Abstract
Background:
Renal sympathetic nerves are involved in the reflective activation of the sympathetic nervous system in circulatory control. Catheter-based renal denervation (RDN) ameliorated treatment-resistant hypertension safely, but 10%–20% of treated patients are nonresponders to radiofrequency denervation. The purpose of this study was to investigate the safety and efficiency of cryoablation for sympathetic denervation in a swine model and to explore a new way of RDN.
Methods:
Seven swines randomly assigned to two groups: Renal cryoablation (CR) group and control group. The control group underwent renal angiogram only. The CR group underwent renal angiogram plus bilateral renal cryoablation. Renal angiograms via femoral were performed before denervation, after denervation and prior to the sacrifice to access the diameter of renal arterial and the pressure of aorta abdominalis. Euthanasia of the swine was performed on 28-day to access norepinephrine (NE) changes of the renal cortex and the changes of renal nerves.
Results:
Cryoablation did not induce severe complications at any time point. There was no significant change in diameter of renal artery. CR reduced systolic blood pressure (BP) from 145.50 ± 9.95 mmHg at baseline to 119.00 ± 14.09 mmHg. There was a slight but insignificant decrease in diastolic BP. The main nerve changes at 28-day consisted of necrosis with perineurial fibrosis at the site of CR exposure in conjunction with the nerve vacuolation. Compared with the control group, renal tissue NE of CR group decreased by 89.85%.
Conclusions:
Percutaneous catheter-based cryoablation of the renal artery is safe. CR could effectively reduce NE storing in the renal cortex, and the efficiency could be maintained 28-day at least.
Keywords: Cryoablation, Renal Sympathetic Denervation, Sympathetic Nerve System
INTRODUCTION
Dysfunction of the automatic nervous system has a pathophysilogical role in the initiation and progression of hypertension, heart failure, atrial fibrillation, and other cardiovascular and metabolic diseases. Accordingly, direct targeting of the sympathetic nervous system is a logical therapeutic approach for the treatment of these dysfunctions. Therefore, surgical sympathectomy was first performed in 1921 and continued into the 1950s.[1] The procedure was associated with a significant reduction in blood pressure (BP). Patients however suffered severe side-effects, which led to the abandonment of the procedure as newer pharmacological interventions were introduced.[2]
With the further understanding of the relationship between automatic nervous system, especially sympathetic system and cardiovascular system, renal afferent and efferent sympathetic nerves are found be involved in the reflective activation of the sympathetic nervous system in circulatory control.[3,4,5] Animal and clinical studies found that denervation of the renal sympathetic nerves can reduce renal and whole body sympathetic activity, which leads to the conclusion that renal sympathetic nerves are potential target for inhibiting hyper activation of the whole sympathetic nervous system to treat the diseases above. Against this background, the concept of sympathectomy was revisited.[6] Rather than nonspecific sympathectomy, renal denervation (RDN), especially catheter-based renal nerve ablation has been proposed as a potential approach targeting patients with chronic sympathetic over activity.
After the first proof-of-principle trial demonstrated catheter-based RDN ameliorated treatment-resistant hypertension safely in 2009,[7] the application of RDN expanded quickly, which led to a proliferation of new devices for RDN. Anecdotal reports online suggest up to 40 RDN devices are currently in development.[8] The majority of these denervation devices have focused upon radiofrequency (RF) catheters. However, according to the literature, about 10%–20% of treated patients are nonresponders to RF denervation, indicated by a postinterventional reduction of systolic BP (SBP) <10 mmHg.[7,9] In addition, recently Medtronic has announced that the Symplicity HTN-3 trial has failed to meet its primary efficacy endpoint of change in office SBP from baseline to 6 months.[10] Besides placebo effect, we tend to believe there may be other reasons as well, such as technique and regeneration of the nerves. Prochnau et al. found that cryoenergy was effective in the treatment of resistant hypertension in nonresponders to RF RDN.[11]
Different from point-by-point approach of RF ablation, we used cryoenergy for sympathetic denervation of the renal arterial circumferentially. The aim of this study was to investigate the safety and efficiency of cryoablation for sympathetic denervation in a swine model, and to explore a new way of RDN.
METHODS
Ethics statement
The experiment scheme and the use of swine were approved by Animal Use and Management Ethics Committee of Fudan University. Experimental design and the implementation process were undertaken in accordance with animal welfare guidelines.
Group setting and animal preparation
Seven swines (mean body weight 45 ± 15 kg), have fourteen renal arteries, randomly assigned into two groups. The control group (n = 3) underwent renal angiogram only. The renal cryoablation (CR) group (n = 4) underwent renal angiogram plus bilateral renal cryoablation. All swine were anesthetized using ketamine (0.3 mg/kg). Ketamine (25 mg/kg) and diazepam (5 mg/kg) were used as an analgesic and all efforts were made to minimize pain. Standard electrocardiographic lead II was continuously recorded to determine the heart rate and rhythm.
Right femoral arteries were cannulated and used for catheter insertion. Every swine served as the controls for plasma hormones and renal function measurements. Then they were given 200 U/kg heparin through catheter to anticoagulate.
Renal cryoablation
Before renal cyoablation or sham procedure, the baseline renal angiograms were obtained to insure that the diameter, length and morphology of renal artery are suitable for cryoablation. After renal angiogram via femoral access, an 11–16 mm (depending on the length of the target artery) tip 7 French (F) cryocatheter was introduced into the renal artery. The balloon was then inflated to the desired size and blocked the blood flow. Complete circumferential denervation was created in both renal arteries by lowering the temperature up to −95°C (ranging between −80°C–110°C) for 3 min each. The balloon was warmed immediately after cryoablation and then was removed. Renal angiogram via femoral performed after denervation to exclude severe vessel dissection and spasm of the cryoablation position. After the procedure, we remove sheath, ligate femoral artery and sew up. After swine were awoken, they were given penicillin 800,000 U intramuscularly for 3 days. The control group underwent the same procedure except ablation.
Detection of the diameter of renal arterial and the pressure of aorta abdominalis
Before renal cryoablation or sham procedure, the baseline renal angiogram and the pressure of aorta abdominalis were obtained. Renal angiogram via femoral performed after denervation and prior to the sacrifice to access the diameter of renal artery and the pressure of aorta abdominalis.
Determination of the proportion of the renal norepinephrine
Renal tissue norepinephrine (NE) values were measured using high-performance liquid chromatography. At the end of the 28-day period, the swine were anesthetized, and six pieces of renal cortex tissue were collected per kidney, and placed in liquid nitrogen. Samples were then homogenized in glutathione and ethylene diamine tetraacetie acid buffer and centrifuged to remove cell parts, and the supernatant was collected and frozen. All steps were performed on ice or in a refrigerated centrifuge.[10]
Analytical methods
Measurement data are expressed as mean ± standard division Qualitative data are expressed as ratios. Values between the two groups were compared using t-tests. P < 0.05 was considered to be statistically significant.
RESULTS
Renal angiogram
Renal angiograms were performed at four time points: At baseline [Figure 1a], after the balloon was inflated [Figure 1b], at the end of the procedure [Figure 1c] and 28-day after renal cryoablation [Figure 1d]. Renal angiogram at all the time points found that cryoablation did not induce hematostenosis, endovascular thrombi, vascular injury or other severe complications. There was no significant change in diameter of renal artery (at baseline: 5.48 ± 0.73 mm vs. 28-day after CR: 5.75 ± 0.69 mm).
Figure 1.
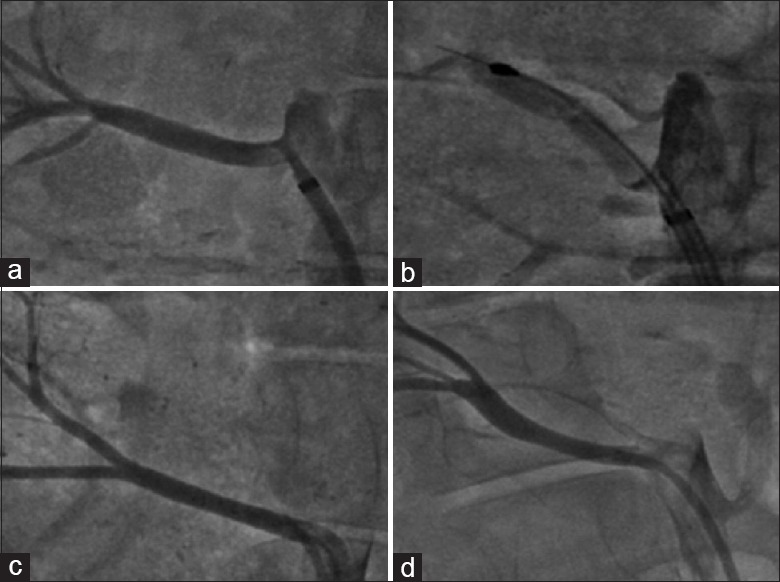
Renal angiography at different time points: (a) At baseline; (b) After the balloon was inflated; (c) At the end of the procedure and (d) 28-day after renal cryoablation.
There were no adverse nephrotoxic or systemic effects seen. The pigs’ serum creatinine, blood urea nitrogen and electrolytes remained unchanged over the study period.
Systolic blood pressure and diastolic blood pressure of aorta abdominalis
CR reduced SBP from 145.50 ± 9.95 mmHg at baseline to 119.00 ± 14.09 mmHg (P < 0.05) at the end of the experimental period. There was a slight but insignificant decrease in diastolic BP (DBP) from 99.75 ± 17.04 mmHg to 91.25 ± 13.89 mmHg [Figure 2].
Figure 2.
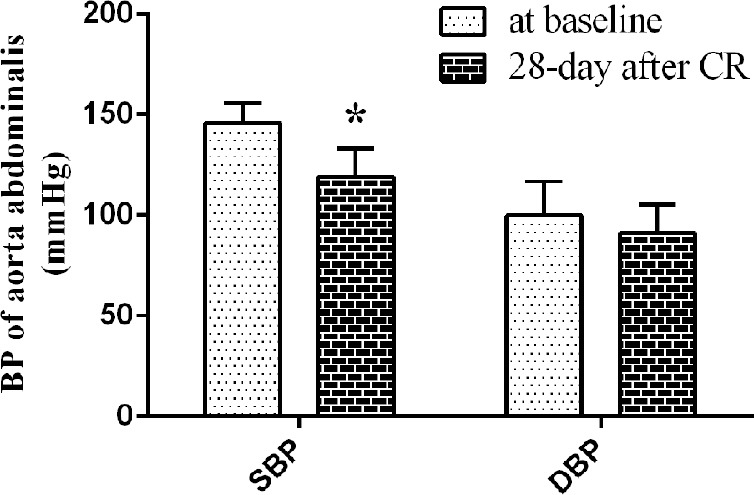
Systolic blood pressure and diastolic blood pressure of aorta abdominalis of CR group (*P < 0.05 vs. baseline).
Histology of renal arteries and nerves
The average depth of CR changes was 4.4 mm on 28-day. And maximum depth of CR injury reached up to 9.5 mm. CR changes in vessels treated consisted of segmental media hyalinization with formation of mature nonstenosing neointima [Figure 3a and 3b].
Figure 3.
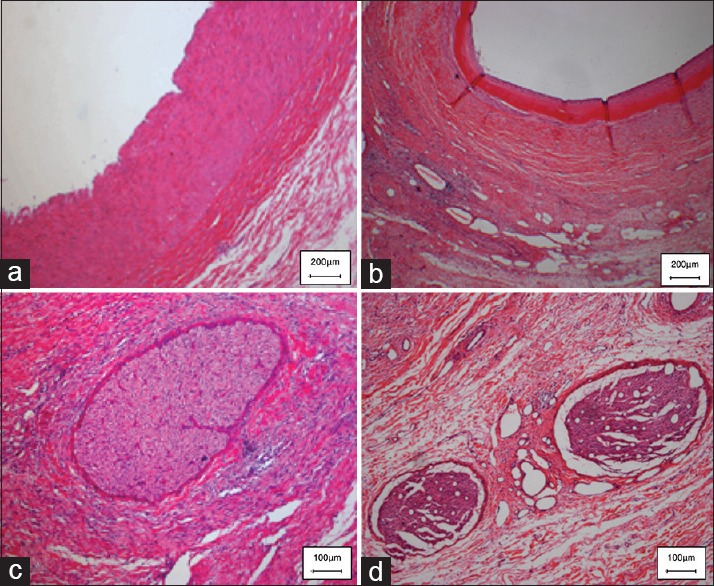
Histopathology (H and E) of renal denervation versus sham procedure at 28-day. The appearances of normal renal arteries and renal nerves from a naïve animal are given in Figure 3a and 3c. (a) The renal arteries (intima and media) appear intact with no evidence of injury or inflammation. (c) The nerve fiber bundles in the sham control appear totally intact. (b) 28 days after CR, there is marked segmental media hyalinization with formation of mature nonstenosing neointima in the vessel; (d) and necrosis with perineurial fibrosis in conjunction with the nerve vacuolation.
All sections of arterial tissue with CR damage showed the presence of one or more nerves in the peri-arterial connective tissue. The appearance of a normal nerve from a naive animal is given in Figure 3c. The main nerve changes at 28-day consisted of necrosis with perineurial fibrosis at the site of CR exposure [Figure 3d] in conjunction with the nerve vacuolation. There was also nerve atrophy and low-grade nerve inflammation extending distal to the levels of CR injury.
Renal norepinephrine
Compared with control group (416.25 ± 7.50 pg/mg renal tissue), renal tissue NE of CR group (42.28 ± 11.27 pg/mg renal tissue) decreased by 89.85%.
DISCUSSION
This study demonstrated the safety and viability, as well as the efficiency and duration of catheter with cryogenic balloon to achieve circumferential sympathetic nerve ablation, with curable injury to renal arteries. Renal angiogram and histology of renal arteries suggested there were no severe device-related artery compliances or adverse nephrotoxic or systemic effects during the study period. CR induced NE content of the renal cortex, which is the surrogate marker for efficacy of RDN, decreased significantly compared with the control group. The efficiency was further validated by histology of renal arteries and nerves. Histology of renal arteries demonstrated complete renal nerve damage in connective tissue around renal artery 28-day after renal cryoablation. CR reduced SBP profoundly; however, DBP were not significantly from CR swine compared with control ones. We propose that increased SBP depends on sympathetic overdrive.
The efferent renal sympathetic nerves innervate the renal arterial resistance vasculature, all tubular segments of the nephron and the juxtaglomerular granular cells. Increased renal sympathetic nerve stimulation results in a rightward shift of the pressure-natriuresis curve.[11] Kidneys influence the central regulation of cardiovascular hemodynamics via afferent fibers that carry impulses centrally to structures that govern global sympathetic tone.[12,13,14,15] Lots of human and animal data strongly suggests that the renal, cardiovascular and central sympathetic systems lead three aspects of closely interconnected. All of these evidences suggest renal sympathetic nerve is a potential target to intervene to treat dysfunctions induced by sympathetic over activation. The anatomical position of renal sympathetic nerves and the development in endovascular catheter technology enabled renal ablation to be performed via minimally invasive approach. Sobotka et al. reduced BP of resistant hypertension patients through RDN by catheter-based approach, which aroused interest in RDN technology. So far, multiple researches have been carried out, and data point to much more indications other than resistant hypertension, including congestive heart failure,[16] central obstructive sleep apnea,[17,18] left ventricular hypertrophy,[19] metabolic syndrome,[20,21] chronic kidney disease[22,23] atrial and/or ventricular arrhythmias[24,25] and other disease states driven by chronic sympathetic hyper activation.
The potential broadending of its application and the considerable economic benefits have stimulated the development of RDN technologies. The majority of evidence for RDN has been derived with RF ablation catheter, among which the Medtronic Symplicity Catheter System is most widely used. Thirteen percent of patients in Symplicity HTN-1 and 16% of patients in Symplicity HTN-2 did not have a decrease in office BP at the 6-month time point after the RDN procedure. The percentage decreased as time went on. However, there was still more than 10% nonresponder 3 years after the operation. The reason of lack of respond is uncertain. Uncomplete nerve ablation is a potential explanation cannot be ignored. Radiologically, good catheter placement and the contact with the vessel wall are important. In addition, renal sympathetic fibers intertwine renal arteries over the adventitia other than parallel to the arteries. And RF ablations are applied discretely. Even ablating the artery in the helical pattern, there are probably functional fibers remained theoretically. The tissue heating during ablation causes a diffuse visceral abdominal pain. A device with higher stability and less side effect is necessary.
To supraventricular arrhythmias patients, the efficiency of cryoablation and RF ablation are counterbalanced, the pain perception is less in the procedure ablated with cryoenergy.[26,27] Cryoenergy should also be used for renal sympathetic denervation effectively.
In this study, we demonstrated the feasibility, safety, and efficiency of cryoablation for sympathetic denervation in a swine model. The cardiovascular system of swine has high similarity with human, and swine RDN model does appear to predict security and efficiency the treatment of refractory hypertension in human subjects. The cryoablation balloon used in this study as long as 11 mm, and with double coil high efficient freezing element performed denervation of the renal arterial circumferentially. Four weeks after the procedure, the NE content of the renal cortex dropped by nearly 90%, which is more significant than 73% reduction induced by frequency ablation. We confirmed the neurohormonal effects by the histology of renal nerves.
Considering the safety of the device, the cryoablation balloon used in this study with double coil high efficient freezing element and rewarm system is able to limit the kidney ischemic duration shorter than 5 min, which is just a quarter of the surmountable period of kidney. Besides, from angiograms of renal arteries at either the end of the procedure or 28-day after renal cryoablation did not find hematostenosis, endovascular thrombi or pseudoaneurysm. In clinical studies, cases of renal artery stenosis within 6 months of RF ablation have been reported. Autopsies in animals after frequency denervation have demonstrated intimal thickening, medial and adventitial fibrosis, and disruption of the external elastic lamina of the renal arteries. In this study, CR changes in vessels just segmental media hyalinization with formation of mature nonstenosing neointima. These are consistent with previous finding that cryolesions are associated with less endothelial damage and thrombus formation as compared with RF energy.[28]
As an animal experiment, this study has a few shortcomings. The content of renal NE is a surrogate biomarker of denervation efficiency. A robust biomarker of successful denervation is currently unavailable. The research period is 28-day, the long-term safety and efficiency are still need further research. The object is normal swine, and it cannot stand for patient completely. As a therapeutic procedure to human, clinical evaluation is required.
Percutaneous catheter-based cryoablation of the renal artery is safe. CR could effectively reduce NE storing in the renal cortex, and the efficiency could maintain 28-day at least. The long-term follow-up of safety and efficiency are undergoing.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: This work was supported by grants from National Natural Science Foundation of China (No. 81370323), the National Basic Research Program of China (No. 2011CB503905) from the Ministry of Science and Technology of China.
Conflict of Interest: None declared.
REFERENCES
- 1.Freis E. New York: Raven Press; 1995. Hypertension: Pathophysiology, Diagnosis and Management; p. 2. [Google Scholar]
- 2.Doumas M, Douma S. Renal sympathetic denervation: The jury is still out. Lancet. 2010;376:1878–80. doi: 10.1016/S0140-6736(10)62111-3. [DOI] [PubMed] [Google Scholar]
- 3.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens. 1984;2:349–59. [PubMed] [Google Scholar]
- 4.Ye S, Ozgur B, Campese VM. Renal afferent impulses, the posterior hypothalamus, and hypertension in rats with chronic renal failure. Kidney Int. 1997;51:722–7. doi: 10.1038/ki.1997.103. [DOI] [PubMed] [Google Scholar]
- 5.Kopp UC, Cicha MZ, Smith LA, Mulder J, Hökfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2-adrenoceptors on renal sensory nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1561–72. doi: 10.1152/ajpregu.00485.2007. [DOI] [PubMed] [Google Scholar]
- 6.Mann J. What's new in hypertension 2009? Nephrol Dial Transplant. 2010;25:37–41. doi: 10.1093/ndt/gfp703. [DOI] [PubMed] [Google Scholar]
- 7.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 8.Mary Stuart. Renal denervation: Device market's gold rush. Medical Devices Today. 2012 [Google Scholar]
- 9.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903–9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 11.Prochnau D, Figulla HR, Surber R. Cryoenergy is effective in the treatment of resistant hypertension in non-responders to radiofrequency renal denervation. Int J Cardiol. 2013;167:588–90. doi: 10.1016/j.ijcard.2012.09.224. [DOI] [PubMed] [Google Scholar]
- 12.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 13.Campese VM, Krol E. Neurogenic factors in renal hypertension. Curr Hypertens Rep. 2002;4:256–60. doi: 10.1007/s11906-002-0016-3. [DOI] [PubMed] [Google Scholar]
- 14.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–9. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 15.Kopp UC. San Rafael, CA: Morgan and Claypool Life Sciences; 2011. Neural Control of Renal Function; p. 87. [PubMed] [Google Scholar]
- 16.Sobotka PA, Krum H, Böhm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012;14:285–92. doi: 10.1007/s11886-012-0258-x. [DOI] [PubMed] [Google Scholar]
- 17.Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60:172–8. doi: 10.1161/HYPERTENSIONAHA.112.191965. [DOI] [PubMed] [Google Scholar]
- 18.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–65. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 19.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Schlaich MP, Hering D, Sobotka P, Krum H, Lambert GW, Lambert E, et al. Effects of renal denervation on sympathetic activation, blood pressure, and glucose metabolism in patients with resistant hypertension. Front Physiol. 2012;3:10. doi: 10.3389/fphys.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: A pilot study. Circulation. 2011;123:1940–6. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 22.Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168:2214–20. doi: 10.1016/j.ijcard.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 23.Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–7. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linz D, Mahfoud F, Schotten U, Ukena C, Hohl M, Neuberger HR, et al. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension. 2013;61:225–31. doi: 10.1161/HYPERTENSIONAHA.111.00182. [DOI] [PubMed] [Google Scholar]
- 25.Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, et al. Renal sympathetic denervation for treatment of electrical storm: First-in-man experience. Clin Res Cardiol. 2012;101:63–7. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- 26.Deisenhofer I, Zrenner B, Yin YH, Pitschner HF, Kuniss M, Grossmann G, et al. Cryoablation versus radiofrequency energy for the ablation of atrioventricular nodal reentrant tachycardia (the CYRANO Study): Results from a large multicenter prospective randomized trial. Circulation. 2010;122:2239–45. doi: 10.1161/CIRCULATIONAHA.110.970350. [DOI] [PubMed] [Google Scholar]
- 27.Malmborg H, Lönnerholm S, Lundqvist CB. A prospective randomised comparison of large-tip cryoablation and 8-mm-tip radiofrequency catheter ablation of atrial flutter. J Interv Card Electrophysiol. 2009;24:127–31. doi: 10.1007/s10840-008-9315-1. [DOI] [PubMed] [Google Scholar]
- 28.Tse HF, Reek S, Timmermans C, Lee KL, Geller JC, Rodriguez LM, et al. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol. 2003;42:752–8. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]


