Abstract
Background:
Despite great reduction of in-stent restenosis, first-generation drug-eluting stents (DESs) have increased the risk of late stent thrombosis due to delayed endothelialization. Arsenic trioxide, a natural substance that could inhibit cell proliferation and induce cell apoptosis, seems to be a promising surrogate of sirolimus to improve DES performance. This randomized controlled trial was to evaluate the efficacy and safety of a novel arsenic trioxide-eluting stent (AES), compared with traditional sirolimus-eluting stent (SES).
Methods:
Patients with symptoms of angina pectoris were enrolled and randomized to AES or SES group. The primary endpoint was target vessel failure (TVF), and the second endpoint includes rates of all-cause death, cardiac death or myocardial infarction, target lesion revascularization (TLR) by telephone visit and late luminal loss (LLL) at 9-month by angiographic follow-up.
Results:
From July 2007 to 2009, 212 patients were enrolled and randomized 1:1 to receive either AES or SES. At 2 years of follow-up, TVF rate was similar between AES and SES group (6.67% vs. 5.83%, P = 0.980). Frequency of all-cause death was significantly lower in AES group (0 vs. 4.85%, P = 0.028). There was no significant difference between AES and SES in frequency of TLR and in-stent restenosis, but greater in-stent LLL was observed for AES group (0.29 ± 0.52 mm vs. 0.10 ± 0.25 mm, P = 0.008).
Conclusions:
After 2 years of follow-up, AES demonstrated comparable efficacy and safety to SES for the treatment of de novo coronary artery lesions.
Keywords: Arsenic Trioxide, Coronary Artery Disease, Drug-eluting Stent
INTRODUCTION
Percutaneous coronary intervention (PCI) has become the most widely used method to treat patients with coronary artery disease. The introduction of drug-eluting stents (DESs) led the interventional cardiology to a new era, in which the PCI became an alternative to coronary artery bypass surgery even in complex lesions.
Paclitaxel, sirolimus, and its derivatives were the most widely used anti-proliferative drugs of DES and their efficacy in inhibiting smooth muscle cell proliferation had been verified in several clinical trials. However, several studies found that both sirolimus and paclitaxel lead to delayed arterial healing[1] and endothelial dysfunction,[2] which could be the major reason of the increasing late and very late stent thrombosis (VLST) events.[3]
A new DES platform combining the approved 316L stainless stent platform with a novel anti-proliferative agent, arsenic trioxide, and a biodegradable poly-l-lactic acid (PLLA) polymer has been developed. Arsenic trioxide, a chemotherapy drug that has been widely used in the treatment of acute promyelocytic leukemia (APL), was proved to inhibit cell growth and induce cell apoptosis.[4] Our previous study had proved this stent system significantly suppressed in-stent restenosis by reducing proliferation and inducing apoptosis of vascular smooth muscle cells (VSMCs) compared to uncoated stents in a rabbit iliac artery injury model.[5] In addition, it also inhibits local inflammatory reactions, which suppresses the endothelial healing.[6]
The purpose of this study was to investigate whether the AES (Amsinomed Medical Company, Beijing, China) is effective and safe as an alternative to current DES in human. The FIREBIRD™ SES (Microport Corporation, Shanghai, China), a widely used DES in China, is selected as the comparison.
METHODS
Study design and population
The trial was a multicenter, perspective, randomized controlled trial designed to compare clinical and angiographic outcomes between arsenic trioxide-eluting stents (AESs) and sirolimus-eluting stents (SES) [Figure 1]. The study protocol was approved by the Ethics Committee at each participating center and was conducted according to the principles of the Declaration of Helsinki regarding investigations in humans.
Figure 1.
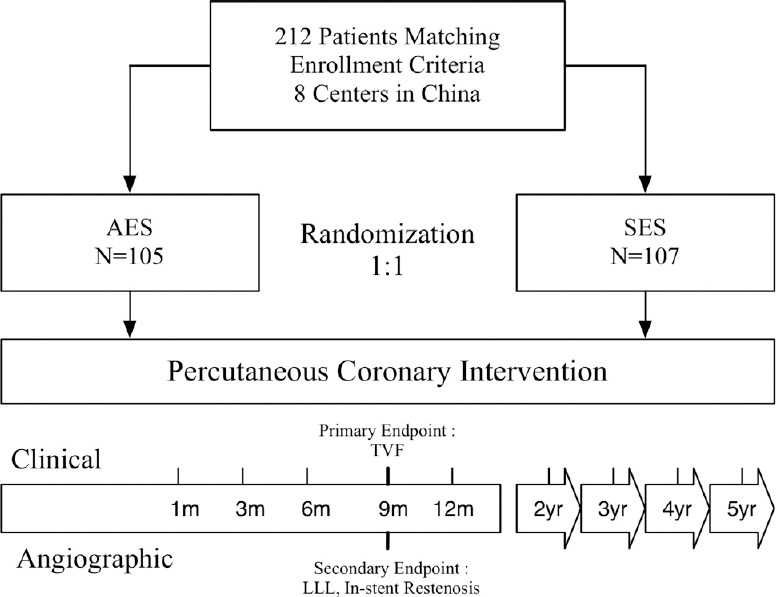
Patient flow chart. Flow chart showed patient flow and follow-up during the study. AES: Arsenic trioxide-eluting stent(s); SES: Sirolimus-eluting stent(s); TVF: Target vessel failure; LLL: Late luminal loss.
Patients ≥18 and <80 years of age were eligible for enrollment if they showed symptoms of angina pectoris and/or signs of ischemia or angina and met the main inclusion criteria of coronary angiography: (1) Lesion length ≤37 mm; (2) reference vessel diameter (RVD) 2.0–4.0 mm; (3) a diameter stenosis ≥70% or total occlusion lesion. No restriction was applied to the total number of treated lesions, treated vessels, or number of stents implanted. Exclusion criteria included cardiac shock, congenital heart disease, severe valvular disease, left ventricular ejection fraction <30%, allergy to antiplatelet drugs, heparin, stainless steel, contrast agents, surgery in 6 weeks, acute myocardial infarction (MI) within 2 weeks, transient ischemic attack or stroke within 7 days, WBC <4.0 × 109/L, PBC <100 × 109/L, 1.5 times higher than normal value of alanine aminotransferases, aspartate aminotransferase, or alkaline phosphate, using thrombolytic agents or GPIIb/IIIa antagonists in 1 week, gastrointestinal and fundus bleeding within 6 months, true bifurcation lesion with side branch diameter >2.00 mm, life expectancy <1 year, stented target lesion, inability or unwillingness to comply with all protocol-required procedure, and a positive pregnancy test within 24 h before enrollment. All patients provided written, informed consent for participation in this trial.
Randomization and procedure
Randomization was carried out after diagnostic coronary angiography and before PCI. Patients were assigned a number from a randomization sequence generated by SAS 9.1 (SAS Institute, North Carolina, USA) and maintained by a statistician who was blinded to the treatments. We randomly assign patients (1:1) to receive an AES or an SES.
Quantitative coronary analysis was required prior to stenting. All coronary stent implantations were performed according to standard techniques. AES were available in diameters of 2.0, 2.5, 2.75, 3.0, 3.5, and 4.0 mm and in lengths of 8, 13, 17, 22, 27, 33, and 37 mm. Both the AES and SES were expanded to achieve <20% residual stenosis by visual estimate in the treated segment, with a combination of the stent deployment balloon and at the operators’ discretion, subsequent postdilatation balloons.
Patients who were not on long-term antiplatelet or aspirin therapy were required to receive a loading dose of aspirin (300 mg/d) and clopidogrel bisulfate (300 mg) at least 24 h before the procedure, followed by 75 mg/d of clopidogrel after procedure. At discharge, patients were maintained on at least 100 mg/d of aspirin and 75 mg/d of clopidogrel for at least 6 months. Heparin was administered throughout the procedure to maintain an activated clotting time ≥230 s.
Follow-up
All patients were scheduled to undergo a repeated angiography at 9 months. Clinical follow-up was performed at 1, 3, 6, 9, 18, 24 months by telephone. At follow-up, patients were specifically questioned regarding any adverse events or angina symptoms or other interventional therapy.
End points and definition
The primary end point was target vessel failure (TVF), a composite of cardiac death, target vessel MI, clinically driven target vessel revascularization (defined as a repeated revascularization due to either a >50% restenosis in the treated lesion with symptoms of ischemia or for a >70% restenosis with or without symptoms within the entire major coronary vessel proximal and distal to a target lesion, including upstream and downstream branches and the target lesion itself). The secondary endpoints included late luminal loss (LLL), procedure success rate, in-stent restenosis rate, target lesion revascularization (TLR), all-cause death, cardiac death or MI. The LLL was defined as the difference between postprocedural and 9-month angiography follow-up minimal lumen diameter. The quantitative coronary angiography measurements were obtained for both the stented segment (“in-stent”) and a segment covering the stented length as well as 5-mm distal and proximal margins (“in-segment”). Restenosis was defined as >50% stenosis of target lesion in target vessel. TLR was defined as any ischemia-driven repeat PCI of the target lesion or bypass surgery of the target vessel. Cardiac death was defined as death due to any of the following: Acute MI; cardiac perforation/pericardial tamponade; arrhythmia or conduction abnormality. The trial was designed to test the efficacy of AES compared with SES with respect to the primary endpoint of TVF. All primary and secondary endpoints were adjudicated by the independent and blinded clinical event committee.
Statistical analysis
The objective of the study was to assess efficacy of AES compared with the SES with regard to the primary endpoint, TVF. The sample size was thus calculated using a t-test. Based on the previous studies, we expect a mean in-stent LLL of 0.40 mm FIREBIRD SES. The standard deviation (SD) for LLL was presumed to be 0.45 mm, so the noninferiority margin was set at 0.15 mm (equaling 35% of 0.45 mm). Based on these assumptions, 102 patients per treatment group needed to be analyzed to provide a statistical power of 0.80 at an alpha level of 0.05.
Continuous variables are presented as mean ± SD, and categorical variables are presented as counts and percentages. Categorical variables were compared using Chi-square and Fisher's exact tests while continuous variables were compared using Student's t-test. Survival and event-free status were assessed using the methods of Kaplan–Meier. All statistical analyses were performed by a physician with the use of R version 3.0.2 (http://www.r-project.org). All reported P values were two-sided, and P < 0.05 were regarded as statistically significant.
RESULTS
Baseline characteristics and procedural results
Between July 2009 and January 2010, 212 patients and 265 lesions were enrolled into this randomized study at eight sites across China. At 24 months, clinical follow-up was available for 105 patients in AES group and 103 patients in SES group. Baseline, procedural, and lesion characteristics of the two randomized groups of patients were similar. The average age of the patients was 58.6 years, and 69.8% of enrolled patients were male. All of them suffered a history of angina pectoris and presented with an abnormal electrocardiogram and abnormal ultrasonic cardiogram [Table 1]. Of a total of 265 treated lesions, approximately half of them are type B2/C lesions with mean RVDs of 3.01 ± 0.36 mm and lesion lengths of 19.97 ± 8.20 mm (AES) and 19.52 ± 8.13 mm (SES). There was no significant difference between the baseline target lesions, but more severe diameter stenosis was observed in AES group (87.09 ± 8.86% vs. 83.36 ± 9.00%, P = 0.001) [Table 2].
Table 1.
Baseline patient characteristics
| Variables | AES (n = 105) | SES (n = 107) | P |
|---|---|---|---|
| Age (years) | 57.9 ± 9.3 | 59.3 ± 9.5 | 0.281 |
| Male, n (%) | 73 (69.5) | 75 (70.1) | 0.928 |
| Body weight (kg) | 69.4 ± 8.5 | 69.7 ± 9.2 | 0.844 |
| Stature (cm) | 167.4 ± 6.4 | 167.7 ± 6.4 | 0.709 |
| Clinical indication | |||
| Angina pectoris, n (%) | 105 (100.0) | 107 (100.0) | 1.000 |
| Duration of angina (m) | 13.9 ± 24.0 | 25.1 ± 44.8 | 0.059 |
| Medication for angina, n (%) | 56 (53.3) | 64 (59.8) | 0.341 |
| Abnormal ECG, n (%) | 105 (100.0) | 107 (100.0) | 1.000 |
| Abnormal UCG, n (%) | 105 (100.0) | 107 (100.0) | 1.000 |
| History of other diseases, n (%) | 50 (47.6) | 63 (58.9) | 0.100 |
| Medication for other diseases, n (%) | 27 (25.7) | 35 (33.0) | 0.263 |
ECG: Electrocardiogram; UCG: Ultrasound cardiogram; AES: Arsenic trioxide-eluting stent; SES: Sirolimus-eluting stent.
Table 2.
Lesion and procedure characteristics
| Variables | AES | SES | P |
|---|---|---|---|
| AHA/ACC lesion classification, n (%) | |||
| Type A | 29 (21.48) | 24 (18.46) | 0.784 |
| Type B1 | 38 (28.15) | 39 (30.00) | |
| Type B2 | 28 (28.74) | 23 (17.69) | |
| Type C | 40 (29.63) | 44 (33.85) | |
| Moderate/heavy calcification, n (%) | 9 (6.67) | 7 (5.38) | 0.857 |
| Ostial lesion, n (%) | 6 (4.44) | 10 (7.69) | 0.394 |
| Total occlusion, n (%) | 13 (9.63) | 5 (3.85) | 0.104 |
| Bifurcation lesions, n (%) | 7 (5.19) | 9 (6.92) | 0.737 |
| Target lesion length (mm) | 19.97 ± 8.20 | 19.52 ± 8.13 | 0.651 |
| Target lesion DS (%) | 87.09 ± 8.86 | 83.36 ± 9.00 | 0.001 |
| RVD (mm) | 3.03 ± 0.36 | 3.00 ± 0.44 | 0.547 |
| Pretreatment for stenting, n (%) | |||
| Predilatation usage | 66 (62.9) | 55 (51.4) | 0.092 |
| Overlapped stent implantation | 20 (19.0) | 14 (13.1) | 0.237 |
| Postdilatation usage | 27 (25.7) | 35 (32.7) | 0.263 |
DS: Diameter stenosis; RVD: Reference vessel diameter; AES: Arsenic trioxide-eluting stent; SES: Sirolimus-eluting stent; ACC: American College of Cardiology; AHA: American Heart Association.
In both groups, a certain proportion of patients were treated with balloon predilatation (62.9% AES vs. 51.4% SES) and postdilatation (25.7% AES vs. 32.7% SES) [Table 2]. Average stent diameter (3.01 ± 0.35 mm AES vs. 3.00 ± 0.43 mm SES) and mean stent length (21.22 ± 5.77 mm AES vs. 22.12 ± 6.59 mm SES) have been illustrated in Table 3. Acute procedural success rate was 100% in both groups.
Table 3.
Stent implantation data
| Variables | AES (n = 157) | SES (n = 150) | P |
|---|---|---|---|
| Stent diameter (mm) | 3.01 ± 0.35 | 3.00 ± 0.43 | 0.690 |
| Stent length (mm) | 21.22 ± 5.77 | 22.12 ± 6.59 | 0.202 |
| Maximal stent dilating pressure (atm) | 14.58 ± 3.59 | 14.18 ± 3.40 | 0.318 |
| Maximal stent dilating duration (s) | 10.27 ± 5.56 | 10.67 ± 5.27 | 0.519 |
| Stent dilating times | 1.39 ± 0.67 | 1.37 ± 0.87 | 0.807 |
AES: Arsenic trioxide-eluting stent; SES: Sirolimus-eluting stent.
Clinical outcomes and angiographic characteristics
The follow-up rate was more than 80% through the 24-month follow-up. The primary endpoint TVF in AES group was stable (6.67%), but an increasing trend was observed in SES group (1.87%–5.83%). No significant difference between two groups was concluded. All cause death rate, as well as cardiac death or MI rate (0 vs. 3.88%, P = 0.058) at 24 months, was lower in AES group compared with SES group (0 vs. 4.85%, P = 0.028). Other adverse events including TLR, cerebral hemorrhage, noncardiac death, stent thrombosis, and angina pectoris were similar in both groups [Table 4]. 85 patients with 101 lesions in AES group (80.95%) and 57 patients with 62 lesions in SES group (53.27%) underwent angiographic follow-up at 9 months after stent implantation. In-segment late loss was 0.20 ± 0.22 mm in AES group and 0.10 ± 0.35 mm SES group. In-stent late loss was 0.29 ± 0.52 mm in AES group and 0.10 ± 0.25 mm in SES group. The difference in in-stent late loss between two groups was 0.18 mm (95% confidence interval: 0.077–0.313 mm), which was larger than the noninferiority margin as 0.15 mm. Binary in-stent and in-segment restenosis rate were not different between the two groups; however, diameter stenosis was statistically greater in AES group than SES group for both in-stent (21.05% ± 18.70% vs. 12.01% ± 16.26%, P = 0.002) and in-segment (22.86% ± 11.34% vs. 17.83% ± 13.59%, P = 0.012) [Table 5].
Table 4.
Clinical outcomes, n (%)
| Variables | AES (n = 105) | SES (n = 107) | P |
|---|---|---|---|
| TVF | |||
| At 270 days | 7/105 (6.67) | 2/107 (1.87) | 0.100 |
| At 18 months | 7/105 (6.67) | 4/102 (3.92) | 0.538 |
| At 24 months | 7/105 (6.67) | 6/103 (5.83) | 0.802 |
| Cardiac death or MI | 0/105 (0) | 4/103 (3.88) | 0.058 |
| TLR | 7/105 (6.67) | 2/103 (1.94) | 0.170 |
| All-cause death | 0/105 (0) | 5/103 (4.85) | 0.028 |
| Noncardiac death | 0/105 (0) | 1/103 (0.97) | 0.495 |
| Stent thrombosis | |||
| Definite ST | 0/105 (0) | 1/103 (0.97) | 0.495 |
| Probable ST | 0/105 (0) | 2/103 (1.94) | 0.244 |
| Possible ST | 0/105 (0) | 1/103 (0.97) | 0.495 |
| Angina pectoris | 42/103 (40.78) | 37/96 (38.54) | 0.747 |
| Cerebral hemorrhage | 1/105 (0.95) | 1/103 (0.97) | 1.000 |
TVF: Target vessel failure; MI: Myocardial infarction; TLR: Target lesion revascularization; ST: Stent thrombosis; AES: Arsenic trioxide-eluting stent; SES: Sirolimus-eluting stent.
Table 5.
Nine-month angiographic follow-up results
| Variables | AES (n = 101) | SES (n = 62) | P |
|---|---|---|---|
| Late luminal loss (mm) | |||
| In-stent | 0.29 ± 0.52 | 0.10 ± 0.25 | 0.008 |
| In-segment | 0.20 ± 0.22 | 0.10 ± 0.35 | 0.026 |
| Binary restenosis rate, n (%) | 10 (9.90) | 1 (1.61) | 0.041 |
| In-stent | 8 (7.92) | 1 (1.61) | 0.155 |
| In-segment | 5 (4.94) | 1 (1.61) | 0.409 |
| Diameter stenosis (%) | |||
| In-stent | 21.05 ± 18.70 | 12.01 ± 16.26 | 0.002 |
| In-segment | 22.86 ± 11.34 | 17.83 ± 13.59 | 0.012 |
AES: Arsenic trioxide-eluting stent; SES: Sirolimus-eluting stent.
DISCUSSION
The main findings of the current study were as below: (1) The efficacy of AES and SES was similar over the follow-up period, with no significant difference observed in the incidence of TVF. (2) AES group has a significant lower rate of all-cause death than SES group, but not in LLL and in-stent restenosis.
Although both SES and paclitaxel-eluting stents have greatly reduced in-stent restenosis, the first generation of DES has led to concern for increased risk of late and VLST due to delayed arterial healing.[7] In spite of the unclear mechanism, both sirolimus and paclitaxel were thought to play an important role on both delayed healing and endothelial dysfunction, which may cause the increasing risk of LST.[8] Despite the lower rate of late adverse events and better endothelial function of the second generation DES, the anti-proliferative drugs they used, such as zotarolimus, everolimus, and biolimus A9, were still sirolimus’ derivatives. All of them are specific inhibitors of the mammalian target of rapamycin (mTOR) signaling pathway, which controls protein synthesis and has an important role in modulating cell division in response to mitogenic stimuli. The inhibition of mTOR pathway could greatly inhibit the proliferation of VSMC,[9] however it could also inhibit the proliferation and migration of endothelial cell (EC), which causes delayed healing and endothelial dysfunction. Hence, the attempt to using a novel drug, which has diverse pathways to inhibiting VSMC proliferation, could possibly reduce the damage to ECs, and leads to better clinical outcomes. Arsenic trioxide, a natural substance that was used in the treatment of APL,[4] also proved to induce cellular apoptosis in VSMCs through intracellular reactive oxygen species formation and free Ca2+ increase in vitro.[10] Previous study suggested that the AES could significantly suppress the in-stent restenosis in animal models as well.[5,6] Besides, the dosage of arsenic trioxide in our eluting stent was only 40 μg, which was much less than the safe amount administered systemically (0.15 mg·kg−1·day−1).[11] In our study, no adverse events correlated with arsenic trioxide toxicity occurred. Though further observation for long-term safety is needed, AES was safe to use on the basis of current evidence.
The primary end point TVF was similar between two groups in 24-month follow-up (6.67% vs. 5.83%, P = 0.802). We note that all TVF (actually all TLR) events occurred within the 9-month follow-up in AES group, in contrast to the gradually increasing incidence in SES group, which could be explained as “late catch-up” phenomenon.[12,13] Although early observation of TLR showed possible greater neointimal hyperplasia with AES, no events after 12 months indicated the potential advantage of AES in long-term efficacy. Notably, all-cause death (0 vs. 4.85%, P = 0.028) and cardiac death or MI events (0 vs. 3.88%, P = 0.058), mostly happening after 1 year, were lower in AES group. Among the four cardiac death events, three of them were attributed to definite or probable late or VLST, although there were no significant differences between two groups. Possible explanation of this finding may be the comparative lower toxicity of arsenic trioxide to EC and rapid elution of the drug from a biodegradable polymer.[6,14,15] Although DES remarkably reduced restenosis and repeated revascularization, the occurrence of late events, such as LST and VLST, was the major limitation of DES.[16] The reduction of cardiac death and MI, which seemed to be mainly driven by a reduction in VLST, could greatly shift the risk/benefit balance in favor of DES. Hence, the less long-term adverse events could be a potential advantage of AES over SES.
In-stent LLL at 9-month angiographic follow-up was significantly higher in patients treated with AES as compared those with SES (0.29 ± 0.52 mm vs. 0.10 ± 0.25 mm, P = 0.008), as well as in-segment LLL (0.20 ± 0.22 mm vs. 0.10 ± 0.35 mm, P = 0.026). The reasons for higher LLL observed with AES in this trial compared with SES are not completely understood. It might be due to differences in biological activity of arsenic trioxide compared with sirolimus, although in vitro cell culture experiments and animal studies would suggest at least equivalent potencies in suppressing smooth muscle cell proliferation and even additionally inducing cell apoptosis.[5]
Another potential reason for the difference is the too short elution time of arsenic trioxide from the PLLA. In previous study, arsenic trioxide could reach a plateau after 8 days in contrast to 14 days of paclitaxel in vitro.[5] More rapid elution time leads to insufficient tissue exposure to the therapeutic agents, resulting inadequate suppression of inflammation and intimal hyperplasia induced by injury responses. The ENDEAVOR zotarolimus-eluting stent (E-ZES), which also has a short release duration, suffered an even higher in-stent LLL of around 0.60 mm and higher TLR rate compared to SES.[17,18] Hence, it has reason to believe that the higher LLL of the AES group is caused by the quick release of drugs and polymers. Third, low rate of angiographic follow-up in SES group (53.27%) may result in an overestimate of the difference between two groups.
Despite the higher restenosis rate, the lower late events and stable TLR rate let us consider AES as a potential “safer” stent compared with its contemporary competitors. Interestingly, in spite of the higher TLR of E-ZES compared with DES in the 1st year, the rates of cardiac death/MI, TLR, and definite/probable stent thrombosis were significantly lower with E-ZES in the 5-year time frame.[17,18,19] Hence, the long-term efficacy and safety of AES are promising, and the attempt to using novel arsenic trioxide as an alternative to traditional sirolimus’ derivatives remains bright prospects.
Our study had several limitations. First, patients included in this study were selected strictly by angiographic and clinical characteristics, so the study population may not represent the real-world patients with coronary artery diseases, including MI. Meanwhile, the history of some risk factors such as diabetes was not included in this study, and it may cause bias of the result. Second, the angiographic follow-up rate was unsatisfactory in SES group and may have resulted in an underestimation of the occurrence of angiographic restenosis in a controlled group. In addition, around 20% of patients were lost to clinical follow-up during the 270 days, and it may also affect the final conclusion. Third, IVUS, OCT, and other intracoronary imaging examinations were not used to evaluate the stent-treated lesion. Besides that, the trial is a lack of a long-term angiographic endpoint, which can be used to confirm the long-term safety of AES. Prospective observational and controlled clinical trials with a larger size may contribute to our understanding of the performance of AES and may provide insight into outcomes of the real-world use of AES. The comparison group used in this study enrolled patients using the first-generation SES, which was in routine clinical use when the study was undertaken while now it is used relatively infrequently in clinical practice.
The 2-year follow-up of the randomized controlled trial demonstrates the efficacy and safety of AES compared with SES for the treatment of de novo coronary lesions. Despite the higher LLL, AES group shows similar TVF rate and significant reduction of all-caused death rate.
ACKNOWLEDGMENTS
We thank the coordinators, data coordinators, and physician operators of the eight centers participating in this trial for their diligence and success with patient consents, data collection, and stent implantation. Participating centers and principal investigators include Zhongshan Hospital of Fudan University (Dr. Jun-Bo Ge), Shanghai Changhai Hospital (Dr. Yong-Wen Qin), Beijing Chaoyang Hospital (Dr. Le-Feng Wang), Wuhan University Renmin Hospital (Dr. Hong Jiang), Beijing Anzhen Hospital (Dr. Zhi-Zhong Li), Xi’an Xijing Hospital (Dr. Hai-Chang Wang), Guangdong General Hospital (Dr. Ji-Yan Chen), Hospital of Chinese Medicine of the Xinjiang Uygur Autonomous Region (Dr. Li-Shu Xu).
Footnotes
Edited by: Li-Min Chen
Source of Support: This work was supported by grants from the National Natural Science Foundation (No. 81370323) and the National Basic Research Program of China (No. 2011CB503905).
Conflict of Interest: None declared.
REFERENCES
- 1.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–42. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Minami Y, Kaneda H, Inoue M, Ikutomi M, Morita T, Nakajima T. Endothelial dysfunction following drug-eluting stent implantation: A systematic review of the literature. Int J Cardiol. 2013;165:222–8. doi: 10.1016/j.ijcard.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 3.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–61. [PubMed] [Google Scholar]
- 5.Yang W, Ge J, Liu H, Zhao K, Liu X, Qu X, et al. Arsenic trioxide eluting stent reduces neointima formation in a rabbit iliac artery injury model. Cardiovasc Res. 2006;72:483–93. doi: 10.1016/j.cardiores.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Shen L, Gong F, Tian W, Li W, Zhang F, Qian J, et al. Anti-inflammatory effects of arsenic trioxide eluting stents in a porcine coronary model. Biomed Res Int 2013. 2013 doi: 10.1155/2013/937936. 937936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: Should we be cautious? Circulation. 2004;109:701–5. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 8.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–10. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–53. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 10.Li JX, Shen YQ, Cai BZ, Zhao J, Bai X, Lu YJ, et al. Arsenic trioxide induces the apoptosis in vascular smooth muscle cells via increasing intracellular calcium and ROS formation. Mol Biol Rep. 2010;37:1569–76. doi: 10.1007/s11033-009-9561-z. [DOI] [PubMed] [Google Scholar]
- 11.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 12.Park KW, Kim CH, Lee HY, Kang HJ, Koo BK, Oh BH, et al. Does “late catch-up” exist in drug-eluting stents: Insights from a serial quantitative coronary angiography analysis of sirolimus versus paclitaxel-eluting stents. Am Heart J. 2010;159:446–453.e3. doi: 10.1016/j.ahj.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sheiban I, Chiribiri A, Galli S, Biondi-Zoccai G, Montorsi P, Beninati S, et al. Sirolimus-eluting stent implantation for bare-metal in-stent restenosis: Is there any evidence for a late catch-up phenomenon? J Cardiovasc Med (Hagerstown) 2008;9:783–8. doi: 10.2459/JCM.0b013e3282fb7882. [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Kawasaki D, Oka K, Akahori H, Fukunaga M, Sawada H, et al. Endothelium-dependent coronary vasomotor response and neointimal coverage of zotarolimus-eluting stents 3 months after implantation. Heart. 2011;97:977–82. doi: 10.1136/hrt.2010.204594. [DOI] [PubMed] [Google Scholar]
- 15.Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: A pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–22. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 16.Wessely R. New drug-eluting stent concepts. Nat Rev Cardiol. 2010;7:194–203. doi: 10.1038/nrcardio.2010.14. [DOI] [PubMed] [Google Scholar]
- 17.Kirtane AJ, Leon MB, Ball MW, Bajwa HS, Sketch MH, Jr, Coleman PS, et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6:325–33. doi: 10.1016/j.jcin.2012.12.123. [DOI] [PubMed] [Google Scholar]
- 18.Kandzari DE, Leon MB, Meredith I, Fajadet J, Wijns W, Mauri L. Final 5-year outcomes from the Endeavor zotarolimus-eluting stent clinical trial program: Comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovasc Interv. 2013;6:504–12. doi: 10.1016/j.jcin.2012.12.125. [DOI] [PubMed] [Google Scholar]
- 19.Kutcher MA. The “final voyage” of the Endeavor stent. JACC Cardiovasc Interv. 2013;6:513–5. doi: 10.1016/j.jcin.2013.03.004. [DOI] [PubMed] [Google Scholar]


