Abstract
Background:
The concept of minimally invasive techniques is to make every effort to reduce tissue damage. Certainly, reducing skin incision is an important part of these techniques. This study aimed to investigate the clinical feasibility of Mast Quadrant-assisted modified transforaminal lumbar interbody fusion (TLIF) with a small single posterior median incision.
Methods:
During the period of March 2011 to March 2012, 34 patients with single-segment degenerative lumbar disease underwent the minimally invasive modified TLIF assisted by Mast Quadrant with a small single posterior median incision (single incision group). The cases in this group were compared to 37 patients with single-segment degenerative lumbar disease in the double incision group. The perioperative conditions of patients in these two groups were statistically analyzed and compared. The Oswestry Disability Index (ODI) scores, Visual Analog Scale (VAS) scores, and sacrospinalis muscle damage evaluation indicators before operation and 3, 12 months postoperation were compared.
Results:
A total of 31 and 35 cases in the single incision and double incision groups, respectively, completed at least 12 months of systemic follow-up. The differences in perioperative conditions between the two groups were not statistically significant. The incision length of the single incision group was significantly shorter than that of the double incision group (P < 0.01). The ODI and VAS scores of patients in both groups improved significantly at 3 and 12 months postoperation. However, these two indicators at 3 and 12 months postoperation and the sacrospinalis muscle damage evaluation indicators at 3 months postoperation did not differ significantly between the two groups (P ≥ 0.05).
Conclusions:
Mast Quadrant-assisted modified TLIF with a small single posterior median incision has excellent clinical feasibility compared to minimally invasive TLIF with a double paramedian incision.
Keywords: Interbody Fusion, Lumbar, Lumbar Fusion, Mast Quadrant Retractor, Minimally Invasive Spine Surgery, Transforaminal Lumbar Interbody Fusion
INTRODUCTION
Several minimally invasive spine technologies are undergoing vigorous development to obtain better practical results and social benefits in the clinical treatment of a variety of spinal disorders. Therefore, a greater number of spinal surgeons and medical facilities are launching minimally invasive spine technologies. Minimal invasiveness is a technology, a concept, a means, and a goal. Minimal invasiveness is not merely a “small incision”; the efforts to reduce wound length and injury to soft tissues, like skin, is also an important component of minimally invasive spine technologies.[1,2,3,4,5,6]
Foley et al.[7,8] first reported minimally invasive transforaminal lumbar interbody fusion (TLIF) in 2002. Minimally invasive TLIF uses a paramedian incision that spares the spinous process and interspinous ligament and retains the corresponding blood supply compared to the traditional open TLIF. The direct exposure of the facet and transverse processes through the sacrospinalis muscle causes very little stripping damage to sacrospinalis muscle, and this exposure is helpful during postoperative recovery. In addition, minimally invasive TLIF directly exposes the transverse process and facet joint via an intramuscular approach, which facilitates the placement of screws along the anatomical direction of the pedicle.[9,10,11,12,13] A review of the relevant literatures from the past 10 years showed that minimally invasive TLIF has primarily used bilateral paramedian incisions during treatment. This approach produces clearer clinical efficacy, but it also has some drawbacks, including the need for bilateral incisions and the effect of surgical scars on appearance.[9,10,11,12,13,14,15,16,17]
This study further investigated the clinical surgical feasibility of Mast Quadrant-assisted minimally invasive modified TLIF with a small single posterior median incision based on previous clinical surgeries using Mast Quadrant-assisted minimally invasive modified TLIF with a double paramedian incision.[14,18] A clinical comparison between these two methods was also conducted.
METHODS
Patient data
From March 2011 to March 2012, 34 patients with single segmental lumbar degenerative disease (18 males and 16 females) for whom conservative treatment was ineffective underwent minimally invasive modified TLIF in Huashan Hospital, Fudan University. The Mast Quadrant-assisted operation with a small single posterior median incision was performed in the single incision group. The clinical manifestations of patients in this group included symptoms of a unilateral or bilateral radiation to a lower extremity with or without obvious low back pain, with symptoms aggravated after walking. Before surgery, patients were clearly confirmed to have lumbar degenerative diseases (e.g., lumbar intervertebral disc herniation, lumbar spinal canal stenosis, and degenerative lumbar spondylolisthesis) using conventional lateral and dynamic lumbar X-ray examination, computed tomography scans of the lumbar spine with two-dimensional reconstruction, and lumbar spine magnetic resonance imaging (MRI) examination. All patients had nonobvious symptom remission or recurrent symptoms after 6 months of regular conservative treatment. Patients in this group were compared to the 37 patients with single-segment degenerative lumbar disease in the double incision group who received the Mast Quadrant-assisted minimally invasive modified TLIF with a double paramedian incision between June 2010 and February 2011 in Huashan Hospital, Fudan University. The average age and average weight of patients in the single incision group were 56.0 ± 13.5 years and 68.3 ± 8.2 kg, respectively, and the average age and average weight of patients in the double incision group were 54.8 ± 12.7 years and 70.3 ± 7.7 kg, respectively. The t-test results of the corresponding data between these two groups showed no significant differences (P values were 0.696 and 0.270, respectively) [Table 1].
Table 1.
Comparison of basic clinical data of patients between the two groups
| Characteristics | Single incision group | Double incision group | P |
|---|---|---|---|
| Number of cases (n) | 34 | 37 | – |
| Age (mean ± SD, years) | 56.0 ± 13.5 | 54.8 ± 12.7 | 0.696 |
| Weight (mean ± SD, kg) | 68.3 ± 8.2 | 70.3 ± 7.7 | 0.270 |
| Male/female (n/n) | 18/16 | 21/16 | – |
| Surgical segment | 4 cases at L3-4, 17 cases at L4-5, 13 cases at L5-S1 | 6 cases at L3-4, 16 cases at L4-5, 15 cases at L5-S1 | – |
| Clinical diagnosis | Lumbar intervertebral disc herniation 8 cases, lumbar spinal canal stenosis 15 cases, and degenerative lumbar spondylolisthesis 11 cases | Lumbar intervertebral disc herniation 10 cases, lumbar spinal canal stenosis 18 cases, degenerative lumbar spondylolisthesis 9 cases | – |
SD: Standard deviation.
Surgical procedure
Modified transforaminal lumbar interbody fusion with a small single posterior median incision
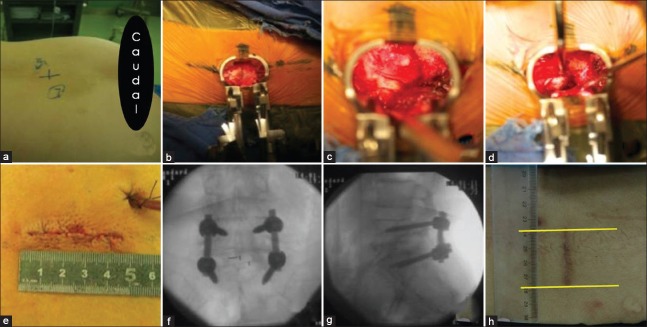
A C-arm fluoroscopy machine (Siemens, Germany) was used to locate surgical segments [Figure 1a]. A posterior median longitudinal incision of approximately 4 cm was made, using the surgical space as the midline. Skin and subcutaneous tissues were sequentially cut to expose the lumbodorsal fascia until it was free 2.0–2.5 cm from the symptomatic side or the side with serious symptoms. A longitudinal cut approximately 3.0–3.5 cm in length was made in the lumbodorsal fascia, and Quadrant retractors were sequentially placed to establish a working channel [Figure 1b]. The lower articular process and lower 1/2–2/3 of the lamina of the upper vertebra on the affected side, ligamentum flavum, and the thicker part of the upper articular process were cut with forceps under direct vision to fully decompress the nerve root and central canal. The affected segment of the intervertebral disc was exposed and excised, the intervertebral space was cleaned, and the intervertebral space was distracted to the appropriate height. The decompressed and excised autologous bone graft was trimmed to bone particles of an appropriate size and was implanted into the anterior disc space. A Capstone intervertebral fusion device (Medtronic Sofamor Danek, USA) filled with autologous bone particles was implanted under direct vision [Figure 1c and 1d]. Pedicle screws and rods (Medtronic Sofamor Danek, USA) were further installed for fixation.[18,19] A longitudinal cut was made in lumbodorsal fascia from the posterior median incision (2.0–2.5 cm) to the contralateral free skin and subcutaneous tissues to establish a working channel for the placement of pedicle screws and rods for fixation on the contralateral side. During the bilateral pedicle screw placement process, the direction of Quadrant retractor should be adjusted in order to obtain sufficient inner inclination for the screw placement. Fenestration and decompression were performed based on the clinical symptoms and radiographic spinal stenosis conditions. After flushing and hemostasis, a negative pressure drainage system was placed on the side of the interbody fusion surgery. The bilateral lumbodorsal fascia was densely sutured, and the incision was closed [Figure 1e]. The pedicle screws and intervertebral fusion device had a good location on X-ray immediately after surgery [Figure 1f and 1g], and the length of the wound was approximately 4.0 cm postoperative 3 months follow-up [Figure 1h].
Figure 1.
Surgical procedure of modified transforaminal lumbar interbody fusion with a small single posterior median incision. (a) Preoperative localization of the surgical segment; (b) 3.0–3.5 cm longitudinal incision was made in the lumbodorsal fascia, and expose the lamina on the symptomatic side; (c and d) Remove part of lamina, decompress the canal, and conduct the interbody fusion; (e) The appearance and length of the wound after closing; (f and g) The anteroposterior and lateral X-ray results immediately after surgery; (h) The appearance and length of the wound at postoperative 3 months follow-up.
Modified transforaminal lumbar interbody fusion with a double paramedian incision
Surgical segments were located by fluoroscopy before surgery. A 3.0–3.5 cm incision was made from the posterior midline to both sides. A longitudinal paramedian incision approximately 3.5 cm in length was made using the surgical space as the midline. Skin and subcutaneous tissues were sequentially incised to expose and cut the lumbodorsal fascia. Quadrant retractors were sequentially placed to establish a working channel. Decompression was performed, and the interbody fusion operation was completed on the side with lower limb symptoms or serious symptoms. Fenestration and decompression were performed based on the contralateral clinical symptoms and imaging manifestations. Bilateral placement of pedicle screws and rods was performed for fixation. After flushing and hemostasis, a negative pressure drainage system was placed on the side of interbody fusion operation, and incisions were sutured layer by layer.[14,18]
Comparison of indicators
Operation indicators: Statistical analysis of the operation time, intraoperative fluoroscopy time, intraoperative blood loss, incision length, and perioperative complications of the two groups.
Functional evaluation indicator: The Oswestry Disability Index (ODI) and Visual Analog Scale (VAS) scores of the two groups before surgery and 3, 12 months postoperation.
Sacrospinalis muscle damage evaluation indicator: Three months postoperation, patients in the two groups received electrophysiological examinations of sacrospinalis muscle at the surgical segment. The MEB9400-K electromyography instrument (Nihon Kohden, Japan) performed surface electromyography of sacrospinalis muscle at the surgical segment. The electromyography indicators of the sacrospinalis muscles, including the average discharge amplitude (AMP, μV) and average discharge frequency (Hz), were measured. In addition, a 3.0 T MRI (TR = 3000, Siemens, Germany) was used to performed continuous scanning of the sacrospinalis muscle at the surgical segment. A multifidus area of 1.5 cm × 1.5 cm at the level of the midline fusion device was symmetrically selected to measure the T2 relaxation time.[14]
Evaluation of fusion using radiological imaging: The Brantigan-Steffee fusion criteria evaluated the fusion conditions of patients in the two groups.[20]
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). The t-test was performed to evaluate changes in surgical indicators, sacrospinalis muscle injury evaluation indicator, VAS scores, and ODI scores before and after surgery of the two groups. The P < 0.05 was considered to be statistically significant.
RESULTS
A total of 31 and 35 cases in the single incision and double incision groups, respectively, completed at least 12 months of systemic follow-up. Five patients of two groups were lost to follow-up in this study. The average operation times of the two groups were 149.2 ± 28.2 min and 155.7 ± 28.6 min, respectively. No significant difference was observed between the groups (P = 0.333). The average intraoperative fluoroscopy time of the two groups was 81.3 ± 11.5 s and 86.3 ± 11.7 s, respectively. No significant difference was observed (P = 0.072). The comparison of intraoperative blood loss and postoperative drainage between the two groups showed no significant differences, with P values of 0.909 and 0.343, respectively. The total surgical incision lengths in the two groups were 4.4 ± 0.7 cm and 7.5 ± 0.4 cm, respectively, which were significantly different (P = 0.000). For the perioperative complications, one case in the single incision group had a postoperative superficial infection at the incision site, which recovered after enhanced dressing, and one case developed pulmonary infection, which recovered after antibiotic treatment. In the double incision group, two cases had pulmonary infections, and one case had fat liquefaction at the wound, which recovered after treatment [Table 2].
Table 2.
Comparison of operative indicators between the two groups
| Category | Single incision group | Double incision group | P |
|---|---|---|---|
| Operation time (mean ± SD, min) | 149.2 ± 28.2 | 155.7 ± 28.6 | 0.333 |
| Intraoperative fluoroscopy time (mean ± SD, s) | 81.3 ± 11.5 | 86.3 ± 11.7 | 0.072 |
| Intraoperative blood loss (mean ± SD, ml) | 169.9 ± 38.9 | 168.7 ± 46.7 | 0.909 |
| Postoperative drainage (mean ± SD, ml) | 103.3 ± 28.9 | 109.8 ± 28.6 | 0.343 |
| Total surgical incision length (mean ± SD, cm) | 4.4 ± 0.7 | 7.5 ± 0.4 | 0.000 |
| Perioperative complication (n) | 2 | 3 | – |
| Nerve root injury | 0 | 0 | |
| Superficial wound infection | 1 | 0 | |
| Other complications | 1 (pulmonary infection) | 3 (2 pulmonary infection, 1 wound fat liquefaction) |
SD: Standard deviation.
The ODI and VAS scores of patients in the two groups before surgery and at 3, 12 months postoperation all significantly improved (all P < 0.05). The ODI and VAS scores at 3 and 12 months postoperation did not show significant differences between the two groups (P ≥ 0.05). The AMP (μV), average discharge frequency of sacrospinalis muscle, and MR T2 relaxation time between groups were not significantly different (P ≥ 0.05) [Table 3].
Table 3.
Comparison of clinical function scores and sacrospinalis muscle damage between the two groups (mean ± SD)
| Category | Single incision group | Double incision group | P |
|---|---|---|---|
| Preoperative VAS scores | 5.0 ± 1.4 | 4.9 ± 1.3 | 0.728 |
| VAS scores at 3 months postoperation | 1.2 ± 0.8 | 1.2 ± 0.8 | 0.718 |
| VAS scores at 12 months postoperation | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.728 |
| Preoperative ODI scores | 59.2 ± 9.5 | 57.4 ± 9.0 | 0.392 |
| ODI scores at 3 months postoperation | 23.4 ± 8.7 | 24.0 ± 8.4 | 0.764 |
| ODI scores at 12 months postoperation | 11.1 ± 2.8 | 11.5 ± 2.6 | 0.478 |
| Sacrospinalis muscle damage evaluation | |||
| Average discharge amplitude (μV) | 202.4 ± 17.6 | 198.6 ± 16.3 | 0.343 |
| Average discharge frequency (Hz) | 98.9 ± 7.3 | 95.9 ± 7.5 | 0.091 |
| MR T2 relaxation time (ms) | 49.6 ± 8.4 | 52.8 ± 7.2 | 0.084 |
SD: Standard deviation; VAS: Visual Analog Scale; ODI: Oswestry Disability Index; MR: Magnetic resonance.
The radiological results of 12 months postoperation showed that there was no Grade A and B of the Brantigan-Steffee fusion criteria in both groups; two cases and three cases of Grade C were in the single incision group and double incision group, respectively. The fusion rate of the two groups was 93.5%, 91.4% (Grade D and E cases), respectively; there were no significant differences between the two groups (P ≥ 0.05).
DISCUSSION
Minimally invasive spine surgery (MISS) is the combination of traditional spinal surgery with minimally invasive technology to ensure the effectiveness of spinal surgery. This technology tries to minimize the interference of surgery on the injury and physiological function of surrounding tissues to achieve smaller incisions, less tissue damage, lighter systemic reactions, faster recovery cycles, and better psychological effects.[1,2,3,4,5,6] Due to the disease characteristics and the treatment requirements of clinical diagnosis and treatment of spinal surgery, spinal surgery has as its “goal” the preservation of the following tissues and structures: Nervous tissues, such as the spinal cord and nerve root, the inherent bony structure of the spine, the muscle fascia surrounding the spine, and the local skin surrounding the surgery site. Therefore, the basic principle of MISS can be summarized as follows: On the bases of the safe and effective decompression of nervous tissues, such as the spinal cord and nerve root, the destruction of inherent bony and ligamentous structures of the spine is minimized as much as possible, the damage of muscle and fascia tissues around the surgical area is reduced, and the length of the skin incision is reduced. In other words, from the earliest total laminectomy for decompression to unilateral lamina fenestration decompression, the destruction of bony and ligamentous structures of the lumbar spine is effectively decreased, which allows minimal invasiveness in the development of lumbar intervertebral disc herniation treatment. The use of the Wiltse paraspinal approach to reduce sacrospinalis muscle damage is minimally invasive, and the transition from longitudinal incision to transverse incision in the anterior cervical spine to effectively minimize skin incision is also minimally invasive.[21,22] However, it must be emphasized that the essence of “minimal invasiveness” is to require less surgical trauma to reach the equivalent or better efficacy than traditional open surgery. If MISS only obtains a “small incision” in appearance using an advanced endoscope, percutaneous surgical technique, or other new high technologies but does not perform effective decompression for important tissues, such as spinal cord and nerve root, or it does not obtain better results than the traditional open surgery due to insufficient decompression or improper surgical operation, then this so-called “minimal invasiveness” is meaningless.[23]
A large number of the past studies have shown that minimally invasive TLIF was better or equal to the traditional open TLIF in clinical efficacy, and most comparative studies have shown that minimally invasive TLIF did not significantly increase the operative-related complications.[9,10,11,12,13,14,15,16,17] In addition, most of the clinical studies have confirmed that minimally invasive lumbar posterior surgery could significantly reduce the paraspinal muscles injuries compared with the traditional open approaches.[12,14,24,25,26] On the bases of clinical performance of the Mast Quadrant-assisted minimally invasive modified TLIF with a double paramedian incision, this study further investigated the clinical surgical feasibility of the minimally invasive modified TLIF with a small single posterior median incision. This study found that the clinical efficacy of this minimally invasive modified TLIF with a small single posterior median incision was the same as that of the previous double paramedian incision. This technology reduced the number of surgical incisions, which may have reduced the overall incidence of wound complications. It also significantly shortened the total surgical incision length. Although this modified clinical operation did not significantly increase clinical efficacy, it effectively shortened the length of surgical incisions and presented an excellent minimally invasive spinal operation concept because this technique allowed the safe and effective decompression of nerve structures such as nerve roots (i.e., the clinical efficacy and perioperative complications between the two groups were not significantly different) and minimized the destruction and damage of inherent bony and ligamentous structures of the spine and muscle fascia tissues (i.e., the excised bony structure was the same, and the exposure and surgery were conducted through a paraspinal approach in which the muscle tissue injury was not increased). This surgical approach significantly shortened the skin incision length and minimized the potential effect of skin scarring on local appearance and the requirement of a second surgery.
It should be noted that Mast Quadrant-assisted modified TLIF with a small single posterior median incision may have some potential shortcomings, such as subcutaneous free might affect the blood supply of the skin, and increase in incision-related complications. However, the total surgical incision length of this technique was just 4.4 ± 0.7 cm, without extensive subcutaneous free. In theory, the influence of the blood supply was relatively small. Moreover, in this study, the patients in the single incision group did not have any wound-related complications. In addition, in order to obtain sufficient inner inclination for the screw placement, it is necessary to adjust the direction of quadrant retractors repeatedly.
In conclusion, through this clinical comparison study, we considered the Mast Quadrant-assisted minimally invasive modified TLIF with a small single posterior median incision to have excellent clinical operation feasibility compared to the minimally invasive TLIF with a double paramedian incision. The minimally invasive modified TLIF with a small single posterior median incision significantly shortened surgical incisions and exhibited similar clinical efficacy. This approach is an excellent concept for minimally invasive surgery, and patients are more willing to accept this technique. Therefore, this technique has clinical value for promotion.
Footnotes
Edited by: Xin Chen
Source of Support: This work was supported by grants from the 2012 Key Medical Projects of Shanghai Committee of Science and Technology (No. 12411951201) and the 2013 Joint Research Projects of Shanghai Municipal Hospital in Emerging Cutting-edge Technology (No. SHDC12013108).
Conflict of Interest: None declared.
REFERENCES
- 1.Jaikumar S, Kim DH, Kam AC. History of minimally invasive spine surgery. Neurosurgery. 2002;51:S1–14. [PubMed] [Google Scholar]
- 2.Thongtrangan I, Le H, Park J, Kim DH. Minimally invasive spinal surgery: A historical perspective. Neurosurg Focus. 2004;16:E13. doi: 10.3171/foc.2004.16.1.14. [DOI] [PubMed] [Google Scholar]
- 3.O’Toole JE, Eichholz KM, Fessler RG. Minimally invasive spine surgery. Preface. Neurosurg Clin N Am. 2006;17:ix–x. doi: 10.1016/j.nec.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Fessler RG, O’Toole JE, Eichholz KM, Perez-Cruet MJ. The development of minimally invasive spine surgery. Neurosurg Clin N Am. 2006;17:401–9. doi: 10.1016/j.nec.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole JE. The future of minimally invasive spine surgery. Neurosurgery. 2013;60(Suppl 1):13–9. doi: 10.1227/01.neu.0000430305.33208.96. [DOI] [PubMed] [Google Scholar]
- 6.Spetzger U, Von Schilling A, Winkler G, Wahrburg J, König A. The past, present and future of minimally invasive spine surgery: A review and speculative outlook. Minim Invasive Ther Allied Technol. 2013;22:227–41. doi: 10.3109/13645706.2013.821414. [DOI] [PubMed] [Google Scholar]
- 7.Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg. 2002;49:499–517. [PubMed] [Google Scholar]
- 8.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26–35. doi: 10.1097/01.BRS.0000076895.52418.5E. [DOI] [PubMed] [Google Scholar]
- 9.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1–6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs RE, Podichetty VK, Santiago P, Sandhu FA, Spears J, Kelly K, et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 11.German JW, Foley KT. Minimal access surgical techniques in the management of the painful lumbar motion segment. Spine (Phila Pa 1976) 2005;30:S52–9. doi: 10.1097/01.brs.0000174501.53285.9d. [DOI] [PubMed] [Google Scholar]
- 12.Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine (Phila Pa 1976) 2006;31:712–6. doi: 10.1097/01.brs.0000202533.05906.ea. [DOI] [PubMed] [Google Scholar]
- 13.Karikari IO, Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: A review of techniques and outcomes. Spine (Phila Pa 1976) 2010;35:S294–301. doi: 10.1097/BRS.0b013e3182022ddc. [DOI] [PubMed] [Google Scholar]
- 14.Wang HL, Lü FZ, Jiang JY, Ma X, Xia XL, Wang LX. Minimally invasive lumbar interbody fusion via MAST Quadrant retractor versus open surgery: A prospective randomized clinical trial. Chin Med J. 2011;124:3868–74. [PubMed] [Google Scholar]
- 15.Seng C, Siddiqui MA, Wong KP, Zhang K, Yeo W, Tan SB, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: A matched-pair comparison study. Spine (Phila Pa 1976) 2013;38:2049–55. doi: 10.1097/BRS.0b013e3182a8212d. [DOI] [PubMed] [Google Scholar]
- 16.Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ, Oglesby M, Pelton MA, et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 2014;14:1694–701. doi: 10.1016/j.spinee.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Eckman WW, Hester L, McMillen M. Same-day discharge after minimally invasive transforaminal lumbar interbody fusion: A series of 808 cases. Clin Orthop Relat Res. 2014;472:1806–12. doi: 10.1007/s11999-013-3366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu FZ, Wang HL, Jiang JJ, Ma X, Xia XL, Wang LX. Mast Quadrant-assisted modified transforaminal lumbar interbody fusion (in Chinese) Chin J Orthop. 2011;31:1072–7. doi: 10.4103/0366-6999.154280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang JY, Ma X, Lü FZ, Wang HL, Chen WJ, Ma XS, et al. The anatomic study and clinical significance of the modified transforaminal lumbar interbody fusion (in Chinese) Chin J Surg. 2009;47:1100–3. [PubMed] [Google Scholar]
- 20.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993;18:2106–7. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 21.Wiltse LL, Spencer CW. New uses and refinements of the paraspinal approach to the lumbar spine. Spine (Phila Pa 1976) 1988;13:696–706. [PubMed] [Google Scholar]
- 22.Hoh DJ, Wang MY, Ritland SL. Anatomic features of the paramedian muscle-splitting approaches to the lumbar spine. Neurosurgery. 2010;66:13–24. doi: 10.1227/01.NEU.0000350866.25760.33. [DOI] [PubMed] [Google Scholar]
- 23.Kim CW, Siemionow K, Anderson DG, Phillips FM. The current state of minimally invasive spine surgery. J Bone Joint Surg Am. 2011;93:582–96. [PubMed] [Google Scholar]
- 24.Stevens KJ, Spenciner DB, Griffiths KL, Kim KD, Zwienenberg-Lee M, Alamin T, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77–86. doi: 10.1097/01.bsd.0000193820.42522.d9. [DOI] [PubMed] [Google Scholar]
- 25.Kim CW. Scientific basis of minimally invasive spine surgery: Prevention of multifidus muscle injury during posterior lumbar surgery. Spine (Phila Pa 1976) 2010;35:S281–6. doi: 10.1097/BRS.0b013e3182022d32. [DOI] [PubMed] [Google Scholar]
- 26.Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: Minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19:316–24. doi: 10.1007/s00586-009-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]