Abstract
BACKGROUND: Despite the best standard treatment, optimal cytoreductive surgery (CRS) and platinum/taxane-based chemotherapy, prognosis of advanced epithelial ovarian carcinoma (EOC) remains poor. Recently, CRS plus hyperthermic intraperitoneal chemotherapy (HIPEC) has been developed to treat peritoneal carcinomatosis (PC). This study was to evaluate the efficacy and safety of CRS+HIPEC to treat PC from advanced/recurrent EOC. METHODS: Forty-six PC patients from advanced EOC (group A) or recurrent EOC (group B) were treated by 50 CRS+HIPEC procedures. The primary endpoints were progression-free survival (PFS) and overall survival (OS); the secondary endpoints were safety profiles. RESULTS: The median OS was 74.0 months [95% confidence interval (CI) 8.5-139.5] for group A versus 57.5 months (95% CI 29.8-85.2) for group B (P = .68). The median PFS was not reached for group A versus 8.5 months (95% CI 0-17.5) for group B (P = .034). Better median OS correlated with peritoneal cancer index (PCI) < 20 (76.6 months for PCI ≤ 20 group vs 38.5 months for PCI > 20 group, P = .01), complete cyroreduction (residual disease ≤ 2.5 mm) [79.5 months for completeness of cytoreduction (CC) score 0-1 vs 24.3 months for CC 2-3, P = .00], and sensitivity to platinum (65.3 months for platinum-sensitive group vs 20.0 for platinum-resistant group, P = .05). Serious adverse events occurred in five patients (10.0%). Multivariate analysis identified CC score as the only independent factor for better survival. CONCLUSION: For advanced/recurrent EOC, CRS+HIPEC could improve OS with acceptable safety.
Introduction
Epithelial ovarian cancer (EOC) causes more deaths than any other malignancy affecting the female reproductive system. In up to 75% of the patients, the disease is diagnosed at an advanced stage, with peritoneal involvement or distant metastasis [International Federation of Gynecology and Obstetrics (FIGO) stage III to IV], and for such patients. the overall 5-year survival rate is less than 20% [1].
The standard treatment of advanced EOC is based on optimal cytoreductive surgery (CRS) to remove all the visible tumors if possible, followed commonly by intravenous platinum/taxane-based chemotherapy [2], [3]. However, even after the best standard treatment, optimal CRS and platinum/taxane-based chemotherapy, 60% to 70% of advanced EOC patients experience a relapse, mostly in the form of peritoneal carcinomatosis (PC) [4]. There has been no curative treatment for EOC PC. Repeated conventional chemotherapy alone or in combination with molecular targeting agents could improve survival and quality of life at the cost of considerable treatment-related adverse events [5], [6].
Over the past three decades, aggressive CRS plus hyperthermic intraperitoneal chemotherapy (HIPEC) has been developed as a comprehensive treatment package integrating multivisceral resections to remove the macroscopic tumor and HIPEC to eradicate the microscopic residual disease. In China, we have conducted a series of preclinical and clinical studies on the feasibility, efficacy, and safety of this multidisciplinary treatment approach in animal models [7] and in clinical setting [8], [9]. The benefits of this treatment package have been demonstrated in PC patients from gastric cancer [9], [10], colorectal cancer [11], pseudomyxoma peritonei, and peritoneal mesothelioma. As a result, a regional PC center has been set up, and a prospective database has been established. In this report, we summarized our experience in 50 CRS+HIPEC procedures for the treatment of 46 EOC patients.
Patients and Methods
Patient Selection
This is a retrospective cohort study on prospectively established database covering 46 consecutive Chinese patients with advanced EOC (FIGO stage IIIc/IV, n = 16, group A) or recurrent EOC with PC (n = 30, group B), aged from 22 to 75 years old (median 57.5 years), treated by 50 CRS+HIPEC procedures from March 2005 to September 2014. Among the 30 patients in the recurrent group were 25 patients at the first recurrence, 3 patients at the second recurrence, and 2 patients at the third recurrence. The inclusion criteria were 1) age 20 to 75 years; 2) Karnofsky performance status > 50; 3) peripheral blood white blood cells count ≥ 3500/mm3 and platelet count ≥ 80,000/mm3; 4) acceptable liver function, with bilirubin ≤ 2 × the upper limit of normal (ULN) and aspartic aminotransferase and alanine aminotransferase ≤ 2 × ULN; 5) acceptable renal function, with serum creatinine ≤ 1.5 mg/dl; and 6) cardiovascular pulmonary and other major organ functions could stand major operation. Major exclusion criteria were 1) age < 20 years or > 75 years; 2) any lung metastasis, liver metastasis, or prominent retroperitoneal lymph node metastasis during preoperative assessment; 3) serum bilirubin level > 3 × ULN; 4) liver enzymes > 3 × ULN; and 5) serum creatinine level > 1.5 mg/dl. Informed consent was obtained from all patients, and the study was approved by the institutional review board and the ethics committee.
CRS plus HIPEC Procedure
All CRS+HIPEC procedures were conducted by a designated team focusing on PC treatment. In brief, the abdominal exploration was performed through a midline xiphoid-pubic incision after general anesthesia, and peritoneal cancer index (PCI) was evaluated and recorded according to Sugarbaker's criteria [12]. Then maximal CRS was performed. The extent of CRS was determined by Sugarbaker's criteria [13] on the completeness of cytoreduction (CC). A score of CC 0 indicates no residual peritoneal disease after CRS; CC 1, less than 2.5 mm of residual disease; CC 2, residual tumor between 2.5 mm and 2.5 cm; and CC 3, more than 2.5 cm of residual tumor or the presence of a sheet of unresectable tumor nodules.
After CRS, open HIPEC was implemented with cisplatin 100 mg/m2 and mitomycin C (MMC) 20 mg/m2 in 35 cases, and paclitaxel 100 mg/m2 and lobaplatin 50 mg/m2 in 15 cases with increased risk of renal dysfunctions, each dissolved in 6 l of heated saline at temperature of 43.0 ± 0.5°C. The total HIPEC time was 60 minutes, after which gastrointestinal anastomoses or stomata were made. After operation, the patient was delivered to the intensive care unit for recovery. When the condition stabilized, the patients were transferred to the surgical oncology ward.
Postoperative Chemotherapy
Postoperative adjuvant chemotherapy was delivered within 2 to 3 weeks after CRS+HIPEC, including platinum/taxane-based systemic chemotherapy (SC) and perioperative intraperitoneal chemotherapy (PIC) through the intraperitoneal chemotherapy port once every 3 to 4 weeks mainly using cisplatin 100 mg/m2 and paclitaxel 100 mg/m2. For platinum-resistant disease, paclitaxel 100 mg/m2 and doxorubicin 35 mg/m2 were used. The median cycles of postoperative adjuvant chemotherapy (SC and/or PIC) were 6 (range, 0-26), and the median cycles of postoperative PIC were 3 (range, 0-9).
Study Endpoints and Definition
The primary endpoints of this study were progression-free survival (PFS) calculated from the date of CRS+HIPEC to the date of disease progression and the overall survival (OS) defined as the time interval from the first treatment to death due to the disease. Patients with recurrent EOC were further divided into platinum-sensitive or -resistant subgroups according to the established criteria [14]. The secondary endpoints were the perioperative serious adverse events (SAEs), defined as complications directly attributable to the treatment within 30 days of CRS+HIPEC, based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [15].
Follow-Up
All patients were regularly followed up once every 3 months for the first 2 years and every 6 months thereafter for detailed information on disease status. Patients alive at the time of analysis were censored at the last follow-up. The follow-up package included physical examination, serum tumor markers (CA125, CEA, and CA19-9), and imaging examination, with the most recent follow-up on February 28, 2015. No patient was lost to follow-up.
Statistical Analysis
The patient information was systematically integrated into a prospectively established database. Data analysis was conducted using the Statistical Package for Social Sciences version 17.0 (SPSS, Inc., Chicago, IL). The numerical data were directly recorded, and the category data were recorded into different categories. The Kaplan-Meier method was used to compare the survival with log-rank test, and multivariate Cox regression analysis was performed to delineate the independent predictors. Subgroup OS comparisons were performed by HIPEC timing (primary versus recurrent), PCI [low PCI (LPCI) versus high PCI (HPCI)] (PCI ≤ 20 defined as LPCI, and PCI > 20 defined as HPCI), and CC score (CC 0-1 vs CC 2-3). A two-sided P < .05 was considered as statistically significant.
Results
Major Clinical and Pathological Characteristics of the Patients
A total of 46 patients with stage IIIc/IV EOC (n = 16) and recurrent EOC (n = 30) were treated with 50 CRS+HIPEC procedures, including 4 patients each receiving 2 CRS+HIPEC procedures due to tumor recurrence. The detailed clinical and pathological characteristics were listed in Table 1, and the major intraoperative parameters and surgical procedures were listed in Table 2.
Table 1.
Major Clinicopathologic Characteristics of the 46 Patients⁎
| Items | Value, n (%) |
|---|---|
| Median age (years) | 58 |
| < 60 | 25 (54.3) |
| ≥ 60 | 21 (45.7) |
| Median Karnofsky performance score (range) | 70 (50-90) |
| HIPEC timing | |
| Primary (IIIc/IV) | 16 (34.8) |
| Recurrent | 30 (65.2) |
| Histopathology | |
| Serous adenocarcinoma | 34 (73.9) |
| Mucinous adenocarcinoma | 11 (23.9) |
| Endometrioid carcinoma | 1 (2.2) |
| Histopathology differentiation | |
| Well differentiated | 11 (23.9) |
| Intermediately/poorly differentiated | 35 (76.1) |
| Comorbidity | |
| Yes | 18 (39.1) |
| No | 28 (60.9) |
| Neoadjuvant chemotherapy for primary EOC PC | |
| Yes | 5 (31.2) |
| No | 11 (68.8) |
| SAE⁎ | |
| Yes | 5 (10.0) |
| No | 45 (90.0) |
| Median postoperative chemotherapy cycles† | 6 |
| < 6 | 17 (37.0) |
| ≥ 6 | 29 (63.0) |
| Median postoperative intraperitoneal chemotherapy cycles† | 3 |
| Median postoperative hospital stay (days) (range)⁎ | 11 (4-43) |
| Median ICU stay (hours) (range)⁎ | 15 (0-89) |
| Median gastric tube removal time (days) (range)⁎ | 5 (2-13) |
| Platinum sensitivity for recurrent patients (n = 30) | |
| Sensitive | 16 (53.3) |
| Nonsensitive | 14 (46.7) |
A total of 50 CRS+HIPEC procedures were performed for 46 patients, including 4 patients each having 2 procedures.
According to the first CRS+HIPEC procedure.
Table 2.
Intraoperative Parameters of the 46 EOC PC Patients
| Items | Value, n (%) |
|---|---|
| PCI | |
| Median PCI (range) | 20 (7-39) |
| ≤ 20 | 24 (52.2) |
| > 20 | 22 (47.8) |
| CC scores | |
| 0-1 | 28 (60.9) |
| 2-3 | 18 (39.1) |
| Surgical procedures; organ resection | |
| Resection of jejunum | 1 (0.2) |
| Resection of ileum | 7 (15.0) |
| Right colectomy | 17 (26.1) |
| Transverse colectomy | 4 (8.7) |
| Descending colectomy | 3 (6.5) |
| Sigmoidectomy | 6 (13.0) |
| Rectectomy | 9 (19.6) |
| Splenectomy | 4 (8.7) |
| Resection of ovarian/fallopian tube | 16 (34.8) |
| Hysterectomy | 7 (15.0) |
| Partial hepatectomy | 1 (0.2) |
| Cholecystectomy | 4 (8.7) |
| Appendectomy | 7 (15.0) |
| Partial gastrectomy | 1 (0.2) |
| Number of organ resected | |
| 0 resection | 15 (32.6) |
| 1-3 resections | 24 (52.2) |
| 4-8 resections | 7 (15.0) |
| Peritonectomy | |
| Greater/lesser/omentum | 46 (100.0) |
| Left diaphragmatic copula | 5 (10.9) |
| Right diaphragmatic copula | 23 (50.0) |
| Right colon gutter | 22 (47.8) |
| Left colon gutter | 17 (37.0) |
| Liver round ligament/sickle ligament | 46 (100.0) |
| Douglas pouch | 23 (50.0) |
| Anterior wall peritoneum | 12 (26.1) |
| Pelvic peritoneum | 34 (73.9) |
| Mesenteric fulguration | 33 (71.7) |
| Peritoneal regions resected | |
| 1-3 regions | 17 (37.0) |
| 4-6 regions | 15 (32.6) |
| 7-10 regions | 14 (30.4) |
| Number of anastomosis | |
| None or stoma only | 30 (65.2) |
| = 1 | 12 (26.1) |
| > 1 | 4 (8.7) |
| Fluid output volume at surgery (median, range) (ml) | |
| Blood loss | 600 (100-2000) |
| Urine output | 1000 (100-3350) |
| Ascites | 1000 (0-4500) |
| ≤ 1000 | 25 (54.3) |
| > 1000 | 21 (45.7) |
| Fluid intake volume at surgery (median, range) | |
| Plasma (ml) | 450 (0-1550) |
| Red blood cells (U)⁎ | 3 (0-16) |
| Cryoprecipitation (U)† | 4 (0-14) |
| Other fluids (ml) | 4000 (2000-8000) |
| CRS+HIPEC duration (median, range) (h) | 7.5 (4.5-13.5) |
1 U = 200 ml.
1 U = 25 ml.
Survival of the Total Population
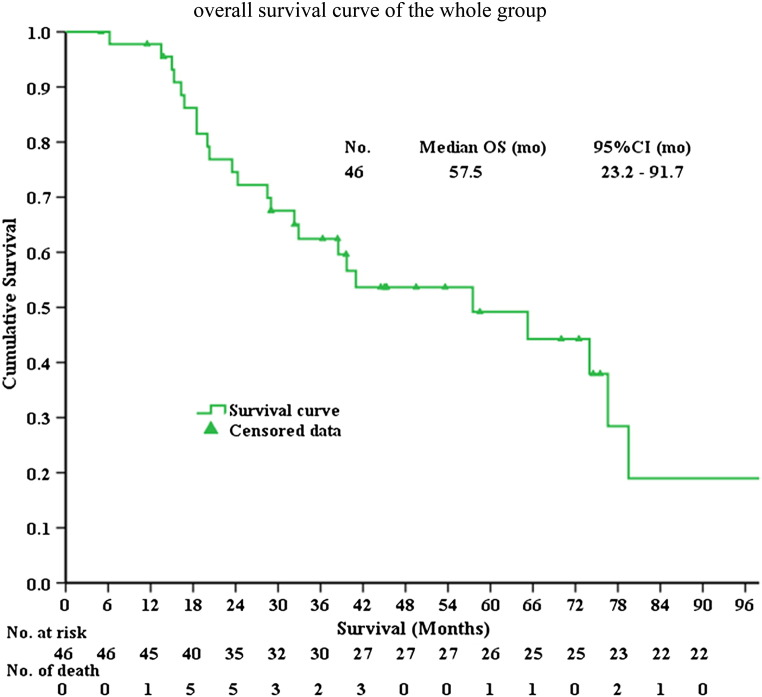
By February 28, 2015, the median follow-up time was 45.8 months (range, 5.0-213.3), and 24 patients (52.1%) were deceased and 22 patients (47.8%) were still alive. The median OS was 57.5 months [95% confidence interval (CI) 23.2-91.7], and the 1-, 3-, 5-year survival rates were 97.8%, 65.2% and 56.5%, respectively (Figure 1).
Figure 1.
The Kaplan-Meier overall survival curve of the whole patients in this study. mo, months.
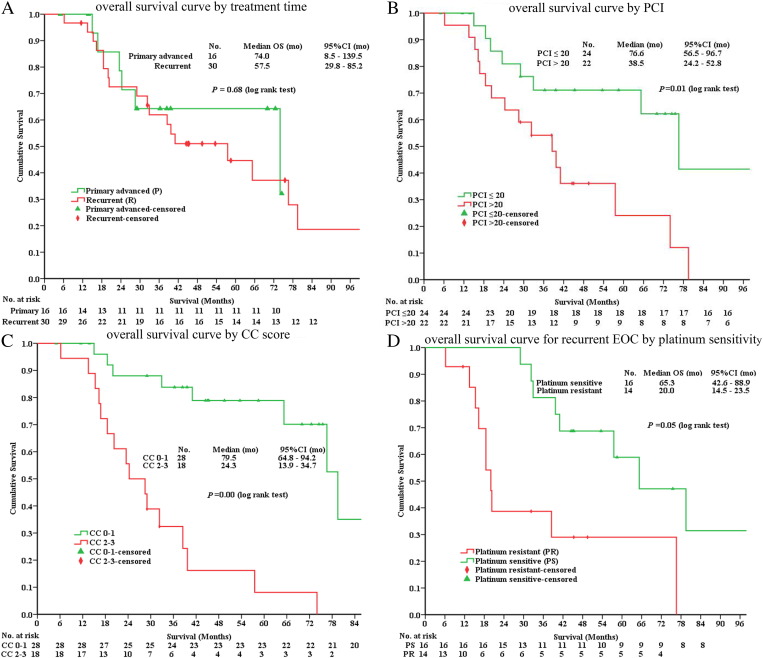
Subgroup analysis was conducted. For group A of 16 patients with advanced EOC, the median PFS was not reached; for group B of 30 patients with recurrent EOC, the median PFS was 8.5 months (95% CI 0-17.5) (P = .034, log-rank test).
The median OS was 74.0 months (95% CI 8.5-139.5) in group A versus 57.5 months (95% CI 29.8-85.2) in group B (P = .68, log-rank test) (Figure 2A). The median OS for patients with LPCI (n = 24) versus HPCI (n = 22) was 76.6 months (95% CI 56.5-96.7) versus 38.5 months (95% CI 24.2-52.8) (P = .01, log-rank test) (Figure 2B). The median OS for patients with CC 0 to 1 versus CC 2 to 3 was 79.5 months (95% CI, 64.8-94.2) versus 24.3 months (95% CI 13.9-34.7) (P = .00, log-rank test) (Figure 2C). For recurrent patients, the median OS was 65.3 months (95% CI 42.6-88.9) for platinum-sensitive patients versus 20.0 months (95% CI 14.5-23.5) for platinum-resistant patients (P = .05, log-rank test) (Figure 2D).
Figure 2.
The Kaplan-Meier overall survival curve by treatment time from first treatment (A), by PCI (B) and by CC score (C) for both primary and recurrent EOC patients, and by platinum sensitivity (D) for recurrent EOC patients.
Special Analysis on Patients with OS over 60 Months
By the last follow-up, there were 9 patients (19.6%) with OS over 60 months (Table 3). Four patients had no evidence of tumor recurrence, with OS of 70.0, 72.5, 75.5, and 122.7 months, respectively. One patient was alive with tumor recurrence, living over 74.5 months by the time of this analysis. The other 4 patients died of tumor recurrence, with a median OS of 75.5 months (range, 65.3-204.0).
Table 3.
Major Clinicopathological Features of 9 Patients with OS > 60 Months
| No. | Age (Years) | P/R | Histopathology | CRS⁎ | HIPEC | PCI | CC | OS/PFS (Months) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | P | Serous-mucinous cystadenocarcinoma, moderately differentiated | Resection of pelvic peritoneum, anterior wall peritoneum, hysterectomy and resection of bilateral ovarian/fallopian tube, mesenteric fulguration | DDP 100 mg/m2, MMC 20 mg/m2 | 15 | 0 | 70.0/70.0, DFS | Comorbidity: poliomyelitis sequel period, polycystic liver, and polycystic kidney |
| 2 | 62 | P | Serous papillary adenocarcinoma, moderately differentiated | Right diaphragmatic copula peritoneum, left/right paracolic sulci peritoneum, pelvic peritoneum, liver round ligament resection, hysterectomy and resection of ovarian/fallopian tube | DDP 100 mg/m2, MMC 20 mg/m2 | 19 | 1 | 74.5/16.5, SWT | Pleurectomy and hyperthermic intrathoracic chemotherapy were conducted 2 years after CRS+HIPEC due to pleural metastasis |
| 3 | 32 | P | Mucinous cystadenocarcinoma, well differentiated | Resection of bilateral ovarian/fallopian tube, pelvic peritoneum, and liver round ligament | DDP 100 mg/m2, MMC 20 mg/m2 | 15 | 0 | 72.5/72.5, DFS | |
| 4 | 49 | R | Serous papillary adenocarcinoma, moderately-poorly differentiated | Sigmoidectomy, rectectomy, resection of pelvic peritoneum | DDP 100 mg/m2, MMC 20 mg/m2 | 11 | 1 | 75.5/72.5, DFS | |
| 5 | 57 | R | Borderline serous cystadenocarcinoma, moderately differentiated | Resection of right paracolic sulci peritoneum, pelvic peritoneum, anterior wall peritoneum | DDP 100 mg/m2, MMC 20 mg/m2 | 16 | 0 | 76.6/18.0, D | |
| 6 | 50 | R | Serous papillary adenocarcinoma, moderately-poorly differentiated | 1st: ileectomy, resection of anterior wall peritoneum 2nd: resection of pelvic peritoneum, anterior wall peritoneum and bilateral fossa iliaca peritoneum, mesenteric fulguration |
1st: DDP 100 mg/m2, MMC 20 mg/m2, 2nd: Lobaplatin 50 mg/m2, TAX 100 mg/m2 | 1st: 20 2nd: 22 | 1st: 1 2nd: 2 |
65.3/8.5, D | Intraperitoneal extensive bowel adhesions were found at the second operation |
| 7 | 67 | R | Endometrioid carcinoma, poorly differentiated | Resection of right paracolic sulci peritoneum, right diaphragmatic copula peritoneum, pelvic peritoneum, anterior wall peritoneum | DDP 100 mg/m2, MMC 20 mg/m2 | 16 | 1 | 204.0/54.0,D | A total of 4 operations and 2 TACEs were implemented from first treatment to death |
| 8 | 51 | R | Borderline mucinous cystadenocarcinoma, moderately differentiated | Resection of left/right diaphragmatic copula peritoneum, left/right paracolic sulci, pelvic peritoneum and bilateral fossa iliaca peritoneum | DDP 100 mg/m2, MMC 20 mg/m2 | 22 | 1 | 79.5/16.0, D | SAE: small intestinal leakage, abdominal infection (Proteus mirabilis infection) |
| 9 | 60 | R | Mucinous cystadenocarcinoma, moderately differentiated | 1st: greater/lesser omentectomy, appendicectomy 2nd: resection of ileocecum, left/right diaphragmatic copula peritoneum and ascending colon |
1st: DDP 100 mg/m2, MMC 20 mg/m2, 2nd: Lobaplatin 50 mg/m2, TAX 100 mg/m2 | 1st: 5 2nd: 8 | 1st: 0 2nd: 0 |
122.7/58.0,DFS | Comorbidity: hypertension; type 2 diabetes mellitus |
P, primary; R, recurrent; DDP, cis-platinum; TAX, paclitaxel; TACE, transcatheter arterial chemoembolization; DFS, disease-free survival; SWT, survival with tumor; D, died.
PFS was calculated from the first CRS+HIPEC in this table.
Greater omentectomy and lesser omentectomy were performed on all the nine patients.
Detailed Description of Four Patients with Repeated CRS+HIPEC
There were four patients that each had two CRS+HIPEC treatments in this study including one primary advanced and three recurrent EOC patients. Two patients survived more than 5 years, and one patient was still alive without tumor with OS of 122.7 months. She reached CC 0 resection in two CRS+HIPEC procedure. The other 2 patients had OS of 38.4 and 38.5 months, respectively. The detailed information was listed in Table 4.
Table 4.
Major Clinicopathological Features on 4 Patients Undergoing Twice CRS+HIPEC Procedures
| No. | Age (Years) | P/R | Histopathology | CRS | HIPEC | PCI | CC | OS/PFS (Months) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | R | Serous papillary adenocarcinoma, moderately-poorly differentiated | 1st: ileectomy, resection of anterior wall peritoneum; 2nd: resection of pelvic peritoneum, anterior wall peritoneum and bilateral fossa iliaca peritoneum, mesenteric fulguration |
1st: DDP 100 mg/m2, MMC 20 mg/m2, 2nd: lobaplatin 50 mg/m2, TAX 100 mg/m2 |
1st: 20, 2nd: 22 | 1st: 1, 2nd: 2 | OS: 65.3, PFS 1: 8.5, PFS 2: 6.0, D | Intraperitoneal extensive bowel adhesions were found at the second operation |
| 2 | 60 | R | Mucinous cystadenocarcinoma, moderately differentiated | 1st: greater/lesser omentectomy, appendicectomy 2nd: resection of ileocecum, left/right diaphragmatic copula peritoneum and ascending colon |
1st: DDP 100 mg/m2, MMC 20 mg/m2, 2nd: lobaplatin 50 mg/m2, TAX 100 mg/m2 |
1st: 5, 2nd: 8 | 1st: 0, 2nd: 0 | OS: 122.7, PFS 1: 58.0, PFS 2: 19.6, DFS | Comorbidity: hypertension; type 2 diabetes mellitus |
| 3 | 61 | P | Serous papillary adenocarcinoma, moderately-poorly differentiated | 1st: right hemicolectomy, splenectomy, cholecystectomy, hysterectomy and resection of bilateral ovarian/fallopian tube. Greater/lesser omentectomy, resection of right diaphragmatic copula peritoneum, right/left paracolic sulci peritoneum, pelvic peritoneum, mesenteric fulguration 2nd: partial gastrectomy, resection of anterior wall peritoneum, bilateral fossa iliaca peritoneum, mesenteric fulguration |
1st: lobaplatin 50 mg/m2, TAX 100 mg/m2 2nd: lobaplatin 50 mg/m2, TAX 100 mg/m2 |
1st: 22, 2nd: 23 | 1st: 1, 2nd: 2 | OS: 38.4, PFS 1: 14.0, PFS 2: 5.1, SWT | |
| 4 | 69 | R | Serous adenocarcinoma, poorly differentiated | 1st: transverse colectomy, resection of right/left diaphragmatic copula peritoneum, right/left paracolic sulci peritoneum, pelvic peritoneum, mesenteric fulguration 2nd: proctosigmoidectomy, resection of bilateral fossa iliaca peritoneum, mesenteric fulguration |
1st: DDP 100 mg/m2, MMC 20 mg/m2, 2nd: lobaplatin 50 mg/m2, TAX 100 mg/m2 |
1st: 15, 2nd: 23 | 1st: 1, 2nd: 2 | OS: 38.5, PFS 1:10.0, PFS 2:8.0, D | Grade 4 myelosuppression during postoperative chemotherapy; comorbidity: hypertension; coronary heart disease |
PFS 1, from the first CRS+HIPEC to disease progression; PFS 2, from the second CRS+HIPEC to disease progression.
SAE Analysis
There was no 30-day perioperative death in this study. SAEs occurred in five patients, including small intestinal leakage in one patient, ascending colon leakage in one patient, protracted postoperative intestinal obstruction in one patient, wound infection with Escherichia coli sepsis in one patient, and pseudomembranous colitis in one patient. The detailed clinical course of these five patients was listed in Table 5.
Table 5.
Detailed Data on 5 Patients with SAE⁎
| No. | Age (Years) | P/R | Histopathology | CRS | HIPEC | PCI | CC | SAE | Treatment | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | R | Serous papillary adenocarcinoma, moderately differentiated | Greater omentectomy, mesenteric fulguration | DDP 100 mg/m2, MMC 20 mg/m2 | 39 | 3 | Ascending colon leakage (POD 8), abdominal MRSA infection (POD 12) | CT | 32.3, D |
| 2 | 51 | R | Borderline mucinous cystadenocarcinoma, moderately differentiated | Resection of left/right diaphragmatic copula peritoneum, left/right paracolic sulci, pelvic peritoneum and bilateral fossa iliaca peritoneum | DDP 100 mg/m2, MMC 20 mg/m2 | 22 | 1 | Small intestinal leakage (POD 6), abdominal infection (Proteus mirabilis infection, POD 15) | CT | 79.5, D |
| 3 | 56 | R | Serous cystadenocarcinoma, poorly differentiated | Left hemicolectomy, left lower quadrant abdominal giant tumor resection, resection of part left upper quadrant of the abdomen peritoneum and round ligament of liver, mesenteric fulguration | Lobaplatin 50 mg/m2, TAX 100 mg/m2 | 19 | 1 | Tumor recurrence, abdominal giant lump, intestinal obstruction (POD 14) | CT | 32.9, D |
| 4 | 55 | R | Serous papillary adenocarcinoma, moderately differentiated | Greater omentectomy, ileectomy, sigmoid colon and rectum resection, peritonectomy of the pelvis, right and left paracolic gutter, round ligament of liver resection | Lobaplatin 50 mg/m2, TAX 100 mg/m2 | 33 | 3 | Infection of incisional wound with Escherichia coli sepsis (POD 14) | CT | 16.3, D |
| 5 | 44 | R | Serous papillary cystadenocarcinoma, poorly differentiated | Greater/lesser omentectomy, splenectomy, cholecystectomy, right hemicolectomy, sigmoidectomy, rectectomy, right diaphragmatic copula, anterior wall and pelvic peritonectomy | Lobaplatin 50 mg/m2, TAX 100 mg/m2, 43 °C, 60 min | 33 | 2 | Pseudomembranous colitis (POD 14) | CT | 6.2, D |
POD, postoperative days; CT, conservative treatment; MRSA, methicillin-resistant Staphylococcus aureus.
Common Terminology Criteria for Adverse Events version 4.0.
A binary logistic regression analysis was conducted to study the correlation of SAEs with major treatment parameters. There were no significant correlations between SAEs and age, FIGO stage, histopathology, organ and peritoneal resection area, PCI score, and CC score.
Tumor Recurrent after CRS+HIPEC
At the time of last follow-up, tumor recurrence was documented in 35 cases, and most patients experienced tumor recurrence in more than 1 site (Table 6). In terms of frequency, 26 (56.5%) patients had peritoneal recurrence including abdominopelvic mass, intestinal obstruction, and malignant ascites; 9 (19.6%) patients had lymphatic metastasis; 6 (13.0%) patients had hematogenous metastasis including liver, spleen, lung, and bone metastasis; and 6 (13.0%) patients had malignant pleural effusion.
Table 6.
The Sites of Recurrence following HIPEC for the 46 Patients
| Item | N (%) |
|---|---|
| Peritoneal recurrence (including abdominopelvic mass, intestinal obstruction, malignant ascites) | 26 (56.5%) |
| Hematogenous (including liver, spleen, lung, bone metastasis) | 6 (13.0) |
| Lymphatic metastasis | 9 (19.6) |
| Malignant pleural effusion | 6 (13.0) |
| Malignant ascites only | 10 (21.7) |
| The second primary tumor | 1 (2.1) |
| Intestinal obstruction | 6 (13.0) |
Univariate and Multivariate Analysis on Predictors of OS
A univariate analysis identified 3 covariates indicative of improved survival, including CC 0 to 1, PCI ≤ 20, and ascites ≤ 1000 ml. Multivariate Cox regression analysis identified CC scores as the only independent predictors for better survival. Compared with CC 2 to 3, CC 0 to 1 was about 7 times (hazard ratio = 7.2, 95% CI 1.9-27.0, P < .01) more likely to improve survival (Table 7).
Table 7.
Univariate and Multivariate Analysis of Independent Factors Influencing OS
| Covariate | Univariate Analysis |
Multivariate Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P | HR | 95% CI | χ2 | P | HR | 95% CI | |
| CC score (CC 0-1 vs CC 2-3) | 17.399 | .000 | 7.937 | 3.003-12.277 | 8.743 | .003 | 7.246 | 1.949-27.027 |
| PCI (≤ 20 vs > 20) | 5.998 | .014 | 2.941 | 1.241-6.993 | 0.005 | .943 | 1.045 | 0.316-3.455 |
| Ascites (≤ 1000 ml vs > 1000 ml) | 3.841 | .050 | 2.398 | 1.000-5.747 | 1.341 | .247 | 1.686 | 0.696-4.082 |
| Age (≥ 60 vs < 60) | 0.726 | .394 | 1.436 | 0.625-3.301 | ||||
| Differentiated (well vs moderate/poorly) | 1.517 | .218 | 2.000 | 0.664-6.024 | ||||
| Comorbidity (yes vs no) | 1.203 | .273 | 1.616 | 0.685-3.809 | ||||
| HIPEC timing (primary vs recurrent) | 0.170 | .680 | 1.220 | 0.475-3.135 | ||||
| NACT (yes vs no) | 0.535 | .465 | 1.456 | 0.532-3.984 | ||||
| PIC (yes vs no) | 0.422 | .516 | 1.315 | 0.575-3.006 | ||||
| SAE (yes vs no) | 1.114 | .291 | 1.610 | 0.664-3.906 | ||||
NACT, neoadjuvant chemotherapy.
Discussion
In this study, we performed 50 CRS+HIPEC procedures as a comprehensive treatment strategy on 46 patients with advanced or recurrent EOC, achieving 1-, 3- and 5-year survival rates of 97.8%, 65.2%, and 56.5%, respectively. Of special note are the nine patients with OS > 5 years, including four patients still disease free at the time of the most recent follow-up. Special analysis of these patients reveals several features. First, these patients were relatively young at the time of treatment. Among the 9 cases, there were 8 patients ≤ 65 years old and only 1 patient aged 67 years. Second, these patients had relatively low PCI at the time of surgery. Among the 9 cases, there were 8 patients with PCI < 20 and only 1 patient with PCI 22. Third, these patients were mostly platinum-sensitive cases. Among the nine cases, eight patients were presumably platinum sensitive and 1 patient platinum resistant. Fourth, most patients had extensive CRS to achieve CC 0 to 1 (n = 8). Only one patient had CC 2 at the second CRS+HIPEC treatment.
As stage IIIc/IV and recurrent EOC are not curable by the current standard treatment of conventional surgery and taxane- and platinum-based SC [3], even with the recent addition of molecular targeting agents [16], [17], many surgical and gynecological oncologists have performed more extensive CRS to minimize tumor burden and HIPEC to eradicate microscopic residual tumors and free cancer cells after operation [18], [19], [20], [21], [22], [23]. This comprehensive treatment package integrates surgery, chemotherapy, and tumor heating and washing in one treatment setting, different from any other previously applied therapies that separate surgical therapy or medical therapy as independent treatments.
There have been several published studies on CRS+HIPEC to treat advanced EOC [24], [25], [26]. These studies achieved median OS of 28.5 to 77.8 months and 5-year survival rates of 28.0% to 72.0%. A multicenter phase II trial from Italy [24] to study upfront CRS+HIPEC for advanced EOC achieved a median PFS of 30.0 months and 5-year OS and PFS rates of 60.7% and 15.2%, respectively. Gonzalez et al. [26] studied the different time points of CRS+HIPEC to treat advanced EOC, and the median OS was 77.8 months for patients treated upfront, 62.8 months at first recurrence, and 35.7 months at second or subsequent recurrence. In our study, the 5-year survival rate for advanced EOC is similar to that reported in the literature.
Many studies also used CRS+HIPEC to treat recurrent EOC [23], [27], [28], achieving median OS of 37.0 to 48.9 months and 5-year survival rates of 35% to 41.3%. Our median OS was relatively higher than others, reaching 57.5 months (95% CI 29.8-85.2) in the recurrent group. Due to the heterogeneity of patient selection and complexity of this therapy strategy, most published reports were phase I [29] and phase II [30] studies, but the most recent phase III prospective randomized trial by Spiliotis et al. [18] on 120 patients with recurrent EOC has provided more convincing evidence. In this study, the mean OS was 26.7 versus 13.4 months for CRS+HIPEC group versus CRS alone group (P < .01). Three-year survival rate was 75% for CRS+HIPEC group versus 18 % for CRS alone group (P < .01).
One of the most important factors to determine the success of CRS+HIPEC is the PCI to reflect the degree of PC. In our study, the median PCI was 20, with 24 patients (52.2%) having PCI ≤ 20 and 22 patients (47.8%) having PCI > 20. We found that patients with PCI ≤ 20 had median OS of 76.6 months, whereas those with PCI > 20 had median OS of only 38.5 months (P = .01). At the same time, univariate analysis identified PCI ≤ 20 as an independent factor for better survival. So far, there has been no clear dividing line to determine the degree of PCI, although most reports used PCI 10 [23], 12 [31], and 15 [32], [33], [34] as the dividing line. All these studies indicated that patients with limited peritoneal cancer (relatively LPCI) could benefit more from this comprehensive treatment, emphasizing the importance of patient selection.
Complete CRS is another important factor for long-term survival. In our study, patients with CC 0 to 1 CRS had median OS of 79.5 months, whereas those with CC 2 to 3 had median OS of only 24.3 months. Multivariate Cox regression analysis also identified CC score as an independent factor for better survival. Compared with CC 2 to 3, CC 0 to 1 is about 7 times (hazard ratio = 7.2, 95% CI 1.9-27.0, P < .01) more likely to improve survival. These findings are in accordance with the literature reports [20], [28]. Of special note is the exhaustive meta-analysis by Bristow et al. [35] on 81 clinical studies totaling 6995 EOC patients treated during the platinum era, which demonstrated that for every 10% increase in the rate of optimal CRS, there is a 3-month OS increase for patients with recurrent EOC. Therefore, every attempt should be tried to achieve maximal CRS. However, extensive surgery means high potential morbidity and mortality. In our experience, no perioperative death occurred, but five patients developed SAE, including two with intestinal fistula, two with severe infection and sepsis, and one patient with intestinal obstruction. Although binary logistic regression analysis revealed no significant correlations between SAEs and the clinical and operation variables, several factors deserve special attention: First, these five patients with SAE were all recurrent EOC; three were platinum sensitive and two were platinum resistant. Second, these patients had high PCI, 4 with PCI over 22 and only 1 with PCI 19. Third, these patients had high CC score, 3 with CC 2 to 3 and 2 with CC 1; there was no CC 0 patient. Fourth, SAE had significant detrimental impact on survival. Therefore, intensive perioperative risk factors management is warranted.
Conclusion
In summary, this retrospective cohort study on a prospectively established database from China has provided evidence that CRS+HIPEC could bring OS benefits calculated from first treatment for advanced and recurrent EOC. More high-level evidence-based clinical studies are needed to validate this strategy.
Conflict of Interest Statement
The authors declare no conflicts of interest in this work.
Acknowledgements
This study was supported by grants from Hubei Province’s Outstanding Medical Academic Leader Program, Science Fund for Doctorate Mentors by China’s Ministry of Education (no. 20120141110042), and Special Fund for Key Academic Discipline Development of Beijing Shijitan Hospital Affiliated to Capital Medical University.
Footnotes
Supported by grants from Hubei Province’s Outstanding Medical Academic Leader Program, Science Fund for Doctorate Mentors by China’s Ministry of Education (no. 20120141110042), and Special Fund for Key Academic Discipline Development of Beijing Shijitan Hospital Affiliated to Capital Medical University.
References
- 1.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Person D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Leitao MM, Jr., Kardos S, Barakat RR, Chi DS. Tertiary cytoreduction in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2004;95:181–188. doi: 10.1016/j.ygyno.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Yabuno A, Fujiwara K. Critical appraisal of bevacizumab in the treatment of ovarian cancer. Drug Des Devel Ther. 2015;9:2351–2358. doi: 10.2147/DDDT.S83275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 7.Tang L, Mei LJ, Yang XJ, Huang CQ, Zhou YF, Yonemura Y, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: evidence from an experimental study. J Transl Med. 2011;9:53. doi: 10.1186/1479-5876-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XJ, Li Y, al-shammaa Hassan AH, Yang GL, Liu SY, Lu YL, Zhang JW, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: results of 21 cases. Ann Surg Oncol. 2009;16:345–351. doi: 10.1245/s10434-008-0226-2. [DOI] [PubMed] [Google Scholar]
- 9.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: results from a Chinese center. J Surg Oncol. 2010;101:457–464. doi: 10.1002/jso.21519. [DOI] [PubMed] [Google Scholar]
- 11.Huang CQ, Feng JP, Yang XJ, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol. 2014;109:730–739. doi: 10.1002/jso.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43:S15–S25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27:239–243. doi: 10.1053/ejso.2000.1038. [DOI] [PubMed] [Google Scholar]
- 14.Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34:S1–S15. doi: 10.1053/j.seminoncol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Available at:
- 16.Damia G, Sessa C. Successes and limitations of targeted cancer therapy in ovarian cancer. Prog Exp Tumor Res. 2014;41:89–97. doi: 10.1159/000355905. [DOI] [PubMed] [Google Scholar]
- 17.Syrios J, Banerjee S, Kaye SB. Advanced epithelial ovarian cancer: from standard chemotherapy to promising molecular pathway targets—where are we now? Anticancer Res. 2014;34:2069–2077. [PubMed] [Google Scholar]
- 18.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22:1570–1575. doi: 10.1245/s10434-014-4157-9. [DOI] [PubMed] [Google Scholar]
- 19.Fagotti A, Costantini B, Petrillo M, Vizzielli G, Fanfani F, Margariti PA, Turco LC, Piovano E, Scambia G. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up. Gynecol Oncol. 2012;127:502–505. doi: 10.1016/j.ygyno.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, Gilly FN. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813–1820. doi: 10.1007/s00268-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 21.Cascales-Campos PA, Gil Martinez J, Galindo Fernandez PJ, Gil Gómez E, Martinez Frutos IM, Parrilla Paricio P. Perioperative fast track program in intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery in advanced ovarian cancer. Eur J Surg Oncol. 2011;37:543–548. doi: 10.1016/j.ejso.2011.03.134. [DOI] [PubMed] [Google Scholar]
- 22.Fagotti A, Paris I, Grimolizzi F, Fanfani F, Vizzielli G, Naldini A, Scambia G. Secondary cytoreduction plus oxaliplatin-based HIPEC in platinum-sensitive recurrent ovarian cancer patients: a pilot study. Gynecol Oncol. 2009;113:335–340. doi: 10.1016/j.ygyno.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Bakrin N, Cotte E, Golfier F, Gilly FN, Freyer G, Helm W, Glehen O, Bereder JM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052–4058. doi: 10.1245/s10434-012-2510-4. [DOI] [PubMed] [Google Scholar]
- 24.Deraco M, Kusamura S, Virzi S, Puccio F, Macri A, Famulari C, Solazzo M, Bonomi S, Iusco DR, Baratti D. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol. 2011;122:215–220. doi: 10.1016/j.ygyno.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Parson EN, Lentz S, Russell G, Shen P, Levine EA, Stewart JH., IV Outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface dissemination from ovarian neoplasms. Am J Surg. 2011;202:481–486. doi: 10.1016/j.amjsurg.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez Bayon L, Steiner MA, Vasquez Jimenez W, Asencio JM, Alvarez de Sierra P, Atahualpa Arenas F, Rodriguez del Campo J, Garcia Sabrido JL. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of advanced epithelial ovarian carcinoma: upfront therapy, at first recurrence, or later? Eur J Surg Oncol. 2013;39:1109–1115. doi: 10.1016/j.ejso.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Fagotti A, Costantini B, Vizzielli G, Perelli F, Ercoli A, Gallotta V, Scambia G, Fanfani F. HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol. 2011;122:221–225. doi: 10.1016/j.ygyno.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Ceelen WP, Van Nieuwenhove Y, Van Belle S, Denys H, Pattyn P. Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol. 2012;19:2352–2359. doi: 10.1245/s10434-009-0878-6. [DOI] [PubMed] [Google Scholar]
- 29.Guardiola E, Chauffert B, Delroeux D, Royer B, Heyd B, Combe M, Benoit L, Causeret S, Demarchi M, Magnin G. Intraoperative chemotherapy with cisplatin and epinephrine after cytoreductive surgery in patients with recurrent ovarian cancer: a phase I study. Anticancer Drugs. 2010;21:320–325. doi: 10.1097/CAD.0b013e328334d953. [DOI] [PubMed] [Google Scholar]
- 30.Deraco M, Rossi CR, Pennacchioli E, Guadagni S, Somers DC, Santoro N, Raspagliesi F, Kusamura S, Vaglini M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: a phase II clinical study. Tumori. 2001;87:120–126. doi: 10.1177/030089160108700302. [DOI] [PubMed] [Google Scholar]
- 31.Cascales-Campos P, Gil J, Parrilla P. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with primary and recurrent advanced ovarian cancer. Eur J Surg Oncol. 2014;40:970–975. doi: 10.1016/j.ejso.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, Tarquini S, Di seri M, Ciardi A, Montruccoli D. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315–325. doi: 10.1002/cncr.23553. [DOI] [PubMed] [Google Scholar]
- 33.Tentes AA, Kakolyris S, Kyziridis D, Karamveri C. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy in the treatment of advanced epithelial ovarian cancer. J Oncol. 2012;2012:358341. doi: 10.1155/2012/358341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, De laco P, Di battista M, Framarini M, Gazzotti F, Trimble EL, Montz FJ. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22:778–785. doi: 10.1097/IGC.0b013e31824d836c. [DOI] [PubMed] [Google Scholar]
- 35.Bristow RE, Tomacruz RS, Armstrong DK, Trimble E.L., Montz F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]