Abstract
Somewhat paradoxically, fracture risk, which depends on applied loads and bone strength, is elevated in both anorexia nervosa and obesity at certain skeletal sites. Factor-of-risk (Φ), the ratio of applied load to bone strength, is a biomechanically-based method to estimate fracture risk; theoretically, higher Φ reflects increased fracture risk.
We estimated vertebral strength [linear combination of integral volumetric BMD (Int.vBMD) and cross-sectional area from QCT], vertebral compressive loads, and Φ at L4 in 176 women (65 anorexia nervosa, 45 lean controls, 66 obese). Using biomechanical models, applied loads were estimated for: 1) standing; 2) arms flexed 90°, holding 5 kg in each hand (holding); 3) 45° trunk flexion, 5 kg in each hand (lifting); 4) 20° trunk right lateral bend, 10 kg in right hand (bending). We also investigated associations of Int.vBMD and vertebral strength with lean mass (from DXA) and visceral adipose tissue (VAT, from QCT).
Women with anorexia nervosa had lower, whereas obese women had similar, Int.vBMD and estimated vertebral strength compared to controls. Vertebral loads were highest in obesity and lowest in anorexia nervosa for standing, holding, and lifting (p<0.0001), but were highest in anorexia nervosa for bending (p<0.02). Obese women had highest Φ for standing and lifting, whereas women with anorexia nervosa had highest Φ for bending (p<0.0001). Obese and anorexia nervosa subjects had higher Φ for holding than controls (p<0.03). Int.vBMD and estimated vertebral strength were associated positively with lean mass (R= 0.28–0.45, p≤0.0001) in all groups combined, and negatively with VAT (R= −[0.36–0.38], p<0.003) within obese group.
Therefore, women with anorexia nervosa had higher estimated vertebral fracture risk (Φ) for holding and bending, due to inferior vertebral strength. Despite similar vertebral strength as controls, obese women had higher vertebral fracture risk for standing, holding, and lifting, due to higher applied loads from higher body weight. Examining the load-to-strength ratio helps explain increased fracture risk in both low-weight and obese women.
Keywords: osteoporosis, biomechanics, Bone QCT
Introduction
Fracture risk is elevated at both extremes of the weight spectrum—in anorexia nervosa and obesity. Women with anorexia nervosa have higher risk of overall fracture, vertebral fractures, and forearm fractures.(1,2) Bone loss is a serious and frequent health problem for individuals with anorexia nervosa, and the spine is the skeletal site most profoundly affected, as manifested by relatively lower areal bone mineral density (aBMD) by DXA T- and Z-scores at the spine than at the hip and radius.(3,4) Obese women often have normal or higher aBMD compared to lean women, yet demonstrate higher fracture rates at certain skeletal sites, such as the humerus, upper arm, elbow, upper leg, and ankle.(5–7) Risk of vertebral fracture in obese women is unclear; some studies have found higher risk in obese women,(8–10) whereas others have not.(7,11)
Areal BMD assessment by DXA has limitations in predicting fracture, and is particularly subject to inaccuracies in the settings of obesity(12,13) and weight fluctuations.(14) Almost 40 percent of postmenopausal women, and up to 60 percent of obese postmenopausal women, who have experienced low-trauma fractures have normal aBMD at the spine and hip.(15) These issues have prompted the investigation of other methods to assess bone strength and fracture risk. High resolution peripheral quantitative CT, which assesses bone microarchitecture, can be performed at the radius and tibia, but not at the spine. In contrast, quantitative computed tomography (QCT) is possible at the spine and is characterized by less error and variability compared to DXA in obese individuals.(12) QCT at the spine can provide measurements of volumetric BMD (vBMD) and cross-sectional area (CSA), which can be used to estimate vertebral strength, according to engineering beam theory as previously published.(16,17) This approach of estimating vertebral strength from QCT measurements of vertebral vBMD and CSA has not been reported in premenopausal women at the extremes of the BMI spectrum, in whom DXA is particularly inaccurate.(12,14)
Body composition significantly influences BMD. Lean mass is positively associated with aBMD.(18–21) Similarly, muscle mass is positively associated with vertebral vBMD in obese premenopausal women.(22) In contrast, the positive association of aBMD with total body fat mass is weaker than with lean mass,(19,21) and higher trunk fat mass is associated with inferior microarchitecture of the iliac crest in premenopausal women.(23) Furthermore, higher quantities of visceral adipose tissue (VAT) are associated with lower vertebral vBMD in obese premenopausal women(22) and healthy adult Chinese men and women,(24) and with impaired microarchitecture of the radius in young obese men.(25) To our knowledge, effects of lean mass and fat mass on vertebral vBMD in anorexia nervosa have not been reported. Moreover, little is known regarding the associations of lean mass and fat mass with vertebral strength across the weight spectrum.
Fracture risk depends not only on BMD, but also on the load applied to the bone. Different activities subject bone to varying forces, and in general, higher body weight increases the forces applied to the bone. The factor-of-risk (Φ), the ratio of applied load to bone strength, is a biomechanically-based approach to estimate fracture risk and has been used to estimate vertebral fracture risk in prior studies.(16,17) Higher Φ values indicate higher fracture risk. Theoretically when Φ>1 (when applied load exceeds bone strength), a fracture will occur. By examining the ratio of applied load to bone strength, Φ for vertebral fracture provides important information regarding fracture risk not provided by aBMD alone.
In this study, our principle objectives were to estimate vertebral strength and utilize the factor-of-risk approach as a conceptual framework to better understand the differences in vertebral fracture risk during different activities in women across the BMI spectrum. We therefore calculated vBMD, CSA, and vertebral strength, as well as Φ for vertebral fracture during different activities at the L4 vertebral body in women with anorexia nervosa, obese women, and lean female controls. Our primary hypotheses were: 1) women with anorexia nervosa would have impaired, whereas obese women would have similar, vertebral strength compared to lean controls, and 2) Φ for vertebral fracture would be higher in anorexia nervosa driven predominantly by inferior vertebral strength, whereas Φ for vertebral fracture in obese women would be higher than controls during some activities, driven by higher loads applied to L4 due to higher body weight. Secondary objectives were to investigate the relationships of vertebral strength and its components, vBMD and CSA, with body composition. We posited that vertebral strength would be associated positively with lean mass, but negatively with VAT.
Materials and Methods
Study Participants
We performed a cross-sectional analysis of 176 premenopausal women (65 with anorexia nervosa, 45 healthy lean, and 66 otherwise healthy obese women) of ages 18–45 years who had undergone quantitative computed tomography (QCT) of the L4 vertebral body. Partners Institutional Review Board had approved all protocols, and informed consent was obtained from all subjects. Vertebral strength estimates and vertebral factor-of-risk data have not been reported previously for any subjects. L4 trabecular BMD, a component of integral volumetric BMD (Int.vBMD) but not Int.vBMD itself, was previously reported for a subset of subjects.(22,26,27) Clinical characteristics and body composition have been reported for a subset of subjects.(18,22,26–32) Subjects did not have renal disease, liver disease, diabetes mellitus (any type), active substance abuse, prior use of medications known to affect bone metabolism, or fractures of the L4 vertebral body. Women with anorexia nervosa met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for diagnosis of anorexia nervosa and had BMIs < 18.5 kg/m2. Subjects with anorexia nervosa could be eumenorrheic, amenorrheic, or receiving exogenous estrogen and/or progestin, provided they met DSM-5 criteria for anorexia nervosa and were low-weight. Lean controls had BMIs between 18.5–24.9 kg/m2. Obese women had BMIs ≥ 30 kg/m2. Lean and obese women were eumenorrheic.
Vertebral Strength, vBMD, and CSA
Volumetric BMD (g/cm3) of the L4 vertebral body was determined from QCT (LightSpeed CT, General Electric, Milwaukee, WI) with the use of a standard calibration phantom (Mindways, Austin, TX). Imaging parameters were as follows: table height 144 cm, 80 kv, 70 mA, scan time 2 seconds, slice thickness 1 cm, and field of view 48 cm. Scans were then reviewed off-line on an IMPAX workstation (AGFA Diagnostic Software, version 4, Afga, Ridgefield Park, NJ), and a region of interest within the trabecular bone of L4 was drawn. As previously described,(22) the trabecular vBMD of L4 was calculated using the mean trabecular density of L4 in Hounsfield units (HU) and the density of the calibration solutions: mg/ml of sample = (CT # of sample − intercept of calibration line)/slope of calibration line. Each subject’s trabecular vBMD was compared to the young female normal reference mean vBMD(33) to calculate the T-score and to the age-matched normal reference mean vBMD(33) to calculate the Z-score. Next, the contour of the L4 vertebral body for each subject was outlined by a single investigator (C.M.G.) in order to determine the L4 vertebral body cross-sectional area (CSA, cm2), as well as integral volumetric BMD (Int.vBMD, g/cm3), which represents the total volumetric BMD (both trabecular and cortical compartments).
Estimated vertebral compressive strength (“vertebral strength”) was determined by the linear combination of Int.vBMD and CSA, as previously published.(16,17)
This relationship is based on engineering beam theory, which makes the following assumptions: 1) the vertebral body is primarily loaded in compression, and 2) vertebral strength is proportional to the structural rigidity of its weakest cross-section. Structural rigidity depends on bone size and elastic modulus,(16) which was estimated from a previously published relationship:
Elastic modulus = −34.7 + 3230 × Int.vBMD(34)
Vertebral strength was then estimated from the previously published equation:
Vertebral Strength = 0.0068 × Elastic Modulus × CSA(17)
This technique of estimating vertebral strength has been validated in vitro using human cadavers.(35)
Applied Loads and Factor-of-Risk (Φ)
For each subject, a biomechanical model of the spine was used to estimate the loads applied in vivo to the L4 vertebral body during four activities of daily life: 1) neutral standing (standing); 2) standing with elbows flexed at 90°, holding 5 kg in each hand (holding); 3) 45° trunk flexion with arms hanging down and 5 kg in each hand (lifting); 4) 20° trunk right lateral bend, 10 kg in right hand (bending). According to previously published methods,(16,36,37) in the biomechanical model, the body was divided into sections, and each section’s length, weight, and center of mass position were estimated, and the muscle forces required to maintain static equilibrium during each activity were calculated. For each activity, the applied load to the L4 vertebral body was determined by totaling all forces (from body weight and muscle loading) in the axial direction of L4.
For each activity, the factor-of-risk (Φ) for vertebral fracture was calculated as the ratio of applied load to the L4 vertebral body during the activity divided by L4 vertebral strength:
Factor-of-risk (Φ) = Applied Load/Vertebral Strength
Higher values of factor-of-risk are associated with greater risk of fracture.(16,17)
Body Composition Evaluation
Total body lean mass and total body fat mass were measured by DXA (Hologic Inc), with coefficients of variation (CV) of 2.4% and 1.7%, respectively, in our laboratory.(38) Subcutaneous adipose tissue (SAT), VAT, and total adipose tissue (TAT) of the abdomen at the level of the L4 vertebral body were measured from QCT, with CV of 2.5% in our laboratory.(31) As previously described,(31) SAT was defined as the area between the outer contour of the abdomen and the inner contour of the back and abdominal wall musculature. VAT was defined as the area within the inner contour containing pixels with attenuation coefficients between −50 and −250 HU. TAT was defined as the sum of SAT and VAT. Analyses were performed using Alice imaging processing software (version 4.3.9; PAREXEL, Waltham, MA).
Statistical Analysis
Statistical analyses were performed using JMP Statistical Discovery Software, version 10 Professional (SAS Institute Inc, Cary, NC). All analyses were adjusted for age. Continuous variables were compared among the three groups with Fisher’s least significant difference test, controlling for age, using standard least squares testing; if overall p value between groups ≤ 0.05, pairwise comparisons were then performed. Further adjustment for multiple comparisons was not necessary because of the use of a preliminary test of significance with three groups.(39) Regression models were constructed and partial correlation coefficients, controlled for age, are reported. Statistical significance was defined as a two-tailed p-value ≤ 0.05. Data are presented as means +/− standard deviations.
Results
Clinical Characteristics
Clinical characteristics are presented in Table 1. BMIs ranged from 13.3–18.49 kg/m2 among women with anorexia nervosa, from 19.2–24.9 kg/m2 among lean controls, and from 30.2–49.7 kg/m2 among obese women. As expected, BMI, total body lean and fat mass, VAT, SAT, and TAT were lowest in anorexia nervosa and highest in obese women (p<0.0001). Mean age differed between the three groups. Therefore, all subsequent analyses were adjusted for age.
Table 1.
Baseline Characteristics
| AN (N=65) |
C (N=45) |
OB (N=66) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Overall ANOVA |
AN vs. C |
AN vs. OB |
C vs. OB |
||||
| Age, years | 25.7 ± 5.7 | 28.0 ± 6.5 | 36.0 ± 6.6 | <0.0001 | 0.035 | <0.0001 | <0.0001 |
| Height, cm | 164.6 ± 6.7 | 166.4 ± 6.6 | 162.4 ± 7.0 | NS | - | - | - |
| Weight, kg | 46.3 ± 5.0 | 61.4 ± 6.5 | 96.9 ± 13.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| BMI, kg/m2 | 17.0 ± 1.1 | 22.1 ± 1.7 | 36.7 ± 4.3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| L4 trabecular BMD, g/cm3 | 131.9 ± 28.8 | 158.6 ± 27.5 | 159.8 ± 25.9 | <0.0001 | <0.0001 | <0.0001 | NS |
| L4 T-score | −1.7 ± 1.1 | −0.7 ± 1.1 | −0.6 ± 1.0 | <0.0001 | <0.0001 | <0.0001 | NS |
| L4 Z-score | −1.7 ± 1.1 | −0.5 ± 1.1 | −0.1 ± 0.9 | <0.0001 | <0.0001 | <0.0001 | 0.038 |
| Total body lean mass, kg | 35.9 ± 4.7 | 42.4 ± 4.5 | 52.9 ± 6.1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Total body fat mass, kg | 9.5 ± 3.2 | 18.2 ± 4.6 | 42.8 ± 8.9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| VAT, cm2 | 16.7 ± 12.5 | 36.1 ± 16.0 | 123.0 ± 51.0 | <0.0001 | 0.016 | <0.0001 | <0.0001 |
| SAT, cm2 | 66.3 ± 33.5 | 179.9 ± 77.2 | 509.9 ± 121.9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| TAT, cm2 | 83.0 ± 40.1 | 214.9 ± 82.6 | 633.0 ± 146.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Vigorous activity, hr/wk | 5.0 ± 7.3 | 5.0 ± 4.4 | 4.0 ± 6.7 | NS | - | - | - |
NS, not significant; AN, anorexia nervosa; C, lean controls; OB, obese; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue, TAT, total adipose tissue
Vertebral Strength, vBMD, and CSA
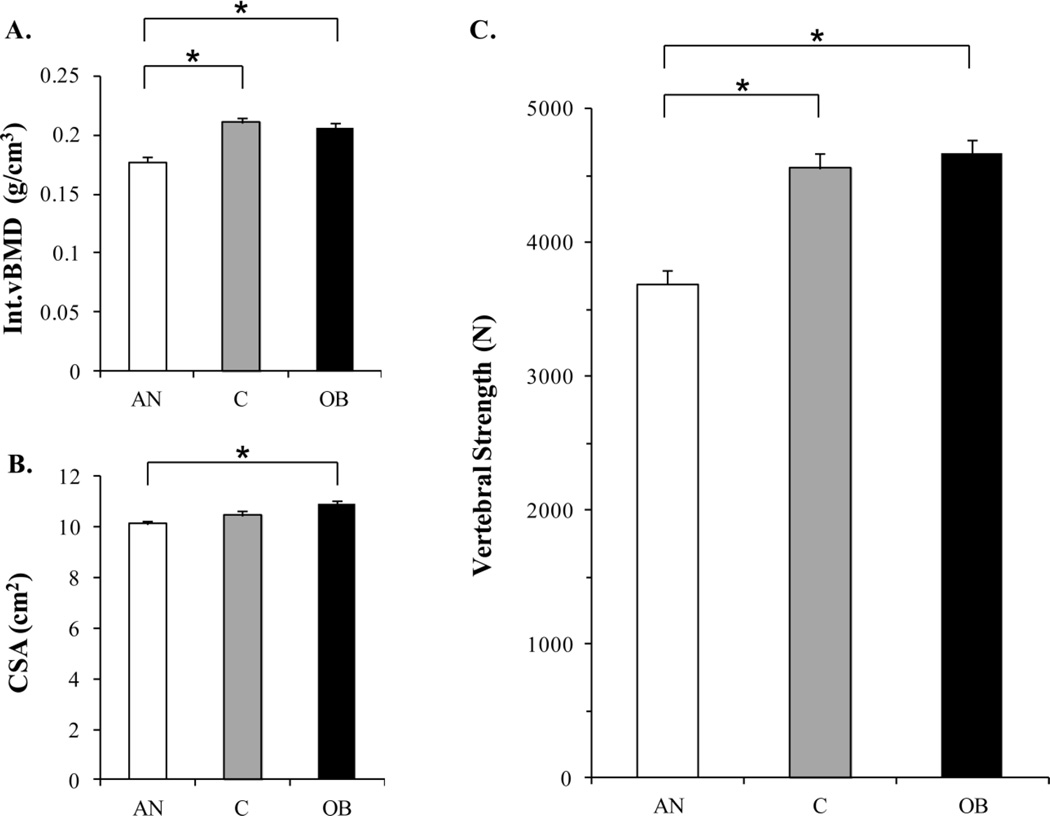
Women with anorexia nervosa had lower Int.vBMD compared to lean and obese women (p<0.0001) and lower CSA compared to obese women (p<0.004; Figure 1). Accordingly women with anorexia nervosa had lower estimated vertebral strength compared to lean and obese women (p<0.0001). In contrast, obese women had similar Int.vBMD, CSA, and estimated vertebral strength as lean controls.
Figure 1.
The linear combination of Int.vBMD (panel A) and CSA (panel B) is used to estimate vertebral strength (panel C). Women with anorexia nervosa had lower Int.vBMD compared to lean controls and obese women, and lower CSA than obese women. Women with anorexia nervosa had lower vertebral strength compared to lean and obese women. Obese women had similar Int.vBMD, CSA, and vertebral strength as lean controls. Results are reported as means +/− standard errors of the mean (SEM). *p<0.004; AN, anorexia nervosa; C, lean controls; OB, obese; Int.vBMD, integral volumetric BMD; CSA, cross-sectional area
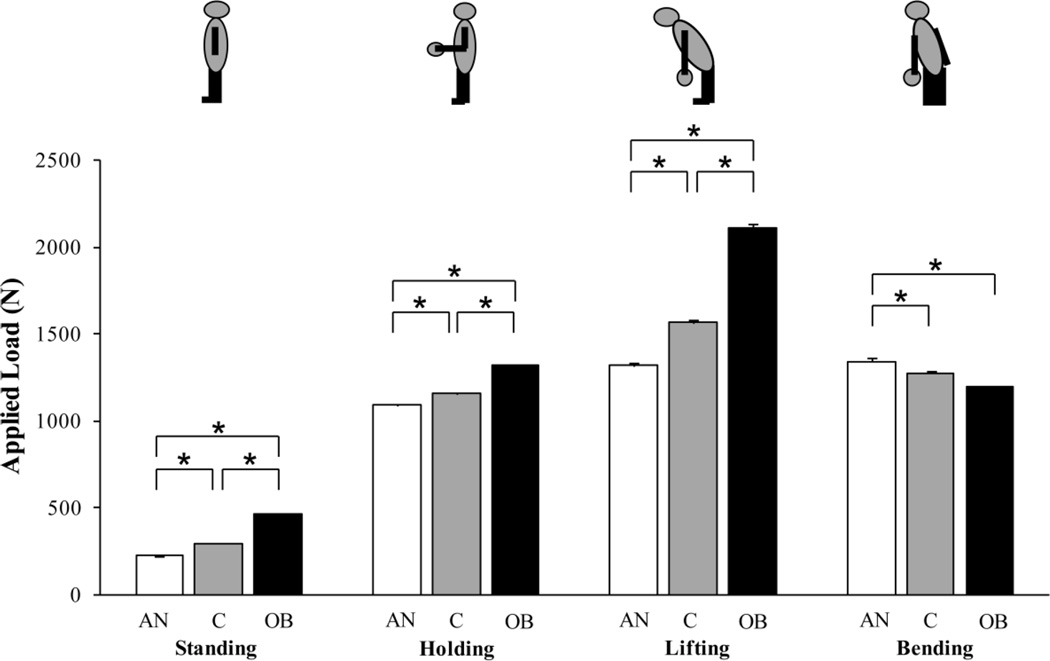
Vertebral Compressive Loading (Applied Load)
Estimated L4 compressive loading was highest in obesity and lowest in anorexia nervosa for standing, holding, and lifting (p<0.0001; Figure 2). In contrast, for lateral bending, L4 compressive loading estimates were higher in women with anorexia nervosa compared to lean and obese women (p<0.02). The biomechanical model demonstrated that for lateral bending, the lower muscular strength of subjects with anorexia nervosa required the activation of additional muscle groups (including the contralateral rectus abdominis and sacrospinalis muscles) in women with anorexia nervosa, but not in lean or obese women, in order to maintain static equilibrium. The activation of these additional muscle groups in subjects with anorexia nervosa thereby caused the applied load to the L4 vertebral body during lateral bending to be higher in women with anorexia nervosa compared to lean and obese women.
Figure 2.
Applied load was highest in obesity and lowest in anorexia nervosa for standing, holding, and lifting. Applied load for bending was higher in women with anorexia nervosa compared to lean controls and obese women. Results are reported as means +/− SEM. *p<0.02; AN, anorexia nervosa; C, lean controls; OB, obese
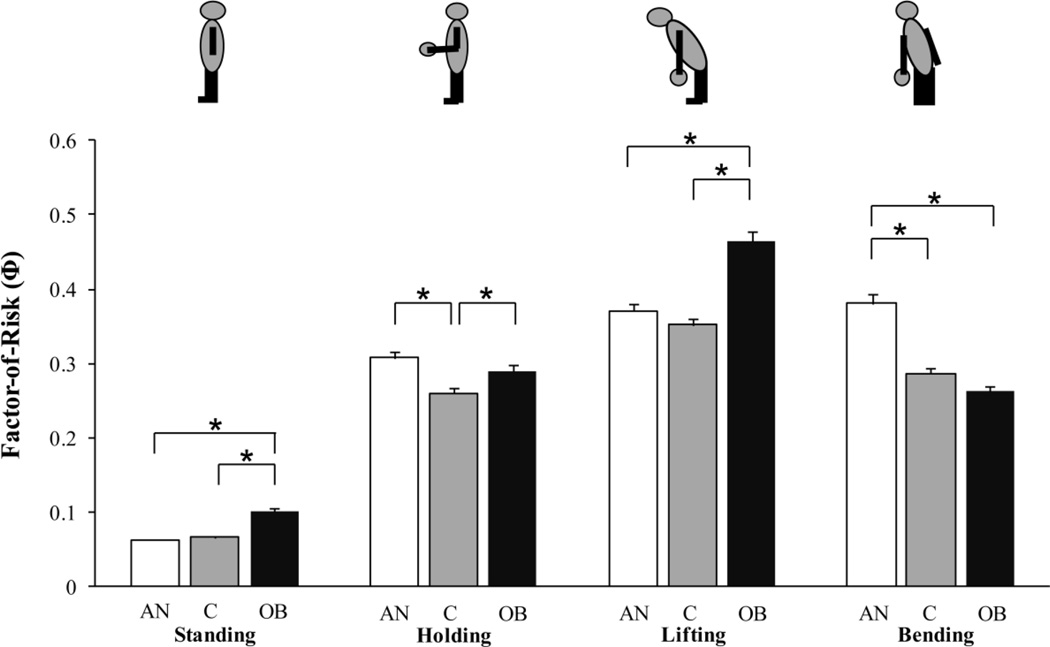
Factor-of-Risk (Φ) for Vertebral Fracture
Obese women had higher vertebral Φ for standing and lifting compared to lean controls and subjects with anorexia nervosa (p<0.0001). In contrast, subjects with anorexia nervosa had higher Φ for lateral bending than lean and obese women (p<0.0001; Figure 3). Both obese women and subjects with anorexia nervosa had higher Φ for holding compared to lean controls (p<0.03).
Figure 3.
Factor-of-risk (Φ) for vertebral fracture is the ratio of applied load to vertebral strength. Obese subjects had higher Φ for standing and lifting compared to lean controls and women with anorexia nervosa. In contrast, women with anorexia nervosa had higher Φ for bending than lean and obese women. Obese subjects and women with anorexia nervosa had higher Φ for holding compared to lean controls. Results are reported as means +/− SEM. *p<0.03; AN, anorexia nervosa; C, lean controls; OB, obese; Φ, factor-of-risk
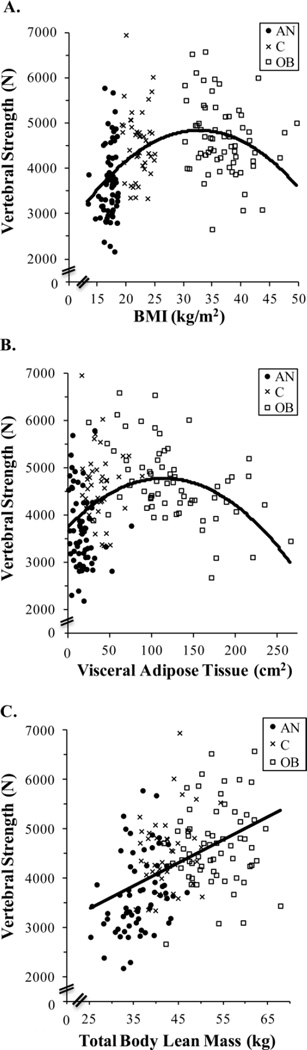
Determinants of Vertebral Strength and Its Components
Across all groups (anorexia nervosa, lean, and obese) combined, vertebral strength was related to BMI (Figure 4A) in an inverted U-shape, such that BMI was negatively associated with vertebral strength within the obese group (R= −0.25, p<0.05). BMI was not associated with Int.vBMD, CSA, or vertebral strength within the anorexia nervosa group or within lean controls.
Figure 4.
BMI (panel A) and VAT (panel B) demonstrated inverted U-shaped relationships with vertebral strength, whereas lean mass (panel C) had a positive linear relationship with vertebral strength. The equation for the displayed curve of Vertebral Strength vs. BMI (panel A) is: Vertebral Strength = −4.187*BMI2 + 273.964*BMI + 355.787 (R2=0.23; p<0.0001 for BMI and BMI2 estimates). The equation for the displayed curve of Vertebral Strength vs. VAT (panel B) is: Vertebral Strength = −0.078*VAT2 + 18*VAT + 3732 (R2=0.14 and p<0.0001 for VAT and VAT2 estimates). The equation for the displayed curve of Vertebral Strenth vs. lean mass (panel C) is: Vertebral Strength = 46.68*lean mass + 2217.91 (R2=0.22; p<0.0001 for lean mass estimate). When the equations for vertebral strength vs. BMI (panel A), VAT (panel B), and lean mass (panel C) were adjusted for log age, the p-value of log age was not significant, the p-values for all estimates of BMI, VAT, and lean mass, respectively, remained <0.0001, and the R2 values were essentially unchanged. VAT, Visceral Adipose Tissue; lean mass, total body lean mass; ●, AN, anorexia nervosa; x, C, lean controls; □, OB, obese
Abdominal VAT (Figure 4B) and TAT assessed by QCT at the level of L4 vertebral body demonstrated inverted U-shaped relationships with both Int.vBMD and vertebral strength. Thus, VAT (R −0.36 to −0.38, p<0.003) and TAT (R= −0.33 to −0.38, p≤0.007) exhibited negative relationships with Int.vBMD and vertebral strength within obese women in linear models; all associations remained significant after controlling for BMI. Moreover, abdominal SAT (R= −0.46, p<0.002) and TAT (R= −0.47, p<0.002) were negatively associated with Int.vBMD within lean controls, which remained significant after controlling for BMI. SAT and TAT trended toward negative associations with vertebral strength within lean controls, which became significant after controlling for BMI (R= −0.30 to −0.35, p<0.05). TAT also trended toward a negative association with vertebral strength within anorexia nervosa, which became significant after controlling for BMI (R= −0.28, p<0.03).
Total body fat mass assessed by DXA was negatively associated with Int.vBMD within obese subjects (R= −0.26, p<0.04); this was not significant after controlling for BMI. In contrast to the multiple fat measures described above, total body lean mass exhibited positive linear relationships with Int.vBMD (R= 0.27), CSA (R= 0.41), and vertebral strength (R= 0.45) (all p≤0.0004) across all groups combined (Figure 4C). Associations of total body lean mass with CSA and vertebral strength remained significant after controlling for BMI. Total body lean mass was associated positively with CSA within each group individually (R= 0.26–0.56, p<0.03) and with vertebral strength within anorexia nervosa group (R= 0.32, p<0.01); all remained significant after adjusting for BMI. Moreover, after controlling for BMI, the association between total body lean mass and vertebral strength became significant within the obese group (R= 0.25, p<0.05) and was positive, in contrast to the negative association between BMI and vertebral strength within obese women.
In summary, after controlling for BMI, total body lean mass was positively, whereas multiple measures of abdominal fat (VAT, SAT, TAT) were negatively, associated with vertebral strength parameters.
Discussion
This is the first report comparing vertebral strength, Int.vBMD, CSA, and factor-of-risk (Φ) for vertebral fracture across the weight spectrum, from anorexia nervosa to obesity. We demonstrated that women with anorexia nervosa had lower Int.vBMD and hence vertebral strength compared to lean and obese women, whereas obese women had similar Int.vBMD, CSA, and vertebral strength compared to lean controls. We employed the factor-of-risk (Φ) for vertebral fracture (applied load to strength ratio) to help explain vertebral fracture risk in women with anorexia nervosa, lean controls, and obese women. Women with anorexia nervosa had higher Φ for vertebral fracture than lean controls for certain activities (lateral bending and holding), which was driven predominantly by inferior vertebral strength. In contrast, despite similar vertebral strength as lean controls, obese women had higher Φ for vertebral fracture for standing and lifting, which was driven by higher applied loads due to their higher body weights. Thus, fracture risk is elevated in both low-weight and obese women, but for different reasons, and differs by activity.
Anorexia nervosa is associated with low aBMD, particularly at the spine,(3,4) and increased fracture risk.(1,2) At the other end of the weight spectrum, obese women often have normal or even higher aBMD compared to controls yet exhibit higher fracture risk at certain skeletal sites.(5–7) Areal BMD has limitations in predicting fracture in obesity(12,13) and with weight fluctuations,(14) as can occur in eating disorders. The shortcomings of aBMD in predicting fracture create the need for understanding bone strength beyond aBMD; however, data regarding vertebral strength in anorexia nervosa and obesity are limited. In this study, we demonstrated that women with anorexia nervosa had inferior Int.vBMD and vertebral strength compared to lean women, but obese women had similar Int.vBMD, CSA, and vertebral strength as controls. These findings suggest that inferior vertebral strength likely contributes to the increased rate of vertebral fracture in anorexia nervosa, whereas in obese women vertebral strength appears similar to controls, although still insufficient with respect to the higher forces applied to their vertebral bodies due to higher body weights.
Fracture risk depends on both bone strength, as well as the loads applied to the bone during different activities, a concept captured by the factor-of-risk (Φ). Given that Φ is the ratio of applied load to bone strength, Φ can be increased by higher applied load values or by lower bone strength values. Among women with anorexia nervosa, the higher Φ for vertebral fracture compared to controls for lateral bending and holding was driven primarily by their inferior vertebral strength. In addition, during lateral bending, the lower muscular strength of women with anorexia nervosa (due to their smaller muscle size) necessitated the recruitment of additional muscles to maintain static equilibrium during this activity. The contraction of these additional muscles led to a higher applied load during lateral bending, leading to an even further increase in the load-to-strength ratio during that activity for women with anorexia nervosa. In contrast, despite similar bone strength compared to controls, obese women had higher Φ for standing and lifting due to higher applied loads due to their higher body weights. The variations in applied load during different activities might help explain why obese women can sustain vertebral fractures despite normal aBMD and why prior studies regarding vertebral fracture risk in obese women have reported conflicting results.(7–11)
BMI is positively associated with BMD in general, but BMI alone does not capture how different components of body mass can impact BMD and bone strength in different ways. Lean mass is positively associated with areal BMD,(19–22) but the association of BMD with fat mass is less clear. Total body fat has been found to be positively associated with aBMD,(19,21) but VAT is negatively associated with vertebral vBMD in obese premenopausal women(22) and healthy Chinese men and women.(24) Little is known regarding the associations of vertebral strength with lean and fat mass. A secondary objective of our study was to explore the relationships of lean and fat mass with vertebral strength, as these might provide insights into vertebral strength not explained by BMI alone. In our study, BMI was negatively associated with vertebral strength within obese women and was not associated with vertebral strength or its components within anorexia nervosa or lean controls. After controlling for BMI, lean mass was associated positively with CSA and vertebral strength within obese women and within anorexia nervosa. This positive association between lean mass and vertebral strength in obese women is particularly interesting, given that BMI was negatively associated with vertebral strength in the obese group. In contrast to the positive relationship of vertebral strength with lean mass, multiple measures of abdominal fat mass (VAT, SAT, TAT) were associated negatively with Int.vBMD and vertebral strength after controlling for BMI. In obese women, VAT and thereby TAT were negative determinants of vertebral strength, driven by the strength component Int.vBMD. Thus, it appears that the negative association of BMI with vertebral strength in obese women is driven by abdominal VAT, whereas lean mass has favorable effects on vertebral strength in obese women. Our findings suggest that lean mass is the component of BMI that contributes positively to vertebral strength, whereas abdominal fat mass is detrimental to vertebral strength. A possible explanation for the beneficial effects of lean mass is that the pull of muscles on bone stimulates adaptive increases in bone density(40) and bone geometry(41) over time. In contrast, abdominal fat appears to have unfavorable effects on bone; possible mechanisms are that VAT is a strong negative determinant of endogenous growth hormone secretion and that VAT might stimulate osteoclastic activity through the release of pro-inflammatory cytokines.(42–44)
Limitations of our study include its cross-sectional nature and lack of prospective fracture data, which limits our ability to confirm our estimates of fracture risk. Of note, vertebral compressive strength determined in vitro from failure loads of human cadavers was strongly correlated with vertebral strength estimated from Int.vBMD and CSA, the method employed in our study.(35) In addition, the biomechanical models for L4 loading only included axial compression. However, in prior studies using finite element analysis of vertebral bodies, the mechanical behaviors of the lumbar vertebra in axial compression and anterior bending were strongly correlated.(45,46) Thus, we believe that the estimated compressive strength values reflect strength during other activities such as bending. Also, the absolute fracture risk was small for all groups; theoretically Φ>1 (when applied load exceeds the bone’s strength) suggests a high risk of fracture, and the mean Φ for any group in our study did not exceed 1. Even though the absolute fracture risk was low in this young, premenopausal population, absolute fracture risk is known to increase with age. Therefore, relative differences that are evident in young populations may become clinically significant as patients age and absolute fracture risk increases.
In conclusion, we are the first to report that the higher vertebral fracture risk during certain activities in women with anorexia nervosa is predominantly due to inferior vertebral strength, whereas the higher fracture risk during some activities in obese women is driven by higher applied loads due to their higher body masses. This may help explain variations in vertebral fracture risk in obesity reported in prior studies. Our data support the notion that fracture risk may depend not only on bone density, but also on applied loads experienced during various activities.
Acknowledgments
Grants: NIH Grants: T32 DK 007028, K24 HL092902, RO1 HL 077674, R01 MH083657, R01 DK052625, RO3-DK59297, M01-RR01066-27S1, M01-RR01066, UL1 RR025758, 8UL1 TR000170, 1UL1TR001102, K23 RR-23090, R01 AR053986, F31AG041629
Footnotes
Authors’ Roles: KNB and KKM conducted the study, collected the data, analyzed and interpreted the data, drafted the manuscript, and take responsibility for the data integrity and accuracy of the data analysis. KKM, MLB, AK, KNB, AGB, and MAB designed the study. AGB and MLB developed the biomechanical model, estimated the applied vertebral loads for all subjects, and interpreted the data. MAB and CMG analyzed QCT scans in order to calculate integral volumetric BMD and cross-sectional area. AK, KKM, MAB, EAL, EM, AVG, KNB, MS, KTE, SE, SLK, JMG, RJK, TW, and ED were involved in the design and/or conduct of prior research protocols from which data were drawn for the current study. KNB, AGB, MLB, KKM, AK, MAB, MS, EAL, VS, MM, and CMG revised the manuscript. All authors reviewed and approved the manuscript.
References
- 1.Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clinic proceedings. 1999;74(10):972–977. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders--a nationwide register study. The International journal of eating disorders. 2002;32(3):301–308. doi: 10.1002/eat.10101. [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Annals of internal medicine. 2000;133(10):790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Archives of internal medicine. 2005;165(5):561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 5.Compston JE, Flahive J, Hosmer DW, et al. Relationship of Weight, Height, and Body Mass Index With Fracture Risk at Different Sites in Postmenopausal Women: The Global Longitudinal Study of Osteoporosis in Women (GLOW) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(2):487–493. doi: 10.1002/jbmr.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(1):223–233. doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- 7.Prieto-Alhambra D, Premaor MO, Fina Aviles F, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 8.Laslett LL, Just Nee Foley SJ, Quinn SJ, Winzenberg TM, Jones G. Excess body fat is associated with higher risk of vertebral deformities in older women but not in men: a cross-sectional study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(1):67–74. doi: 10.1007/s00198-011-1741-8. [DOI] [PubMed] [Google Scholar]
- 9.Pirro M, Fabbriciani G, Leli C, et al. High weight or body mass index increase the risk of vertebral fractures in postmenopausal osteoporotic women. Journal of bone and mineral metabolism. 2010;28(1):88–93. doi: 10.1007/s00774-009-0108-0. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S, Kuroda T, Saito M, Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(1):69–76. doi: 10.1007/s00198-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 11.Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. The American journal of medicine. 2011;124(11):1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(1):119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javed F, Yu W, Thornton J, Colt E. Effect of fat on measurement of bone mineral density. International journal of body composition research. 2009;7(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2005;8(1):31–38. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- 15.Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(2):292–297. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 16.Bruno AG, Broe KE, Zhang X, et al. Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(3):562–569. doi: 10.1002/jbmr.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouxsein ML, Melton LJ, 3rd, Riggs BL, et al. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(9):1475–1482. doi: 10.1359/jbmr.060606. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann KN, Fazeli PK, Lawson EA, et al. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. The Journal of clinical endocrinology and metabolism. 2014;99(12):4664–4673. doi: 10.1210/jc.2014-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho-Pham LT, Nguyen UD, Nguyen TV. Association between lean mass, fat mass, and bone mineral density: a meta-analysis. The Journal of clinical endocrinology and metabolism. 2014;99(1):30–38. doi: 10.1210/jc.2014-v99i12-30A. [DOI] [PubMed] [Google Scholar]
- 20.Lu LJ, Nayeem F, Anderson KE, Grady JJ, Nagamani M. Lean body mass, not estrogen or progesterone, predicts peak bone mineral density in premenopausal women. The Journal of nutrition. 2009;139(2):250–256. doi: 10.3945/jn.108.098954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37(4):474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Bredella MA, Torriani M, Ghomi RH, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–754. doi: 10.1016/j.bone.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. The Journal of clinical endocrinology and metabolism. 2013;98(6):2562–2572. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Ma X, Xue P, et al. Associations between fat distribution and volumetric bone mineral density in Chinese adults. Endocrine. 2014;47(3):862–868. doi: 10.1007/s12020-014-0252-8. [DOI] [PubMed] [Google Scholar]
- 25.Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. The Journal of clinical endocrinology and metabolism. 2012;97(11):4115–4122. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2005;90(3):1428–1433. doi: 10.1210/jc.2004-1181. [DOI] [PubMed] [Google Scholar]
- 27.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity. 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredella MA, Gerweck AV, Barber LA, et al. Effects of growth hormone administration for 6 months on bone turnover and bone marrow fat in obese premenopausal women. Bone. 2014;62:29–35. doi: 10.1016/j.bone.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. European journal of endocrinology / European Federation of Endocrine Societies. 2012;166(4):601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin E, Bredella MA, Gerweck AV, et al. Effects of growth hormone withdrawal in obese premenopausal women. Clinical endocrinology. 2013;78(6):914–919. doi: 10.1111/cen.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity. 2010;18(11):2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bredella MA, Gill CM, Keating LK, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity. 2013;21(12):2458–2464. doi: 10.1002/oby.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genant HK, Cann CE, Pozzi-Mucelli RS, Kanter AS. Vertebral mineral determination by quantitative CT: clinical feasibility and normative data. Journal of Computer Assisted Tomography. 1983;7(3):554. [Google Scholar]
- 34.Kopperdahl DL, Morgan EF, Keaveny TM. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(4):801–805. doi: 10.1016/S0736-0266(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 35.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744–750. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 36.Iyer S, Christiansen BA, Roberts BJ, Valentine MJ, Manoharan RK, Bouxsein ML. A biomechanical model for estimating loads on thoracic and lumbar vertebrae. Clinical biomechanics. 2010;25(9):853–858. doi: 10.1016/j.clinbiomech.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson DE, D'Agostino JM, Bruno AG, Manoharan RK, Bouxsein ML. Regressions for estimating muscle parameters in the thoracic and lumbar trunk for use in musculoskeletal modeling. Journal of biomechanics. 2012;45(1):66–75. doi: 10.1016/j.jbiomech.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S. Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: a randomized controlled trial. Jama. 2004;292(2):210–218. doi: 10.1001/jama.292.2.210. [DOI] [PubMed] [Google Scholar]
- 39.Marcus R, Peritz E, Gabriel KR. On Closed Testing Procedures with Special Reference to Ordered Analysis of Variance. Biometrika. 1976;63(3):655–660. [Google Scholar]
- 40.Looker AC, Flegal KM, Melton LJ., 3rd Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18(3):307–313. doi: 10.1007/s00198-006-0241-8. [DOI] [PubMed] [Google Scholar]
- 41.Beck TJ, Oreskovic TL, Stone KL, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(6):1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 42.Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. The Journal of clinical endocrinology and metabolism. 2008;93(5):1931–1938. doi: 10.1210/jc.2007-2191. [DOI] [PubMed] [Google Scholar]
- 43.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 44.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochemical and biophysical research communications. 2005;337(2):422–429. doi: 10.1016/j.bbrc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 45.Graeff C, Chevalier Y, Charlebois M, et al. Improvements in vertebral body strength under teriparatide treatment assessed in vivo by finite element analysis: results from the EUROFORS study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(10):1672–1680. doi: 10.1359/jbmr.090416. [DOI] [PubMed] [Google Scholar]
- 46.Crawford RP, Keaveny TM. Relationship between axial and bending behaviors of the human thoracolumbar vertebra. Spine. 2004;29(20):2248–2255. doi: 10.1097/01.brs.0000142435.90314.3b. [DOI] [PubMed] [Google Scholar]