Abstract
Background
This study investigated the relationship between changes in lung function (as measured by forced expiratory volume in one second [FEV1]) and the St. George’s Respiratory Questionnaire (SGRQ) and economically significant outcomes of exacerbations and health resource utilization, with an aim to provide insight into whether the effects of COPD treatment on lung function and health status relate to a reduced risk for exacerbations.
Methods
A systematic literature review was conducted in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to identify randomized controlled trials of adult COPD patients published in English since 2002 in order to relate mean change in FEV1 and SGRQ total score to exacerbations and hospitalizations. These predictor/outcome pairs were analyzed using sample-size weighted regression analyses, which estimated a regression slope relating the two treatment effects, as well as a confidence interval and a test of statistical significance.
Results
Sixty-seven trials were included in the analysis. Significant relationships were seen between: FEV1 and any exacerbation (time to first exacerbation or patients with at least one exacerbation, p = 0.001); between FEV1 and moderate-to-severe exacerbations (time to first exacerbation, patients with at least one exacerbation, or annualized rate, p = 0.045); between SGRQ score and any exacerbation (time to first exacerbation or patients with at least one exacerbation, p = 0.0002) and between SGRQ score and moderate-to-severe exacerbations (time to first exacerbation or patients with at least one exacerbation, p = 0.0279; annualized rate, p = 0.0024). Relationships between FEV1 or SGRQ score and annualized exacerbation rate for any exacerbation or hospitalized exacerbations were not significant.
Conclusions
The regression analysis demonstrated a significant association between improvements in FEV1 and SGRQ score and lower risk for COPD exacerbations. Even in cases of non-significant relationships, results were in the expected direction with few exceptions. The results of this analysis offer health care providers and payers a broader picture of the relationship between exacerbations and mean change in FEV1 as well as SGRQ score, and will help inform clinical and formulary-making decisions while stimulating new research questions for future prospective studies.
Keywords: COPD, Exacerbations, FEV1, SGRQ, Health resource utilization, Regression analysis
Background
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airway obstruction related to chronic inflammatory responses in the lungs with symptoms including disabling dyspnea, fatigue, and persistent cough with excessive sputum. Exacerbations are characterized by a sustained acute worsening of respiratory symptoms beyond daily fluctuations, which leads to changes in medication use. Due to the disease symptoms, COPD patients often have a reduced capacity for physical activity and this may worsen potential systemic manifestations of the disease, such as cardiovascular and psychiatric comorbidities. The global prevalence of COPD is estimated to be 9.2 % [1] with variable estimates, ranging from 3.9 % [2] in the Netherlands to 20.9 % in the US, [3] when reported by country. Therefore, COPD presents a major clinical and humanistic burden, [4] despite the availability and use of standard treatments, which aim to relieve symptoms and slow disease progression [5].
This heavy disease toll inevitably focuses interest on how patients are treated and the extent to which medications produce meaningful benefits. Assessment of such value in clinical trials has traditionally relied on measures of lung function (such as forced expiratory volume in one second [FEV1]), symptom control, health status, and rates of exacerbation over a period of up to one year. Exacerbations are a particularly important marker, not least because they are a key driver of health resource use (HRU), such as emergency department visits, antibiotic use and hospitalization. Evidence of this includes the fact that an exacerbation can cost upwards of $7,000 each, depending on its severity and whether the patient is hospitalized [6]. Unsurprisingly, payers tend to focus on this outcome in their formulary considerations, with the expectation that decreased exacerbation rates will likely result in lower costs for their plan.
The clinical and economic importance of exacerbations in COPD invites questions about their inter-relationship with other well-established measures of treatment effect. These include, for example, persistent and/or uncontrolled disease symptoms and health status as measured by the St. George’s Respiratory Questionnaire [SGRQ] – which captures symptoms, impact on patient well-being, and activities of daily living. Additionally, clinically relevant improvements in lung function measures such as FEV1, are often required by regulators for certain drug approval processes. Of note, previous studies have looked at the link between FEV1 and SGRQ score [7, 8] but their relationship to longer-term outcomes, such as exacerbations and HRU, is not well-known and/or accepted, and this may account for why they have received comparatively less consideration from clinicians and payers.
Against this background, the current study aimed to investigate the relationship between changes in FEV1 and SGRQ score and economically significant outcomes of exacerbations and HRU, by conducting a systematic literature review (SLR) and regression analysis of relevant studies of pharmacological interventions for COPD. The results of this analysis will help the interpretation of clinical trial results and provide insights into whether or how the effects of COPD treatment seen in such studies relate to long-term clinical benefits.
Methods
Literature review
Search strategy
We systematically reviewed MEDLINE- (via PubMed), Embase-, and the Cochrane Central Register of Controlled Trials (CENTRAL) -indexed literature published from January 1, 2002 through October 1, 2014. The search algorithms used keywords for COPD paired with terms for the endpoints of interest--SGRQ, FEV1, exacerbations, and HRU. Limits included clinical trials on humans published in English.
Study selection
Following the literature search, all titles and abstracts identified from MEDLINE, Embase, and CENTRAL were manually reviewed against the inclusion and exclusion criteria using PICOS (Patient, Interventions, Comparisons, Outcomes, Study Design)-related elements. Studies were required to report on at least 20 adult COPD patients, to evaluate pharmacologic treatments labeled for or intended for use as treatment of COPD with any comparator treatment, to report mean change in either FEV1 or SGRQ score and either COPD exacerbations or any HRU endpoint, and to be a randomized controlled trial (RCT). A single investigator screened all abstracts identified through the searches, according to the specified inclusion and exclusion criteria. The full-text articles of accepted studies that passed abstract screening were retrieved for further review. Screening was conducted by a single investigator using the same inclusion and exclusion criteria that had been applied at the abstract level. All excluded studies were confirmed by a second, senior investigator and any discrepancies between the two investigators were resolved by involvement of a third investigator.
Data extraction process
The results of all accepted studies identified as part of the SLR were extracted by a single investigator trained in the critical assessment of evidence, with validation performed by a senior investigator. Trial quality and risk of bias were assessed during extraction for each included study using the Jadad quality score assessment.
Statistical analysis
The analyses relating measures of FEV1 and SGRQ total score to exacerbations and HRU followed the meta-analyses methods outlined by Johnson et al. [9] Each trial supplied one or more pairs of data points on the treatment effects of interest. These predictor/outcome pairs from each of the studies were analyzed using sample-size weighted regression analyses, which estimated a regression slope relating the two treatment effects, as well as a confidence interval and a test of statistical significance. In general, the predictor was a relative treatment effect for change in SGRQ or trough FEV1, and the outcome was a log-relative-risk or log-rate for exacerbations. Pre-bronchodilator FEV1 was considered as equivalent to trough FEV1 for analysis, while post-bronchodilator measures and FEV1 that was unspecified were not included. Primary analyses were designed to avoid the use of an intercept in the regressions, but fit was superior with an intercept included.
For the analyses of patients experiencing at least one exacerbation, studies were included if they reported on exacerbations of all severities. For analyses of patients experiencing at least one moderate-to-severe exacerbation, studies were included if they reported on exacerbations that required antibiotics, oral corticosteroids (OCS), and/or hospitalization. Data on time to first exacerbation or the number of patients with at least one exacerbation were combined for analysis. COPD exacerbations reported as an adverse event were not included in analysis. All studies reporting data at timepoints ≥24 weeks were eligible for inclusion in the analyses. Separate analyses were conducted for all timepoints ≥24 weeks and ≥48 weeks.
Results
Literature review
The literature review identified 67 trials reporting endpoints of interest at timepoints ≥24 weeks that were eligible for inclusion in the regression analysis. Fig. 1 outlines the overall search hits and study attrition during screening and analysis.
Fig. 1.
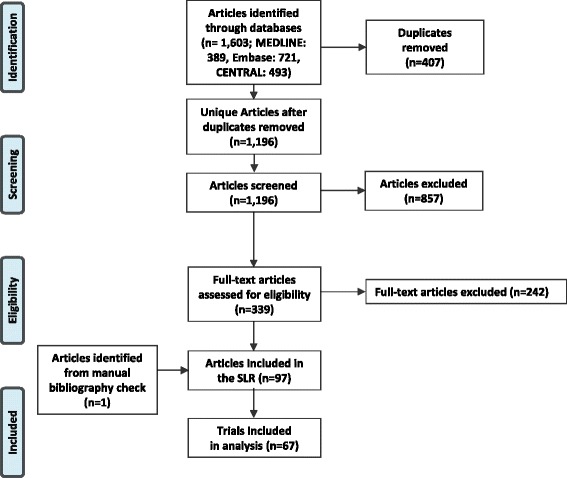
Study Attrition in the Systematic Literature Review
Regression analysis
In the figures representing the analyses, each point in the plot represents a study comparison for two effects. For instance, the point in the middle of Fig. 2 is from Bateman et al. [10] and represents their findings in the comparison of tiotropium 5 mg (via the Respimat® inhaler) vs. placebo. In this example, the difference between the two treatments in trough FEV1 change was -0.10, and the hazard ratio (HR) for any exacerbation risk was 0.693 (for a log-HR of -0.37). Each study with two arms (one treatment comparison, e.g. treatment A vs. treatment B) and with sufficient data contributed one data point to the analysis; studies with three arms (two treatment comparisons, e.g. A vs. B and A vs. C) contributed two data points.
Fig. 2.
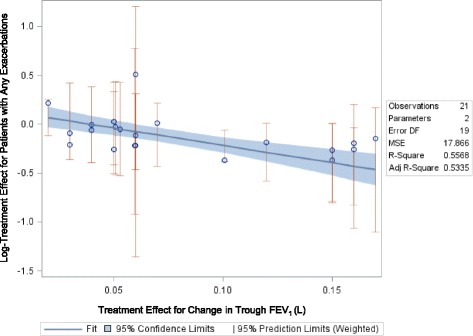
Relationship between Mean Change in Trough FEV1 and Relative Risk for Any Exacerbation
Any given slope can be interpreted by determining what difference between treatments in log-exacerbation risk one would expect given the difference in trough FEV1 change. The predicted log-relative-risk of exacerbation in studies like Bateman 2010 is:
Or
As exp (-0.22) = 0.80, we can predict that the relative risk of exacerbation in studies like Bateman 2010 will be 20 % lower for active treatment than for control. As noted above and in the plot, in Bateman 2010 the relative risk of any exacerbation was actually slightly lower than this value (0.693).
Relationships with exacerbations at ≥48 weeks
Forced Expiratory Volume in One Second (trough FEV1)
Mean Change in Trough FEV1 and COPD Patients’ Risk for Any Exacerbation
The relationship between relative treatment effects on change in FEV1 and any exacerbation was of moderate strength and was statistically significant (slope: -3.56, p = 0.0001; Fig. 2) when defining the exacerbation outcome as time to first exacerbation or the number of patients with at least one exacerbation. No relationship was found (slope: 0.078, p = 0.9199) between treatment effects on FEV1 and annualized exacerbation rate. Figure 2 plots the relationship between the mean difference in trough FEV1 and relative risk for any exacerbation and Table 1 shows the raw trial data contributing to this analysis.
Table 1.
Study Data for Trials Reporting Mean Change in Trough FEV1 and Patients Experiencing Any Exacerbation
| Author, Year | Treatment | Time point (weeks) | N Randomized | Definition of exacerbation | Annual exacerbation rate | N with any exacerbation | Comparison data for Time to first exacerbation (Hazard ratio) | Mean change in Trough FEV1 (L) | Comparison data for Trough FEV1 (treatment difference) |
|---|---|---|---|---|---|---|---|---|---|
| Bateman, 2010 [10] | Tiotropium 5 ug | 48 | 1989 | B+ | 0.12 | 685 | Tio5 vs. Placebo: 0.69 | 0.119 | -- |
| Placebo | 48 | 2002 | 0.15 | 842 | 0.018 | -- | |||
| Calverley, 2010 [12] | Beclomethasone/formoterol pMDI 400/24 μg | 48 | 237 | NR | 0.074 | 64 | -- | 0.077 | B/F pMDI vs. F-DPI: 0.051 |
| Budesonide/formoterol DPI)800/24 μg | 48 | 242 | 0.033 | 64 | -- | 0.08 | B/F dry vs. F-DPI: 0.053 | ||
| Formoterol DPI 12 μg | 48 | 239 | 0.04 | 66 | -- | 0.026 | -- | ||
| Chapman, 2011 [13] | Indacaterol, 150 μg | 52 | 420 | A | -- | -- | Ind150 vs. Placebo: 0.82 | 0.12 | -- |
| Indacaterol, 300 μg | 52 | 418 | -- | -- | Ind300 vs. Placebo: 0.86 | 0.13 | -- | ||
| Placebo | 52 | 425 | -- | -- | -0.04 | -- | |||
| Dahl, 2010 [14] | Indacaterol 300 μg | 52 | 437 | A | -- | -- | Inda300 vs. Placebo: 0.77 | -- | Inda300 vs. Placebo: 0.16 |
| Indacaterol 600 μg | 52 | 428 | -- | -- | Inda600 vs. Placebo: 0.69 | -- | Inda600 vs. Placebo: 0.15 | ||
| Formoterol | 52 | 435 | -- | -- | F vs. Placebo: 0.77 | -- | F vs. Placebo: 0.05 | ||
| Placebo | 52 | 432 | -- | -- | -- | -- | |||
| Decramer, 2013 [15] | Tiotropium bromide 18 μg | 26 | 1721 | C | -- | -- | Tio18 vs. Inda150: 0.81 | -- | Tio18 vs. Inda150: 0.02 |
| Indacaterol maleate 150 μg once-daily | 26 | 1723 | -- | -- | -- | -- | |||
| Tiotropium bromide 18 μg | 52 | 1721 | 0.07 | 547 | -- | 0.092 | -- | ||
| Indacaterol maleate 150 μg once-daily | 52 | 1723 | 0.1 | 619 | -- | 0.073 | -- | ||
| Dusser, 2006 [16] | Tiotropium 18 μg once daily | 48 | 500 | C | -- | 248 | -- | -- | -- |
| Placebo | 48 | 510 | -- | 305 | -- | -- | Tio18 vs. Placebo: 0.12 | ||
| Ferguson, 2008 [17] | Fluticasone propionate/salmeterol (FSC) 250/50 | 52 | 394 | C | -- | 343 | -- | -0.012 | -- |
| Salmeterol 50 μg | 52 | 388 | -- | 335 | -- | -0.082 | -- | ||
| van Grunsven, 2003 [18] | Fluticasone propionate (Flixotides) 250 μg bid | 103 | 24 | D | -- | 5 | -- | -0.12 | F250 vs. Placebo: 0.06 |
| Placebo bid | 103 | 24 | -- | 3 | -- | -0.17 | -- | ||
| Vincken, 2002 [19] | Tiotropium 18 μg qd in the morning | 52 | 356 | B | -- | 125 | -- | 0.12 | -- |
| Ipratropium 40 μg qid | 52 | 179 | -- | 82 | -- | -0.03 | -- | ||
| Wouters, 2005 [20] | Salmeterol/fluticasone (3 month run in period of salmeterol 50 μg and fluticasone 500 μg bid) | 52 | 189 | E | -- | 115 | -- | -0.04 | S/F vs. S: 0.05 |
| Salmeterol (3 month run in period of salmeterol 50 μg and fluticasone 500 μg bid) | 52 | 184 | -- | 109 | -- | -0.1 | -- | ||
| Zhou, 2006 [21] | Theophylline | 52 | 57 | C | -- | 26 | -- | 0.0063 | -- |
| Placebo | 52 | 53 | -- | 30 | -- | -0.0533 | -- | ||
| Dransfield, 2013 [22] | Vilanterol 25 μg | 52 | 409 | A | -- | 203 | -- | -0.04 | -- |
| Fluticasone furoate 50 μg + Vilanterol 25 μg | 52 | 408 | -- | 190 | -- | 0 | -- | ||
| Fluticasone furoate 100 μg + Vilanterol 25 μg | 52 | 403 | -- | 161 | -- | 0.02 | -- | ||
| Fluticasone furoate 200 μg + Vilanterol 25 μg | 52 | 402 | -- | 178 | -- | 0.02 | -- | ||
| Vilanterol 25 μg | 52 | 409 | -- | 197 | -- | -0.02 | -- | ||
| Fluticasone furoate 50 μg + Vilanterol 25 μg | 52 | 412 | -- | 198 | -- | 0.02 | -- | ||
| Fluticasone furoate 100 μg + Vilanterol 25 μg | 52 | 403 | -- | 177 | -- | 0.01 | -- | ||
| Fluticasone furoate 200 μg + Vilanterol 25 μg | 52 | 409 | -- | 160 | -- | 0.01 | -- |
Exacerbation Definitions:
A:Symptom deterioration requiring antibiotics, systemic corticosteroids, and/or hospitalization
B:A complex of respiratory events lasting ≥3 days
B+:A complex of respiratory events lasting ≥3 days requiring treatment
C:Worsening of at least two symptoms for at least two days
D:Having two of the following three symptoms: increased cough, wheezing and/or dyspnea; change in sputum color; use of bronchodilator rescue medication
E:If a patient has in ≥2 consecutive days used ≥3 extra inhalations of salbutamol per 24 hours above their reference rescue value
-- = Not Reported
Mean Change in Trough FEV1 and COPD Patients’ Risk for Moderate-to-Severe Exacerbations
The relationship between relative treatment effects on change in FEV1 and moderate-to-severe exacerbations was of moderate strength and was statistically significant (slope: -1.46, p = 0.045; Fig. 3) when defining the exacerbation outcome either as time to first exacerbation, the number of patients with at least one exacerbation, or as annualized exacerbation rates. Figure 3 shows the relationship between the mean difference in trough FEV1 and the relative risk for a moderate-to-severe exacerbation. Table 2 shows the raw trial data contributing to this analysis.
Fig. 3.
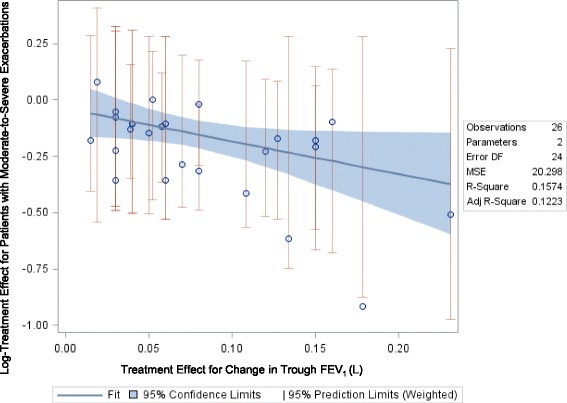
Relationship between Mean Change in Trough FEV1 and Risk for a Moderate-to-Severe Exacerbation
Table 2.
Study Data for Trials Reporting Mean change in FEV1 and Patients Experiencing Moderate-to-Severe COPD Exacerbation
| Author, Year | Treatment | Time point (weeks) | N Randomized | Annual exacerbation rate (M-S) | N with M-S exacerbation | Comparison data for Time to first exacerbation (Hazard ratio) | Mean change in Trough FEV1 (L) | Comparison data for Trough FEV1 (treatment difference) |
|---|---|---|---|---|---|---|---|---|
| Anzueto, 2009 [23] | Fluticasone propionate/salmeterol 250 mcg/50 mcg bid | 52 | 394 | 1.1 | 208 | FP250 + S50 vs. S50: 0.73 | -0.017 | -- |
| Salmeterol 50 mcg bid | 52 | 403 | 1.59 | 234 | -- | -0.097 | -- | |
| Bateman, 2010 [10] | Tiotropium 5 μg orally inhaled once daily | 48 | 670 | 0.93 | 249 | -- | 0.08 | Tio5 vs. Placebo: 0.127 |
| Tiotropium 10 μg orally inhaled once daily | 48 | 667 | 1.02 | 246 | -- | 0.11 | Tio10 vs. Placebo: 0.150 | |
| Placebo | 48 | 653 | 1.91 | 288 | -- | -0.04 | ||
| Dahl, 2010 [14] | Indacaterol 300 μg | 52 | 437 | 0.6 | 133 | -- | -- | Inda300 vs. Placebo: 0.16 |
| Indacaterol 600 μg | 52 | 428 | 0.57 | 116 | -- | -- | Inda600 vs. Placebo: 0.15 | |
| Formoterol | 52 | 435 | 0.56 | 126 | -- | -- | F vs. Placebo: 0.05 | |
| Placebo | 52 | 432 | 0.74 | 145 | -- | -- | ||
| Donohue, 2014 [24] | UMEC/VI 125/25 mcg | 52 | 226 | -- | 30 | UMEC/VI vs. Placebo: 0.6 | 0.18 | UMEC/VI vs. Placebo: 0.231 |
| UMEC 125 mcg | 52 | 227 | -- | 34 | UMEC vs. Placebo: 0.4 | 0.13 | UMEC vs. Placebo: 0.178 | |
| Placebo | 52 | 109 | -- | 26 | -- | -0.05 | -- | |
| Ferguson, 2008 [17] | Fluticasone propionate/salmeterol (FSC) 250/50 | 52 | 394 | 1.06 | 211 | FP/S vs. S: 0.75 | -0.012 | -- |
| Salmeterol 50 μg | 52 | 388 | 1.53 | 230 | -- | -0.082 | -- | |
| Kerwin, 2012 [25] | NVA237 50 μg qd | 52 | 529 | 0.54 | NVA vs. Placebo: 0.66 | 0.112 | NVA vs. Placebo: 0.108 | |
| Tiotropium 18 μg qd | 52 | 268 | -- | -- | NVA vs. Tio: 1.1 | 0.092 | NVA vs. Tio: 0.019 | |
| Placebo | 52 | 269 | 0.8 | -- | -- | -0.097 | ||
| Sharafkhaneh, 2012 [26] | Budesonide/formoterol pMDI 160/4.5 μg x 2 inhalations bid (320/9 μg) | 52 | 407 | 0.867 | 169 | -- | 0.07 | -- |
| Budesonide/formoterol pMDI 80/4.5 μg x 2 inhalations bid (160/9 μg) | 52 | 408 | 0.952 | 173 | -- | 0.07 | -- | |
| Formoterol DPI 4.5 μg x 2 inhalations bid (9 μg) | 52 | 404 | 1.171 | 182 | -- | 0.04 | -- | |
| Tang, 2013 [27] | Tiotropium 5 μg (2 x 2.5 μg/puff) | 48 | 167 | -- | 58 | Tio5 vs. Placebo: 0.54 | -- | Tio5 vs. Placebo: 0.134 |
| Placebo (2 puffs) | 48 | 171 | -- | 83 | -- | -- | -- | |
| Tashkin, 2008 [11] | Tiotropium 18 μg once daily; followed by 40 μg of ipratropium four times daily for 30 days after 4 years of treatment. | 206 | 2987 | -- | 2001 | -- | 0.03 | -- |
| Placebo once daily; followed by 40 μg of ipratropium four times daily for 30 days after 4 years of treatment. | 206 | 3006 | -- | 2049 | -- | -0.05 | -- | |
| Calverley, 2009 [28] | Roflumilast 500 mcg once per day | 52 | 765 | 1.08 | 344 | ROLF500 vs. Placebo (Trial 1): 0.88 | 0.046 | ROLF500 vs. Placebo (Trial 1): 0.039 |
| Placebo | 52 | 758 | 1.27 | 389 | -- | 0.008 | -- | |
| Roflumilast 500 mcg once per day | 52 | 772 | 1.21 | 373 | ROLF500 vs. Placebo (Trial 2): 0.89 | 0.033 | ROLF500 vs. Placebo (Trial 2): 0.058 | |
| Placebo | 52 | 796 | 1.49 | 432 | -- | -0.025 | -- | |
| Dransfield, 2013 [22] | Vilanterol 25 μg | 52 | 409 | 1.05 | -- | FF200 + V vs. V: 0.9 | -0.04 | -- |
| Fluticasone furoate 50 μg + Vilanterol 25 μg | 52 | 408 | 0.92 | -- | FF100 + V vs. V: 0.7 | 0 | -- | |
| Fluticasone furoate 100 μg + Vilanterol 25 μg | 52 | 403 | 0.7 | -- | FF50 + V vs. V: 0.9 | 0.02 | -- | |
| Fluticasone furoate 200 μg + Vilanterol 25 μg | 52 | 402 | 0.9 | -- | -- | 0.02 | -- | |
| Vilanterol 25 μg | 52 | 409 | 1.14 | -- | FF200 + V vs. V: 0.7 | -0.02 | -- | |
| Fluticasone furoate 50 μg + Vilanterol 25 μg | 52 | 412 | 0.92 | -- | FF100 + V vs. V: 0.8 | 0.02 | -- | |
| Fluticasone furoate 100 μg + Vilanterol 25 μg | 52 | 403 | 0.9 | -- | FF50 + V vs. V: 0.9 | 0.01 | -- | |
| Fluticasone furoate 200 μg + Vilanterol 25 μg | 52 | 409 | 0.79 | -- | -- | 0.01 | -- | |
| Jones, 2011 [29] | Aclidinium 200 μg | 52 | 627 | 167 | Aclid200 vs. Placebo (Trial 1): 0.00 | -0.013 | ||
| Placebo | 52 | 216 | 0.46 | 55 | -- | -0.065 | -- | |
| Aclidinium 200 μg | 52 | 600 | 199 | -- | -0.009 | -- | ||
| Placebo | 52 | 204 | 0.8 | 81 | -- | -0.024 | -- |
M-S = moderate-to-severe
-- = Not reported
St. George’s respiratory questionnaire
Mean Change in SGRQ Total Score and COPD Patients’ Risk for Any Exacerbations
The relationship between relative treatment effects for change in SGRQ score and any exacerbation was of moderate strength (slope: 0.112, p = 0.0002; Fig. 4) and was statistically significant when defining the exacerbation outcome as time to first-exacerbation or the number of patients with at least one exacerbation. The relationship was weaker and not statistically significant (slope: 0.014, p = 0.2825) when examining annualized exacerbation rates. Figure 4 shows the relationship between the mean difference in SGRQ score and relative risk for any exacerbation and Table 3 shows the raw trial data contributing to this analysis.
Fig. 4.
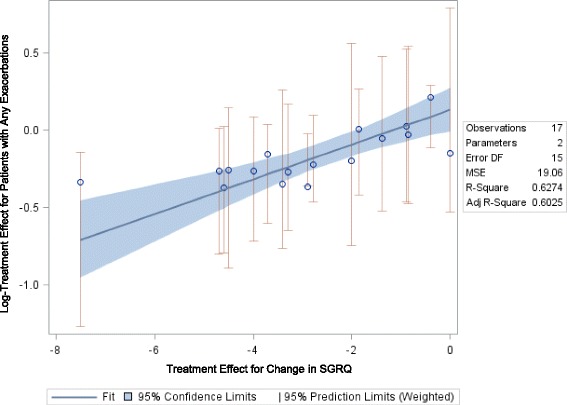
Relationship between Mean Change in SGRQ Total Score and Risk for Any Exacerbation
Table 3.
Study Data for Trials Reporting Mean change in SGRQ Total Score and Patients Experiencing Any COPD Exacerbation
| Author, year | Treatment | Time point (weeks) | N Randomized | Definition of exacerbation | Annual exacerbation rate (any) | N with any exacerbation | Comparison data for Time to first exacerbation (Hazard ratio) | Mean change in SGRQ Total Score | Comparison data for SGRQ (treatment difference) |
|---|---|---|---|---|---|---|---|---|---|
| Bateman, 2010 [10] | Tiotropium 5 μg | 48 | 1989 | B+ | 0.69 | 685 | Tio vs. placebo: 0.93 | -4.7 | Tio5 vs. placebo: -2.9 |
| Placebo | 48 | 2002 | 0.87 | 842 | -1.8 | -- | |||
| Calverley, 2003 [30] | Budesonide/formoterol 320/9 mg (bid) | 52 | 254 | A | 1.38 | -- | B + F vs. B: 0.77 | -- | B + F vs. B: -4.5 |
| Budesonide 400 mg (bid) | 52 | 257 | 1.6 | -- | B + F vs. F: 0.71 | -- | B + F vs. F: -3.4 | ||
| Formoterol 9 mg (bid) | 52 | 255 | 1.85 | -- | B + F vs. Placebo: 0.72 | -- | B + F vs. Placebo: -7.5 | ||
| Placebo | 52 | 256 | 1.8 | -- | -- | -- | -- | ||
| Calverley, 2010 [12] | Beclomethasone/formoterol pMDI 400/24 μg | 48 | 237 | NR | 0.414 | 64 | -- | -3.75 | -- |
| Budesonide/formoterol DPI 800/24 μg | 48 | 242 | 0.423 | 64 | -- | -4.28 | -- | ||
| Formoterol DPI 12 μg | 48 | 239 | 0.431 | 66 | -- | -2.9 | -- | ||
| Casaburi, 2002 [31] | Tiotropium 18 μg | 52 | 550 | B | 0.76 | 198 | -- | -3.2 | -- |
| Placebo | 52 | 371 | 0.95 | 156 | -- | 0.5 | -- | ||
| Chapman, 2011 [13] | Indacaterol, 150 μg | 52 | 420 | A | -- | -- | Ind150 vs. Placebo: 0.82 | -7.5 | -- |
| Indacaterol, 300 μg | 52 | 418 | -- | -- | Ind300 vs. Placebo: 0.86 | -5.5 | -- | ||
| Placebo | 52 | 425 | -- | -- | -- | -5.5 | -- | ||
| Dahl, 2010 [14] | Indacaterol 300 μg | 52 | 437 | A | -- | -- | Inda300 vs. Placebo: 0.77 | -6.5 | Inda300 vs. Placebo: -4.7 |
| Indacaterol 600 μg | 52 | 428 | -- | -- | Inda600 vs. Placebo: 0.69 | -7.2 | Inda600 vs. Placebo: -4.6 | ||
| Formoterol | 52 | 435 | -- | -- | F vs. Placebo: 0.77 | -7 | F vs. Placebo: -4 | ||
| Placebo | 52 | 432 | -- | -- | -- | -1.7 | -- | ||
| Decramer, 2013 [15] | Tiotropium bromide 18 μg | 26 | 1721 | C | -- | -- | -- | -5.2 | -- |
| Indacaterol maleate 150 μg once-daily | 26 | 1723 | -- | -- | -- | -4.5 | -- | ||
| Tiotropium bromide 18 μg | 52 | 1721 | 0.61 | 547 | -- | -4.9 | -- | ||
| Indacaterol maleate 150 μg once-daily | 52 | 1723 | 0.79 | 619 | -- | -4.5 | -- | ||
| Ferguson, 2008 [17] | Fluticasone propionate/salmeterol (FSC) 250/50 | 52 | 394 | C | 4.82 | 343 | -- | -3.49 | FP/S vs. S: -1.86 |
| Salmeterol 50 μg | 52 | 388 | 5.78 | 335 | -- | -1.86 | -- | ||
| Vincken, 2002 [19] | Tiotropium 18 μg qd in the morning | 52 | 356 | B | 0.73 | 125 | -- | -3.74 | Tio18 vs. Ipra40: -3.3 |
| Ipratropium 40 μg qid | 52 | 179 | 0.96 | 82 | -- | -0.44 | -- | ||
| Wedzicha, 2014 [32] | beclomethasone dipropionate/formoterol fumarate (BDP/FOR) 100/6 μg, 2 inhalations BID | 48 | 602 | F | 0.8 | 264 | BDP + F vs. F: 0.8 | -3.55 | BDP/F vs. F: -2.78 |
| Formoterol fumarate (FOR) 12 μg, 1 inhalation BID | 48 | 597 | 1.12 | 294 | -- | -0.77 | -- | ||
| Wouters, 2005 [20] | Salmeterol/fluticasone (3 month run in period of salmeterol 50 μg and fluticasone 500 μg bid) | 52 | 189 | E | -- | 115 | -- | 2.4 | S/F vs. S: -0.89 |
| Salmeterol (3 month run in period of salmeterol 50 μg and fluticasone 500 μg bid) | 52 | 184 | -- | 109 | -- | 3.2 | -- |
Exacerbation Definitions:
A:Symptom deterioration requiring antibiotics, systemic corticosteroids, and/or hospitalization
B:A complex of respiratory events lasting ≥3 days
B+:A complex of respiratory events lasting ≥3 days requiring treatment
C:Worsening of at least two symptoms for at least two days
E:If a patient has in ≥2 consecutive days used ≥3 extra inhalations of salbutamol per 24 hours above their reference rescue value
F:An acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication
-- = Not reported
Mean Change in SGRQ Total Score and COPD Patients’ Risk for Moderate-to-severe Exacerbations
The relationship between relative treatment effects for change in SGRQ score and a moderate-to-severe exacerbation was of moderate strength and was statistically significant when defining the exacerbation outcome as either the number of patients with at least one exacerbation (slope: 0.046, p = 0.0279, Fig. 5) or as an annualized exacerbation rate (slope: 0.056, p = 0.0024, figure not shown). Figure 5 shows the relationship between the mean difference in SGRQ score and the relative risk for a moderate-to-severe exacerbation and Table 4 shows the raw trial data contributing to this analysis.
Fig. 5.
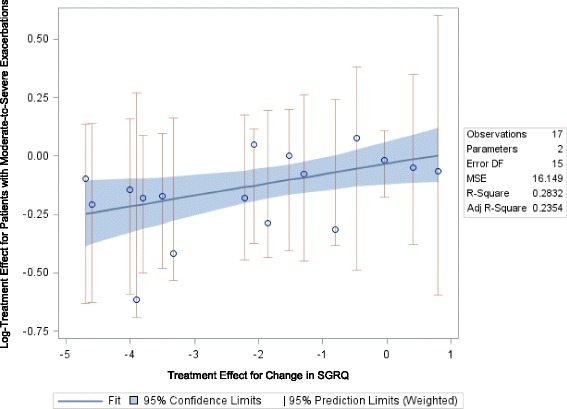
Relationship between Mean Change in SGRQ Total Score and Risk for a Moderate-to-severe Exacerbation
Table 4.
Study Data for Trials Reporting Mean change in SGRQ Total Score and Patients Experiencing Moderate-to-severe COPD Exacerbation
| Author, Year | Treatment | Time point (weeks) | N Randomized | Annual exacerbation rate (M-S) | N with M-S exacerbation | Comparison data for Time to first exacerbation (Hazard ratio) | Mean change in SGRQ Total Score | Comparison data for SGRQ (treatment difference) |
|---|---|---|---|---|---|---|---|---|
| Anzueto, 2009 [23] | Fluticasone propionate/salmeterol 250 mcg/50 μg bid | 52 | 394 | 1.1 | 208 | FP250 + S50 vs. S50: 0.73 | 2.49 | FP250 + S50 vs. S50: -0.81 |
| Salmeterol 50 μg bid | 52 | 403 | 1.59 | 234 | -- | 3.28 | -- | |
| Bateman, 2010 [20] | Tiotropium 5 μg orally inhaled once daily | 48 | 670 | 0.93 | 249 | -- | -5.1 | Tio5 vs. Placebo: -3.5 |
| Tiotropium 10 μg orally inhaled once daily | 48 | 667 | 1.02 | 246 | -- | -5.5 | Tio10 vs. Placebo: -3.8 | |
| Placebo | 48 | 653 | 1.91 | 288 | -- | -1.6 | -- | |
| Dahl, 2010 [14] | Indacaterol 300 μg | 52 | 437 | 0.6 | 133 | -- | -6.5 | Inda300 vs. Placebo: -4.7 |
| Indacaterol 600 μg | 52 | 428 | 0.57 | 116 | -- | -7.2 | Inda600 vs. Placebo: -4.6 | |
| Formoterol | 52 | 435 | 0.56 | 126 | -- | -7 | F vs. Placebo: -4 | |
| Placebo | 52 | 432 | 0.74 | 145 | -- | -1.7 | -- | |
| Ferguson, 2008 [17] | Fluticasone propionate/salmeterol (FSC) 250/50 | 52 | 394 | 1.06 | 211 | FP + S vs. S: 0.75 | -3.49 | FP/S vs. S: -1.86 |
| Salmeterol 50 μg | 52 | 388 | 1.53 | 230 | -- | -1.86 | -- | |
| Hagedorn, 2013 [33] | Salmeterol xinafoate/fluticasone propionate via a single inhaler (SFC) | 52 | 108 | 0.81 | 42 | -- | -1.8 | -- |
| Salmeterol xinafoate/fluticasone propionate via separate inhalers (Sal/FP) | 52 | 106 | 0.98 | 44 | -- | -2.6 | -- | |
| Kerwin, 2012 [25] | NVA237 50 μg qd | 52 | 529 | 0.54 | -- | NVA vs. Placebo: 0.66 | -- | NVA vs. Placebo: -3.32 |
| Tiotropium 18 μg qd | 52 | 268 | -- | NVA vs. Tio: 1.1 | -- | NVA vs. Tio: -0.48 | ||
| Placebo | 52 | 269 | 0.8 | -- | -- | -- | -- | |
| Sharafkhaneh, 2012 [26] | Budesonide/formoterol pMDI 160/4.5 μg x 2 inhalations bid (320/9 μg) | 52 | 407 | 0.867 | 169 | -- | -7.2 | -- |
| Budesonide/formoterol pMDI 80/4.5 μg x 2 inhalations bid (160/9 μg) | 52 | 408 | 0.952 | 173 | -- | -5.5 | -- | |
| Formoterol DPI 4.5 μg x 2 inhalations bid (9 μg) | 52 | 404 | 1.171 | 182 | -- | -5.9 | -- | |
| Tang, 2013 [27] | Tiotropium 5 μg (2 x 2.5 μg/puff) | 48 | 167 | -- | 58 | Tio5 vs. Placebo: 0.54 | -7.1 | Tio5 vs. Placebo: -3.9 |
| Placebo (2 puffs) | 48 | 171 | -- | 83 | -- | -3.3 | -- | |
| Tashkin, 2008 [11] | Tiotropium 18 μg once daily; followed by 40 μg of ipratropium four times daily for 30 days after 4 years of treatment. | 206 | 2987 | -- | 2001 | -- | -1.25 | -- |
| Placebo once daily; followed by 40 μg of ipratropium four times daily for 30 days after 4 years of treatment. | 206 | 3006 | -- | 2049 | -- | -1.21 | -- | |
| Wedzicha, 2008 [34] | Salmeterol 50 μg + fluticasone propionate 500 μg bid | 104 | 658 | -- | 408 | -- | -1.7 | -- |
| Tiotropium bromide 18 μg once daily | 104 | 665 | -- | 392 | -- | 0.37 | S + F vs. Tio18: -2.07 | |
| Jones, 2011 [29] | Aclidinium 200 μg | 52 | 627 | -- | 167 | Aclid200 vs. Placebo (trial 1): 1.00 | -- | Aclid200 vs. Placebo (trial 1): -1.53 |
| Placebo | 52 | 216 | -- | 55 | -- | -- | -- | |
| Aclidinium 200 μg | 52 | 600 | -- | 199 | -- | -- | Aclid200 vs. Placebo (trial 2): -2.21 | |
| Placebo | 52 | 204 | -- | 81 | -- | -- | -- |
M-S = moderate-to-severe
-- = Not reported
Relationship between FEV1 and SGRQ and Hospitalized COPD Exacerbations
There were insufficient data to analyze association with all-cause hospitalizations, and the annualized and patient-level data were combined for the analysis of hospitalizations due to exacerbations. Additionally, relative effects for the number of patients with an exacerbation were combined with annualized exacerbation rates to facilitate analyses.
FEV1 and SGRQ
For both SGRQ score and FEV1, the plots indicate a somewhat weaker relationship with exacerbations resulting in hospitalization (compared to the findings for exacerbations overall). Results were not statistically significant (FEV1 slope: -1.49, p-value = 0.174 [Fig. 6]; SGRQ slope: 0.0518, p = 0.126 [Fig. 7]) for either relationship.
Fig. 6.
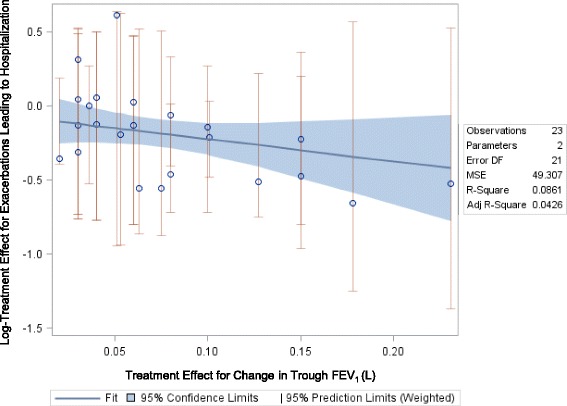
Relationship between Mean Change in FEV1 and Risk for Hospitalization
Fig. 7.
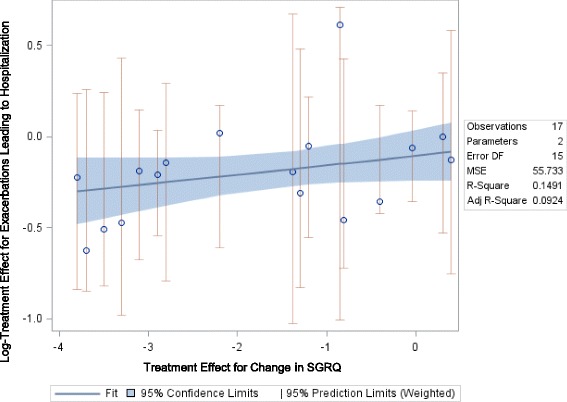
Relationship between Mean Change in SGRQ and Risk for Hospitalization
Impact of including All timepoints >24 weeks
Expanding the data set from outcomes reported at >48 weeks to include outcomes reported at >24 weeks showed similar directionality but weaker results compared with the long-term analysis data of both SGRQ score and FEV1 (data not shown).
Discussion
Our systematic literature review and regression analysis demonstrated that beneficial mean change in either FEV1 or SGRQ total score was associated with a lower risk for exacerbations. Specifically, it showed that in randomized trials of COPD drug treatments lasting ≥48 weeks, there was generally a relationship between relative efficacy in improving FEV1 and SGRQ total score and relative efficacy for lowering exacerbation risk. The majority of analyses showed the same trend towards a relationship between positive changes in FEV1 and SGRQ score and exacerbation risk, even though results did not always reach statistical significance. Of note, there was no relationship shown between mean change in FEV1 and annualized exacerbation rate, despite this relationship being moderate and statistically significant when the risk of experiencing at least one exacerbation in patients was analyzed. The mean change in SGRQ total score was not significantly related to the rate of exacerbations across all severities but had a moderate, statistically significant relationship with the rate of moderate-to-severe exacerbations. The relationship between FEV1 and SGRQ score and hospitalizations was less clear, and further research is needed in this area.
To our knowledge, the literature review and regression analysis we conducted is the first such study to evaluate the inter-relationship that health status and lung function have with exacerbation risk. It provides a more rigorous examination of a relationship between laboratory values and exacerbations than has been done in the past, as, unlike former studies, it correlates relative treatment effects instead of absolute ones, thus lowering the possibility of ecological bias. However, as this analysis used only aggregated patient data from published trials, we cannot assume that any statistical association observed between arm-level variables may be translated to patient-level associations. Therefore, our findings cannot be used to predict any outcome at the patient-level. Additionally, our analysis may be limited by the available data for the surrogate measures given the trials reported FEV1 in several different ways. Since our analysis was limited to trough or prebronchodilator FEV1 data, analysis using other measures of FEV1 could yield different results. Similarly, regarding exacerbation severity, we categorized exacerbations based on the definitions reported by study authors using a standardized approach as defined in our methods section. However, in some cases definitions were not reported so we relied on author-defined groupings of any or moderate-to-severe exacerbations.
Our research may have important implications for regulatory assessment of drugs intended to help reduce the risk of exacerbations in COPD and, in particular, the evidence considered in such deliberations. Currently, to gain marketing approval for this indication, such treatments have to be tested in long-term, parallel trials, which represent a logistic and economic burden on the sponsoring organization. Because of this, few trials of COPD drugs are powered to identify a significant difference in the reduced risk of exacerbations. It is for this reason that to date very few drugs have been approved for reducing exacerbations on the basis of prospective 1–2 year parallel trials, usually in patients with history of acute exacerbations in the prior year. Our study suggests changes in FEV1 and SGRQ might serve as reliable surrogate markers of patients’ likelihood of experiencing an exacerbation. If so, these measures could allow future trials to be shorter and more manageable while still offering key insights into treatments’ longer-term efficacy. Since exacerbations can be costly to health plans, payers should consider the effect of medications on these surrogate markers, even when long-term RCTs cannot be carried out. Also, confirmation of our results would broaden the application of data already available from published shorter-term studies. This is especially important since the trials used to inform regulatory approval were powered on each specific drug’s expected effect on the acute exacerbation rate and all but one [11] were small and had very selective entry criteria. This contrasts with the trials contributing data for our review and analysis, since these were broader and more inclusive (e.g. with regards to disease duration and reversibility, comorbidities, interventions, and concomitant therapies) and collectively more representative of the general COPD population seen in everyday clinical practice. Therefore, these collated data sources potentially allow more generalizable conclusions to be drawn regarding whether or how standard short-term endpoints assessed in trials relate to effects on exacerbations.
Conclusions
In conclusion, this study demonstrates a significant association between improvements in FEV1 and SGRQ total score and lower risk for COPD exacerbations. We believe that the results of our study offer providers and payers a more informed picture of the inter-relationship between exacerbations and both FEV1 and SGRQ score, which will aid clinical and formulary decisions while stimulating research questions for future prospective studies.
Acknowledgements
The authors would like to acknowledge Abhishek Kavati for his contributions to critical review of the manuscript.
Funding
Funding for this manuscript was provided by Novartis.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in one second
- HR
hazard ratio
- HRU
health resource use
- OCS
oral corticosteroids
- PICOS
Patient, Interventions, Comparisons, Outcomes, Study Design
- RCT
randomized controlled trial
- SGRQ
St. George’s Respiratory Questionnaire
- SLR
systematic literature review
Footnotes
Competing interests
Amber L. Martin, Kyle Fahrbach, Teresa K. Wilcox, and Sarah M. Cadarette are employees of Evidera which received funding from Novartis Pharmaceuticals Corporation to conduct the study on which this manuscript is based.
Jessica Marvel is an employee and stockholder of Novartis Pharmaceuticals Corporation.
James F. Donohue is a Member or Chair of the following Data and Safety Monitoring Boards: Teva, Pearl, AZ, Otsuka, Novartis, Insmed, National Institutes of Health and a paid consultant for the following companies; Novartis, GSK, BI, AstraZeneca, Sunovion, Biomark.
Author contributions
ALM and SMC consulted on the study design, carried out the review, maintained the dataset, and drafted the manuscript. JM formed the research questions and contributed to study design. KF performed the statistical analysis and contributed to data refinement and study design. TKW contributed to study design and helped refine manuscript focus. JFD participated in study design and provided clinical insight. All authors contributed to interpreting the data and read and approved the final manuscript.
Contributor Information
Amber L. Martin, Phone: (781) 960-0231, Email: amber.martin@evidera.com
Jessica Marvel, Email: jessica.marvel@novartis.com.
Kyle Fahrbach, Email: kyle.fahrbach@evidera.com.
Sarah M. Cadarette, Email: sm.cadarette@gmail.com
Teresa K. Wilcox, Email: terry.Wilcox@evidera.com
James F. Donohue, Email: james_donohue@med.unc.edu
References
- 1.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 2.Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: prevalence, incidence and survival. Respir Med. 2011;105:1872–1884. doi: 10.1016/j.rmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpin DM, Tashkin DP. Defining disease modification in chronic obstructive pulmonary disease. COPD. 2009;6:211–225. doi: 10.1080/15412550902918402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.How Is COPD Treated? [http://www.nhlbi.nih.gov/health/health-topics/topics/copd/treatment]. Accessed 10 Nov 2015.
- 6.Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7:214–228. doi: 10.3109/15412555.2010.481697. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161. doi: 10.1186/1465-9921-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: A systematic review. Respir Res. 2011;12:40. doi: 10.1186/1465-9921-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KR, Ringland C, Stokes BJ, Anthony DM, Freemantle N, Irs A, Hill SR, Ward RL. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol. 2006;7:741–746. doi: 10.1016/S1470-2045(06)70800-2. [DOI] [PubMed] [Google Scholar]
- 10.Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, Pavia D, Zhong NS. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104:1460–72. [DOI] [PubMed]
- 11.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54.
- 12.Calverley PM, Kuna P, Monso E, Costantini M, Petruzzelli S, Sergio F, Varoli G, Papi A, Brusasco V. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104:1858–68. [DOI] [PubMed]
- 13.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long-term safety and efficacy of indacaterol, a long-acting (beta)2-agonist, in subjects with COPD: A randomized, placebo-controlled study. Chest. 2011;140:68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- 14.Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Bleasdale P, Owen R, Higgins M, Kramer B. Efficacy of a new once-daily long-acting inhaled (beta)2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–9. [DOI] [PubMed]
- 15.Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, Cameron R, Shoaib M, Lawrence D, Young D, McBryan D. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): A randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524–33. [DOI] [PubMed]
- 16.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–555. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med. 2008;102:1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 18.van Grunsven P, Schermer T, Akkermans R, Albers M, van den Boom G, van Schayck O, van Herwaarden C, van Weel C. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med. 2003;97:1303–12. [DOI] [PubMed]
- 19.Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, Cornelissen PJ, Dutch/Belgian Tiotropium Study G. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–16. [DOI] [PubMed]
- 20.Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AF, Pasma HR, Hensing CA, Creutzberg EC, Group CS. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60:480–7. [DOI] [PMC free article] [PubMed]
- 21.Zhou Y, Wang X, Zeng X, Qiu R, Xie J, Liu S, Zheng J, Zhong N, Ran P. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology. 2006;11:603–10. [DOI] [PubMed]
- 22.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Sanford L, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–23. [DOI] [PubMed]
- 23.Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, Knobil K, O'Dell D, Kalberg C, Crater G. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6:320–9. [DOI] [PubMed]
- 24.Donohue JF, Niewoehner D, Brooks J, O’Dell D, Church A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: Results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15:78. doi: 10.1186/1465-9921-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerwin E, Hebert J, Gallagher N, Martin C, Overend T, Alagappan VKT, Lu Y, Banerji D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: The GLOW2 study. Eur Respir J. 2012;40:1106–14. [DOI] [PMC free article] [PubMed]
- 26.Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106:257–268. doi: 10.1016/j.rmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Massey D, Zhong NS. Evaluation of the efficacy and safety of tiotropium bromide (5 microg) inhaled via Respimat in Chinese patients with chronic obstructive pulmonary disease. Chin Med J (Engl) 2013;126:3603–3607. [PubMed] [Google Scholar]
- 28.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Rennard SI, Agusti A, Chanez P, Magnussen H, Fabbri L, Donohue JF, Bateman ED, Gross NJ, Lamarca R, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res. 2011;12:55. [DOI] [PMC free article] [PubMed]
- 30.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 31.Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T., Jr A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 32.Wedzicha JA, Singh D, Vestbo J, Paggiaro PL, Jones PW, Bonnet-Gonod F, Cohuet G, Corradi M, Vezzoli S, Petruzzelli S, Agusti A. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108:1153–62. [DOI] [PubMed]
- 33.Hagedorn C, Kassner F, Banik N, Ntampakas P, Fielder K. Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Respir Med. 2013;107:542–549. doi: 10.1016/j.rmed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, Investigators I. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. [DOI] [PubMed]


