Abstract
Background:
Recent studies reported that percutaneous coronary intervention with stent implantation was safe and feasible for the treatment of left main coronary artery (LMCA) disease in select patients. However, it is unclear whether drug-eluting stents (DESs) have better outcomes in patients with LMCA disease compared with bare-metal stent (BMS) during long-term follow-up in Chinese populations.
Methods:
From a perspective multicenter registry, 1136 consecutive patients, who underwent BMS or DES implantation for unprotected LMCA stenosis, were divided into two groups: 1007 underwent DES implantation, and 129 underwent BMS implantation. The primary outcome was the rate of major adverse cardiac events (MACEs), including cardiovascular (CV) death, myocardial infarction (MI), and target lesion revascularization (TLR) at 5 years postimplantation.
Results:
Patients in the DES group were older and more likely to have hyperlipidemia and bifurcation lesions. They had smaller vessels and longer lesions than patients in the BMS group. In the adjusted cohort of patients, the DES group had significantly lower 5 years rates of MACE (19.4% vs. 31.8%, P = 0.022), CV death (7.0% vs. 14.7%, P = 0.045), and MI (5.4% vs. 12.4%, P = 0.049) than the BMS group. There were no significant differences in the rate of TLR (10.9% vs. 17.8%, P = 0.110) and stent thrombosis (4.7% vs. 3.9%, P = 0.758). The rates of MACE (80.6% vs. 68.2%, P = 0.023), CV death (93.0% vs. 85.3%, P = 0.045), TLR (84.5% vs. 72.1%, P = 0.014), and MI (89.9% vs. 80.6%, P = 0.029) free survival were significantly higher in the DES group than in the BMS group. When the propensity score was included as a covariate in the Cox model, the adjusted hazard ratios for the risk of CV death and MI were 0.41 (95% confidence interval [CI]: 0.21–0.63, P = 0.029) and 0.29 (95% CI: 0.08–0.92, P = 0.037), respectively.
Conclusions:
DES implantation was associated with more favorable clinical outcomes than BMS implantation for the treatment of LMCA disease even though there was no significant difference in the rate of TLR between the two groups.
Keywords: Bare-metal Stent, Drug-eluting Stent, Left Main Coronary Artery Disease, Percutaneous Coronary Intervention
INTRODUCTION
Left main coronary artery (LMCA) disease has been associated with poor prognoses when treated medically, especially during the period of bare-metal stents (BMS). LMCA is often accompanied by a series of complications such as in-stent restenosis for the treatment of percutaneous coronary intervention (PCI). Therefore, the current guidelines for coronary revascularization recommend coronary artery bypass grafting as the standard treatment for LMCA disease.[1,2,3] However, drug-eluting stents (DESs) and adjunctive pharmacological therapy have led to re-evaluation of the potential use of PCI for the treatment of LMCA disease, and several studies have reported that this procedure was feasible and had favorable mid-term outcomes.[4,5,6] The development of DES, which is associated with significantly lower rates of restenosis and repeat revascularization, has made PCI in patients with LMCA disease more feasible.[7,8,9,10] Based on the improved clinical outcomes after treatment, the American College of Cardiology/American Heart Association guidelines include a Class IIa recommendation for PCI for unprotected LMCA (ULMCA) disease.[2]
There is still uncertainty about the long-term safety of DES implantation for the treatment of LMCA disease. In particular, late stent thrombosis (ST) has been reported to occur more frequently after DES implantation than after BMS implantation.[11,12,13,14] The US Food and Drug Administration has warned that the risk of ST may outweigh the benefits of DES implantation for off-label use such as the treatment of ULMCA stenosis.[15] Mid-term pilot studies found that DES implantation was safe and effective for the treatment of ULMCA stenosis, compared to BMS implantation; however, these studies had small sample sizes, were based at single centers, and had relatively short follow-up periods.
METHODS
Study population
This study included 1159 consecutive patients who underwent PCI for LMCA lesions at Shenyang Northern Hospital and Nanjing First Hospital between January 2003 and March 2009, of which 1020 underwent DES implantation, and 139 underwent BMS implantation. All the included patients had clinical symptoms and signs of myocardial ischemia and had an angiographically documented ULMCA lesion deemed suitable for stenting. The reasons for BMS implantation rather than DES implantation included lack of clinical evidence for the use of DES for the treatment of ULMCA disease, lack of a suitably sized DES, inability to afford long-term dual antiplatelet therapy, and acute myocardial infarction (MI) caused by ULMCA disease. Patients with cardiogenic shock, contraindications to aspirin or clopidogrel therapy, or planned upcoming noncardiac surgery were excluded. The local institutional ethics committees approved the use of patient data for this study.
Percutaneous coronary intervention procedure
A glycoprotein IIb/IIIa inhibitor was administered as needed during the PCI procedure based on the angiographic findings and operator judgment. Predilatation was routinely performed. The stent length and location were selected to provide coverage for the full length of each lesion, extending 1–2 mm proximal and distal to each lesion with a stent-to-vessel ratio of 1:1. For ostial LMCA lesions, stents were positioned to protrude into the aorta by 1–2 mm. For LMCA bifurcation lesions, stent positioning was determined by the operator based on the lesion type. For distal LMCA lesions with involvement of either the left anterior descending or the left circumflex ostium, and for lesions involving a side branch of ≤2.5 mm diameter, provisional T-stenting was performed. For true bifurcation lesions involving two major branches, two stent procedures such as T-stenting, modified T-stenting, kissing stenting, or crush stenting were performed as previously described. Most of the crush techniques were modified as follows: The side branch stent was placed with a small protrusion (1–2 mm) into the main vessel, or a balloon in the main vessel (0.5 mm undersized) was simultaneously dilated when the side branch stent was deployed. After stent implantation, high-pressure dilatation was routinely performed, including final kissing balloon dilatation after distal LMCA bifurcation stenting. For patients with multiple lesions, treatment strategies were at the discretion of the operators.
Medication protocols
Loading doses of aspirin (300 mg) and clopidogrel (300–600 mg) were administered before the procedure. Clopidogrel was continued at a maintenance dose of 150 mg/d for 2–4 weeks after the procedure, followed by 75 mg/d for 3–6 months after BMS implantation or 12–24 months after DES implantation. Aspirin was continued at a dose of 300 mg/d for one month after the procedure and then 100 mg/d indefinitely.
Clinical outcomes and definitions
The primary clinical outcome was the rate of major adverse cardiac events (MACEs) including cardiovascular (CV) death, MI, and target lesion revascularization (TLR). The secondary clinical outcome was the rate of ST. All deaths were considered to be CV deaths unless another cause was clearly documented. MI was defined as an elevation of the creatine kinase-MB level to more than 5 times the upper limit of normal coupled with typical chest pain lasting >30 min or new ischemic changes on the electrocardiogram. TLR was defined as repeat target-lesion-related intervention or bypass surgery. In-stent restenosis was defined as a ≥50% diameter stenosis at the site of the stented lesion. All patients were followed-up at 5 years via telephone call, clinic visit or rehospitalization.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD) and compared between groups using the t-test. Categorical data were expressed as percentages and compared between groups using the Chi-square or Fisher's exact tests. It was prospectively determined that missing observations would be imputed using the last-observation-carried-forward method. The cumulative incidences of events were calculated using the Kaplan–Meier method. Estimated Kaplan–Meier survival curves were compared using the log-rank test.
To reduce the impact of treatment selection bias and potentially confounding variables in an observational study, we performed rigorous adjustments for significant differences in characteristics of patients by use of the weighted Cox proportional-hazards regression models using the inverse-probability-of-treatment weighting (IPTW). With that technique, weights for patients receiving BMS were the inverse of (one – propensity score), and weights for patients receiving DES were the inverse of the propensity score. The propensity scores were estimated by multiple logistic regression analysis. To create the propensity score, multiple imputation with Markov Chain Monte Carlo methods was used to fill out incomplete baseline variables with the assumption that data were missing at random. All prespecified covariates were included in the full unmatched models for treatment with DES versus BMS [Table 1]. The discrimination and calibration abilities of each propensity score model were assessed by means of the C statistic and the Hosmer-Lemeshow statistic. After all the propensity score matches had been performed, the baseline variables were compared between the two groups. Continuous variables were compared using the paired t-test or Wilcoxon signed-rank test. The procedure yielded 129 well-matched pairs. The Kaplan–Meier method was used to plot the estimated incidences of MACE, CV death, TLR, and MI in these groups of patients. Differences between groups were analyzed using the log-rank test.
Table 1.
Clinical, angiographic, and procedural characteristics before and after propensity score matching
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| BMS (n = 129) | DES (n = 1007) | P | BMS (n = 129) | DES (n = 129) | P | |
| Age, years | 62.1 ± 11.6 | 64.4 ± 10.8 | 0.038 | 62.1 ± 11.6 | 63.74 ± 9.75 | 0.474 |
| Male, n (%) | 98 (76.0) | 782 (77.7) | 0.666 | 98 (76.0) | 100 (77.5) | 0.768 |
| Hypertension, n (%) | 73 (56.6) | 620 (61.6) | 0.275 | 73 (56.6) | 84 (65.1) | 0.161 |
| Diabetes mellitus, n (%) | 31 (24.0) | 288 (28.6) | 0.277 | 31 (24.0) | 40 (31.0) | 0.210 |
| Smoking history, n (%) | 52 (40.3) | 429 (42.6) | 0.620 | 52 (40.3) | 63 (48.8) | 0.168 |
| Hyperlipidemia, n (%) | 44 (31.1) | 443 (44.6) | 0.033 | 44 (31.1) | 43 (33.3) | 0.895 |
| Renal dysfunction, n (%) | 4 (3.1) | 75 (7.5) | 0.100 | 4 (3.1) | 3 (2.3) | 1.000 |
| Family history of CHD, n (%) | 14 (11.0) | 79 (13.4) | 0.241 | 14 (11.0) | 16 (12.4) | 0.698 |
| UAP, n (%) | 99 (76.7) | 764 (75.9) | 0.827 | 99 (76.7) | 102 (79.1) | 0.653 |
| STEMI, n (%) | 26 (20.2) | 144 (14.3) | 0.079 | 26 (20.2) | 17 (13.2) | 0.133 |
| NSTEMI, n (%) | 6 (4.7) | 56 (5.6) | 0.668 | 6 (4.7) | 6 (4.7) | 1.000 |
| LVEF (%) | 57.8 ± 12.0 | 56.5 ± 12.4 | 0.298 | 57.8 ± 12.0 | 56.4 ± 12.6 | 0.351 |
| Lesion location, n (%) | ||||||
| Ostium and shaft | 95 (73.6) | 653 (64.8) | 0.047 | 95 (73.6) | 90 (69.8) | 0.490 |
| Bifurcation | 34 (26.4) | 354 (35.2) | 34 (26.4) | 39 (30.2) | ||
| Multivessel disease, n (%) | 64 (49.6) | 589 (58.5) | 0.055 | 64 (49.6) | 71 (55.0) | 0.383 |
| Multivessel treatment, n (%) | 31 (24.0) | 311 (30.9) | 0.110 | 31 (24.0) | 38 (29.5) | 0.325 |
| Reference vessel diameter, mm | 3.9 ± 0.6 | 3.5 ± 0.4 | <0.001 | 3.9 ± 0.6 | 3.8 ± 0.4 | 0.738 |
| Lesion length, mm | 18.1 ± 7.5 | 26.5 ± 11.4 | <0.001 | 18.1 ± 7.5 | 20.5 ± 8.9 | 0.191 |
BMS: Bare-metal stent; DES: Drug-eluting stent; CHD: Coronary heart disease; NSTEMI: Non-ST elevation myocardial infarction; STEMI: ST elevation myocardial infarction; LVEF: Left ventricular ejection fraction; UAP: Unstable angina pectoris.
Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) software. A two-tailed P < 0.05 was considered statistically significant. The authors had full access to all the data and took full responsibility for its integrity. All the authors have read and agree with the manuscript as written.
RESULTS
Baseline characteristics
The 1136 patients, who met the inclusion criteria, represented 98.0% of the 1159 patients originally enrolled in the registry and underwent stent implantation for the treatment of ULMCA disease in the absence of cardiogenic shock during the study period. The study population included 1007 patients (88.6%) who underwent DES implantation and 129 patients (11.4%) who underwent BMS implantation.
Table 1 shows the baseline characteristics, according to the type of stent used (DES vs. BMS), before and after propensity score matching. Before matching, there were no significant differences in baseline characteristics between the two groups, except that patients in the DES group were older (P = 0.038), were more likely to have hyperlipidemia (P = 0.037), bifurcation lesions (P = 0.047), smaller vessels (P < 0.001), and longer lesions (P < 0.001) than patients in the BMS group. After propensity score matching, all the recorded baseline characteristics were similar between DES and BMS groups.
Clinical outcomes
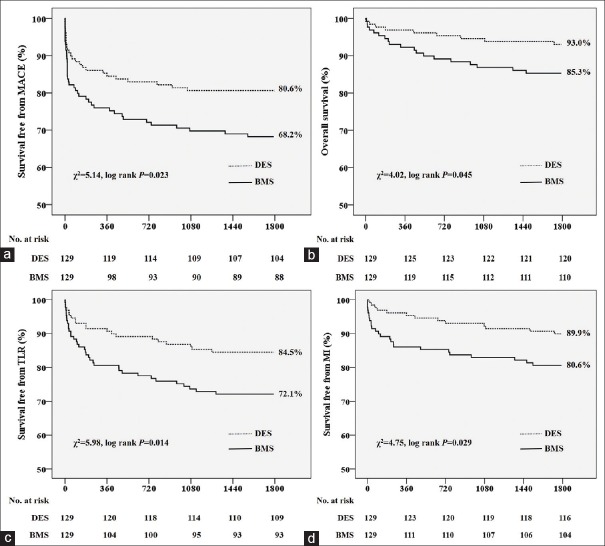
Clinical outcome information was obtained for all patients at 5 years. As shown in Table 2, in the adjusted cohort of patients, the rate of MACE was significantly lower in the DES group than in the BMS group (19.4% vs. 31.8%, P = 0.022). The DES group also had significantly lower 5-year rates of CV death (7.0% vs. 14.7%, P = 0.045) and MI (5.4% vs. 12.4%, P = 0.049) than the BMS group. There were no significant differences in the rates of TLR (10.9% vs. 17.8%, P = 0.110) and ST (4.7% vs. 3.9%, P = 0.758). Kaplan–Meier analyses of the 5-year survival free from MACE, CV death, TLR, and MI are shown in Figure 1. The rate of MACE-free survival was significantly higher in the DES group than in the BMS group (80.6% vs. 68.2%, P = 0.023). The DES group also had significantly higher 5-year survival-free rates from CV death (93.0% vs. 85.3%, P = 0.045), TLR (84.5% vs. 72.1%, P = 0.014), and MI (89.9% vs. 80.6%, P = 0.029) than the BMS group.
Table 2.
Clinical outcomes at 5 years for the adjusted data set, n (%)
| Outcomes | BMS (n = 129) | DES (n = 129) | P |
|---|---|---|---|
| MACE | 41 (31.8) | 25 (19.4) | 0.022 |
| MI | 16 (12.4) | 7 (5.4) | 0.049 |
| TLR | 23 (17.8) | 14 (10.9) | 0.110 |
| CV death | 19 (14.7) | 9 (7.0) | 0.045 |
| Stent thrombosis | 5 (3.9) | 6 (4.7) | 0.758 |
MACE: Major adverse cardiac event; MI: Myocardial infarction; CV: Cardiovascular; TLR: Target-lesion revascularization; BMS: Bare-metal stent; DES: Drug-eluting stent.
Figure 1.
Cumulative 5-year incidences of major adverse cardiovascular events (a); cardiovascular death (b); target lesion revascularization (c); and myocardial infarction (d) in patients who underwent drug-eluting stent implantation and bare-metal stent implantation.
Cox multivariable regression models were used to correct for differences and independent predictors of CV death and MI between groups [Table 3]. After correcting for the independent predictors of adverse events, the adjusted hazard ratio (HR) for the risk of CV death after DES implantation relative to BMS implantation was 0.51 (95% confidence interval [CI]: 0.30–0.86, P = 0.045) and the adjusted HR for the risk of MI after DES implantation relative to BMS implantation was 0.31 (95% CI: 0.09–1.03, P = 0.065).
Table 3.
Predictors of CV death and MI on the multivariable Cox proportional-hazards analysis
| Items | Hazard ratio (95% CI) | P |
|---|---|---|
| CV death | ||
| Diabetes | 2.61 (1.27–5.61) | 0.029 |
| Male | 0.74 (0.26–2.14) | 0.580 |
| Age | 1.02 (0.98–1.06) | 0.361 |
| LVEF | 0.94 (0.93–0.99) | 0.017 |
| Lesion length | 0.98 (0.93–1.04) | 0.502 |
| Multivessel disease | 0.93 (0.41–2.07) | 0.654 |
| Renal dysfunction | 1.13 (0.72–3.06) | 0.653 |
| Reference vessel diameter | 0.69 (0.48–1.27) | 0.328 |
| DES versus BMS | 0.51 (0.30–0.86) | 0.045 |
| IPTW adjusted | ||
| DES versus BMS | 0.41 (0.21–0.63) | 0.029 |
| MI | ||
| Diabetes | 2.86 (1.08–7.56) | 0.031 |
| Male | 1.86 (0.65–5.38) | 0.259 |
| Age | 1.00 (0.92–1.08) | 0.918 |
| LVEF | 0.96 (0.92–0.99) | 0.033 |
| Lesion length | 0.96 (0.87–1.05) | 0.367 |
| Multivessel disease | 0.92 (0.34–2.49) | 0.864 |
| Renal dysfunction | 0.62 (0.19–2.70) | 0.716 |
| Reference vessel diameter | 0.33 (0.12–0.88) | 0.035 |
| DES versus BMS | 0.31 (0.09–1.03) | 0.065 |
| IPTW adjusted | ||
| DES versus BMS | 0.29 (0.08–0.92) | 0.037 |
CV: Cardiovascular; LVEF: Left ventricular ejection fraction; DES: Drug-eluting stent; BMS: Bare-metal stent; IPTW: Inverse-probability-of-treatment weighting; MI: Myocardial infarction; CI: Confidence interval.
When the IPTW adjustment was included as a covariate in the model, the adjusted HRs for the risk of CV death and MI were 0.41 (95% CI: 0.21–0.63, P = 0.029) and 0.29 (95% CI: 0.08–0.92, P = 0.037), respectively [Table 3]. These results show a slight increase in statistical significance after adjustment by IPTW.
DISCUSSION
The most important findings of the present study are: (1) The long-term rate of TLR is similar after DES and BMS implantation but the rate of survival free from TLR was higher after DES implantation than BMS, and (2) even after several statistical adjustments for possible confounders, DES implantation was associated with lower risks of CV death and MI than BMS implantation.
Percutaneous coronary intervention is increasingly performed for the treatment of lesions previously considered to be contraindications, like ULMCA stenosis. DES implantation is used with increasing frequency because it is associated with lower rates of restenosis than BMS implantation when used for the treatment of standard coronary lesions, but superiority of DES over BMS implantation for the treatment of ULMCA lesions has not been clearly established.
Although DES implantation has been shown to be procedurally feasible for the treatment of ULMCA disease, there is limited information available regarding long-term outcomes.[16,17,18,19,20,21,22] Most previously reported studies had small sample sizes, no control groups, and short follow-up periods. Longer-term follow-up and a control group of patients who undergo BMS implantation are required to fully assess the safety of DES implantation compared to BMS implantation because the relative rates of various complications may differ after one year.[23] This study may have the longest observation period to date among studies comparing outcomes after DES and BMS implantation for ULMCA stenosis in China. In our study group, 27% of patients had diabetes, 56% had multivessel coronary disease, and 32% had lesions at the LMCA bifurcation. This large registry of consecutive patients recruited at multiple centers may represent real-world clinical practice. In our study, 2-Stage adjustments using the multivariable Cox modeling with IPTW were performed to overcome the limitations of the observational study. The IPTW method may avoid the possibility that the benefit of DES was overestimated as a result of the residual confounding variables related to the selection of lower-risk population by propensity score matching.
This study found that DES implantation was safe for the treatment of ULMCA lesions and showed lower risks of CV death and MI compared to BMS implantation. Our results are consistent with those of large registry studies of patients undergoing off-label DES implantation, which found that DES implantation was not associated with increased long-term rates of CV death or MI in patients with complex coronary lesions, compared with BMS implantation.[24,25,26] For example, a large registry study of 13,353 patients in Ontario found that the 3-year mortality rate in a propensity-matched population was significantly higher after BMS implantation than after DES implantation.[8] The comparable incidences of CV death and MI between use of the two stent types may be due, at least in part, to the offsetting risks of restenosis and ST. Because restenosis or repeat revascularization of the LMCA may result in death or MI,[25] the higher risk of restenosis after BMS implantation may counterbalance the potential risk of ST-mortality after DES implantation.[26]
The results of some recent pooled analyses and a large registry study suggest that DES implantation may be associated with an increased risk of late mortality as a result of very late ST, compared with BMS implantation.[12,13,23,27] However, in the present study, the rate of ST at 5-year follow-up was comparable between the DES group and BMS group. The reason for the different result may be the possibility that very late ST has limited the use of DES implantation for the treatment of ULMCA lesions.
Two-stage adjustments were performed using multivariable Cox modeling with propensity score matching to overcome the limitations associated with observational bias in our study. This method of statistical analysis may avoid the possibility that the benefit of DES implantation was overestimated as a result of the residual confounding related to the selection of a lower-risk population by propensity score matching, which was used in recent analyses of registry studies.[28,29,30] The multivariable Cox proportional-hazards analysis indicated that DES versus BMS showed a trend in reducing CV death and MI while not reducing TLR, which is unexpected. However, some possible explanations may be considered. First of all, the two participating centers started to enroll patients when the use of DES was low in China. This scenario, in which BMS were the only stents available, could have common ground with the ascending phase of the learning curve for ULMCA PCI. Thus, in our experience, DES could have taken advantage of its enhanced and improved use on the plateau of the learning curve and from a general technical improvement in PCI stenting. In addition, patients treated with DES could have benefited from a closer angiographic follow-up than those treated with BMS, suggesting the need for routine angiographic follow-up after ULMCA treatment.
Finally, the most important limitation of the present study is the lack of random assignment to treatment groups. Evaluation of the impact of a specific treatment using a registry can lead to incorrect conclusions because of the influence of unassessed confounding variables such as comorbidities, terminal illness, socioeconomic status, and intravascular ultrasound guidance. In this study, the stent type used was not assigned randomly but was decided according to the individual patient characteristics, resulting in an unavoidable risk of bias regarding treatment selection and prognosis. To partly compensate for the baseline and angiographic differences between the patient groups, we performed extensive adjustments using both multivariate analysis and propensity scores, thereby reducing the likelihood of residual selection bias. However, it is impossible to know if these adjustments are appropriate or if the relevant characteristics have been correctly identified. Only randomization can provide an unbiased estimation of the effects of a treatment.
Patients, who underwent DES implantation at a later phase of the study, could have benefited from advances in PCI techniques and adjunctive medications. No statistical method can completely eliminate this limitation.
In conclusion, our findings show that DES implantation is safe and effective in the long-term for the treatment of ULMCA stenosis compared with BMS implantation. In combination with previously reported registry data, our findings indicate that PCI with DES is a promising treatment for ULMCA stenosis.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. Authors/Task Force Members. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: A report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–53. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri M, Barragan P, Sainsous J, Bayet G, Simeoni JB, Roquebert PO, et al. Unprotected left main coronary artery stenting: Immediate and medium-term outcomes of 140 elective procedures. J Am Coll Cardiol. 2000;35:1543–50. doi: 10.1016/s0735-1097(00)00588-x. [DOI] [PubMed] [Google Scholar]
- 5.Black A, Cortina R, Bossi I, Choussat R, Fajadet J, Marco J. Unprotected left main coronary artery stenting: Correlates of midterm survival and impact of patient selection. J Am Coll Cardiol. 2001;37:832–8. doi: 10.1016/s0735-1097(00)01176-1. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Hong MK, Lee CW, Kim JJ, Song JK, Kang DH, et al. Elective stenting of unprotected left main coronary artery stenosis: Effect of debulking before stenting and intravascular ultrasound guidance. J Am Coll Cardiol. 2001;38:1054–60. doi: 10.1016/s0735-1097(01)01491-7. [DOI] [PubMed] [Google Scholar]
- 7.Chieffo A, Stankovic G, Bonizzoni E, Tsagalou E, Iakovou I, Montorfano M, et al. Early and mid-term results of drug-eluting stent implantation in unprotected left main. Circulation. 2005;111:791–5. doi: 10.1161/01.CIR.0000155256.88940.F8. [DOI] [PubMed] [Google Scholar]
- 8.Valgimigli M, van Mieghem CA, Ong AT, Aoki J, Granillo GA, McFadden EP, et al. Short-and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of left main coronary artery disease: Insights from the Rapamycin-eluting and Taxus Stent Evaluated at Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH) Circulation. 2005;111:1383–9. doi: 10.1161/01.CIR.0000158486.20865.8B. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Kim YH, Lee BK, Lee SW, Lee CW, Hong MK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: Comparison with bare metal stent implantation. J Am Coll Cardiol. 2005;45:351–6. doi: 10.1016/j.jacc.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Park DW, Lee SW, Yun SC, Lee CW, Hong MK, et al. Long-term safety and effectiveness of unprotected left main coronary stenting with drug-eluting stents compared with bare-metal stents. Circulation. 2009;120:400–7. doi: 10.1161/CIRCULATIONAHA.108.800805. [DOI] [PubMed] [Google Scholar]
- 11.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 12.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 13.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 14.Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108–13. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 15.Farb A, Boam AB. Stent thrombosis redux - The FDA perspective. N Engl J Med. 2007;356:984–7. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 16.Pandya SB, Kim YH, Meyers SN, Davidson CJ, Flaherty JD, Park DW, et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis a meta-analysis. JACC Cardiovasc Interv. 2010;3:602–11. doi: 10.1016/j.jcin.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price MJ, Cristea E, Sawhney N, Kao JA, Moses JW, Leon MB, et al. Serial angiographic follow-up of sirolimus-eluting stents for unprotected left main coronary artery revascularization. J Am Coll Cardiol. 2006;47:871–7. doi: 10.1016/j.jacc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Palmerini T, Marzocchi A, Marrozzini C, Ortolani P, Saia F, Savini C, et al. Comparison between coronary angioplasty and coronary artery bypass surgery for the treatment of unprotected left main coronary artery stenosis (the Bologna Registry) Am J Cardiol. 2006;98:54–9. doi: 10.1016/j.amjcard.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 19.Lee MS, Kapoor N, Jamal F, Czer L, Aragon J, Forrester J, et al. Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2006;47:864–70. doi: 10.1016/j.jacc.2005.09.072. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Dangas GD, Solinas E, Aoki J, Parise H, Kimura M, et al. Effectiveness of drug-eluting stent implantation for patients with unprotected left main coronary artery stenosis. Am J Cardiol. 2008;101:801–6. doi: 10.1016/j.amjcard.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Buszman PE, Kiesz SR, Bochenek A, Peszek-Przybyla E, Szkrobka I, Debinski M, et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol. 2008;51:538–45. doi: 10.1016/j.jacc.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 22.Meliga E, Garcia-Garcia HM, Valgimigli M, Chieffo A, Biondi-Zoccai G, Maree AO, et al. Longest available clinical outcomes after drug-eluting stent implantation for unprotected left main coronary artery disease: The DELFT (Drug Eluting stent for LeFT main) Registry. J Am Coll Cardiol. 2008;51:2212–9. doi: 10.1016/j.jacc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–19. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 24.Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030–9. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 25.Tu JV, Bowen J, Chiu M, Ko DT, Austin PC, He Y, et al. Effectiveness and safety of drug-eluting stents in Ontario. N Engl J Med. 2007;357:1393–402. doi: 10.1056/NEJMoa071076. [DOI] [PubMed] [Google Scholar]
- 26.Marroquin OC, Selzer F, Mulukutla SR, Williams DO, Vlachos HA, Wilensky RL, et al. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358:342–52. doi: 10.1056/NEJMoa0706258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone GW, Ellis SG, Colombo A, Dawkins KD, Grube E, Cutlip DE, et al. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007;115:2842–7. doi: 10.1161/CIRCULATIONAHA.106.687186. [DOI] [PubMed] [Google Scholar]
- 28.Seung KB, Park DW, Kim YH, Lee SW, Lee CW, Hong MK, et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781–92. doi: 10.1056/NEJMoa0801441. [DOI] [PubMed] [Google Scholar]
- 29.Normand SL. Evaluating the optimal timing of angiography: Landmark or off the mark? Circulation. 2007;116:2656–7. doi: 10.1161/CIRCULATIONAHA.107.741132. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]