Abstract
Background:
Current randomized trials have demonstrated the effects of short-term rosuvastatin therapy in preventing contrast-induced acute kidney injury (CIAKI). However, the consistency of these effects on patients administered different volumes of contrast media is unknown.
Methods:
In the TRACK-D trial, 2998 patients with type 2 diabetes and concomitant chronic kidney disease (CKD) who underwent coronary/peripheral arterial angiography with or without percutaneous intervention were randomized to short-term (2 days before and 3 days after procedure) rosuvastatin therapy or standard-of-care. This prespecified analysis compared the effects of rosuvastatin versus standard therapy in patients exposed to (moderate contrast volume [MCV], 200–300 ml, n = 712) or (high contrast volume [HCV], ≥300 ml, n = 220). The primary outcome was the incidence of CIAKI. The secondary outcome was a composite of death, dialysis/hemofiltration or worsened heart failure at 30 days.
Results:
Rosuvastatin treatment was associated with a significant reduction in CIAKI compared with the controls (2.1% vs. 4.4%, P = 0.050) in the overall cohort and in patients with MCV (1.7% vs. 4.5%, P = 0.029), whereas no benefit was observed in patients with HCV (3.4% vs. 3.9%, P = 0.834). The incidence of secondary outcomes was significantly lower in the rosuvastatin group compared with control group (2.7% vs. 5.3%, P = 0.049) in the overall cohort, but it was similar between the patients with MCV (2.0% vs. 4.2%, P = 0.081) or HCV (5.1% vs. 8.8%, P = 0.273).
Conclusions:
Periprocedural short-term rosuvastatin treatment is effective in reducing CIAKI and adverse clinical events for patients with diabetes and CKD after their exposure to a moderate volume of contrast medium.
Keywords: Chronic Kidney Disease, Contrast-induced Acute Kidney Injury, Rosuvastatin
INTRODUCTION
Contrast-induced acute kidney injury (CIAKI), which is characterized by the development of acute renal insufficiency after exposure to a radiocontrast agent, is a major complication that has adverse outcomes after contrast medium injections.[1] Although CIAKI is generally transient and clinically asymptomatic, it is associated with prolonged hospital stays, increased health care costs and higher mortality rates, particularly in patients with diabetes and/or chronic kidney disease (CKD).[2,3,4]
In the multicenter, randomized TRACK-D trial,[5] we have demonstrated the safety and efficacy of short-term rosuvastatin in preventing CIAKI in patients with moderate or severe CKD and diabetes, which was consistent with the results of several recently published randomized trials.[6,7,8,9] Due to the different inclusion criteria, the mean contrast volumes varied in previous studies. Given that contrast volume is an independent predictor of CIAKI,[10] it is important to know if the efficacy of statin in CIAKI prevention is consistent across different stratifications of contrast volume. In this prespecified analysis of the TRACK-D trial, we sought to explore the efficacy of short-term rosuvastatin treatment in preventing CIAKI in patients with contrast administration ≥200 ml.
METHODS
Study population
The TRACK-D trial design has been previously described in detail.[5] Briefly, this investigator-initiated, prospective, randomized controlled trial enrolled patients with type 2 diabetes mellitus (DM) and Stage 2 or 3 CKD undergoing coronary/peripheral arterial diagnostic angiography, left ventriculography, or percutaneous coronary intervention (PCI) at 53 Chinese centers. The primary exclusion criteria were hypersensitivity to contrast medium or statins, type 1 DM, ketoacidosis, lactic acidosis, Stage 0 or 1 CKD, Stage 4 or 5 CKD, acute ST-segment elevation myocardial infarction within the previous 4 weeks, Class IV heart failure (as defined by the New York Heart Association [NYHA] functional classification system), hemodynamic instability, administration of iodinated contrast medium during the 2 weeks before randomization, low-density lipoprotein cholesterol (LDL-C) concentration <1.82 mmol/L, and hepatic dysfunction or renal artery stenosis (unilateral >70% or bilateral >50%). All patients provided written, informed consent before study enrollment, and the study was approved by the Ethics Committees at all participating centers.
Study treatment
Patients were randomized to treatment with either rosuvastatin (Crestor; Astra Zeneca, UK), 10 mg every evening or blank control from 2 days before to 3 days after contrast medium administration. Statin therapy was resumed in both groups 3 days after contrast medium administration. Hydration therapy was administered at the physicians’ discretion. The iso-osmolar, nonionic contrast medium iodixanol (Visipaque, GE Healthcare) was administered during all procedures.
Study outcomes
The primary outcome was the development of CIAKI, which was defined as an increase in serum creatinine concentration ≥0.5 mg/dl (44.2 mmol/L) or ≥25% above baseline at 72 h after exposure to the contrast medium. The secondary outcome was a composite of adverse clinical events at 30 days after exposure to the contrast medium, including all-cause death, dialysis/hemofiltration or worsening heart failure, which was defined as a deteriorated NYHA functional class (i.e. a class change ≥1).
Statistical analysis
Analyses were performed based on the modified intention-to-treat population, which was defined as all patients who underwent randomization and had at least one assessment of renal function after being administered the contrast medium. Comparisons among normally distributed continuous variables, which were expressed as the means ± standard deviation, were performed using t-tests. The Chi-squared test or the Fisher's exact test was used for the categorical data, which were expressed as percentages. Time-to-events curves were calculated using the Kaplan–Meier method and compared with log-rank tests. All P values were two-tailed, and statistical significance was defined as a P ≤ 0.05. All statistical analyses were performed using SAS software, version 9.13 (SAS Institute Inc., Cary, NY, USA).
RESULTS
Patients
Of the 2998 patients included in the TRACK-D study, 932 (31.1%) were administered a contrast medium volume ≥200 ml. These patients were stratified into two groups: The moderate contrast volume group (MCV, 200–300 ml, n = 712) and the high contrast volume (HCV) group (HCV, ≥300 ml, n = 220). In the MCV group, 357 (52.7%) patients were allocated in the rosuvastatin arm, and 355 (47.3%) were allocated in the control arm. For the HCV patients, 118 (53.6%) were allocated in the rosuvastatin arm, and 102 (46.4%) were allocated in the control arm. Baseline clinical characteristics, procedural results, and in-hospital medications were comparable between the rosuvastatin and control groups in both patient stratifications, as shown in Table 1.
Table 1.
Baseline characteristics
| Parameters | Moderate contrast volume (n = 712) | High contrast volume (n = 220) | ||||
|---|---|---|---|---|---|---|
| Rosuvastatin (n = 357) | Control (n = 355) | P | Rosuvastatin (n = 118) | Control (n = 102) | P | |
| Age (years) | 61.8 ± 8.5 | 61.4 ± 8.7 | 0.907 | 61.0 ± 9.2 | 61.5 ± 8.1 | 0.571 |
| Men, n (%) | 244 (68.3) | 263 (74.1) | 0.091 | 87 (73.7) | 76 (74.5) | 0.895 |
| Risk factors, n (%) | ||||||
| Body mass index | 25.5 ± 2.9 | 25.5 ± 2.8 | 0.732 | 25.6 ± 2.9 | 26.1 ± 2.5 | 0.384 |
| Current smoker | 114 (31.9) | 122 (34.4) | 0.383 | 41 (34.7) | 43 (42.2) | 0.269 |
| Diabetes history, n (%) | ||||||
| <5 years | 187 (52.4) | 197 (55.5) | 0.598 | 60 (50.8) | 48 (47.1) | 0.507 |
| 5–10 years | 71 (19.9) | 71 (20.0) | 27 (22.9) | 20 (19.6) | ||
| ≥10 years | 99 (27.7) | 87 (24.5) | 31 (26.3) | 34 (33.3) | ||
| Hypertension, n (%) | 255 (71.4) | 270 (76.1) | 0.247 | 96 (81.4) | 73 (71.6) | 0.086 |
| Prior MI, n (%) | 92 (25.8) | 74 (20.8) | 0.120 | 33 (28.0) | 25 (24.5) | 0.562 |
| CAD family history, n (%) | 21 (5.9) | 18 (5.1) | 0.634 | 8 (6.8) | 6 (5.9) | 0.786 |
| Hypercholesteremia, n (%) | 28 (7.8) | 21 (5.9) | 0.310 | 7 (5.9) | 2 (2.0) | 0.138 |
| Hypertriglyceridemia, n (%) | 35 (9.8) | 30 (8.5) | 0.531 | 13 (11.0) | 8 (7.8) | 0.424 |
| Clinical presentation | ||||||
| Acute coronary syndromes, n (%) | 304 (85.2) | 300 (84.7) | 0.810 | 102 (86.4) | 87 (85.3) | 0.807 |
| SBP, mm Hg | 138.8 ± 20.8 | 140.9 ± 21.2 | 0.561 | 143.3 ± 20.1 | 144.0 ± 26.1 | 0.206 |
| DBP, mm Hg | 81.2 ± 13.0 | 81.7 ± 13.7 | 0.861 | 85.0 ± 12.7 | 83.3 ± 14.4 | 0.383 |
| NYHA class, n (%) | ||||||
| Class I | 292 (81.8) | 290 (81.7) | 0.852 | 101 (85.6) | 86 (84.3) | 0.593 |
| Class II | 59 (16.5) | 57 (16.1) | 16 (13.6) | 16 (15.7) | ||
| Class III | 6 (1.7) | 8 (2.3) | 1 (0.8) | 0 (0) | ||
| Ejection fraction, % | 62.1 ± 8.8 | 62.4 ± 8.0 | 0.095 | 61.9 ± 8.8 | 62.1 ± 8.7 | 0.859 |
| Procedural results | ||||||
| Diseased vessel number, n (%) | ||||||
| 0 | 5 (1.4) | 10 (2.8) | 0.254 | 3 (2.5) | 2 (2.0) | 0.880 |
| 1 | 60 (16.8) | 69 (19.4) | 16 (13.6) | 12 (11.8) | ||
| ≥2 | 292 (81.8) | 276 (77.7) | 99 (83.9) | 88 (86.3) | ||
| PCI, n (%) | 328 (91.9) | 317 (89.3) | 0.238 | 102 (86.4) | 90 (88.2) | 0.690 |
| Contrast volume, ml | 212.1 ± 22.6 | 210.7 ± 22.1 | 0.210 | 347.2 ± 86.1 | 343.2 ± 67.3 | 0.336 |
| Medications, n (%) | ||||||
| ACEI | 241 (67.5) | 223 (62.8) | 0.189 | 88 (74.6) | 69 (67.6) | 0.257 |
| ARB | 83 (23.2) | 87 (24.5) | 0.694 | 18 (15.3) | 29 (28.4) | 0.041 |
| Antibiotic | 78 (21.8) | 65 (18.3) | 0.239 | 33 (28.0) | 18 (17.6) | 0.070 |
| Insulin | 125 (35.0) | 118 (33.2) | 0.618 | 38 (32.2) | 40 (35.71) | 0.574 |
| Diuretic | 78 (21.8) | 71 (20.0) | 0.544 | 28 (23.7) | 23 (22.5) | 0.836 |
| Calcium antagonist | 136 (38.1) | 137 (38.6) | 0.892 | 55 (46.6) | 46 (45.1) | 0.822 |
| β-bloker | 291 (81.5) | 286 (80.6) | 0.747 | 100 (84.7) | 84 (82.4) | 0.632 |
| Heparin | 260 (72.8) | 277 (78.0) | 0.107 | 92 (78.0) | 88 (86.3) | 0.111 |
| Digitalis | 38 (10.6) | 33 (9.3) | 0.548 | 12 (10.2) | 12 (11.8) | 0.705 |
| Hydration | 189 (52.9) | 190 (53.5) | 0.877 | 77 (65.3) | 65 (63.7) | 0.813 |
Data were expressed as n (%) or mean ± SD. MI: Myocardial infarction; CAD: Coronary artery disease; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; NYHA: New York Heart Association; PCI: Percutanenous coronary intervention; ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor II blocker; SD: Standard deviation.
Laboratory test results
Baseline and postprocedural serum creatinine concentrations were comparable between the rosuvastatin and control groups. Rosuvastatin treatment was associated with significant decreases in serum LDL-C and high sensitive C-reactive protein (hsCRP) compared to the control groups in both stratifications [Table 2].
Table 2.
Laboratory results
| Parameters | Moderate contrast volume (n = 712) | High contrast volume (n = 220) | ||||
|---|---|---|---|---|---|---|
| Rosuvastatin (n = 357) | Control (n = 355) | P | Rosuvastatin (n = 118) | Control (n = 102) | P | |
| Serum creatinine, μmol/L | ||||||
| Baseline | 92.9 ± 20.1 | 94.1 ± 19.3 | 0.897 | 92.9 ± 18.9 | 95.8 ± 19.8 | 0.832 |
| Day 2 postprocedure | 93.4 ± 24.1 | 95.4 ± 26.7 | 0.498 | 93.3 ± 23.9 | 94.3 ± 20.2 | 0.411 |
| Day 3 postprocedure | 92.2 ± 23.5 | 95.8 ± 32.8 | 0.168 | 92.9 ± 23.1 | 94.6 ± 19.4 | 0.188 |
| eGFR, ml/min | ||||||
| Baseline | 76.8 ± 15.7 | 76.6 ± 16.2 | 0.809 | 79.3 ± 21.4 | 75.4 ± 17.6 | 0.598 |
| Day 2 postprocedure | 77.5 ± 19.0 | 77.3 ± 22.0 | 0.766 | 79.5 ± 21.4 | 77.3 ± 18.5 | 0.535 |
| Day 3 postprocedure | 78.4 ± 18.6 | 77.2 ± 20.0 | 0.809 | 80.4 ± 22.3 | 77.2 ± 18.2 | 0.307 |
| Hemoglobin, g/dl | 135.2 ± 14.9 | 135.0 ± 14.7 | 0.684 | 139.5 ± 17.6 | 134.8 ± 15.1 | 0.246 |
| WBC count, 109 | 6.9 ± 1.7 | 6.9 ± 2.0 | 0.213 | 6.8 ± 1.8 | 7.3 ± 2.1 | 0.104 |
| Platelet count, 109 | 194.0 ± 51.5 | 198.2 ± 49.7 | 0.734 | 205.3 ± 57.4 | 200.3 ± 42.7 | 0.066 |
| Fasting blood glucose, mmol/L | ||||||
| Baseline | 7.6 ± 2.9 | 7.6 ± 2.8 | 0.345 | 7.7 ± 3.4 | 7.4 ± 3.3 | 0.580 |
| Day 3 postprocedure | 7.2 ± 2.5 | 7.2 ± 2.9 | 0.874 | 7.0 ± 1.8 | 7.3 ± 2.7 | 0.074 |
| Total cholesterol, mg/dl | ||||||
| Baseline | 4.7 ± 1.2 | 4.6 ± 1.2 | 0.405 | 4.7 ± 1.4 | 4.7 ± 1.3 | 0.293 |
| Day 3 postprocedure | 3.9 ± 1.0 | 4.8 ± 1.1 | 0.038 | 3.9 ± 1.2 | 4.7 ± 1.0 | 0.012 |
| HDL-c, mg/dl | ||||||
| Baseline | 1.2 ± 0.4 | 1.3 ± 0.7 | 0.075 | 1.3 ± 0.8 | 1.3 ± 0.4 | 0.715 |
| Day 3 postprocedure | 1.2 ± 0.3 | 1.3 ± 0.5 | 0.398 | 1.2 ± 0.3 | 1.3 ± 0.7 | 0.414 |
| LDL-c, mg/dl | ||||||
| Baseline | 2.6 ± 0.9 | 2.5 ± 0.7 | 0.135 | 2.6 ± 0.9 | 2.6 ± 0.7 | 0.199 |
| Day 3 postprocedure | 2.1 ± 0.7 | 2.6 ± 0.6 | 0.019 | 2.1 ± 0.6 | 2.5 ± 0.7 | 0.022 |
| hsCRP, mg/L | ||||||
| Baseline | 1.7 ± 4.0 | 1.8 ± 8.3 | 0.358 | 1.9 ± 4.3 | 1.8 ± 1.2 | 0.310 |
| Day 3 postprocedure | 1.4 ± 4.0 | 1.7 ± 2.3 | 0.005 | 1.3 ± 3.0 | 2.1 ± 3.1 | 0.016 |
Data were expressed as mean±SD. eGFR: Estimated glomerular filtration rate; WBC: White blood cell; HDL: High density lipoprotein; LDL: Low density lipoprotein; hsCRP: High sensitive C-reactive protein; SD: Standard deviation.
Clinical outcomes
The incidence of CIAKI in the rosuvastatin treated patients was significantly lower than that of patients in the control group (2.1% vs. 4.4%, P = 0.050). For the MCV patients, short-term rosuvastatin treatment was associated with a significantly lower incidence of CIAKI compared to the controls (1.7% vs. 4.5%, relative risk [RR]: 0.373, 95% confidence interval [CI]: 0.148–0.942, P = 0.029). However, the benefit of rosuvastatin was not observed in the HCV patients (3.4% vs. 3.9%, RR: 0.949, 95% CI: 0.243–3.704, P = 0.834).
The incidence of secondary outcomes was significantly lower in the rosuvastatin group compared with the control group (2.7% vs. 5.3%, P = 0.049) in the overall cohort, but the incidence was similar between the groups of MCV patients (2.0% vs. 4.2%, RR: 0.464, 95% CI: 0.192–1.124, P = 0.081) or HCV patients (5.1% vs. 8.8%, RR: 0.576, 95% CI: 0.212–1.564, P = 0.273).
There were no interactions between the rosuvastatin treatment and contrast volume stratifications in terms of primary and secondary outcomes [Table 3].
Table 3.
Primary and secondary outcomes for different contrast volume stratifications
| Parameters | Rosuvastatin (n = 475) | Control (n = 457) | P | P for interaction |
|---|---|---|---|---|
| Primary outcome | ||||
| Overall | 10 (2.1) | 20 (4.4) | 0.050 | 0.319 |
| MCV (n = 712) | 6 (1.7) | 16 (4.5) | 0.029 | |
| HCV (n = 220) | 4 (3.4) | 4 (3.9) | 0.834 | |
| Secondary outcome | ||||
| Overall | 13 (2.7) | 24 (5.3) | 0.049 | 0.780 |
| MCV (n = 712) | 7 (2.0) | 15 (4.2) | 0.081 | |
| HCV (n = 220) | 6 (5.1) | 9 (8.8) | 0.273 |
Data were expressed as n (%). MCV: Moderate contrast volume; HCV: High contrast volume.
Time-to-event curves showed that rosuvastatin was associated with a nonsignificant reduction in the number of adverse clinical events in both the MCV [Figure 1a] and HCV [Figure 1b] stratifications.
Figure 1.
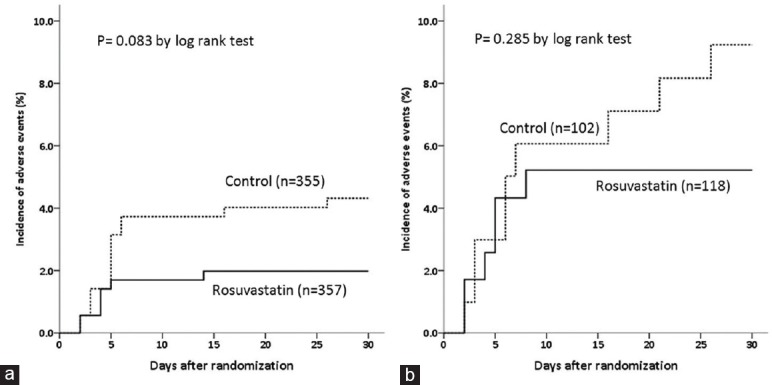
Time-to-event curves comparing adverse clinical events, a composite of death, dialysis/hemofiltration or worsened heart failure between the rosuvastatin and control groups of patients with moderate (a) and high volumes (b) of contrast medium administration.
DISCUSSION
TRACK-D trial was the first large randomized, multicenter, prospective study to evaluate the safety and efficacy of statin therapy in preventing CIAKI in diabetic patients with mild-to-moderate CKD. In this prespecified analysis of TRACK-D trial, we found that periprocedural administration of rosuvastatin (i.e. 10 mg daily for a short duration of 5 days) reduced the incidence of CIAKI in patients with type 2 diabetes and CKD who received moderate or high volumes (≥200 ml) of contrast administration, particularly in the MCV (200–300 ml) stratification.
Contrast-induced acute kidney injury, which is characterized by the development of acute renal insufficiency after exposure to radiocontrast, is the third leading cause of hospital-acquired acute renal injury, accounting for approximately 11% of all cases.[11] Although CIAKI is generally benign in most instances, it is associated with lengthened hospital stays, increased health care costs, and increased risk of death.[2,3,4] CIAKI greatly decreases the efficacy of PCI and results in adverse short- and long-term outcomes.
Several strategies, including hydration, using iso-osmolar contrast, and limiting the amount of administered contrast volume, have become well-established methods of preventing CIAKI. In recent years, periprocedural short-term statin treatment has been emerged as a novel strategy for preventing CIAKI. Several studies have reported that statins may be effective in preventing CIAKI. However, the results from early studies have varied,[7,8,9,12,13,14] primarily because of underpowered sample sizes or nonrandomized study designs. The TRACK-D study and simultaneously published PRATO-ACS study, both of which were well designed randomized trials, demonstrated that short-term rosuvastatin therapy is effective in preventing CIAKI and that the effects are consistent across all prespecified subgroups.[5,6] Based on this new evidence, statins treatment was recommended as a useful prophylactic measure for CIAKI in the updated European Society of Cardiology/European Association for Cardio-thoracic Surgery guidelines for myocardial revascularization.[15]
The mechanism of statin in CIAKI prevention remains unknown. In the present study, patients who developed CIAKI had higher postprocedural hsCRP levels, and the preventive effect of rosuvastatin on CIAKI development was accompanied by a significant decrease in postprocedural hsCRP levels. Inflammation may contribute to the pathogenesis of CIAKI, and renal protection by rosuvastatin during exposure to contrast medium could be due to attenuation of inflammatory responses, a phenomenon observed in the JUPITER trial.[16] In addition to their intended impact on blood cholesterol levels, statins have been found to have multiple nonlipid-lowering effects, which include enhancements of endothelial nitric oxide production, anti-inflammatory and anti-oxidative actions.[17] Given their pleiotropic effects, statins could decrease acute renal injury after contrast administration through two major pathways. First, statins may modulate the kidney hypoperfusion after contrast administration by downregulating angiotensin receptors and decreasing synthesis of endothelin-1.[18,19] Therefore, these drugs may prevent CIAKI by decreasing the period of renal hypoperfusion and ischemia. Second, toxic damage to tubular cells by oxygen-free radicals and pro-inflammatory cytokines, with subsequent cytoplasmic vacuolization, necrosis, interstitial inflammation, and tubular obstruction by protein precipitates, may be decreased by the anti-inflammatory effects of statins, which inhibit tissue factor expression by macrophages and prevent the activation of nuclear factor-kB,[20] a transcription factor that acts on genes encoding for pro-inflammatory mediators, availability, attenuating production of reactive oxygen species, decreasing expression of endothelial adhesion molecules, increasing nitric oxide bioavailability, attenuating production of reactive oxygen species.
Increased contrast medium administration, which is common in complex PCI procedures, is an independent predictor of CIAKI.[10,21] Hydration is an effective strategy to attenuate the adverse effects caused by contrast medium.[15] However, in patients with heart failure or renal insufficiency, hydration should be carefully used. The results from the present study showed that short-term periprocedural rosuvastatin treatment was associated with a significant reduction in CIAKI and adverse clinical events for patients who received moderate or high volumes (≥200 ml) of contrast. Although the benefit of rosuvastatin was obvious in the patients who received moderate volumes of contrast (200–300 ml), there was no interaction between statin treatment and contrast volume stratifications, which indicated the consistent effects of rosuvastatin in both contrast volume stratifications. The less beneficial effects of rosuvastatin observed in patients who received high volume contrast administration (≥300 ml) might be attributed to the small sample size and low rosuvastatin dose (10 mg/day).
The present study had several limitations. First, the data presented in this study were derived from a prespecified but postrandomized analysis. Although the baseline characteristics were well matched between the two arms, interpretations of the results should be cautious due to the potential bias caused by the underpowered sample size and nonstratified randomization. Second, the definitions of moderate or HCV were determined by the investigators, but these definitions were not based on the receiver operating characteristics curve analysis. Thus, these definitions might not reflect the real risk of CIAKI and the procedural complexities.
In conclusion, periprocedural short-term rosuvastatin treatment is effective in reducing CIAKI and adverse clinical events for patients with diabetes and CKD after their exposure to moderate volumes of contrast medium during coronary/peripheral arterial angiography or percutaneous intervention.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: This study was supported by grants from the Key Technologies R and D Project of Liaoning Province (No. 2013225089) and Key Project of National 12th Five-Year Research Program of China (No. 2012ZX09303016-002).
Conflict of Interest: None declared.
REFERENCES
- 1.Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122:2451–5. doi: 10.1161/CIRCULATIONAHA.110.953851. [DOI] [PubMed] [Google Scholar]
- 2.Gruberg L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52:409–16. doi: 10.1002/ccd.1093. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: A clinical and evidence-based approach. Circulation. 2006;113:1799–806. doi: 10.1161/CIRCULATIONAHA.105.595090. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62–70. doi: 10.1016/j.jacc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome) J Am Coll Cardiol. 2014;63:71–9. doi: 10.1016/j.jacc.2013.04.105. [DOI] [PubMed] [Google Scholar]
- 7.Patti G, Ricottini E, Nusca A, Colonna G, Pasceri V, D’Ambrosio A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty – Contrast-induced nephropathy] trial. Am J Cardiol. 2011;108:1–7. doi: 10.1016/j.amjcard.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology. 2010;61:711–4. doi: 10.1177/0003319710364216. [DOI] [PubMed] [Google Scholar]
- 9.Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial – A randomized controlled study. Am Heart J. 2008;155:499.e1–8. doi: 10.1016/j.ahj.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz MA, Maxwell PR, Green K, Hughes DW, Talbert RL. Pravastatin versus simvastatin for prevention of contrast-induced nephropathy. J Cardiovasc Pharmacol Ther. 2011;16:376–9. doi: 10.1177/1074248410394362. [DOI] [PubMed] [Google Scholar]
- 13.Attallah N, Yassine L, Musial J, Yee J, Fisher K. The potential role of statins in contrast nephropathy. Clin Nephrol. 2004;62:273–8. doi: 10.5414/cnp62273. [DOI] [PubMed] [Google Scholar]
- 14.Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O’Donnell MJ, et al. Statin therapy reduces contrast-induced nephropathy: An analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–9. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 17.Deo SH, Fisher JP, Vianna LC, Kim A, Chockalingam A, Zimmerman MC, et al. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H377–85. doi: 10.1152/ajpheart.00289.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichiki T, Takeda K, Tokunou T, Iino N, Egashira K, Shimokawa H, et al. Downregulation of angiotensin II type 1 receptor by hydrophobic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:1896–901. doi: 10.1161/hq1201.099430. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, Sánchez-Pascuala R, Hernández G, Díaz C, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–9. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering – Are they clinically relevant? Eur Heart J. 2003;24:225–48. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang XC, Fu XH, Wang YB, Jia XW, Wu WL, Gu XS, et al. Prediction of contrast-induced nephropathy in diabetics undergoing elective percutaneous coronary intervention: Role of the ratio of contrast medium volume to estimated glomerular filtration rate. Chin Med J. 2011;124:892–6. [PubMed] [Google Scholar]


