Abstract
BACKGROUND: Kim et al. report two patients with melanoma metastases to the brain that responded to treatment with RRx-001 and whole brain radiotherapy (WBRT) without neurologic or systemic toxicity in the context of a phase I/II clinical trial. RRx-001 is an reactive oxygen and reactive nitrogen species (ROS/RNS)-dependent systemically nontoxic hypoxic cell radiosensitizer with vascular normalizing properties under investigation in patients with various solid tumors including those with brain metastases. SIGNIFICANCE: Metastatic melanoma to the brain is historically associated with poor outcomes and a median survival of 4 to 5 months. WBRT is a mainstay of treatment for patients with multiple brain metastases, but no significant therapeutic advances for these patients have been described in the literature. To date, candidate radiosensitizing agents have failed to demonstrate a survival benefit in patients with brain metastases, and in particular, no agent has demonstrated improved outcome in patients with metastatic melanoma. Kim et al. report two patients with melanoma metastases to the brain that responded to treatment with novel radiosensitizing agent RRx-001 and WBRT without neurologic or systemic toxicity in the context of a phase I/II clinical trial.
Introduction
Brain metastases are a common complication of melanoma and constitute a significant cause of morbidity and mortality [[1], [2], [3]]. Their incidence in melanoma is on the rise due to increased patient survival from improved treatment options with targeted therapeutics (e.g., selective BRAF inhibitors like vemurafenib or dabrafenib) and immune checkpoint inhibitors (e.g., ipilimumab and nivolumab) [[4], [5], [6], [7]]. In the setting of disseminated disease, the goal of treatment is not necessarily to eradicate the brain lesions but rather to control them, thereby conserving or prolonging neurologic function, performance status, and potentially survival.
Associations between treatment of brain metastases and survival are confounded by the high risk of death from progressive systemic disease burden [8]. The impact on survival may, therefore, be more easily assessable in terms of a neurologic versus nonneurologic cause of death. Although the large phase II BREAK-MB trial with dabrafenib in BRAF-mutated tumors and smaller studies with ipilimumab have demonstrated improved intracranial response rates, especially compared with conventional chemotherapies (Table 1), the cornerstone of treatment for melanoma brain metastases is radiation therapy (RT). In general, whole brain radiotherapy (WBRT) is administered for multiple brain metastases (≥ 3-4 lesions), whereas stereotactic radiosurgery (SRS) is administered for nonresectable single or oligometastases. To date, no reported randomized clinical trials of WBRT or SRS have been conducted for brain metastases from melanoma. A randomized phase III study evaluating the benefit of WBRT versus observation following local treatment of melanoma brain metastases is currently under way [9]. In addition, no randomized clinical trials have been reported with WBRT or SRS in patients with four or more metastatic lesions to the brain. However, melanoma is a particularly radioresistant tumor, leading some authors to question the benefit of low-dose WBRT [10], in addition to the known neurocognitive sequelae of treatment [11].
Table 1.
Pertinent Studies with Immunotherapy, Chemotherapy, and/or Radiation in Melanoma Brain Metastases
| Study | Regimen | Radiation Therapy | Phase | No. Patients | Overall Intracranial Response Rate % | Progression-Free Survival or Median Time to Progression | Median Overall Survival Months |
|---|---|---|---|---|---|---|---|
| BREAK-MB Long et al. 2012[5], [32] |
Dabrafenib | None | II | Cohort A (no prior treatment): 74 Cohort B (prior treatment): 65 |
39 (2 CR, 27 PR) 31 (0 CR, 20 PR) |
6.3 mo (A) and 4.5 mo (B) | 13.1 (A) and 12.9 (B) |
| Mornex et al. 200333 | Fotemustine + WBRT | 37.5 Gy in 15 fractions | III | 37 | 10 | – | 2.9 (NS) |
| Margolin et al. 20124 | Ipilimumab | None | II | Cohort A (without corticosteroids): 51 Cohort B (with corticosteroids): 21 |
10 (5 PR) 5 (1 PR) |
– | 7 (A) and 4 (B) |
| NIBIT-M1 Di Giacomo et al. 201234 |
Ipilimumab + fotemustine | None | II | 20 with asymptomatic brain mets | 40 (2 irCR, 6 irPR) | – | – |
| Agarwala et al. 200414 | Temozolomide | None | II | 117 | 7 (1 CR, 7 PR) | 1.2 mo | 3.2 |
| Hwu et al. 200535 | Temozolomide + thalidomide | None | II | 15 | 12 (2 CR, 1 PR) | – | 5 |
| Atkins et al. 200836 | Temozolomide + thalidomide + WBRT | 30 Gy in 10 fractions | II | 39 | 7.6 (1 CR, 2 PR) | 1.8 mo | 4 |
| Margolin et al. 200237 | Temozolomide + WBRT | 30 Gy in 10 fractions | I/II | 31 | 9.7 (1 CR, 2 PR) | 2 mo | 6 |
irCR, immune-related complete response; irPR, immune-related partial response; CR, complete response; PR, partial response; (NS), not significant; WBRT, whole brain radiotherapy.
Given the relative radioresistance of the disease [[6], [12]], intensification of radiation treatment with radiosensitizing agents is a logical consideration [[13], [14]]. To date, there have been no reports of a candidate radiosensitizer that conclusively improves outcomes of patients with brain metastases from melanoma or any solid tumor histology.
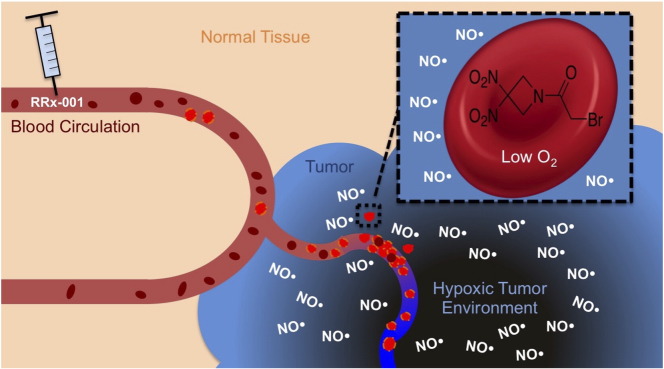
A new agent under investigation, RRx-001, demonstrates evidence of antitumor effect in patients with a wide variety of malignancies, including melanoma. RRx-001 is a small molecule sourced from the aerospace industry with a novel chemical structure (Figure 1). It is under investigation as a single agent, chemosensitizer, and radiosensitizer in several clinical trials. RRx-001 mediates an increase of tumor blood flow and subsequent oxygenation in tumors, which may enhance radiosensitization [[15], [16]]. The mechanism is, at least in part, nitric oxide (NO) related. Under hypoxic conditions, NO production via constitutive nitric oxide synthase (NOS) enzymes is decreased, with a resultant increase in deoxyhemoglobin-mediation NO production, which converts nitrite (NO2−) to NO. RRx-001, which binds to hemoglobin, greatly accelerates the NO2− to NO conversion of deoxyhemoglobin (Figure 2) [[17], [18]]: the result is that, unlike other nitric oxide donors (e.g., nitroglycerine), NO acts locally, not systemically, and especially under hypoxic conditions such as in primary tumors and metastases [19]. The radiosensitizing effects of nitric oxide are attributable to multiple factors including vasodilation, vascular stabilization, and inhibition of DNA repair enzymes [20].
Figure 1.
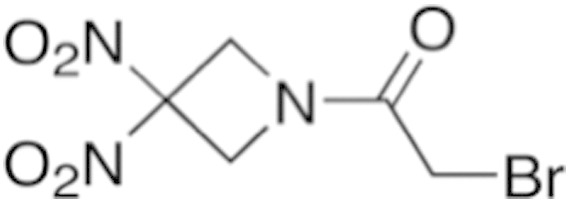
Chemical structure of RRx-001.
Figure 2.
After injection in the bloodstream, RRx-001 penetrates the red cell membrane and binds to a specific residue on hemoglobin. These RRx-001–modified red blood cells home to the hypoxic tumor vasculature. Under hypoxic conditions common to tumors, RRx-001 accelerates deoxyhemoglobin-mediated NO production.
This report describes the initial two patients with adequate follow-up treated on BRAINSTORM, a phase 1/2 dose-escalation clinical trial of RRx-001 in combination with WBRT in patients with solid tumor brain metastases. Both patients responded at the lowest dose level of 5 mg/m2 in the absence of neurologic toxicity.
Results
Case 1
A 62-year-old white man was initially diagnosed with cutaneous melanoma of the left arm for which he underwent wide local excision and sentinel lymph node biopsy, demonstrating melanoma (nodular type, Breslow depth 3.5 mm without ulceration) and one of five lymph nodes positive. Completion axillary lymph node dissection revealed no further disease. He presented 3 years later with biopsy-proven recurrence in the left axilla. Staging workup revealed no other sites of disease. He underwent reoperative axillary lymph node dissection, which demonstrated melanoma involving the subcutaneous tissue (measuring 2 cm in greatest diameter), and seven lymph nodes negative for neoplasm. The patient underwent adjuvant axillary radiation and did well until 5 months later when he developed new-onset cough with a computed tomography of the chest revealing new pulmonary nodules measuring up to 1.9 cm, confirmed by biopsy to be melanoma, as well as soft tissue nodules in the anterior abdomen.
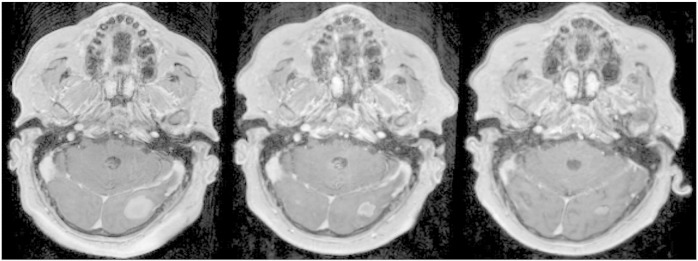
Soon thereafter, the patient noted decreased visual acuity and central and right-sided visual field deficit. Brain magnetic resonance imaging (MRI) revealed the presence of a 2.2 × 2.6 × 2.6–cm mass in the left occipital lobe and a 3.3 × 2.7 × 2.2–cm mass in the right occipital lobe, as well as three other supratentorial lesions. All lesions were hemorrhagic with surrounding vasogenic edema. Dexamethasone 4 mg TID was started without improvement in symptoms. The patient was enrolled on clinical trial and received a single peripheral intravenous dose of RRx-001 (5 mg/m2) 4 days before the initiation of WBRT. Twenty-four hours after the first dose of RRx-001, dynamic contrast-enhanced brain MRI (DCE-MRI) revealed a decrease in ktrans in four of five lesions, suggesting a reduction in vessel leakiness after exposure to the drug. He was then treated with RRx-001 at 5 mg/m2 in combination with WBRT (30 Gy in 10 fractions). Within 48 hours, the patient’s visual symptoms began to improve. RRx-001 was well-tolerated with mild, transient infusion site pain and without any signs of systemic or neurologic toxicity. By the end of treatment, the patient reported improved visual symptoms with near resolution of visual field deficit, and dexamethasone was tapered following completion of WRBT. Four weeks after the completion of RRx-001 and WBRT, a contrasted brain MRI confirmed a decrease in the size of the largest left and right occipital masses and evolving hemorrhagic change in the other three lesions consistent with resolving hemorrhage. The patient reported full physical function with no impairment of activities of daily living, as reported by the Barthel ADL Index, which was unchanged from baseline. Self-reported quality of life using the Functional Assessment of Cancer Therapy instrument revealed stability in all four reported subscales (physical, social, emotional, and functional) 1 month after treatment compared with baseline. Four months after completion of treatment, continued response was seen in all evaluable lesions (left occipital lobe lesion now measuring 0.9 × 0.6 × 1.0 cm and right occipital lobe lesion measuring 2.3 × 2.2 × 1.5 cm), in addition to a decrease in associated edema of all lesions that were also improved compared with before (Figure 3). A new 5-mm lesion was discovered at 4 months, possibly from seeding due to persistent systemic disease.
Figure 3.
Left panel is a representative axial image from a T1 gadolinium-enhanced brain MRI of the patient’s most symptomatic left occipital lesion before treatment. Middle panel demonstrates marked response of the lesion 1 month following 2 weeks of WBRT plus RRx-001, which was accompanied by resolution of the visual field deficits. Right panel demonstrates further response of the lesion 4 months after WBRT plus RRx-001.
Case 2
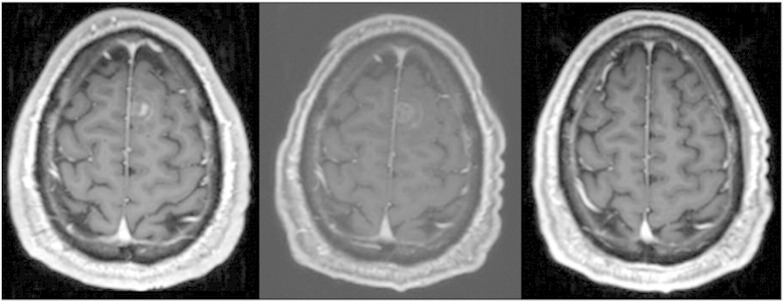
The second patient was a 40-year-old white man initially diagnosed with a melanoma of the upper back 5 years ago (Clark level IV, Breslow depth 1.5 mm) arising from a compound nevus. He underwent wide local excision and sentinel lymph node biopsy with axillary dissection of the right and left axilla, revealing 1 of 21 lymph nodes and 1 of 18 lymph nodes involved, respectively. He elected to undergo surveillance and remained disease free until 5 years later when he developed progressive right arm pain. Restaging confirmed recurrent metastases involving the superior mediastinum, multiple subcutaneous sites, retroperitoneal adenopathy, and at least 18 new asymptomatic brain metastases, most measuring 2 to 3 mm with the largest, partially hemorrhagic lesion measuring 1.7 cm. He was enrolled on study and received RRx-001 (5 mg/m2) 4 days before WBRT, followed by 2 weeks of WBRT and twice weekly RRx-001 at the same dose, per protocol. Twenty-four hours after administration of the drug, a slight reduction in ktrans was noted in the largest evaluable lesion. Subcentimeter lesions could not be quantitatively evaluated with DCE-MRI because of their small size. He had no significant infusion site pain during treatment. At 1 month posttreatment, no significant change in the lesions was seen. The patient reported full physical function and ability to perform activities of daily living 1 month after the end of treatment (as measured by the Barthel ADL index), which was unchanged from baseline. He had no new neurologic symptoms. Self-reported quality of life using Functional Assessment of Cancer Therapy demonstrated decline in the four measured subscales 1 month after treatment in the setting of progressive systemic disease. Serial brain reimaging revealed partial response intracranially 3 months after treatment, with disappearance of three lesions and shrinkage in all other lesions. Four months after the end of treatment, marked response was seen in the brain, confirming the findings at 3 months, with complete disappearance of all but 5 of the initial 18 visualized lesions and size reduction in the remaining 5 (Figure 4).
Figure 4.
Left panel is a representative image from a T1 gadolinium-enhanced brain MRI demonstrating a subcentimeter enhancing brain metastasis before treatment. Middle panel demonstrates the appearance of the lesion 1 month after the end of WBRT + RRx-001, which is not significantly changed in size. Right panel demonstrates complete disappearance of the lesion 4 months after the end of treatment.
Discussion
Poor outcomes among patients with brain metastases, with an overall median survival of 1 to 2 months without treatment and 3 to 8 months with treatment, have led to their exclusion from most clinical trials, making this a relatively understudied patient population [[21], [22], [23]]. Despite the relative radioresistance of melanoma [[24], [25]], RT remains the primary treatment option due to the failure of systemic chemotherapy to control or prevent brain metastases, which shelter behind the sanctuary of the Blood Brain Barrier (BBB) [26]. For example, the response rates of temozolomide in the brain are less than 10% (Table 1). Although emerging data from retrospective studies suggest that stereotactic radiosurgery is effective in combination with checkpoint inhibitors in brain metastases [27] from multiple tumor types, and newer molecular agents such as the BRAF inhibitor dabrafenib are associated with higher response rates and better survival (Table 1), prospective studies are required to evaluate whether these new regimens are comparable or superior to WBRT.
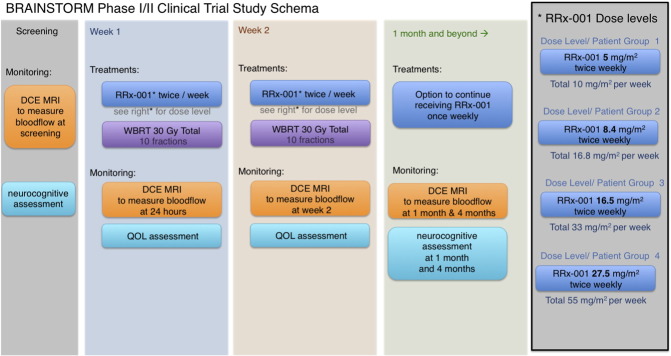
BRAINSTORM is a phase I/II dose escalation study of IV RRx-001, a novel, systemically nontoxic agent with evidence of chemo- and radiosensitizing properties [28], administered twice weekly for 2 weeks in combination with WBRT (30 Gy in 10 fractions), with the option to continue RRx-001 once weekly as maintenance after WBRT (Figure 5).
Figure 5.
BRAINSTORM phase I/II study schema.
The primary end point of the trial is to determine the maximum tolerated dose of RRx-001 in combination with WBRT. Secondary end points include best overall response rate, intracranial progression-free and overall survival, as well as measurement of neurocognitive status and tumor blood flow with serial DCE-MR imaging.
This report demonstrates the radiosensitization potential of RRx-001 even at the lowest dose of twice weekly 5 mg/m2 in the first and third patients with brain metastases treated on the BRAINSTORM clinical trial. The second patient with metastatic melanoma died unexpectedly before response could be assessed with adequate follow-up, possibly because of an acute, unrelated cardiopulmonary event. Treatment for the two patients with adequate follow-up was well tolerated, with mild transient localized infusion pain as the only adverse event. In addition, patients reported complete maintenance of their physical function before and after treatment. The observed enhanced radiation response, resulting in neurological improvement and significant tumor shrinkage at 3- and 4-month assessments, may correlate with the RRx-001–induced increase in tumor blood flow and, by extrapolation, tumor oxygenation. The relationship between tumor perfusion and radiosensitization will be further evaluated on this trial via serial perfusion MRI.
Radiation sensitivity is significantly enhanced in the presence of oxygen. Cells that are anoxic during irradiation are only about one third as sensitive to its cytotoxic effects as their oxygenated counterparts [[29], [30]]; hypoxia is therefore associated with an increased risk of disease recurrence [31]. However, over the last four to five decades, hypoxia-targeted therapies that locally or systemically increase tumor oxygenation including hyperbaric oxygen, pretreatment transfusion, hyperfractionation, carbogen breathing, and electron-affinic and hypoxic-cell sensitizers together with RT consistently have failed to demonstrate a significant benefit [[6], [12]].
By contrast, this report suggests that RRx-001, at the lowest dose of 5 mg/m2 administered twice weekly, is safe as expected and active when used with WBRT for melanoma brain metastases.
In conclusion, RRx-001 is a well-tolerated agent with radiosensitizing potential that may improve clinical outcomes in patients with metastases from melanoma and other cancers. The partial responses and the promising findings from advanced neuroimaging from these two patients heighten interest in further investigation of RRx-001 with dose escalation as planned in the BRAINSTORM trial.
Experimental Procedures
The two cases reported in this article were observed in the ongoing phase I/II clinical trial BRAINSTORM (clinical trial registration identifier, NCT02215512). The methods in this study were carried out in accordance with approved guidelines. The necessary informed consents were obtained. The relevant Institutional Review Boards at University of Michigan Medical School approved all experimental protocols.
Patients with pathologic confirmation of a solid tumor malignancy and a clinical history consistent with metastatic disease to the brain that can be imaged by MRI, with adequate laboratory values; an ECOG score of 0 to 2 (Eastern Cooperative Oncology Group Performance Status); an estimated life expectancy of at least 12 weeks; and discontinuation of systemic chemotherapy, other biologic therapy, or investigational agent at least 2 weeks before intervention were recruited onto this dose escalation study at the University of Michigan to receive one of four dose levels of RRx-001 (5, 8.4, 16.5, and 27.5 mg/m2 twice weekly) in combination with WBRT. Patients received intravenous RRx-001 4 days before WBRT (30 Gy in 10 daily fractions), then twice weekly during WBRT for 5 total doses. The patients described in this report received an RRx-001 dose of 5 mg/m2 twice weekly. Patients underwent DCE-MRI pretreatment, 24 hours after RRx-001 before WBRT, just before the last fraction of WBRT + RRx-001, and 1 month and 4 months after WBRT. Quantitative imaging parameters including mean ktrans and tumor size were analyzed at each time point. Patients were followed serially with neurologic and functional assessments, and dose-limiting toxicity was evaluated within the 28 days following completion of treatment.
Acknowledgements
The authors disclose that the clinical trial in which these cases were observed is funded by EpicentRx, Inc.
Footnotes
Author contributions: The authors declare that each author contributed to the work resulting in this research.
The authors disclose that the clinical trial in which these cases were observed is funded by EpicentRx, Inc.
References
- 1.Sampson JH, Carter JH, Jr, Friedman AH, Seigner HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 2.Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 3.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 4.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 5.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Alkali A. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 6.Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830–833. [PubMed] [Google Scholar]
- 7.Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol. 2015;33(30):3475–3484. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khuntia D. Contemporary review of the management of brain metastasis with radiation. Adv Neurosci. 2015 [Article ID 372856, 13 pages] [Google Scholar]
- 9.Fogarty GB, Hong A, Jacobsen KD, Reisse CH, Shivalingam B, Burmeister B, Haydu LE, Paton E, Thompson JF. Accrual to a randomised trial of adjuvant whole brain radiotherapy for treatment of melanoma brain metastases is feasible. BMC Res Notes. 2014;7:412. doi: 10.1186/1756-0500-7-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal S, Silk AW, Tian S, Mehner J, Danish S, Ranjan S, Kaufman HL. Clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol. 2015;1(5):668–676. doi: 10.1001/jamaoncol.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan AJ. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;31(1):11–13. doi: 10.1200/JCO.2012.46.0410. [DOI] [PubMed] [Google Scholar]
- 12.Dossgg LL, Memula N. The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys. 1982;8:1131–1134. doi: 10.1016/0360-3016(82)90060-8. [DOI] [PubMed] [Google Scholar]
- 13.Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control. 2009;16(3):248–255. doi: 10.1177/107327480901600307. [DOI] [PubMed] [Google Scholar]
- 14.Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brian metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Oronsky BT, Knox SJ, Scicinski J. Six degrees of separation: the oxygen effect in the development of radiosensitizers. Transl Oncol. 2011;4(4):189–198. doi: 10.1593/tlo.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oronsky BT, Oronsky NC, Fanger GR, Oronsky AL, Lybeck MMC, Lybeck HE, Caroen SZ, Parker CW, Scicinski J. A review of two promising radiosensitizers in brain metastases: RRx-001 and 2-deoxyglucose. J Cancer Sci Ther. 2015;7(5):137–141. [Google Scholar]
- 17.Fens M, Larkin SK, Morris CR, Fitch B, Scicinski J, Oronsky B, Kuypers FA. vol. 118. 2011. NO or no NO, increased reduction of nitrite to nitric oxide by modified red blood cells; p. 2125. (ASH Annual Meeting Abstracts). [Google Scholar]
- 18.Scicinski J, Oronsky B, Taylor M, Luo G, Musick T, Marini J, Adams CM, Fitch WL. Preclinical evaluation of the metabolism and disposition of RRx-001, a novel investigative anticancer agent. Drug Metab Dispos. 2012;40:1810–1816. doi: 10.1124/dmd.112.046755. [DOI] [PubMed] [Google Scholar]
- 19.Scicinski J, Oronsky B, Ning S, Knox S, Peehl D, Kim MM, Langecker P, Fanger G. NO to cancer: the complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015;2(6):1–8. doi: 10.1016/j.redox.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oronsky BT, Knox SJ, Scicinski JJ. Is nitric oxide (NO) the last word in radiosensitization? A review. Transl Oncol. 2012;5(2):66–71. doi: 10.1593/tlo.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117:2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 22.Abrey LE. Inclusion of patients with brain metastases in clinical trials. Clin Investig. 2011;1(8):1065–1068. [Google Scholar]
- 23.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin N Am. 2003;21:1–23. doi: 10.1016/s0733-8619(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 24.Khan N, Khan MK, Almasan A, Singh AD, Macklis R. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys. 2011;80(3):645–654. doi: 10.1016/j.ijrobp.2010.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MK, Khan N, Almasan A, Macklis R. Future of radiation therapy for malignant melanoma in an era of newer, more effective biological agents. Onco Targets Ther. 2011;4:137–148. doi: 10.2147/OTT.S20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glantz MJ, Kim L, Choy H, Akerley W. Concurrent chemotherapy and radiotherapy in patients with brain tumors. Oncology. 1999;13(10 Suppl. 5):78–82. [PubMed] [Google Scholar]
- 27.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid T, Dad S, Korn R, Oronsky B, Knox S, Scicinski J. Two case reports of resensitization to previous chemotherapy with the novel hypoxia-activated hypomethylating anticancer agent RRx-001 in metastatic colorectal cancer patients. Case Rep Oncol. 2014;7(1):79–85. doi: 10.1159/000358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 30.Wouters A, Pauweis B, Lardon F, Vermorken JB. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist. 2007;12(6):690–712. doi: 10.1634/theoncologist.12-6-690. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JD, Baer K, Lee J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res. 1983;43(4):1523–1528. [PubMed] [Google Scholar]
- 32.Long GV, Trefzer U, Davies MA. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [Epub 2012 Oct 8] [DOI] [PubMed] [Google Scholar]
- 33.Mornex F., Thomas L. Mohr P et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma Melanoma Res. 2003 Feb;13(1):97–103. doi: 10.1097/00008390-200302000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Di Giacomo A.M., Ascierto P.A., Pilla L. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012 Sep;13(9):879–886. doi: 10.1016/S1470-2045(12)70324-8. Epub 2012 Aug 13. [DOI] [PubMed] [Google Scholar]
- 35.Hwu W.J., Lis E., Menell J.H. Temozolomide plus thalidomide in patients with brain metastases from melanoma: a phase II study. Cancer. 2005 Jun 15;103(12):2590–2597. doi: 10.1002/cncr.21081. [DOI] [PubMed] [Google Scholar]
- 36.Atkins M.B., Sosman J.A., Agarwala S. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008 Oct 15;113(8):2139–2145. doi: 10.1002/cncr.23805. [DOI] [PubMed] [Google Scholar]
- 37.Margolin K., Atkins B., Thompson A. Temozolomide and whole brain irradiation in melanoma metastatic to the brain: a phase II trial of the Cytokine Working Group. J Cancer Res Clin Oncol. 2002 Apr;128(4):214–218. doi: 10.1007/s00432-002-0323-8. Epub 2002 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]