Abstract
Background:
Percutaneous coronary intervention (PCI) through transradial approach (TRA) has shown to be safe and effective as transfemoral approach (TFA) among unselected patients. However, very few studies have compared the outcomes between TRA and TFA specifically in patients with a history of coronary artery bypass grafting surgery (CABG).
Methods:
A total of 404 post-CABG patients who had undergone angiography or PCI were included in the study. The primary endpoint was defined as angiographic success and procedure success. The secondary endpoint was defined as in-hospital net adverse clinical events (NACEs), which included all cause of death, myocardial infarction (MI), stroke, repeat revascularization, and major bleeding. Patients were followed-up for 1-year. Major adverse cardiovascular events (MACEs), which included death, MI, and repeat revascularization, at 1-year follow-up were also compared.
Results:
The angiographic success was reached by 97.4% in the TRA group compared with 100% in the TFA group (P = 0.02). The procedure success was achieved in 99.1% in the TRA group and 97.9% in the TFA group (P = 0.68). The incidence rates of in-hospital NACE (2.7% vs. 2.7%, P = 1.00) and 1-year MACE (11.5% vs. 12.0%, P = 0.88) were similar between TRA and TFA. Meanwhile, TRA was associated with a lower rate of Bleeding Academic Research Consortium ≥2 bleeding (P = 0.02). In patients undergoing graft PCI, the procedure success was similar between TRA and TFA (100.0% vs. 98.7%, P = 1.00). The procedure time (25.0 min vs. 27.5 min, P = 0.53) was also similar. No significant difference was detected between TRA and TFA in terms of in-hospital NACE (0 vs. 0, P = 1.00) and 1-year MACE (21.4% vs. 10.3%, P = 0.19).
Conclusions:
Compared with TFA, TRA had lower angiographic success but had a similar procedure success in post-CABG patients. TRA was also associated with decreased bleeding and shortened hospital stay.
Keywords: Coronary Angioplasty, Coronary Artery Bypass Surgery, Transfemoral, Transradial
INTRODUCTION
Transradial approach (TRA) percutaneous coronary intervention (PCI) is widely used in many countries because of its great superiorities,[1] such as easy hemostasis, early ambulation, and better recovery. Compared with the transfemoral approach (TFA), the rates of access site complications and access site-related major bleeding are lower with TRA. In addition, the decreased rate of bleeding would transform into better survival in certain high-risk patients.[2] People with histories of coronary artery bypass grafting (CABG) surgery usually have severe atherosclerosis and complex lesions, and also at higher risk of adverse cardiovascular events. The femoral route provides enough space and great backups for the catheterizations of the grafts. However, recent studies showed that finishing the angiography and the intervention of the grafts is feasible through the radial route.[3] Given the limited knowledge about the procedure and clinical outcomes between TRA and TFA in post-CABG patients, this study compared the feasibility and safety of TRA for CABG vessels with TFA.
METHODS
Patient selection and data collection
From June 1, 2006 to April 30, 2011, 404 post-CABG patients who had undergone PCI with stent implantation (either in the native vessel or the graft) were included in the study. Data were analyzed based on retrospective extraction. Patient baseline characteristics and clinical outcomes were extracted from the medical records. Patient procedure outcomes were collected from the digital database of the catheter laboratory. The study protocol was approved by the Ethics Committee of FuWai Hospital. Patient information was de-identified prior to analysis.
Antiplatelet and anticoagulation treatment
Aspirin and clopidogrel were pretreated before the procedure. Exactly 300 mg of aspirin and 300 mg of clopidogrel were loaded if patients did not take the drugs consecutively for 7 days. Loading dose of heparin based on weight (100 IU/kg) was administrated intravenously at the start of the procedure, and low-molecular-weight heparin as well as glycoprotein (GP) IIb/IIIa inhibitor was used at the doctor's discretion. A sustained dual antiplatelet therapy was prescribed for at least 1-year for patient receiving drug-eluting stents (DESs) and for 1-month for patients receiving bare metal stents.
Route selection and hemostasis
All the interventions were performed by experienced doctors who had rich expertise and techniques both in TRA and TFA. The selection of the route was at the doctor's discretion. Allen's test was routinely checked before doctor's decision, and patients with failed Allen's test or weak radial pulse were assigned to the TFA group. The hemostasis method for the TRA was relatively easier compared with that of TFA. A plastic clamp was placed over the puncture site, and patients could ambulate right after the procedure. However, the puncture site should be wrapped with a bandage and compressed with sandbag with TFA. Patients need to stay in bed for at least 1 day.
Endpoint definitions and follow-up checkups
The primary endpoint was defined as angiographic success and procedure success. The secondary endpoint was defined as in-hospital net adverse clinical events (NACEs) and 1-year major adverse cardiovascular events (MACEs). NACE was a composite endpoint of all cause death, myocardial infarction (MI), stroke, repeat revascularization, and major bleeding. In addition, MACE was a composite of death, MI and repeat revascularization. Angiographic success was defined as the successful angiography of the native artery and graft. Procedure success referred to the successful intervention of the target lesions with residual stenosis <30% by visual estimation. MI was defined as new onset of chest pain accompanied with an elevation of troponin I more than 3 times the 99th percentile upper reference limit. Stroke was diagnosed by computed tomography scans. Bleeding was defined using “Bleeding Academic Research Consortium (BARC)” definitions.[4] Major bleeding referred to bleeding meeting BARC ≥3 grade and was distinguished as either access site related or nonaccess site related. Vascular complications included large hematoma, arteriovenous fistula, pseudo aneurysm, and retroperitoneal hematoma. Follow-up checkups were performed at 6 months and 1-year by telephone. The occurrence of death was determined by the death certificate from the police office, and the occurrence of MI or repeat revascularization was determined by the diagnosis certificate from the treating hospital.
Statistics
Continuous variables were expressed as mean values ± standard deviation, and compared with Student's t-test. Categorical variables were shown as frequencies and proportions, and compared with Chi-square test or Fisher's exact test. The 1-year cumulative incidence of MACE was estimated by Kaplan–Meier curves and compared with log-rank test. Cox proportional hazards model was performed to determine the predictors of major adverse outcomes. The variables included in the model were as follows: Age, gender, prior MI, prior PCI, prior stroke, diabetes mellitus, hypertension, hyperlipidemia, peri-procedure medication, hemoglobin, sheath size, coronary angiography, use of intra-aortic balloon pump, left ventricle ejection fraction (LVEF), thrombolysis in MI (TIMI) flow after PCI, DES, total length of stent, and mean diameter of stent. Data were analyzed in the SAS software, version 9.13 (SAS Institute, USA). A two-sided P < 0.05 indicated statistical significance.
RESULTS
Baseline characteristics
A total of 404 post-CABG patients were included in the analysis. A total of 113 patients (28%) had TRA PCI, and the rest (72%) had TFA PCI. The baseline characteristics are shown in Table 1. No significant difference was detected with regard to age, gender, prior MI, prior PCI, and diabetes between TRA and TFA. However, patients in the TRA group had a higher rate of hypertension and hyperlipidemia (P < 0.05). Patients’ indications for PCI were similar between TRA and TFA. The mean value of LVEF and the use of peri-procedure medication were also similar.
Table 1.
Baseline characteristics
| Variable | TRA (n = 113) | TFA (n = 291) | P |
|---|---|---|---|
| Age (years) | 62.76 ± 9.16 | 62.75 ± 9.47 | 0.9935 |
| Age ≥ 80 years | 112 (99.1) | 285 (97.9) | 0.3849 |
| Male | 91 (80.5) | 232 (79.7) | 0.8556 |
| Prior MI, n (%) | 46 ( 40.7) | 129 (44.3) | 0.5089 |
| Prior PCI, n (%) | 36 (31.9) | 74 (25.4) | 0.1969 |
| Prior stroke, n (%) | 9 (8.0) | 10 (3.4) | 0.0658 |
| Diabetes, n (%) | 41 (36.3) | 85 (29.2) | 0.1718 |
| Hypertension, n (%) | 84 (74.3) | 182 (62.5) | 0.0227 |
| Hyperlipidemia, n (%) | 75 (66.4) | 154 (52.9) | 0.0136 |
| Diagnosis, n (%) | |||
| STEMI | 5 (4.4) | 11 (3.8) | 0.7680 |
| NSTEMI | 8 (7.1) | 12 (4.1) | 0.2190 |
| Unstable angina | 69 (61.1) | 168 (57.7) | 0.5421 |
| Stable angina | 30 (26.5) | 88 (30.2) | 0.4642 |
| LVEF (%) | 58.69 ± 7.34 | 58.46 ± 7.92 | 0.7926 |
| LVEF < 50% | 11 (9.8) | 33 (11.8) | 0.5733 |
| Hemoglobin (g/L) | 137.39 ± 13.64 | 137.44 ± 14.83 | 0.9788 |
| Peri-procedural medication, n (%) | |||
| GP IIb/IIIa inhibitor | 6 (5.3) | 9 (3.1) | 0.3066 |
| Warfarin | 1 (0.9) | 5 (1.7) | 0.5130 |
| LMWH | 90 (79.6) | 224 (77.0) | 0.5601 |
| Fondaparinux | 2 (1.8) | 5 (1.7) | 0.9715 |
Data represented as n (%) or mean ± SD. GP IIb/IIIa inhibitor: Glycoprotein IIb/IIIa inhibitor; LMWH: Low weight molecule heparin; LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; NSTEMI: Non-ST-segment elevation myocardial infarction; PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TFA: Transfemoral approach; TRA: Transradial approach; SD: Standard deviation.
Angiographic characteristics and procedure outcomes
Angiographic characteristics and procedure outcomes are shown in Table 2. No significant difference was found in terms of the mean number, type or severity of the atherosclerosis of the grafts between the TRA and TFA group. Meanwhile, the proportion of the native vessel PCI, graft PCI, or both were similar between the TRA and TFA groups. The mean TIMI flows before and after the procedure was similar between TRA and TFA. DES was used in more than 90% in both groups. The mean diameter of the stents was larger in the TRA group, but no significant difference was detected with regard to the mean number and total length of stents for each patient.
Table 2.
Angiographic characteristics and procedure outcomes
| Variable | TRA (n = 113) | TFA (n = 291) | P |
|---|---|---|---|
| Graft | |||
| Number of grafts | 2.42 ± 0.83 | 2.55 ± 0.83 | 0.1453 |
| IMA graft, n (%) | 95 (84.1) | 258 (88.7) | 0.2217 |
| SVG graft, n (%) | 99 (87.6) | 261 (89.7) | 0.5515 |
| Graft stenosis, n (%) | 85 (75.2) | 225 (77.3) | 0.6556 |
| Intervention, n (%) | |||
| Native vessel | 84 (75.0) | 211 (73.0) | 0.7103 |
| Graft | 24 (21.4) | 69 (23.9) | 0.5958 |
| Native vessel + graft | 4 (3.6) | 9 (3.1) | 0.8191 |
| Device | |||
| Sheath size | 6.03 ± 0.28 | 6.07 ± 0.47 | 0.3114 |
| Sheath size ≥ 7F, n (%) | 6 (5.3) | 25 (8.6) | 0.2497 |
| DES, n (%) | 111 (98.2) | 288 (99.0) | 0.5604 |
| Number of stent | 1.95 ± 1.14 | 1.79 ± 0.98 | 0.2103 |
| Stent diameter (mm) | 3.13 ± 0.56 | 3.04 ± 0.56 | 0.0488 |
| Total length of stent (mm) | 43.79 ± 30.69 | 42.47 ± 28.82 | 0.6864 |
| IABP support | 2 (1.8) | 4 (1.4) | 0.7720 |
Data represented as n (%) or mean ± SD. IMA: Internal mammary artery; LM: Left main disease; IABP: Intra-aortic balloon pump; SVG: Saphenous vein graft; TFA: Transfemoral approach; TIMI: Thrombolysis in myocardial infarction; TRA: Transradial approach; SD: Standard deviation; DES: Drug-eluting stent.
Clinical outcomes
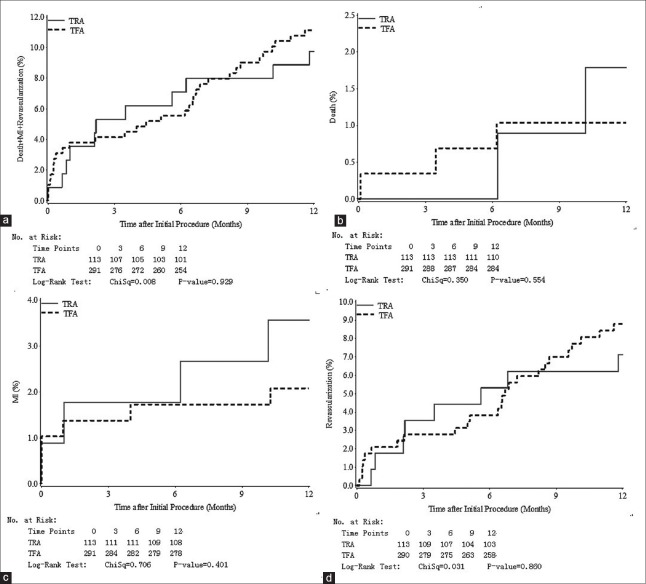
Angiographic success was higher in the TFA group (100% vs. 97.4%, P = 0.02). Three patients (2.6%) had been transferred to the TFA group because of failed catheterization: One was due to the spasm of the radial artery, two were due to the tortuosities of the upper arms. No patient was transferred to the TRA group from the TFA group. Procedure success was similar between the TRA group and the TFA group (99.1% vs. 97.9%, P = 0.68). The incidence rates of in-hospital NACE (2.7% vs. 2.7%, P = 1.00) were similar between the TRA group and the TFA group. One-year rates of MACE (11.5% vs. 12.0%, P = 0.88) were also similar. Patients in the TRA group had a shortened postprocedure stay (3 (2–4) days vs. 3 (2–5) days, P = 0.03). The detailed outcomes are shown in Table 3. The Kaplan–Meier curves of 1-year MACE are shown in Figure 1.
Table 3.
Short- and medium-term clinical outcomes
| Variables | TRA (n = 113) | TFA (n = 291) | P |
|---|---|---|---|
| Angiographic success | 110 (97.4) | 291 (100) | 0.0210 |
| Procedure success | 112 (99.1) | 285 (97.9) | 0.6791 |
| In-hospital outcomes | |||
| NACE | 3 (2.7) | 8 (2.7) | 1.0000 |
| All-cause death | 0 (0.0) | 1 (0.3) | 1.0000 |
| MI | 1 (0.9) | 3 (1.0) | 1.0000 |
| Stroke | 0 (0.0) | 0 (0.0) | 1.0000 |
| Repeat revascularization | 0 (0.0) | 2 (0.7) | 1.0000 |
| BARC ≥ 2 grade bleeding | 10 (8.8) | 54 (18.6) | 0.0118 |
| Access site related bleeding | 8 (7.1) | 40 (13.7) | 0.0517 |
| Access site complications | 8 (7.1) | 32 (11.0) | 0.2226 |
| Total hospital stay | 7 (5,10) | 7 (5, 9) | 0.6253 |
| Postprocedure stay | 3 (2,4) | 3 (2, 5) | 0.0256 |
| One-year outcomes | |||
| MACE | 13 (11.5) | 35 (12.0) | 0.8837 |
| All-cause death | 2 (1.8) | 3 (1.0) | 0.6220 |
| MI | 4 (3.5) | 6 (2.1) | 0.4757 |
| Repeat revascularization | 13 (11.5) | 28 (9.6) | 0.8103 |
Data represented as n (%) or mean ± SD. BARC: Bleeding Academic Research Consortium; MACE: Major adverse cardiovascular event; MI: Myocardial infarction; NACE: Net adverse clinical events; TFA: Transfemoral approach; TRA: Transradial approach; SD: Standard deviation.
Figure 1.
(a) Curves for time to major adverse cardiovascular events according to transradial or transfemoral approach at 12 months of follow-up; (b) Curves for time to death according to transradial or transfemoral approach at 12 months of follow-up; (c) Curves for time to MI according to transradial or transfemoral approach at 12 months of follow-up; (d) Curves for time to repeat revascularization according to transradial or transfemoral approach at 12 months of follow-up. MI: Myocardial infarction; TFA: Transfemoral approach; TRA: Transradial approach.
A total of 28 (24.8%) patients in the TRA group and 78 (26.8%) patients in the TFA group underwent graft intervention. Major outcomes of TRA and TFA for patients undergoing graft intervention are shown in Table 4. The procedure success was similar between TRA and TFA (100% vs. 98.7%, P = 1.00). The procedure time (25 (16–39) minutes vs. 27.5 (15–40) minutes, P = 0.53) and access site complications (7.1% vs. 7.7%, P = 1.00) were also similar. No significant difference was detected between TRA and TFA in terms of in-hospital NACE (0 vs. 0, P = 1.00) and 1-year MACE (21.4% vs. 10.3%, P = 0.19) in these patients.
Table 4.
Outcomes for patients undergoing graft intervention
| Variable | TRA (n = 28) | TFA (n = 78) | P |
|---|---|---|---|
| Procedure success | 28 (100) | 77 (98.7) | 1.0000 |
| Procedure time (min) | 25 (16, 39) | 27.5 (15, 40) | 0.5271 |
| Access site complications | 2 (7.1) | 6 (7.7) | 1.0000 |
| In-hospital NACE | 0 (0) | 0 (0) | 1.0000 |
| 1-year MACE | 6 (21.4) | 8 (10.3) | 0.1904 |
Data represented as n (%) or median (interquartile range). MACE: Major adverse cardiovascular event; NACE: Net adverse clinical event; TRA: Transradial approach; TFA: Transfemoral approach.
Access site complications occurred more in the TFA group than in the TRA group, although the difference did not reach statistical significance (11.0% vs. 7.1%, P = 0.22). Severe complications, such as arteriovenous fistula, pseudo aneurysm, and retroperitoneal hematoma, were found only in the TFA group. BACR ≥2 grade bleeding was significantly higher in the TFA group (18.6% vs. 8.8%, P = 0.012), and access site-related bleeding was more frequently found in the TFA group (7.1% vs. 13.7%, P = 0.052). Cox proportional hazards model showed that the TFA was an independent predictor of BARC ≥2 bleeding (heart rate: 2.41, 95% confidence interval: 1.14–5.10) [Table 5].
Table 5.
Multivariable regression analysis
| Outcomes | HR (95% CI) | P |
|---|---|---|
| In-hospital outcomes | ||
| NACE | 0.83 (0.17–4.10) | 0.8214 |
| Access site complications | 1.67 (0.71–3.93) | 0.2375 |
| BARC ≥ 2 grade bleeding | 2.41 (1.14–5.10) | 0.0216 |
| One-year outcomes | ||
| MACE | 1.01 (0.80–1.27) | 0.9355 |
BARC: Bleeding Academic Research Consortium; CI: Confidence interval; MACE: Major adverse cardiovascular event; NACE: Net adverse clinical event; HR: Hazard ratio.
DISCUSSION
The main findings of this study showed that in patients with histories of CABG, TRA is feasible and effective in the angiography and intervention. The short- and medium-term outcomes are comparable between TRA and TFA, except that TRA is associated with a lower rate of access site related bleeding and a shortened postprocedure stay.
Patients with a history of CABG usually have severe coronary lesions and are at high risk of cardiovascular events. Although the graft works well right after the bypass surgery, the long-term patency of the graft raises concerns. Saphenous vein graft (SVG) and the internal mammary artery (IMA) are estimated to block up again within 10 years in 40% and 15% of the post-CABG patients, respectively.[5] A second CABG surgery was not suggested because of the serious chest tissue adhesion and the increased risk of death after the surgery.[6] However, PCI was still effective in treating occluded grafts.
Transradial approach PCI has been increasingly used since its first successful application in 1993[7] not only because of the easier puncturing and hemostasis, but also for the better survival rate in certain patients.[8,9] Han et al. found similar rates of short-term major adverse cardiac and cerebrovascular events between TRA and TFA (1.5% vs. 5.4%, P = 0.479) in post-CABG patients undergoing angiography or PCI.[10] Bundhoo et al. and Ziakas et al. reported similar short-term death and MACE in post-CABG patients undergoing SVG PCI.[11,12]. Consistent with these results, the present study showed similar procedure success and short-term clinical outcomes between TRA and TFA. Our study was also the first to report the 1-year outcomes between the two groups, whereas no difference was detected in those patients undergoing angiography or PCI.
Access site-related bleeding accounts for approximately 50%-80% of all major bleeding events in patients undergoing PCI.[13] A recently published meta-analysis showed that the introduction of TRA decreased the risk of access site related bleeding by 73%.[14] This reduction could lead to better patient outcomes.[15,16] More BARC 2 bleeding was detected in the TFA group than in the TRA group, indicating the benefit of TRA in reducing nuisance bleeding, even under the frequent use of GP IIb/IIIa inhibitors.[17]
The rates of access site complications were also similar between TRA and TFA (7.1% vs. 11.0%, P = 0.22). However, severe complications occurred only in the TFA group, and two of them led to transfusion with great cost. The postprocedure stay was shortened by almost 1-day in the TRA group compared with that in the TFA group, which is attributed to patients’ earlier ambulation. One concern about TRA is the crossover rate. However, Sanmartin et al. showed similar crossover rates between TRA and TFA (4.0% vs. 1.3%, P = 0.28) in post-CABG patients.[18] In addition, only three patients (2.6%) had transferred from TRA to TFA in the present study.
Some researchers have concerns regarding the complete getting through or the backups of the catheters in the angiography or the intervention of the grafts with TRA. If the graft was left IMA, the radial artery approach through the right hand may be difficult because of the multiple angulations in the route and the absence of adequate support. However, with the development of catheters, experienced doctors can conquer the obstacles. Through proper seating, and adequate guide support, the angiography or the intervention of the graft is feasible. Cha et al., Valsecchi and Vassileva both reported the feasibility of the right hand TRA for the angiography of the left IMA.[19,20] Meanwhile, we observed a high rate of angiographic success and procedure success with right-hand TRA for the left IMAs.
The retrospective design was the natural weakness of our study. The selection of the route was not randomized but at the doctor's discretion, which may result in selection bias. However, most of the patients’ baseline characteristics were similar between the TRA and TFA groups, and a multivariable regression analysis was performed to adjust for potential confounders. All the PCIs were conducted only in one hospital, which may restrict the extrapolation of the result to the general condition. Considering that all the interventionists had great expertise on TRA, in the present study, further investigation is needed to determine the performance success for TRA beginners.
In conclusion, transradial approach PCI showed great feasibility and safety in patients with histories of CABG. The procedure success was similar between TRA and TFA. However, TRA was associated with a lower rate of severe vascular complications and access site-related bleeding.
Footnotes
Edited by: Li-Shao Guo
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodés-Cabau J, Larose E, et al. Transradial approach for coronary angiography and interventions: Results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3:1022–31. doi: 10.1016/j.jcin.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Amoroso G, Kiemeneij F. Transradial access for primary percutaneous coronary intervention: The next standard of care? Heart. 2010;96:1341–4. doi: 10.1136/hrt.2010.196824. [DOI] [PubMed] [Google Scholar]
- 3.Burzotta F, Trani C, Hamon M, Amoroso G, Kiemeneij F. Transradial approach for coronary angiography and interventions in patients with coronary bypass grafts: Tips and tricks. Catheter Cardiovasc Interv. 2008;72:263–72. doi: 10.1002/ccd.21567. [DOI] [PubMed] [Google Scholar]
- 4.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 5.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a department of veterans affairs cooperative study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 6.Verheul HA, Moulijn AC, Hondema S, Schouwink M, Dunning AJ. Late results of 200 repeat coronary artery bypass operations. Am J Cardiol. 1991;67:24–30. doi: 10.1016/0002-9149(91)90093-z. [DOI] [PubMed] [Google Scholar]
- 7.Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn. 1993;30:173–8. doi: 10.1002/ccd.1810300220. [DOI] [PubMed] [Google Scholar]
- 8.Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (rival): A randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 9.Joyal D, Bertrand OF, Rinfret S, Shimony A, Eisenberg MJ. Meta-analysis of ten trials on the effectiveness of the radial versus the femoral approach in primary percutaneous coronary intervention. Am J Cardiol. 2012;109:813–8. doi: 10.1016/j.amjcard.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Han H, Zhou Y, Ma H, Liu Y, Shi D, Zhao Y, et al. Safety and feasibility of transradial approach for coronary bypass graft angiography and intervention. Angiology. 2012;63:103–8. doi: 10.1177/0003319711408863. [DOI] [PubMed] [Google Scholar]
- 11.Bundhoo SS, Earp E, Ivanauskiene T, Kunadian V, Freeman P, Edwards R, et al. Saphenous vein graft percutaneous coronary intervention via radial artery access: Safe and effective with reduced hospital length of stay. Am Heart J. 2012;164:468–72. doi: 10.1016/j.ahj.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Ziakas A, Klinke P, Mildenberger R, Fretz E, Williams M, Della Siega A, et al. A comparison of the radial and the femoral approach in vein graft PCI. A retrospective study. Acute Card Care. 2005;7:93–6. doi: 10.1080/14628840510011270. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand OF, Bélisle P, Joyal D, Costerousse O, Rao SV, Jolly SS, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: A systematic review and hierarchical Bayesian meta-analysis. Am Heart J. 2012;163:632–48. doi: 10.1016/j.ahj.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: A systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–40. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364–9. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, et al. Comparison of 3-year clinical outcomes after transradial versus transfemoral percutaneous coronary intervention. Cardiovasc Interv Ther. 2012;27:84–92. doi: 10.1007/s12928-012-0098-z. [DOI] [PubMed] [Google Scholar]
- 17.Kajiya T, Agahari F, Wai KL, Tai BC, Lee CH, Chan KH, et al. A single-center experience of transitioning from a routine transfemoral to a transradial intervention approach in ST-elevation myocardial infarction: Impact on door-to-balloon time and clinical outcomes. J Cardiol. 2013;62:12–7. doi: 10.1016/j.jjcc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Sanmartin M, Cuevas D, Moxica J, Valdes M, Esparza J, Baz JA, et al. Transradial cardiac catheterization in patients with coronary bypass grafts: Feasibility analysis and comparison with transfemoral approach. Catheter Cardiovasc Interv. 2006;67:580–4. doi: 10.1002/ccd.20633. [DOI] [PubMed] [Google Scholar]
- 19.Cha KS, Kim MH, Hung JS, Woo JS, Kim YD, Kim JS. Nonselective left internal mammary artery angiography during right transradial coronary angiography: A simple, rapid, and safe technique. Angiology. 2001;52:773–9. doi: 10.1177/000331970105201107. [DOI] [PubMed] [Google Scholar]
- 20.Valsecchi O, Vassileva A. Safety and feasibility of selective angiography of left internal mammary artery grafts via right transradial approach. Indian Heart J. 2010;62:255–7. [PubMed] [Google Scholar]