Abstract
Background:
Several platelet function tests are currently used to measure responsiveness to antiplatelet therapy. This study was to compare two tests, light transmittance aggregometry (LTA) and modified thrombelastography (mTEG), for predicting clinical outcomes in Chinese patients after percutaneous coronary intervention (PCI).
Methods:
Prospective, observational, single-center study of 789 Chinese patients undergoing PCI was enrolled. This study was investigated the correlations between the two tests and performed receiver operating characteristic curve (ROC) analysis for major adverse cardiovascular events (MACEs) at 1-year follow-up.
Results:
MACEs occurred in 32 patients (4.1%). Correlations were well between the two tests in the adenosine diphosphate induced platelet reactivity (Spearman r = 0.733, P < 0.001). ROC-curve analysis demonstrated that LTA (area under the curve [AUC]: 0.677; 95% confidence interval [CI]: 0.643–0.710; P = 0.0009), and mTEG (AUC: 0.684; 95% CI: 0.650–0.716; P = 0.0001) had moderate ability to discriminate between patients with and without MACE. MACE occurred more frequently in patients with high on-treatment platelet reactivity (HPR) when assessed by LTA (7.4% vs. 2.7%; P < 0.001), and by TEG (6.7% vs. 2.6%; P < 0.001). Kaplan–Meier analysis demonstrated that HPR based on the LTA and mTEG was associated with almost 3-fold increased risk of MACE at 1-year follow-up.
Conclusions:
The correlation between LTA and mTEG is relatively high in Chinese patients. HPR measured by LTA and mTEG were significantly associated with MACE in Chinese patients undergoing PCI.
Keywords: Clopidogrel, High On-treatment Platelet Reactivity, Light Transmittance Aggregometry, Thrombelastography
INTRODUCTION
Dual antiplatelet therapy with aspirin and clopidogrel reduces atherothrombotic complications in patients with acute coronary syndrome (ACS) or those undergoing percutaneous coronary intervention (PCI) with stenting.[1,2] However, the individual response to dual antiplatelet therapy is not uniform, and consistent findings across multiple investigations support the association between high on-treatment platelet reactivity (HPR) and the occurrence of ischemic events.[3,4,5,6] Therefore, HPR to adenosine diphosphate (ADP) assessed by several platelet function tests is a major risk factor for the occurrence of ischemic events following PCI.
The prognostic value of platelet function testing and the concept of HPR play important roles in personalized antiplatelet therapy strategies.[7,8] Light transmittance aggregometry (LTA) was the most widely used platelet function test and has been considered as the “gold standard” method. Currently, clinical studies have demonstrated a close relation between HPR by the criterion of LTA during clopidogrel therapy and the occurrence of adverse cardiovascular events.[9,10] Modified thrombelastography (mTEG) combined with the platelet-mapping assay, which made use of the whole blood for providing a point-of-care assay, was implemented in the current studies to assess its reliability as a monitoring tool for analyzing the response to antiplatelet therapy. The PREPARE POSTSTENTING study reported increased ADP-induced platelet aggregation measured by mTEG in patients suffering ischemic events compared with patients without ischemic events.[11] However, there are still some unsolved issues to support implementation of platelet function testing in routine clinical practice.[12,13] For example, there is no consensus regarding the most appropriate method to quantify the magnitude of on-treatment platelet reactivity, and there has been no head-to-head comparison between LTA and mTEG for their ability to predict cardiovascular events in Chinese patients undergoing PCI. Thus, in the present study, we directly compared the ability of the two tests with predicting clinical outcomes in Chinese patients undergoing PCI with clopidogrel.
METHODS
Study population
Patients presenting to the Fuwai Hospital between January 1, 2012 and November 30, 2012 were considered for enrollment in our prospective, observational, clinical trial. Consecutive patients were assessed for eligibility for enrollment based on the following inclusion criteria: Age of >18 years, had undergone coronary angiography or an uneventful PCI, and could be followed-up for >1-year after PCI. The major exclusion criteria were hemodynamic instability, active bleeding and bleeding diatheses, oral anticoagulation therapy, use of intensified antiplatelet agents other than standard dual antiplatelet therapy, contraindication to antiplatelet therapy, noncardiac disease with a life expectancy of <1-year, or inability to follow the protocol.
Patients who have not used antiplatelet drugs need to receive a 300 mg loading dose of clopidogrel and aspirin at least 12 h before PCI. Patients who have used antiplatelet drugs before elective PCI over 6 days, still need to receive a 100 mg/day maintenance-dose of aspirin and 75 mg/day of clopidogrel on the day of PCI. Then daily thereafter PCI, all patients were given a 100 mg/day maintenance-dose of aspirin for life and 75 mg/day of clopidogrel for at least 1-year. The Institutional Review Board approved the study protocol, and the patients provided written informed consent for participation and agreed to platelet function testing.
Platelet function measurement
Light transmittance aggregometry
Blood samples were drawn into vacutainer tubes containing 0.5 ml of sodium citrate 3.2% (Becton-Dickinson, San Jose, CA, USA) and processed within 2 h.[14,15] Platelet-rich plasma was obtained as a supernatant after centrifuging the blood at 120 × g for 5 min. The remaining blood was further centrifuged at 1200 × g for 10 min to obtain platelet-poor plasma. Platelet aggregation was assessed at 37°C with an AggRam aggregometer (Helena Laboratories, Corp., Beaumont, TX, USA). Platelets were stimulated with 5 μmol/L ADP. Aggregation was expressed as the maximum percent change in light transmittance from baseline, with platelet-poor plasma as a reference.
Thrombelastograph platelet-mapping assay
Blood was collected at least 6 h after using clopidogrel in a vacutainer tube containing 3.2% trisodium citrate. The vacutainer tube was filled to capacity and inverted 3–5 times to ensure complete mixing of the anticoagulant. mTEG® uses four channels to detect the effects of antiplatelet therapy acting via the arachidonic acid and ADP pathways. A detailed description of this method has been outlined previously.[16] The TEG hemostasis analyzer (Haemonetics Corp., Massachusetts, USA) and automated analytical software were used to measure the physical properties.
Clinical outcomes
Primary endpoints were defined as major adverse cardiovascular events (MACEs), which were a composite of death, myocardial infarction (MI), unplanned target vessel revascularization (TVR), and stent thrombosis. MI was detected as of the rise and/or fall of cardiac biomarker values (preferably troponin) with at least one value above the 99th percentile of the upper reference limit.[17] Unplanned TVR was defined as any repeat PCI or surgical bypass of any segment of the target vessel (the entire major coronary vessel proximal and distal to the target lesion, which includes upstream and downstream branches and the target lesion itself).[18] Stent thrombosis was defined as definite stent thrombosis according to the Academic Research Consortium.[18] Patients were contacted by telephone at 1, 6, and 12 months to identify the occurrence of adverse events, which were obtained and reviewed by two physicians blinded to the study who adjudicated events.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and compared using the Student's t-test or one-way analysis of variance test, as appropriate. Categorical variables were expressed as frequencies and percentages and were compared with a Chi-square test (χ2) or Fisher's exact test. Correlations between results obtained by the two platelet function tests were evaluated using Spearman correlation coefficient (r). Clinical follow-up was censored at the day of the first cardiovascular event corresponding to the clinical endpoints. For subjects without a clinical event, clinical follow-up was censored either at the last clinic visit after 12 months of taking clopidogrel or at the day of clopidogrel discontinuation. Ability of the assays to discriminate between patients with and without MACE at 12 months was evaluated by receiver operating characteristic (ROC) curve analysis. The optimal cut-off value was calculated by determining the value providing the greatest sum of sensitivity and specificity. ROC curve analysis was performed using MedCalc version 9.2.0.1 (MedCalc Software, Mariakerke, Belgium).
Survival analysis for patients with and without HPR, according to the ROC of the specific test, was performed using the Kaplan–Meier method and the differences between groups were assessed by the log-rank test. Multivariate Cox regression analysis was performed to identify independent correlates of 1-year MACE and to adjust for potential confounders: Age, sex, body mass index (BMI), ACS, coronary artery disease history, smoking status, hypertension, hypercholesterolemia, diabetes status, and multi-vessel disease. Hazard ratios (HRs) were presented with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA), and a two-tailed P < 0.05 was considered significant.
RESULTS
Study population and demographics
Totally, 789 consecutive patients were enrolled in our study, which was detailed described in Figure 1. Baseline clinical and procedural characteristics for 789 patients are presented in Table 1. 487 patients (61.6%) were ACSs, and 787 patients (99.7%) were implanted drug-eluting stent. During 1-year follow-up, MACE occurred in 26 patients (3.3%), including 5 patients (0.63%) with recurrent MI, 3 patients (0.38%) with stent thrombosis, and 32 patients (4.1%) with TVR. No death occurred within 1-year follow-up.
Figure 1.
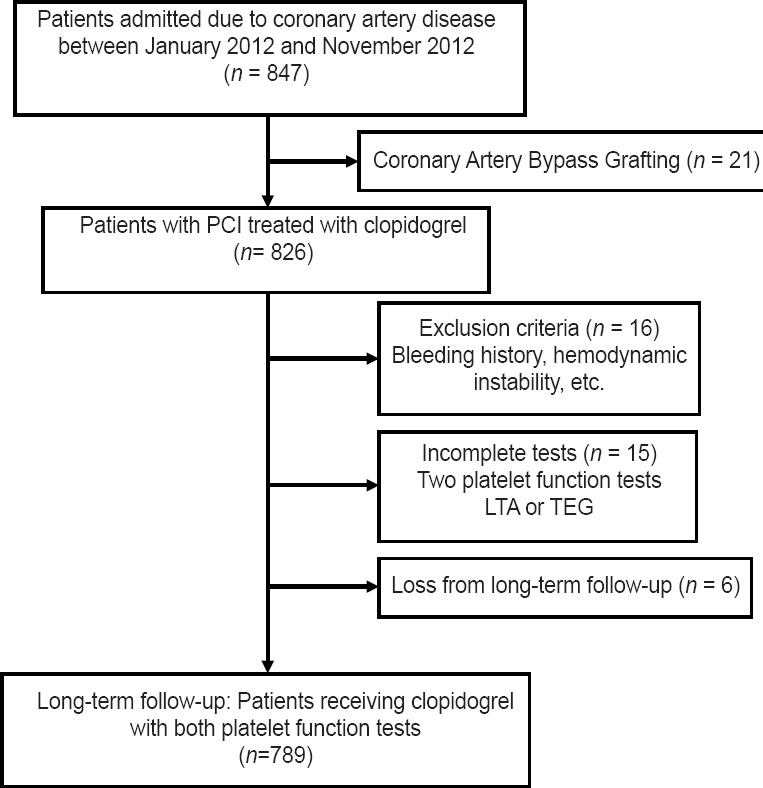
Flow diagram describing the study population. PCI: Percutaneous coronary intervention; LTA: Light transmittance aggregometry; TEG: Thrombelastography.
Table 1.
Baseline characteristics of the total population
| Characteristics | Patients (n = 789) |
|---|---|
| Age, years | 58 ± 10 |
| Male, n (%) | 578 (73.2) |
| BMI, kg/m2 | 26.1 ± 3.1 |
| Risk factor, n (%) | |
| Hypertension | 496 (62.8) |
| Dyslipidemia | 644 (81.5) |
| Diabetes mellitus | 243 (30.8) |
| Current smoking | 273 (34.6) |
| CHD family history | 169 (21.4) |
| Previous history, n (%) | |
| MI | 147 (18.6) |
| PCI | 114 (14.4) |
| Coronary artery bypass grafting | 5 (0.6) |
| Index clinical presentation, n (%) | |
| ACS | 487 (61.6) |
| Unstable angina | 342 (43.3) |
| NSTEMI | 119 (15.1) |
| STEMI | 49 (6.2) |
| Stable angina | 257 (32.5) |
| Laboratory measurement | |
| Hemoglobin, g/L | 137 ± 15 |
| PLT, ×103/mm3 | 207 ± 55 |
| hs-CRP, mg/dl | 3.34 ± 3.75 |
| LDL, mg/dl | 2.45 ± 1.02 |
| HDL, mg/dl | 1.05 ± 0.32 |
| Glucose, mg/dl | 5.99 ± 4.34 |
| Left ventricular ejection fraction, % | 62 ± 7 |
| Infarct-related artery, n (%) | |
| Left anterior descending | 684 (86.6) |
| Left circumflex artery | 506 (64.1) |
| Right coronary artery | 553 (70.0) |
| Left main | 50 (6.3) |
| Intervention methods, n (%) | |
| DES | 787 (99.7) |
| Bare metal stent | 2 (0.3) |
| Concomitant medications, n (%) | |
| On heparin | 691 (87.5) |
| On GP IIb/IIIa | 13 (1.6) |
| On statin | 760 (96.2) |
| On ARB or ACEI | 425 (53.8) |
| On beta-blocker | 699 (88.5) |
| On calcium channel blocker | 354 (44.8) |
| On proton pump inhibitor | 193 (24.4) |
| Platelet function tests, % | |
| ADP-aggregation by LTA | 37.7 ± 21.6 |
| ADP-inhibition by TEG | 47.4 ± 28.5 |
CHD: Coronary heart disease; ACS: Acute coronary syndrome; NSTEMI: Non-ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; PLT: Platelets count; hs-CRP: High-sensitivity C-reactive protein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; GP: Glucose protein; ARB: Angiotensin receptor blocker; ACEI: Angiotensin-converting-enzyme inhibitor; ADP: Adenosine diphosphate; LTA: Light transmittance aggregometry; TEG: Thrombelastography; BMI: Body mass index; PCI: Percutaneous coronary intervention; MI: Myocardial infarction; DES: Drug-eluting stent.
Adenosine diphosphate–induced platelet aggregation
The ADP-induced platelet aggregation by the two tests showed a strong correlation (Spearman r = 0.733, P < 0.001) [Figure 2]. Table 2 displays the area under the curve (AUC) and optimal cut-off value for every test. ROC curve analysis demonstrated that LTA (AUC: 0.677; 95% CI: 0.643–0.710; P = 0.0009) and mTEG (AUC: 0.684; 95% CI: 0.650–0.716; P = 0.0001) were able to distinguish between patients with and without MACE at 1-year follow-up [Figure 3]. The value of ADP-induced platelet aggregation ≥53.2% by LTA was the optimal cut-off point to predict 1-year MACE with sensitivity of 62.5% and specificity of 72.4%, and the value of ADP-induced platelet inhibition ≤32% by mTEG was the optimal cut-off point to predict 1-year MACE with sensitivity of 68.8% and specificity of 65.3% [Table 2].
Figure 2.
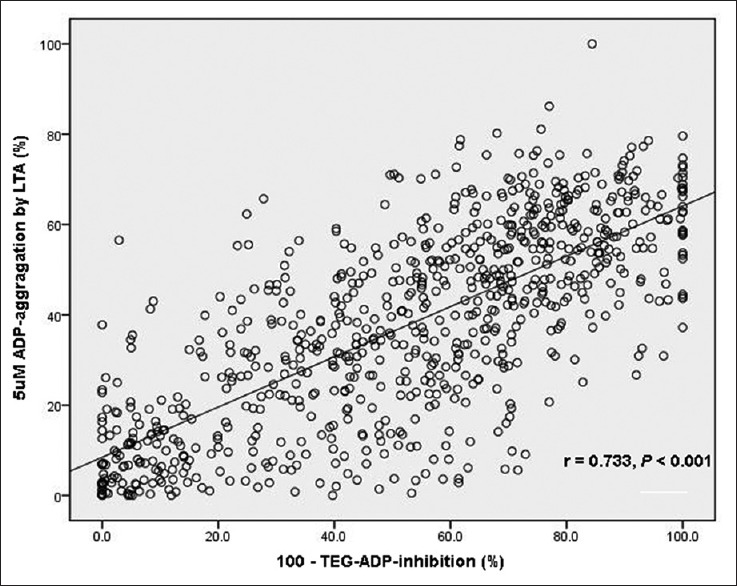
Linear regression model representing correlation between LTA and modified TEG. ADP: Adenosine diphosphate; LTA: Light transmittance aggregometry; TEG: Thrombelastograph.
Table 2.
Area under the ROC curve for prediction of the composite outcome
| Values by test | LTA | TEG |
|---|---|---|
| AUC, % (95% CI) | 0.677 (0.643–0.710) | 0.684 (0.650–0.716) |
| Sensitivity, % (95% CI) | 62.5 (43.7–78.9) | 68.8 (50.0–83.9) |
| Specificity, % (95% CI) | 72.4 (69.1–75.5) | 65.3 (61.7–68.7) |
| Optimal cut-off, % | >53.2 | ≤32 |
| NPV, % | 97.9 | 98.0 |
| PPV, % | 8.7 | 7.7 |
| P | 0.0009 | 0.0001 |
AUC: Area under the curve; CI: Confidence interval; NPV: Negative predictive value; PPV: Positive predictive value; LTA: Light transmittance aggregometry; TEG: Thrombelastography; ROC: Receiver operating characteristic.
Figure 3.
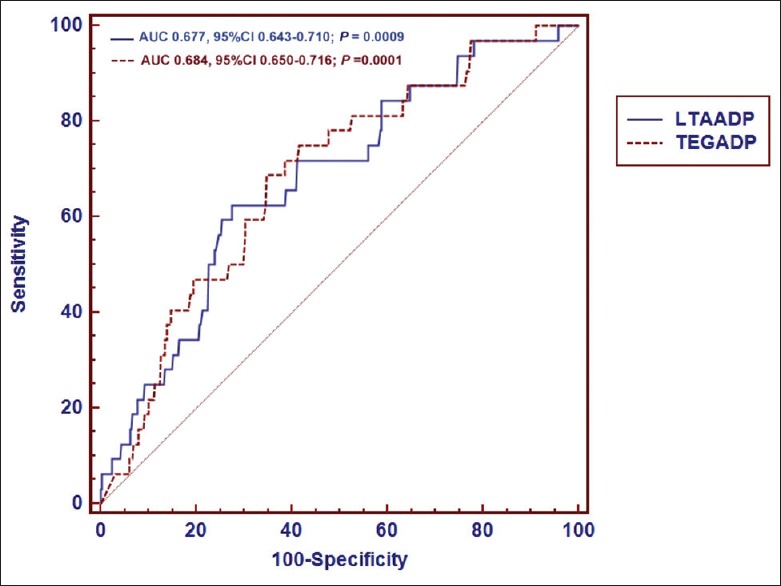
Receiver operating characteristic curve for MACE at 1-year follow-up. Combined receiver-operating characteristic curve for LTA and TEG for MACE at 1-year follow-up. An area of 0.677 and an area of 0.684 were observed below the curves of LTA and TEG, respectively, with P < 0.001 for both areas. LTA: Light transmittance aggregometry; TEG: Thrombelastograph; ADP: Adenosine diphosphate; AUC: Area under the curve; CI: Confidence interval.
Relationship between high on-treatment platelet reactivity and clinical outcomes
High on-treatment platelet reactivity according to the cut-off value (ADP-aggregation ≥ 53.2%) by LTA was found in 229 (29.0%) of the enrolled patients (n = 789), and HPR according to the cut-off value (ADP-inhibition ≤ 32%) by mTEG was found in 285 (36.1%) of the enrolled patients (n = 789). At 1-year follow-up, MACE occurred more frequently in 17 patients (7.4%) with HPR compared with 15 patients (2.7%) without HPR by LTA, and in 19 patients (6.7%) with HPR compared with 13 patients (2.6%) without HPR by TEG. The incidence of MACE during 1-year follow-up for patients with and without HPR according to each platelet function test is depicted in Figure 4.
Figure 4.
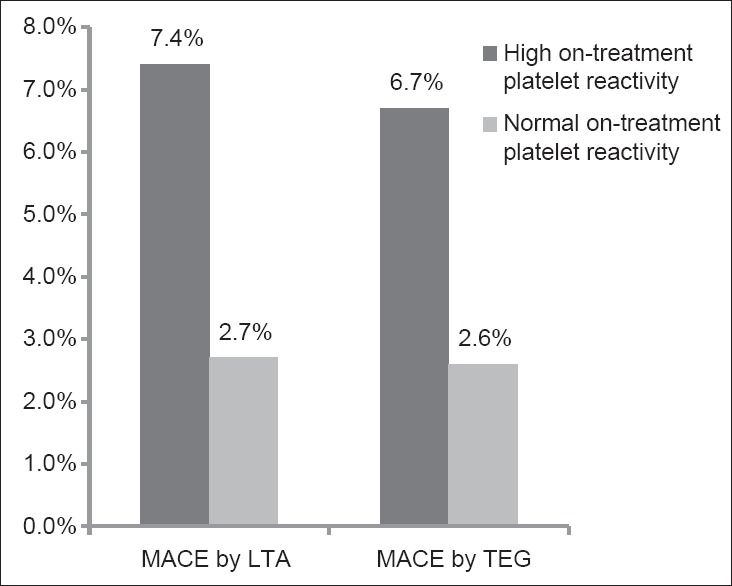
Incidence of major adverse cardiac events according to on-treatment platelet reactivity (high vs. normal) by LTA and modified TEG. MACE: Major adverse cardiac events; LTA: Light transmittance aggregometry; TEG: Thrombelastograph.
In the Kaplan–Meier analysis of patients with and without HPR, the survival rate free from MACE was significantly lower in patients with HPR when measured with LTA and mTEG as compared with patients without HPR [Figure 5]. In the univariate analysis, age, sex, BMI, ACS, coronary artery disease history, smoking status, hypertension, hypercholesterolemia, diabetes status, and multi-vessel disease had no impact on clinical outcomes. In the multivariate Cox regression analysis, patients in the HPR were at significantly higher risk for MACE according to the LTA (HR: 2.752; 95% CI: 1.360–5.569, P = 0.002) [Figure 5a] and the mTEG assay (HR: 2.601; 95% CI: 1.279–5.290, P = 0.005) [Figure 5b].
Figure 5.
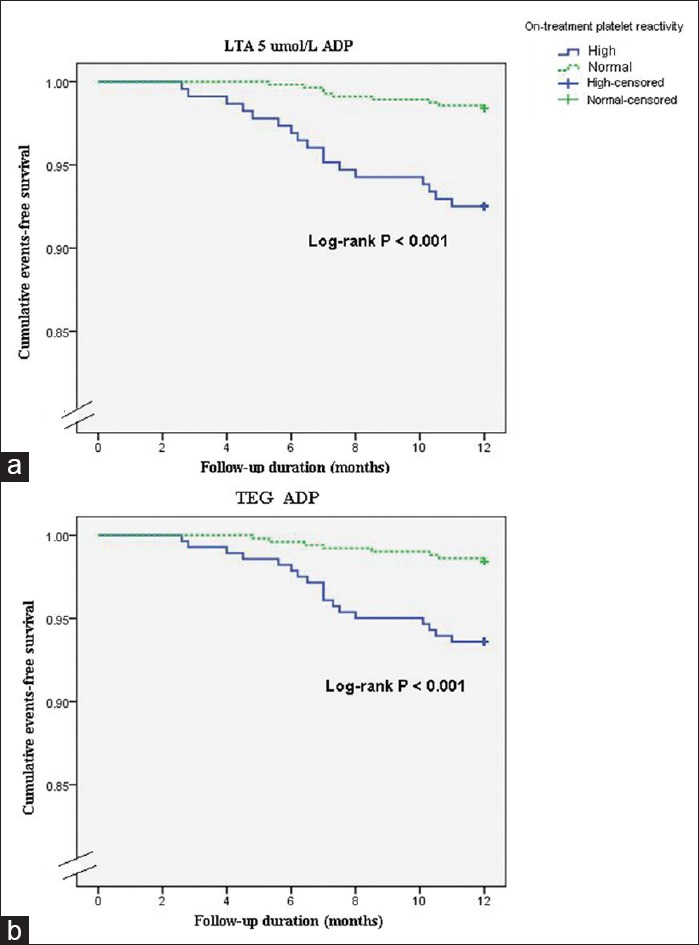
Kaplan–Meier analysis for cumulative event occurrence over 1 year. Kaplan–Meier analysis is for the cumulative risk of major adverse cardiac events in patients with and without high on-treatment platelet reactivity as measured by two platelet function tests (a: LTA; b: TEG). LTA: Light transmittance aggregometry; TEG: Thrombelastograph; ADP: Adenosine diphosphate.
DISCUSSION
To the best of our knowledge, this is the largest study to compare LTA and mTEG for their ability to predict clinical outcomes in Chinese patients after PCI with clopidogrel. Both platelet function tests showed wide individual variability in the response to clopidogrel. There was a strong correlation between the two tests in ADP-induced platelet reactivity. LTA and mTEG discriminated between patients with and without MACE at 1-year follow-up after PCI: HPR when assessed by LTA, and by mTEG was significantly associated with about a 2.8-fold, and a 2.6-fold increased risk of MACE.
There are several methods to measure platelet function. LTA has been the most widely used technique and has clearly demonstrated the relationship between HPR and subsequent atherothrombotic events.[11,19] Bliden et al.[6] evaluated ADP-induced platelet reactivity by LTA in patients undergoing PCI and found that their cut-off value for MACE at 1-year follow-up was 50%; However, Breet et al.[9] found that the cut-off value of 5 μmol/L ADP-induced platelet reactivity by LTA for 1-year death, MI, ST, and stroke was 42.9%. As we known, major limitation in the routine use of LTA are the long sample processing time and the need for specialized technicians.[7,20] Therefore, some new, more easy-to-use platelet function tests have been introduced. mTEG is easy to operate and test platelet function to determine hemostatic status.[21] Our study revealed that mTEG is capable of identifying patients who are at risk for ischemic events undergoing PCI. The present study confirms high linear correlation between LTA and mTEG (r = 0.733, P < 0.001), and consistent with the results of the previous study (r = 0.821, P < 0.001).[6]
Despite growing evidence that HPR is associated with adverse clinical outcome, platelet function testing is not widely implemented in clinical practice due to a lack of consensus on the optimal methods and on the optimal cut-off values of the different tests to identify patients at higher risk. In the light of our study data, our study provides additional evidence, including optimal cut-off values, that the two tests might be used in Chinese patients undergoing PCI (LTA and mTEG). Our optimal diagnostic cut-off value of LTA was ADP-induced platelet aggregation ≥53.2%, and of mTEG was ADP-inhibition ≤32% in Chinese patients for MACE at 1-year follow-up. Meanwhile, the present study also demonstrated that the prevalence of the consensus-defined HPR by the two tests was 29.0%–36.1% from our study after a maintain-dose clopidogrel in Chinese patients undergoing PCI. The ethnic background of the study subjects may play an important role. A high frequency of cytochrome P450 2C19 loss-of-function allele carriage was observed (50%–70%) in the East Asian population.[22,23] These polymorphisms are associated with decreased response to clopidogrel.[24,25] Furthermore, other risk factors such as diabetes mellitus and poor left ventricular ejection fraction have also been demonstrated to be associated with HPR.[26,27]
The incidence of MACE was only 4.1% at 1-year follow-up in Chinese patients. Based on the results of our study, LTA and mTEG are the efficient platelet function tests to identify patients with HPR who are at increased risk for MACE comparing with patients without HPR (6.7%–7.4% vs. 2.6%–2.7% for 1-year follow-up). HPR based on LTA was associated with about 2.8-fold increased risk of MACE at 1-year follow-up, and about 2.6-fold increased risk of MACE based on mTEG. Similar to our study, Breet et al.[9] evaluated ADP-induced platelet reactivity by LTA in patients undergoing PCI and found that HPR was also associated with about 2.09-fold increased risk of composite of all-cause death, nonfatal MI, stent thrombosis, and stroke at 1-year follow-up. Therefore, it seems plausible to screen those patients with HPR undergoing PCI with stenting to provide additional treatment such as double-dosing clopidogrel or new P2Y12 inhibition agents (prasugrel and ticagrelor), to prevent recurrent adverse cardiovascular events and to improve clinical outcomes in our population.
Limitations
First, the current study was a prospective observational study, and the study suggests that HPR was associated with increased ischemic events after PCI. This is the largest and most comprehensive set of Chinese data comparing of mTEG and LTA in predicting clinical outcome in PCI-treated patients with clopidogrel. However, the results were from single-center research in China, the effectiveness of two platelet function tests for predicting long-term clinical outcome will need to expand the patients enrolled from different areas of whole China. Second, because we did not measure clopidogrel active metabolite and P2Y12 receptor occupancy together, our results cannot support the superiority of LTA to mTEG in measuring the antiplatelet effect of clopidogrel. Finally, the follow-up in our study was only 1-year after PCI, and a longer follow-up will be continued in the future.
In conclusion, the correlation between LTA and mTEG is relatively well in Chinese patients. Chinese patients undergoing PCI with HPR on clopidogrel measured by LTA and mTEG were significantly associated with MACE. Therefore, the effectiveness of two platelet function tests was moderately validated for predicting long-term clinical outcomes.
ACKNOWLEDGMENTS
We are grateful to the Department of Cardiology, Cardiovascular Institute of Fuwai Hospital for its help in recruiting patients. We thank all members who contributed to the study.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This work was supported by grants from the National Research Key Project of the Twelfth Five-year Plan of Republic of China (No. 2011BAI11B07), National Natural Science Foundation of China (81170194) and National Special Fund for Healthcare Research in the Public Interests of China (201402001).
Conflict of Interest: None declared.
REFERENCES
- 1.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet. 2001;358:527–33. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 3.Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 4.Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29:992–1000. doi: 10.1093/eurheartj/ehn046. [DOI] [PubMed] [Google Scholar]
- 5.Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: A multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–11. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: Is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–66. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–34. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–62. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 10.Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–23. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: Results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–6. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Krishna V, Diamond GA, Kaul S. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. the role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven? Circulation. 2012;125:1288–303. doi: 10.1161/CIRCULATIONAHA.111.075242. [DOI] [PubMed] [Google Scholar]
- 13.Sibbing D, Byrne RA, Bernlochner I, Kastrati A. High platelet reactivity and clinical outcome-fact and fiction. Thromb Haemost. 2011;106:191–202. doi: 10.1160/TH11-01-0040. [DOI] [PubMed] [Google Scholar]
- 14.Kim IS, Jeong YH, Kang MK, Koh JS, Park Y, Hwang SJ, et al. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J Thromb Thrombolysis. 2010;30:486–95. doi: 10.1007/s11239-010-0484-2. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Jeong YH, Kim IS, Yun SE, Kwon TJ, Hwang SJ, et al. The concordance and correlation of measurements by multiple electrode and light transmittance aggregometries based on the pre-defined cutoffs of high and low on-treatment platelet reactivity. Platelets. 2012;23:290–8. doi: 10.3109/09537104.2011.614974. [DOI] [PubMed] [Google Scholar]
- 16.Hobson AR, Petley GW, Dawkins KD, Curzen N. A novel fifteen minute test for assessment of individual time-dependent clotting responses to aspirin and clopidogrel using modified thrombelastography. Platelets. 2007;18:497–505. doi: 10.1080/09537100701329162. [DOI] [PubMed] [Google Scholar]
- 17.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, et al. Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 19.Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48:1742–50. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 20.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489–93. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 21.Madsen EH, Saw J, Kristensen SR, Schmidt EB, Pittendreigh C, Maurer-Spurej E. Long-term aspirin and clopidogrel response evaluated by light transmission aggregometry, VerifyNow, and thrombelastography in patients undergoing percutaneous coronary intervention. Clin Chem. 2010;56:839–47. doi: 10.1373/clinchem.2009.137471. [DOI] [PubMed] [Google Scholar]
- 22.Kang MK, Jeong YH, Yoon SE, Koh JS, Kim IS, Park Y, et al. Pre-procedural platelet reactivity after clopidogrel loading in korean patients undergoing scheduled percutaneous coronary intervention. J Atheroscler Thromb. 2010;17:1122–31. doi: 10.5551/jat.4564. [DOI] [PubMed] [Google Scholar]
- 23.Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, et al. Genetic variation in metabolizing enzyme and transporter genes: Comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929–40. doi: 10.1177/0091270009355161. [DOI] [PubMed] [Google Scholar]
- 24.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 25.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 26.Stein B, Weintraub WS, Gebhart SP, Cohen-Bernstein CL, Grosswald R, Liberman HA, et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation. 1995;91:979–89. doi: 10.1161/01.cir.91.4.979. [DOI] [PubMed] [Google Scholar]
- 27.Song YB, Hahn JY, Gwon HC, Chang SA, Lee SC, Choe YH, et al. A high loading dose of clopidogrel reduces myocardial infarct size in patients undergoing primary percutaneous coronary intervention: A magnetic resonance imaging study. Am Heart J. 2012;163:500–7. doi: 10.1016/j.ahj.2011.12.007. [DOI] [PubMed] [Google Scholar]


