Vagelos P. R. Acetyl CoA carboxylase, II. Deomonstration of biotin-protein and biotin carboxylase subunits. Proc. Natl Acad. Sci. USA 63, 1319–1326 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E. Jr. & Waldrop G. L. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41, 407–435 (2002). [DOI] [PubMed] [Google Scholar]
- Bianchi A. et al. Identification of an isozymic form of acetyl-CoA carboxylase. J. Biol. Chem. 265, 1502–1509 (1990). [PubMed] [Google Scholar]
- Abu-Elheiga L. et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl Acad. Sci. USA 97, 1444–1449 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R. W., Boone A. N., Elliott J. E., Kulpa J. E. & Lee W. M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 34, 223–227 (2006). [DOI] [PubMed] [Google Scholar]
- Munday M. R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 30, 1059–1064 (2002). [DOI] [PubMed] [Google Scholar]
- Shi S., Chen Y., Siewers V. & Nielsen J. Improving production of malonyl coenzyme A-derived metabolites by abolishing Snf1-dependent regulation of Acc1. MBio 5, e01130–01114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. S. et al. Crystal structure of the alpha(6)beta(6) holoenzyme of propionyl-coenzyme A carboxylase. Nature 466, 1001–1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. S., Ge P., Zhou Z. H. & Tong L. An unanticipated architecture of the 750-kDa alpha6beta6 holoenzyme of 3-methylcrotonyl-CoA carboxylase. Nature 481, 219–223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop G. L., Rayment I. & Holden H. M. Three-dimensional structure of the biotin carboxylase subunit of acetyl-CoA carboxylase. Biochemistry 33, 10249–10256 (1994). [DOI] [PubMed] [Google Scholar]
- Athappilly F. K. & Hendrickson W. A. Structure of the biotinyl domain of acetyl-coenzyme A carboxylase determined by MAD phasing. Structure 3, 1407–1419 (1995). [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang Z., Shen Y. & Tong L. Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase. Science 299, 2064–2067 (2003). [DOI] [PubMed] [Google Scholar]
- Bilder P. et al. The structure of the carboxyltransferase component of acetyl-coA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry 45, 1712–1722 (2006). [DOI] [PubMed] [Google Scholar]
- Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol. Life Sci. 70, 863–891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Maurice M. et al. Domain architecture of pyruvate carboxylase, a biotin-dependent multifunctional enzyme. Science 317, 1076–1079 (2007). [DOI] [PubMed] [Google Scholar]
- Tran T. H. et al. Structure and function of a single-chain, multi-domain long-chain acyl-CoA carboxylase. Nature 518, 120–124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W. et al. Induced polymerization of mammalian acetyl-CoA carboxylase by MIG12 provides a tertiary level of regulation of fatty acid synthesis. Proc. Natl Acad. Sci. USA 107, 9626–9631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray H., Moreau K., Dizin E., Callebaut I. & Venezia N. D. ACCA phosphopeptide recognition by the BRCT repeats of BRCA1. J. Mol. Biol. 359, 973–982 (2006). [DOI] [PubMed] [Google Scholar]
- Davies S. P., Sim A. T. & Hardie D. G. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur. J. Biochem. 187, 183–190 (1990). [DOI] [PubMed] [Google Scholar]
- Ha J., Daniel S., Broyles S. S. & Kim K. H. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 269, 22162–22168 (1994). [PubMed] [Google Scholar]
- Cho Y. S. et al. Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK. Biochem. Biophys. Res. Commun. 391, 187–192 (2010). [DOI] [PubMed] [Google Scholar]
- Ficarro S. B. et al. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20, 301–305 (2002). [DOI] [PubMed] [Google Scholar]
- Woods A. et al. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J. Biol. Chem. 269, 19509–19515 (1994). [PubMed] [Google Scholar]
- Holt L. J. et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovich L. et al. Kinetic and structural analysis of a new group of Acyl-CoA carboxylases found in Streptomyces coelicolor A3(2). J. Biol. Chem. 277, 31228–31236 (2002). [DOI] [PubMed] [Google Scholar]
- Wei J. & Tong L. Crystal structure of the 500-kDa yeast acetyl-CoA carboxylase holoenzyme dimer. Nature 526, 723–727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernado P. Effect of interdomain dynamics on the structure determination of modular proteins by small-angle scattering. Eur. Biophys. J. 39, 769–780 (2010). [DOI] [PubMed] [Google Scholar]
- Smith J. L., Skiniotis G. & Sherman D. H. Architecture of the polyketide synthase module: surprises from electron cryo-microscopy. Curr. Opin. Struct. Biol. 31, 9–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T., Leibundgut M. & Ban N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008). [DOI] [PubMed] [Google Scholar]
- Brignole E. J. & Smith S. Asturias FJ. Conformational flexibility of metazoan fatty acid synthase enables catalysis. Nat. Struct. Mol. Biol. 16, 190–197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieker M., Tanovic A. & Marahiel M. A. Nonribosomal peptide synthetases: structures and dynamics. Curr. Opin. Struct. Biol. 20, 234–240 (2010). [DOI] [PubMed] [Google Scholar]
- Patel M. S., Nemeria N. S., Furey W. & Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem. 289, 16615–16623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J. et al. Protein complex expression by using multigene baculoviral vectors. Nat. Methods 3, 1021–1032 (2006). [DOI] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Y., Cowtan K. & Main P. Combining constraints for electron-density modification. Methods Enzymol. 277, 53–64 (1997). [DOI] [PubMed] [Google Scholar]
- Winn M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W. G. & Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008). [DOI] [PubMed] [Google Scholar]
- Krissinel E. & Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D 60, 2256–2268 (2004). [DOI] [PubMed] [Google Scholar]
- Pape T. & Schneider T. R. HKL2MAP: a graphical user interface for macromolecular phasing with SHELX programs. J. Appl. Crystallogr. 37, 843–844 (2004). [Google Scholar]
- Olsen J. V. et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal. 3, ra3 (2010). [DOI] [PubMed] [Google Scholar]
- Bian Y. et al. An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome. J. Proteomics 96, 253–262 (2014). [DOI] [PubMed] [Google Scholar]
- Cantin G. T. et al. Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J. Proteome Res. 7, 1346–1351 (2008). [DOI] [PubMed] [Google Scholar]
- McCoy A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J. & Grishin N. V. AL2CO: calculation of positional conservation in a protein sequence alignment. Bioinformatics 17, 700–712 (2001). [DOI] [PubMed] [Google Scholar]
- Sievers F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov M. V. et al. New developments in the program package for small-angle scattering data analysis. J. Appl. Crystallogr. 45, 342–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skou S., Gillilan R. E. & Ando N. Synchrotron-based small-angle X-ray scattering of proteins in solution. Nat. Protoc. 9, 1727–1739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques D. A. & Trewhella J. Small-angle scattering for structural biology-Expanding the frontier while avoiding the pitfalls. Protein Sci. 19, 642–657 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev P. V., Volkov V. V., Sokolova A. V., Koch M. H. J. & Svergun D. I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 (2003). [Google Scholar]
- Fischer H., Neto M. D., Napolitano H. B., Polikarpov I. & Craievich A. F. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J. Appl. Crystallogr. 43, 101–109 (2010). [Google Scholar]
- Scheres S. H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabini R. et al. Xmipp: an image processing package for electron microscopy. J. Struct. Biol. 116, 237–240 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7 and Supplementary Table 1