Abstract
Background:
High peritoneal transport status was previously thought to be a poor prognostic factor in peritoneal dialysis (PD) patients. However, its effect on diabetic nephropathy PD patients is unclear in consideration of the adverse impact of diabetes itself. The purpose of this study was to investigate the influence of peritoneal transport characteristics on nutritional status and clinical outcome in diabetic nephropathy patients on PD.
Methods:
One hundred and two diabetic nephropathy patients on PD were enrolled in this observational cohort study. According to the initial peritoneal equilibration test result, patients were divided into two groups: Higher transport group (HT, including high and high average transport) and lower transport group (LT, including low and low-average transport). Demographic characteristics, biochemical data, dialysis adequacy, and nutritional status were evaluated. Clinical outcomes were compared. Risk factors for death-censored technique failure and mortality were analyzed.
Results:
Compared with LT group (n = 37), serum albumin was significantly lower and the incidence of malnutrition by subjective global assessment was significantly higher in HT group (n = 65) (P < 0.05). Kaplan–Meier analyses showed that death-censored technique failure and mortality were significantly increased in HT group compared with that in LT group. On multivariate Cox analyses, higher peritoneal transport status and lower residual renal function (RRF) were independent predictors of death-censored technique failure when adjusted for serum albumin and total weekly urea clearance (Kt/V). Independent predictors of mortality were advanced age, anemia, hypoalbuminemia, and lower RRF, but not higher peritoneal transport status.
Conclusions:
Higher peritoneal transport status has an adverse influence on nutrition for diabetic nephropathy patients on PD. Higher peritoneal transport status is a significant independent risk factor for death-censored technique failure, but not for mortality in diabetic nephropathy patients on PD.
Keywords: Diabetic Nephropathy, Nutrition, Outcome, Peritoneal Dialysis, Peritoneal Equilibration Test
INTRODUCTION
Recently, it was reported that the total prevalence of diabetes mellitus in the Chinese adult population is 11.6%.[1] Diabetes has become the second most common cause of end-stage renal disease (ESRD) in China.[2] With the rapidly growing use of peritoneal dialysis (PD) as renal replacement therapy in China, the issue of the survival rate of diabetic nephropathy patients on PD has been raised. CANUSA and some other studies have suggested that a high peritoneal transport status is associated with higher mortality and technique failure in PD patients.[3,4,5] However, some recent studies demonstrated that a high peritoneal transport status by itself was not an independent risk factor for mortality and technique failure.[6,7,8] The reasons for these conflicting observations are not clear. To our knowledge, all of the above-mentioned studies included diabetic and nondiabetic patients as study subjects. Actually, many studies have confirmed that diabetes itself is an independent risk factor for mortality in PD patients.[9,10] In addition, diabetic patients are more prone to malnutrition relative to nondiabetic patients.[11] Therefore, we chose patients with diabetic nephropathy as research subjects to evaluate the influence of peritoneal transport characteristics on nutritional status and clinical outcome in this specific population on PD.
METHODS
Subjects
All patients who commenced PD between January 1, 2005 and March 31, 2013 in The First Affiliated Hospital of Zhejiang University, School of Medicine, were eligible for the study. Inclusion criteria were as follows: (a) The primary cause of ESRD was diabetic nephropathy with type 2 diabetes; (b) age ≥18 years at the start of PD and survival for at least 3 months from the first PD therapy; (c) a peritoneal equilibration test (PET) had been performed within the first 6 months of PD commencement; (d) PD modality was continuous ambulatory PD (CAPD) or daytime ambulatory PD. The PD patients who transferred from HD or failed renal transplantation were excluded in this study. Eventually, a total of 102 patients were enrolled in the study from all of the 1115 PD patients. There were 59 males and 43 females with the age range of 25–88 years. Dianeal PD solution (containing 1.5% and 2.5% glucose) was used in this study (Baxter, China).
Data collection
Baseline patient demographic characteristics were collected including gender, age, body mass index, and history of cardio-cerebrovascular disease (CVD). CVD was defined as myocardial infarction, angina, congestive heart failure, and cerebrovascular event. Clinical and biochemical data at the initial PET of PD (baseline) included blood pressure, PD dose, daily transperitoneal glucose exposure, urine volume, peritoneal ultrafiltration volume, electrolytes, intact parathyroid hormone, glycated hemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hsCRP). The protein losses through peritoneal dialysate and urine were measured from the collection of 24-h peritoneal dialysate effluent and urine. Adequacy of dialysis was estimated by measurement of total weekly urea clearance (Kt/V) and total weekly creatinine clearance (TCcr) per 1.73 m2 body surface area. Residual renal function (RRF) was estimated by calculating the average residual renal clearance of urea and creatinine as described by van Olden et al.[12]
Peritoneal transport characteristics and grouping
Peritoneal transport characteristic was evaluated by a standard PET which was performed with the 4-h dialysate/plasma creatinine ratio (D/Pcr) used to classify a patient as high (H), high average (HA), low average (LA), or low (L). According to Twardowski, H was defined by D/Pcr as 0.81–1.03, HA as 0.65–0.80, LA as 0.50–0.64 and L as 0.34–0.49. On the basis of initial PET result, patients were divided into two groups: Higher transport group (HT, including H and HA) and lower transport group (LT, including L and LA).
Nutrition assessment
Serum albumin (ALB), hemoglobin (Hb), daily protein intake (DPI), normalized protein equivalent of total nitrogen appearance (nPNA), and subjective global assessment (SGA) were measured to determine the nutritional status during the initial PET and the last follow-up. SGA scores as proposed by the CANUSA Peritoneal Dialysis Study Group,[13] were coded according to a seven-point graded scale (1–2, severe malnutrition; 3–5, mild to moderate malnutrition; 6–7, normal nutritional status).
Statistical analysis
Results are expressed as mean ± standard deviation for continuous data and as frequencies and percentages for categorical data. The differences between the groups were calculated by unpaired t-test, Chi-square test, or Mann–Whitney test as appropriate. A paired t-test was used to determine differences in the measured parameters between the two periods. Correlation between two continuous variables was expressed as Pearson's correlation coefficient. Survival curves were constructed by the Kaplan–Meier method and compared by the log-rank test. In the analysis of patient survival, the end event was the death. In calculating technique survival, end event was defined as a transfer to hemodialysis. The censored events for both patient and technique survival were renal transplantation, move to another center, or “still on PD” at March 31, 2014. In addition, for patient survival, the censored data included switching to hemodialysis, and for technique survival, death as the censored data. Univariate and multivariate Cox proportional hazard regression models were used to analyze the risk factors for mortality and technique failure. Variables with P < 0.1 in the univariate Cox analysis were presented further to the multivariable Cox regression analysis using backward stepwise elimination based on the likelihood ratio. Data were analyzed using the software package SPSS for Windows release 20.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Demographic and baseline clinical data
Among the 102 patients, 65 patients were classified as higher peritoneal transporters (HT group, including H 8 patients and HA 57 patients). The other 37 patients were classified as lower peritoneal transporters (LT group, including LA 32 patients and L 5 patients). In all of the evaluated items below, glucose exposure, 24-h dialysate protein, and TCcr were significantly higher in HT group compared with those in LT group. In the meantime, HT group had lower peritoneal ultrafiltration volume than LT group. There were no significant differences between two groups for other items [Table 1].
Table 1.
Baseline demographic and clinical characteristics of patients in the higher and lower transport group
| Parameters | HT (n = 65) | LT (n = 37) | P |
|---|---|---|---|
| Age (years) | 59.7 ± 11.8 | 60.9 ± 11.4 | 0.606 |
| Gender (male/female, n) | 36/29 | 23/14 | 0.505 |
| BMI (kg/m2) | 22.8 ± 3.1 | 21.6 ± 3.1 | 0.076 |
| Initial PET time (months) | 2.8 ± 1.4 | 2.6 ± 1.4 | 0.363 |
| Systolic pressure (mmHg) | 138 ± 14 | 136 ± 12 | 0.461 |
| Diastolic pressure (mmHg) | 81 ± 10 | 78 ± 11 | 0.133 |
| History of CVD (%) | 29.2 | 24.3 | 0.593 |
| CAPD/DAPD (n) | 38/27 | 27/10 | 0.143 |
| PD dose (L/day) | 6.5 ± 1.5 | 6.1 ± 1.6 | 0.223 |
| Peritoneal ultrafiltration volume (ml/day) | 298 ± 281 | 438 ± 201 | 0.008 |
| Glucose exposure (g/day) | 105 ± 31 | 93 ± 26 | 0.031 |
| 24 h urine protein (g/day) | 2.41 ± 1.58 | 2.79 ± 2.40 | 0.387 |
| 24 h dialysate protein (g/day) | 6.27 ± 2.08 | 5.19 ± 2.14 | 0.015 |
| Calcium (mmol/L) | 2.26 ± 0.21 | 2.25 ± 0.24 | 0.783 |
| Phosphate (mmol/L) | 1.45 ± 0.31 | 1.49 ± 0.29 | 0.541 |
| iPTH (ng/L) | 203 ± 138 | 181 ± 110 | 0.404 |
| HbA1c (g/L) | 7.1 ± 0.7 | 6.9 ± 0.7 | 0.191 |
| hsCRP (mg/L) | 5.9 ± 1.5 | 5.3 ± 1.8 | 0.079 |
| Total Kt/V | 2.1 ± 0.5 | 2.0 ± 0.5 | 0.596 |
| TCcr (L·week−1·1.73 m−2) | 67 ± 18 | 58 ± 13 | 0.012 |
| RRF (ml·min−1·1.73 m−2) | 3.1 ± 1.7 | 3.2 ± 1.6 | 0.759 |
BMI: Body mass index; PET: Peritoneal equilibration test; CVD: Cardio-cerebrovascular disease; CAPD: Continuous ambulatory peritoneal dialysis; DAPD: Daytime ambulatory peritoneal dialysis; iPTH: Intact parathyroid hormone; HbA1c: Glycated hemoglobin A1c; hsCRP: High-sensitivity C-reactive protein; RRF: Residual renal function; LT: Lower peritoneal transport group; HT: Higher peritoneal transport group; PD: Peritoneal dialysis; TCcr: Total clearance of creatinine.
By the end of the study, 22 patients had died (16 in HT group, 6 in LT group), 28 transferred to hemodialysis, 6 had received a renal transplantation, and 3 were lost to follow-up. The most common cause of death was cardio-cerebrovascular events (68.2%). And the most common cause of technique failure was fluid overload (32.1%). Other cause of technique failure included peritonitis (21.4%), inadequate dialysis (14.3%), catheter-related causes (10.8%), and pleuroperitoneal communication or subjective factors (21.4%).
Nutritional status
The median follow-up time was 22.6 months. There were no significant differences between the two groups with respect to PD time, Hb, baseline ALB, and baseline nPNA (P > 0.05). However, we observed a significant difference in the levels of ALB, DPI, nPNA, and SGA between two groups over time. Compared with LT group, ALB, DPI, and nPNA were significantly lower in HT group at the last follow-up. Meanwhile, the incidence of malnutrition by SGA in HT group was significantly higher than LT group at the last follow-up [Table 2].
Table 2.
Comparison of nutritional status in the higher and lower transport group over time
| Group | Follow-up | PD time (months) | ALB (g/L) | Hb (g/L) | DPI·(g kg−1·d−1) | nPNA (g·kg−1·d−1) | Malnutrition by SGA | |
|---|---|---|---|---|---|---|---|---|
| Patients | Rate (%) | |||||||
| HT (n = 65) | Initial PET | 2.8 ± 1.4 | 34.7 ± 4.5 | 98 ± 10 | 0.84 ± 0.16 | 0.89 ± 0.22 | 24 | 36.9 |
| Last follow-up | 20.9 ± 13.3 | 34.5 ± 3.9 | 97 ± 11 | 0.88 ± 0.25 | 0.86 ± 0.18* | 29 | 44.6 | |
| LT (n = 37) | Initial PET | 2.6 ± 1.4 | 35.9 ± 5.9 | 98 ± 11 | 0.94 ± 0.22† | 0.92 ± 0.24 | 11 | 29.7 |
| Last follow-up | 25.5 ± 14.6 | 37.0 ± 3.3† | 101 ± 9 | 1.04 ± 0.19† | 0.97 ± 0.15*† | 9 | 24.3† | |
*P<0.05 versus before values in the same group; †P<0.05 versus HT group. PET: Peritoneal equilibration test; ALB: Serum albumin; Hb: Hemoglobin; DPI: Daily protein intake; nPNA: Normalized protein equivalent of total nitrogen appearance; SGA: Subjective global assessment; LT: Lower peritoneal transport group; HT: Higher peritoneal transport group; PD: Peritoneal dialysis.
Correlation analysis of dialysate/plasma creatinine ratio and nutritional status
Pearson's correlation analysis showed that the D/Pcr was negatively correlated with ALB and DPI [Figure 1]. Meanwhile, the D/Pcr was positively correlated with hsCRP and 24-h dialysate protein [Figure 1]. In addition, ALB was positively correlated with DPI (r = 0.301, P < 0.05), and negatively correlated with hsCRP (r = −0.216, P < 0.05).
Figure 1.
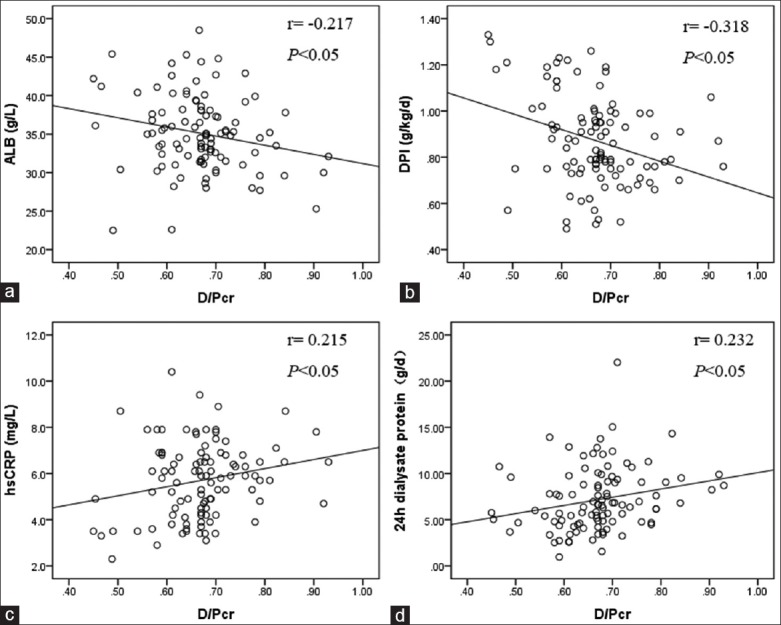
Correlation between D/Pcr and ALB (a); DPI (b); hsCRP (c) and 24 h dialysate protein (d). D/Pcr: Dialysate/plasma creatinine ratio; ALB: Serum albumin; DPI: Daily protein intake; hsCRP: high-sensitivity C-reactive protein.
Death-censored technique survival and patient survival
Death-censored technique survival rates at 1, 2, and 3 years were 91.8%, 71.0%, and 50.1%, respectively, in the HT group; 97.3%, 90.9%, and 82.4% in the LT group, respectively. Compared with LT group, death-censored technique failure was significantly increased in HT group (log-rank P = 0.025) [Figure 2]. Cumulative proportional patient survival at 1, 2, and 3 years were 90.2%, 77.6%, and 61.1%, respectively, in the HT group; 94.4%, 90.8%, and 84.3%, respectively, in the LT group. A higher risk of mortality in HT group was observed than in LT group (log-rank P = 0.047) [Figure 3].
Figure 2.
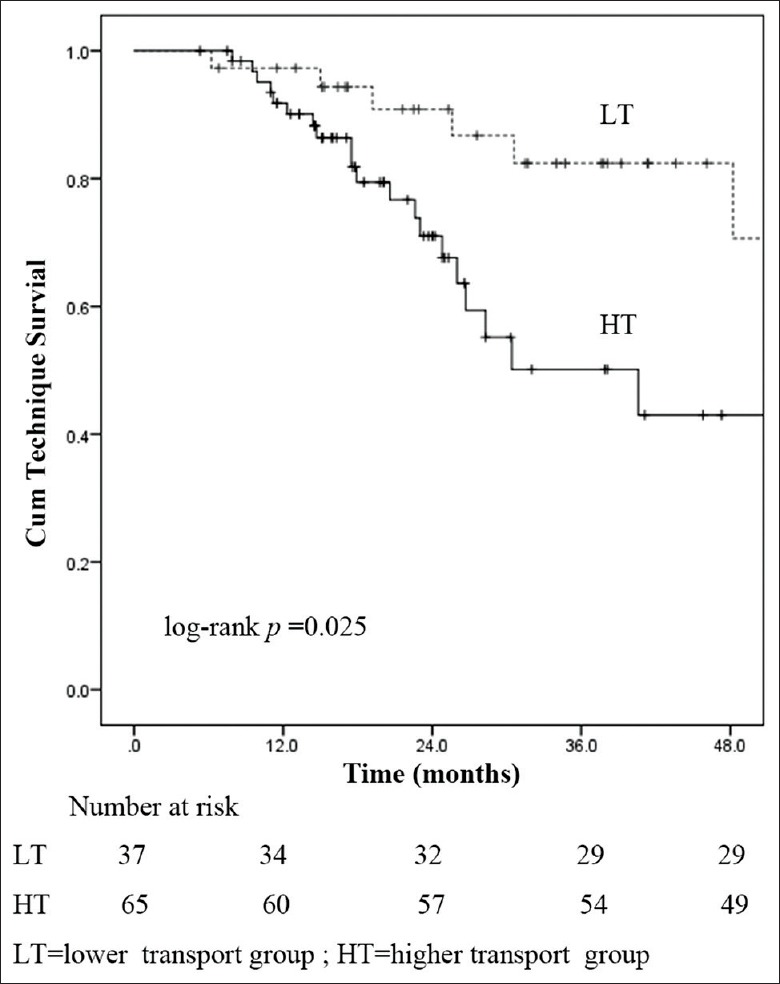
Technique survival curves for peritoneal transport characteristics.
Figure 3.
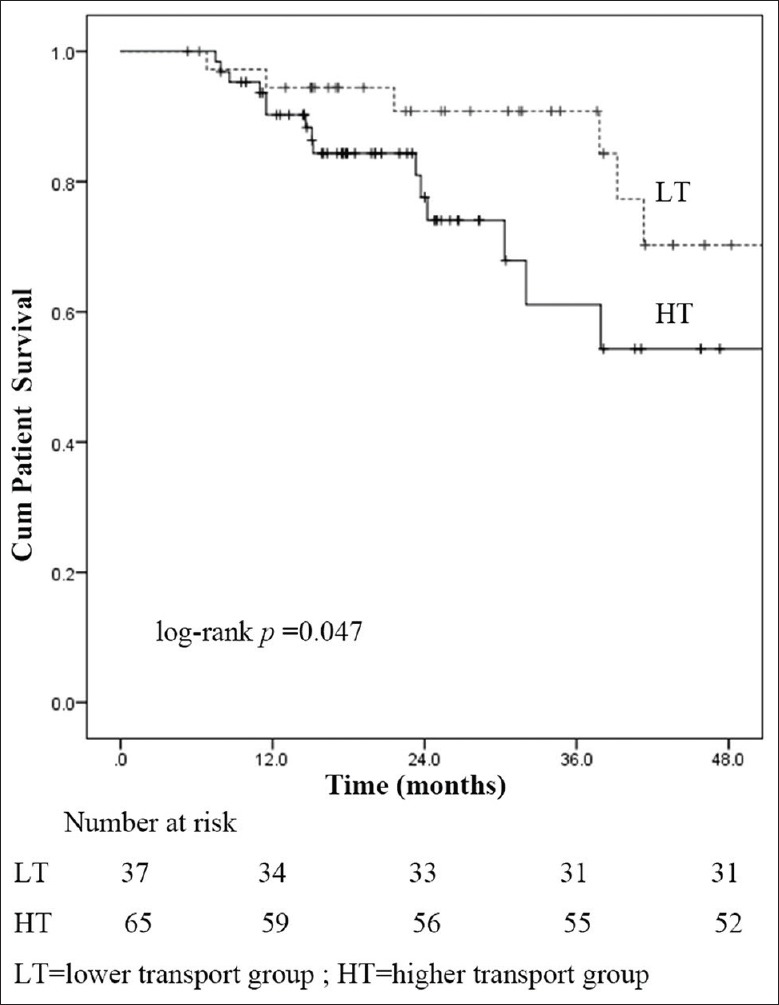
Patient survival curves for peritoneal transport characteristics.
Risk factors for death-censored technique failure and mortality
For the analysis of risk factors for death-censored technique failure, variables with P < 0.1 in the univariate Cox analysis were higher peritoneal transport status, ALB, RRF, and Kt/V. After multivariate Cox proportional hazards model analysis, higher peritoneal transport status (hazard ratio [HR]: 2.311; 95% confidence interval [CI]: 1.003–5.323) and RRF (HR: 0.718; 95% CI: 0.532–0.968) were independent predictor of death-censored technique failure [Table 3]. Screening with univariate Cox analysis, possible risk factors contributing to mortality were age, higher peritoneal transport status, ALB, Hb, HbA1c, TCcr, RRF, and nPNA. However, after multivariate Cox proportional hazards model analysis, mortality was independently predicted by advanced age, anemia, hypoalbuminemia, and lower RRF, but not higher peritoneal transport status [Table 4].
Table 3.
Predictors of death-censored technical failure by multivariate Cox regression analysis
| Factors | B | SE | Wald | P | HR | HR 95% CI |
|---|---|---|---|---|---|---|
| HT | 0.838 | 0.426 | 3.873 | 0.049 | 2.311 | 1.003-5.323 |
| RRF | –0.331 | 0.153 | 4.714 | 0.030 | 0.718 | 0.532-0.968 |
HT: Higher peritoneal transport status; RRF: Residual renal function; CI: Confidence interval; SE: Standard error; HR: Hazard ratio.
Table 4.
Predictors of mortality by multivariate Cox regression analysis
| Factors | B | SE | Wald | P | HR | HR 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.071 | 0.021 | 11.523 | 0.001 | 1.074 | 1.031-1.119 |
| HT | 0.956 | 0.516 | 3.431 | 0.064 | 2.602 | 0.946-7.157 |
| ALB | –0.140 | 0.058 | 5.724 | 0.017 | 0.870 | 0.775-0.975 |
| Hb | –0.065 | 0.023 | 8.300 | 0.004 | 0.937 | 0.896-0.979 |
| RRF | –0.396 | 0.174 | 5.155 | 0.023 | 0.673 | 0.478-0.947 |
HT: Higher peritoneal transport status; ALB: Serum albumin; Hb: Hemoglobin; RRF: Residual renal function; SE: Standard error; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
Peritoneal transport characteristics are assessed primarily on the capability of peritoneal small-solute clearances. Patients with higher peritoneal transport status tend to have enhanced clearance of small solutes, and easier to achieve the objective dialysis adequacy target. However, the ADEMEX trial has demonstrated that no survival advantage was obtained with increases in peritoneal small-solute clearances within usual PD dosing regimens.[14] To our knowledge, the role of peritoneal transport characteristics on technique failure and mortality of PD patients remains controversial. Furthermore, it was reported that the survival of diabetic PD patients is inferior to nondiabetic PD patients in China.[15] Besides, diabetic patients are more prone to malnutrition relative to nondiabetic patients. We therefore performed a study to evaluate the influence of peritoneal transport characteristics on nutritional status and clinical outcome in Chinese diabetic nephropathy patients on PD. We found that although the adequacy of PD assessed by TCcr in HT group was higher than LT group, the ALB was lower, and the incidence of malnutrition by SGA was higher in HT group. And this situation reached a significant difference with the extension of time on PD. The D/Pcr was found having a negative correlation with ALB in this cohort of PD patients. These findings suggest that higher peritoneal transport status has an adverse influence on nutrition. The mechanism of nutritional status affected by higher peritoneal transport characteristic in PD patients is not yet entirely clear. Currently, several postulated mechanisms are as follows. First, high transporters be more prone to ultrafiltration dysfunction as a result of rapid reabsorption of glucose from the dialysate. Decreased ultrafiltration volume can lead to hypertension, fluid overload, inhibition of appetite, and nutrient malabsorption.[16] Second, high transporters have greater peritoneal losses of protein. These mechanisms had been also confirmed in our study. We observed that there was a significantly positive correlation between ALB and DPI. And DPI was significantly lower in HT group than LT group in our follow-up. We found that the D/Pcr was positively correlated with 24 h dialysate protein, and the peritoneal loss of protein was significantly higher in HT group compared with LT group. Furthermore, we observed that high transporters had greater systemic exposure to glucose (P > 0.05). Patients with higher peritoneal transport status are more prone to using higher glucose-containing dialysate due to less ultrafiltration. And HT characteristics itself can induce rapid reabsorption of glucose. That means more difficult to control the glucose level resulting in increased consumption of protein and a negative balance of metabolic in diabetes patients.
Our study demonstrated that hypoalbuminemia was a significant and independent predictor of mortality in diabetic nephropathy patients on PD (HR: 0.870, 95% CI: 0.775–0.975, P = 0.017). This result was consistent with previous studies on general PD patients.[17,18] Hypoalbuminemia is not only an indicator of malnutrition, but also related to clinical complications and more vulnerable to micro-inflammation. It can also result in ultrafiltration dysfunction because of decreased plasma osmolality, which further affecting the prognosis of patients. Recently, a 5-year clinical cohort study completed by the First Affiliated Hospital of Sun Yat-Sen University demonstrated that hypoalbuminemia was an independent risk factor of mortality in Chinese CAPD patients with diabetes.[19] Meanwhile, they found higher HbA1c at the initiation of CAPD was also a risk factor for mortality in patients with diabetes. However, in our study, HbA1c was not significantly different between the two groups of patients. The Cox regression analysis also failed to prompt HbA1c as an independent risk for mortality. This was consistent with the result of a recent multicenter clinical study in Turkey.[9] It has been suggested that uremia can interfere with the assays that measure HbA1c. One of the proposed mechanisms is that uremic acidosis can increase the rate of glycosylation of HbA1c. Meanwhile, HbA1c value can be influenced by either shortening of the life span of erythrocytes or the changing proportion of young to old erythrocytes by erythropoietin use.[20] The assessment of glycemic control by HbA1c in these patients might lead to underestimation. Therefore, there is a need for a much better understanding of the role of HbA1c and its target in PD patients.
On the Kaplan–Meier survival analysis, we observed that death-censored technique failure and mortality were significantly increased in HT group compared with LT group. We further analyzed the independent risk factor for clinical outcomes by univariate and multivariate Cox regression analyses. Higher peritoneal transport status presented independently predictive power for death-censored technique failure (HR: 2.311; 95% CI: 1.003–5.323). And the most common cause of technique failure was fluid overload in our follow-up (32.1%). Our findings suggested that higher peritoneal transport status resulting in fluid overload was an important factor for technique failure in diabetic nephropathy patients on PD. Diabetic PD patients had been reported to have higher peritoneal transport status and be more fluid overloaded as compared to nondiabetics.[21] Contreras-Velázquez et al. demonstrated that the diabetic PD patients have higher mesothelial loss, higher mesothelial basement membrane thickening, higher proportion of vascular wall thickening/sclerosis, and higher proportion of inflammatory infiltrate than nondiabetic PD patients.[22] These peritoneal histological changes in diabetic PD patients can lead to higher peritoneal transport status, reducing ultrafiltration capacity and ultimately leading to increased technique failure. After adjusting for classic mortality risk factors on the multivariate Cox analysis, mortality was independently predicted by advanced age, anemia, hypoalbuminemia, and lower RRF. However, higher peritoneal transport status was no longer an independent predictor of mortality in this cohort of PD patients. Our findings about the relationship between the peritoneal transport status and patient survival were similar to that of some earlier reports.[6,7,8] There are several potential explanations for this founding. First, Yang et al.[23] demonstrated that higher peritoneal transport is not a significant independent risk factor for mortality in patients on automated PD (APD). Although the Australian and New Zealand study showed that high transport status was independently predictive of mortality and death-censored technique failure for patients on CAPD, but not for those received APD.[4] One possible explanation for these results is that the APD may possibly eliminate the consequence of an inadequate ultrafiltration due to an HT status. As such, these results suggested that the poor outcome associated with the HT status may relate more to fluid overload rather than the HT status itself. Second, Chung et al.[24] demonstrate that there are at least three different types of high peritoneal transport status. The early inherent Type I which is associated with comorbidity and inflammation is a risk factor for mortality. The early inherent Type II with a large peritoneal surface area has a good prognosis, in general. And the late acquired Type III is not necessarily associated with poor outcome if fluid balance has been controlled using APD or icodextrin-based PD solution. Therefore, in spite of the same high peritoneal transport characteristics, different types have different clinical outcomes.
It should be noted that there are some limitations in our study. It is a single-center study and thus center-specific effects cannot be excluded. And selection biases cannot be avoided due to the limited number of patients. Consequently, the influence of higher peritoneal transport status for all-cause mortality in diabetic nephropathy patients on PD need to be further confirmed in a larger study sample or multi-center, randomized, controlled trials.
In conclusion, our findings showed that higher peritoneal transport status is associated with an increased risk for malnutrition and technical failure in diabetic nephropathy patients on PD. And fluid overload is an important factor which led to technique failure in this population. Although our study did not suggest higher peritoneal transport status itself a determinant factor on the mortality of this population, more effective fluid control using APD and icodextrin-based PD solutions may have important clinical benefits.
Footnotes
Edited by: Li-shao Guo
Source of Support: This work was supported by a grant from the National Natural Science Foundation of China (No. 81170707).
Conflict of Interest: None declared.
REFERENCES
- 1.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Zuo L, Wang M. Chinese Association of Blood Purification Management of Chinese Hospital Association. Current burden and probable increasing incidence of ESRD in China. Clin Nephrol. 2010;74(Suppl 1):S20–2. [PubMed] [Google Scholar]
- 3.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998;9:1285–92. doi: 10.1681/ASN.V971285. [DOI] [PubMed] [Google Scholar]
- 4.Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006;17:271–8. doi: 10.1681/ASN.2005050566. [DOI] [PubMed] [Google Scholar]
- 5.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006;17:2591–8. doi: 10.1681/ASN.2006030194. [DOI] [PubMed] [Google Scholar]
- 6.Lee CC, Chen KH, Tian YC, Weng CM, Yang CW, Hung CC. Initial high peritoneal transport status is not a predictor of mortality in peritoneal dialysis patients. Ren Fail. 2010;32:788–95. doi: 10.3109/0886022X.2010.493981. [DOI] [PubMed] [Google Scholar]
- 7.Perl J, Huckvale K, Chellar M, John B, Davies SJ. Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol. 2009;4:1201–6. doi: 10.2215/CJN.01910309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes MJ, Bajo MA, Hevía C, Del Peso G, Ros S, de Miguel AG, et al. Inherent high peritoneal transport and ultrafiltration deficiency: Their mid-term clinical relevance. Nephrol Dial Transplant. 2007;22:218–23. doi: 10.1093/ndt/gfl529. [DOI] [PubMed] [Google Scholar]
- 9.Ozener C, Arikan H, Karayaylali I, Utas C, Bozfakioglu S, Akpolat T, et al. The impact of diabetes mellitus on peritoneal dialysis: The Turkey Multicenter Clinic Study. Ren Fail. 2014;36:149–53. doi: 10.3109/0886022X.2013.843275. [DOI] [PubMed] [Google Scholar]
- 10.Unsal A, Koc Y, Basturk T, Sakaci T, Ahbap E, Sinangil A, et al. Clinical outcomes and mortality in peritoneal dialysis patients: A 10-year retrospective analysis in a single center. Clin Nephrol. 2013;80:270–9. doi: 10.5414/CN107711. [DOI] [PubMed] [Google Scholar]
- 11.Park SW, Seo JJ, Bae HS, Kim JY, Kim CD, Park SH, et al. Difficulty in improving malnutrition and low-grade inflammation in diabetic patients on peritoneal dialysis. Ther Apher Dial. 2008;12:475–83. doi: 10.1111/j.1744-9987.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 12.van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1996;7:745–50. doi: 10.1681/ASN.V75745. [DOI] [PubMed] [Google Scholar]
- 13.Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 14.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–20. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 15.Zhong H, Liu F, Fu P, Sha ZH, Tang XH, Qin M, et al. A comparison of clinical characteristics and survival between diabetic nephropathy patients and non-diabetic nephropathy patients undergoing peritoneal dialysis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:442–6. [PubMed] [Google Scholar]
- 16.Tinroongroj N, Jittikanont S, Lumlertgul D. Relationship between malnutrition-inflammation syndrome and ultrafiltration volume in continuous ambulatory peritoneal dialysis patients. J Med Assoc Thai. 2011;94(Suppl 4):S94–100. [PubMed] [Google Scholar]
- 17.Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. Am J Kidney Dis. 2011;58:418–28. doi: 10.1053/j.ajkd.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leinig CE, Moraes T, Ribeiro S, Riella MC, Olandoski M, Martins C, et al. Predictive value of malnutrition markers for mortality in peritoneal dialysis patients. J Ren Nutr. 2011;21:176–83. doi: 10.1053/j.jrn.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Yi C, Liu X, Guo Q, Yang R, Cao P, et al. Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: A 5-year clinical cohort study. Diabetes Res Clin Pract. 2013;100:354–61. doi: 10.1016/j.diabres.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 21.Gan HB, Chen MH, Lindholm B, Wang T. Volume control in diabetic and nondiabetic peritoneal dialysis patients. Int Urol Nephrol. 2005;37:575–9. doi: 10.1007/s11255-005-1202-4. [DOI] [PubMed] [Google Scholar]
- 22.Contreras-Velázquez JC, Soto V, Jaramillo-Rodríguez Y, Samaniego-Ríos LI, Quiñones-Pérez V, Avila M, et al. Clinical outcomes and peritoneal histology in patients starting peritoneal dialysis are related to diabetic status and serum albumin levels. Kidney Int Suppl. 2008;108:S34–41. doi: 10.1038/sj.ki.5002599. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Fang W, Bargman JM, Oreopoulos DG. High peritoneal permeability is not associated with higher mortality or technique failure in patients on automated peritoneal dialysis. Perit Dial Int. 2008;28:82–92. [PubMed] [Google Scholar]
- 24.Chung SH, Heimbürger O, Lindholm B. Poor outcomes for fast transporters on PD: The rise and fall of a clinical concern. Semin Dial. 2008;21:7–10. doi: 10.1111/j.1525-139X.2007.00327.x. [DOI] [PubMed] [Google Scholar]


