Abstract
Background:
Phloroglucinol plays an important role in oxidative stress and inflammatory responses. The effects of phloroglucinol have been proven in various disease models. The aim of the present study was to investigate the efficacy and possible mechanisms of phloroglucinol in the treatment of interstitial cystitis (IC).
Methods:
Thirty-two female Sprague-Dawley (SD) rats were used in this study. IC was induced by intraperitoneal injection of cyclophosphamide (CYP). Rats were randomly allocated to one of four groups (n = 8 per group): A control group, which was injected with saline (75 mg/kg; i.p.) instead of CYP on days 1, 4, and 7; a chronic IC group, which was injected with CYP (75 mg/kg; i.p.) on days 1, 4, and 7; a high-dose (30 mg/kg) phloroglucinol-treated group; and a low-dose (15 mg/kg) phloroglucinol-treated group. On day 8, the rats in each group underwent cystometrography (CMG), and the bladders were examined for evidence of oxidative stress and inflammation. Statistical analysis was performed by analysis of variance (ANOVA) followed by least square difference multiple comparison post-hoc test.
Results:
Histological evaluation showed that bladder inflammation in CYP-treated rats was suppressed by phloroglucinol. CMG revealed that the CYP treatment induced overactive bladder in rats that was reversed by phloroglucinol. Up-regulated tumor necrosis factor-α and interleukin-6 expression in the CYP-treated rats were also suppressed in the phloroglucinol treated rats. CYP treatment significantly increased myeloperoxidase activity as well as the decreased activities of catalase of the bladder, which was reversed by treatment with phloroglucinol.
Conclusions:
The application of phloroglucinol suppressed oxidative stress, inflammation, and overactivity in the bladder. This may provide a new treatment strategy for IC.
Keywords: Antioxidant, Interstitial Cystitis, Inflammation, Phloroglucinol, Rats
INTRODUCTION
Interstitial cystitis (IC) is a chronic disease characterized by suprapubic and/or bladder pain related to bladder filling, accompanied by urinary frequency, urgency, and nocturia.[1] Recent evidence demonstrates that IC may be present in >2% of females, and the estimated ratio of male to female is 1:5.[2] The pathogenesis of IC is still uncertain, and several factors have been suggested, including inflammation, mast cell activation, autoimmune mechanisms, genetic predisposition, and neurogenic causes.[3,4]
Several studies have shown that proinflammatory cytokines induced functional changes in C-fiber afferents that led to these relatively unexcitable afferents becoming hyperactive or hyperexcitable.[3,4] It was reported that an increase in reactive oxygen species (ROS) leads to bladder edema, inflammation and extravasation, indicating that ROS may play a critical role in a rat model of cyclophosphamide (CYP)-induced cystitis.[5]
Symptoms related to IC considerably decrease patients’ quality-of-life, and its treatment remains a substantial difficulty for urologists. There are few effective treatments for IC, and these treatments are often accompanied by side effects.[6] Therefore, the development of alternative and effective treatments for this serious condition is necessary.
Phloroglucinol (1,3,5-trihydroxybenzene) is a nonatropinic antispasmodic agent that is used in the clinic to treat the acute pain related to functional disorders of the digestive tract or the bile duct.[7] Several studies have demonstrated that phloroglucinol has anti-inflammatory and antioxidant properties in vitro and in vivo.[8,9] Because inflammatory cell infiltration and ROS play important roles in IC, it is reasonable to speculate that phloroglucinol may have protective effects on the CYP-induced bladder injury by inhibiting these two events.
In this study, using the model of CYP-induced cystitis, we examined whether phloroglucinol was able to protect the bladder against CYP-induced injury and explored whether the beneficial effects of phloroglucinol on the bladder were related to the inhibition of proinflammatory cytokines. As phloroglucinol has been shown to ameliorate oxidative stress by increasing the activity of catalase (CAT),[10,11] we therefore evaluated the effect of phloroglucinol on CAT activity.
METHODS
Animals
Thirty-two female Sprague-Dawley (SD) rats (200–250 g) were provided by the Laboratory Animal Center of Peking University Health Science Center. Rats were housed in a temperature-controlled (22–24°C) and light-controlled (12 h light/dark cycle) room and were given rodent chow and tap water ad libitum. All procedures were approved by the Ethics Committee of Experimental Animals of The Peking University Health Science Center and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Drugs
Phloroglucinol (>99% purity) was purchased from Sigma Chemical Company (St. Louis, MO, USA).
Induction of chronic interstitial cystitis
Chronic CYP-cystitis was induced by the administration of CYP (75 mg/kg; intraperitoneal [i.p.]) on days 1, 4, and 7.[12] Damage of the bladder mucosa leads to impaired urothelial barrier function. Frequent micturition increased permeability of the bladder wall, bladder inflammation, and mast cell activation were induced in IC rats.
Experimental protocols
Rats were randomly allocated to one of four groups (n = 8 per group): (i) A control group, which was injected with saline (75 mg/kg; i.p.) instead of CYP on days 1, 4, and 7; (ii) a chronic IC group, which was injected with CYP (75 mg/kg; i.p.) on days 1, 4, and 7; (iii) a high-dose (H-) phloroglucinol-treated group; and (iv) a low-dose (L-) phloroglucinol-treated group. The high- and low-dose phloroglucinol-treated groups were administered 30 and 15 mg/kg, i.p. of phloroglucinol from days 1 to 7, respectively, starting from the same day that the rats were first administered CYP to induce chronic cystitis. The phloroglucinol dose was selected based on the results of previous studies and a preliminary experiment.[11]
Cystometrography
On day 8, cystometrography (CMG) was performed in all rats.[4] The rats were anesthetized with urethane (1.2 g/kg, subcutaneously), and a suprapubic midline laparotomy was performed to expose the bladder; then, a polyethylene tube was inserted into the bladder through the bladder dome. The polyethylene tube was connected to a syringe pump and pressure transducer through a three-way stopcock. CMG was performed using a saline infusion at a rate of 10 mL/h. Baseline pressure (BP), peak voiding pressure (PP), and intercontraction interval (ICI) were recorded.
Histopathological examination
After the CMG procedure, bladder tissues were harvested and divided equally into two individual sections. Half of each tissue sample was frozen at −80°C for the measurement of antioxidant enzyme activity, Western blotting, and real-time reverse transcription polymerase chain reaction. The other half of the tissue sample was fixed in 4% paraformaldehyde and embedded in paraffin before being cut into 5-μm sections and stained with hematoxylin and eosin following standard protocols.
Histopathological damage scoring based on the following features was performed: Epithelial damage, hemorrhage, inflammatory cell infiltration, and edema. Each criterion was evaluated (0: Normal, 1: Mild, 2: Moderate, 3: Severe). The maximum score was calculated as 12.
Mast cell counting
The method for acidified toluidine blue staining was as described previously.[13] The number of toluidine blue-stained mast cells in two nonadjacent tissue sections was counted by two individuals who were blinded to the experimental conditions and recorded as the mean number of sections for each animal.[12]
Bladder myeloperoxidase activity measurement
Bladder myeloperoxidase (MPO) activity was assayed using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as previously described.[14]
Determination of catalase activity in the bladder
We measured the CAT levels in bladder tissues. Briefly, we homogenized bladders and centrifuged them at 3000 r/min for 15 min. We collected the supernatants and measured the protein content using commercialized assay kits (Nanjing Jiancheng Bioengineering Institute). We measured CAT activity by employing hydrogen peroxide to generate water and oxygen.[15] The results are expressed as units per milligram of protein. We performed all spectrophotometric numerical readings using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The protein concentrations in the bladder homogenate samples were determined using a BCA Protein Assay Kit (CoWin Bioscience, Beijing, China).
Quantitative real-time reverse transcription polymerase chain reaction analysis
Total RNA was extracted from the bladders using Eastep Universal RNA Extraction Kit (Promega, Madison, WI, USA) according to the manufacturer's protocol. Total RNA (2 μg) was reverse transcribed to generate first-strand cDNA using the GoScript™ Revere System according to its protocol. The primer pairs were as follows: For tumor necrosis factor-α (TNF-α), 5ꞌ-AGCAGATGGGCTGTACCT-3ꞌ and 5ꞌ-ACATGGGCTCATACCAGG-3ꞌ; for interleukin-6 (IL-6), 5ꞌ-ACAGAAGGAGTGGCTAAG-3ꞌ and 5ꞌ-TTAGATACCCATCGACAG-3ꞌ; for IL-1β, 5ꞌ-TGTCTGACCCATGTGAGC-3ꞌ and 5ꞌ-CTTTCATCACACAGGACAGG-3ꞌ; and for glyceraldehydes-3-phosphate dehydrogenase, 5ꞌ-CTCTGCTCCTCCCTGTTCT-3ꞌ and 5ꞌ-CTTGACTGTGCGTGACT-3ꞌ. Data were analyzed using the 2−ΔΔCT method.[16]
Western blotting
Tumor necrosis factor-α and IL-6 expression in the bladder were detected using immunoblot analysis, as previously described.[17] Briefly, we loaded samples (40 μg protein), separated them on 10% sodium dodecyl sulfate-polyacrylamide gels, and transferred them to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked for 1 h with nonfat milk and incubated overnight at 4°C with the primary antibodies, whose concentrations were as follows: Rabbit polyclonal anti-rat TNF-α (1:500; Abcam), rabbit monoclonal anti-rat IL-6 (1:500; Abcam), or mouse polyclonal anti-rat glyceraldehyde-3-phosphate dehydrogenase antibody (1:800; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Next, we incubated the membranes for 1 h at room temperature with goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated secondary antibodies. The protein bands were detected by chemiluminescence technology and analyzed using the Image J software (http://rsb.info.nih.gov/ij/download/).
Statistical analysis
Statistical analyses were performed using SPSS 14 for Windows (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ± standard error (SE). Statistical analysis was performed by analysis of variance (ANOVA) followed by least square difference multiple comparison post-hoc test. P < 0.05 was considered statistically significant.
RESULTS
Cystometrography
As shown in Table 1 and Figure 1, there were statistically significant differences in PP and ICI between the CYP-treated and control rats (P < 0.01). The administration of phloroglucinol (15 or 30 mg/kg) improved the PP and ICI in the CYP-treated rats (P < 0.01). The rats in all four groups had no significant differences in BP (P > 0.05).
Table 1.
Cystometrogram results in four groups
| Groups | BP (mmHg) | PP (mmHg) | ICI (s) |
|---|---|---|---|
| Control | 6.19 ± 0.94 | 33.75 ± 1.83 | 380.37 ± 39.82 |
| CYP | 6.93 ± 1.11 | 15.63 ± 1.99* | 83.13 ± 10.43* |
| H-phloroglucinol | 6.56 ± 1.01 | 26.50 ± 3.96† | 194.75 ± 79.88† |
| L-phloroglucinol | 6.67 ± 1.31 | 20.00 ± 2.56† | 207.70 ± 32.08† |
The results are the means ± SE for 8 rats in each group. *P<0.01 versus control group; †P<0.01 versus CYP group. SE: Standard error; BP: Baseline pressure; PP: Peak voiding pressure; ICI: Intercontraction interval; CYP: Cyclophosphamide.
Figure 1.
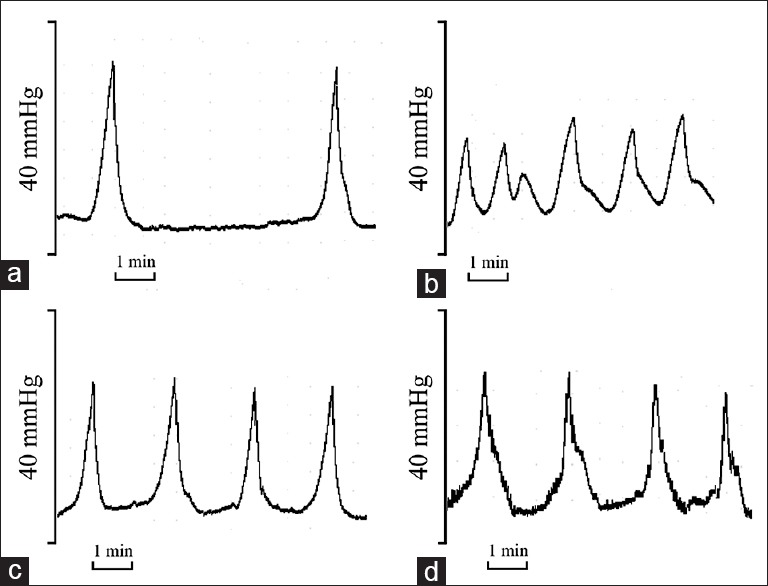
Representative cystometrogram (CMG). (a) Control rats; (b) cyclophosphamide (CYP)-treated rats; (c) H-phloroglucinol-treated rats and (d) L-phloroglucinol-treated rats. The CMG showed that intercontraction interval was significantly reduced, peak voiding pressure was significantly reduced in CYP-treated rats, and these effects were ameliorated by phloroglucinol treatment. Bar indicates 1 min.
Histopathological evaluation
Bladders from the rats of the control group showed normal histology. CYP treatment significantly increased epithelial damage, hemorrhage, cellular infiltration, and edema. In contrast, in the phloroglucinol-treated bladders, edema, hemorrhage, inflammatory cell infiltration were mitigated, and the urothelium appeared to be well-preserved. These results indicated that phloroglucinol treatment mitigated the severity of the rat IC induced by CYP.
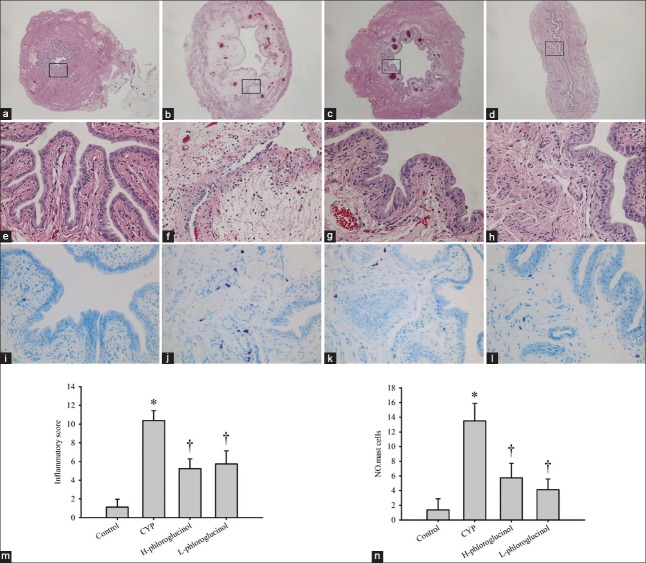
Based on the semiquantitative histopathological scores of all four groups, phloroglucinol treatment significantly reduced the CYP-induced increase in histopathological scores (P < 0.01, Figure 2).
Figure 2.
Representative images of H and E stained (original magnification: [a-d] ×20 and [e-h] ×200) and toluidine blue stained (original magnification: ×200) bladders; (a, e, i) Control rats; (b, f, j) cyclophosphamide (CYP)-treated rats; (c, g, k) H-phloroglucinol-treated rats; (d, h, l) L-phloroglucinol-treated rats; (m) Inflammatory score; (n) Number of mast cells. Phloroglucinol treatment mitigated the severity of the rat of interstitial cystitis. CYP-treatment increases the number of mast cells, which was reversed by phloroglucinol treatment. Values are the mean ± standard error, n = 8. *P < 0.01 (vs. the control group); †P < 0.01 (vs. the CYP-treated group).
Mast cell counting
Mast cells play a critical role in the pathogenesis and pathophysiology of IC. Our results showed that a few mast cells in the bladders of the control group were observed. There was a significant increase in both granulated and degranulated mast cells in the CYP-treated group compared with the control group (P < 0.01), which were markedly decreased by phloroglucinol (15 or 30 mg/kg) treatment (P < 0.01; Figure 2).
Effect of phloroglucinol on the activities of myeloperoxidase and catalase in bladder tissue
Bladder MPO activity was used to assess inflammatory responses in bladders. As shown in Table 2, CYP treatment significantly increased the MPO activity and decreased the CAT activity in bladder tissues (P < 0.01). The effects of the CYP on the activities of MPO were reversed by treatment with phloroglucinol (15 or 30 mg/kg) (P < 0.01, P < 0.05); phloroglucinol improved the CAT activity at the high dosage of 30 mg/kg (P < 0.01), and there was a tendency for the low dosage of phloroglucinol (15 mg/kg) to increase CAT activity, but the trend did not reach statistical significance (P > 0.05, Table 2).
Table 2.
MPO and CAT activities in rat bladder tissues of four groups
| Items | Control | CYP | H-phloroglucinol | L-phloroglucinol |
|---|---|---|---|---|
| MPO, U/g wet tissue | 0.63 ± 0.12 | 1.43 ± 0.23* | 1.07 ± 0.21† | 1.24 ± 0.13‡ |
| CAT, U/mg protein | 16.77 ± 1.26 | 9.15 ± 0.94* | 12.58 ± 2.01† | 10.21 ± 1.61 |
The results are expressed as the mean ± standard error for 8 rats in each group. *P<0.01 versus control group; †P<0.01 versus CYP group; ‡P<0.05 versus CYP group. CYP: Cyclophosphamide; MPO: Myeloperoxidase; CAT: Catalase.
Expression of tumor necrosis factor-α, interleukin-6, and interleukin-1β
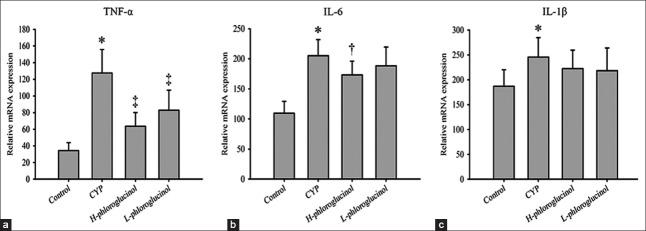
We quantified the TNF-α, IL-6, and IL-1β mRNA levels in the bladder. CYP treatment significantly increased the expression of all of these proinflammatory molecules. The expression of TNF-α decreased after phloroglucinol (15 or 30 mg/kg) treatment compared with the CYP-treated group (P < 0.01); only high-dose phloroglucinol (30 mg/kg) treatment significantly reduced the expression of IL-6 (P < 0.05), and although there was a tendency for phloroglucinol (15 or 30 mg/kg) to reduce the expression of IL-1β, the trend did not reach statistical significance (P > 0.05, Figure 3).
Figure 3.
Reverse transcription polymerase chain reaction of inflammatory factor mRNA in the bladder. Relative quantities of tumor necrosis factor-α (TNF-α) (a), interleukin-6 (IL-6) (b), and interleukin-1β (IL-1β) (c) mRNA were normalized to glyceraldehydes-3-phosphate dehydrogenase mRNA. Values are expressed as the mean ± standard error, n = 8. *P < 0.01 (vs. the control group); †P < 0.05 (vs. the cyclophosphamide (CYP)-treated group), ‡P < 0.01 (vs. the CYP-treated group).
We examined the protein expression of TNF-α and IL-6 in bladder tissues from each group using Western blot analyses. CYP treatment induced the most substantial upregulation of TNF-α and IL-6 expression, which was significantly greater than that observed in the control rats (P < 0.01). Treatment with phloroglucinol (15 or 30 mg/kg) suppressed these increases (P < 0.01, Figure 4).
Figure 4.
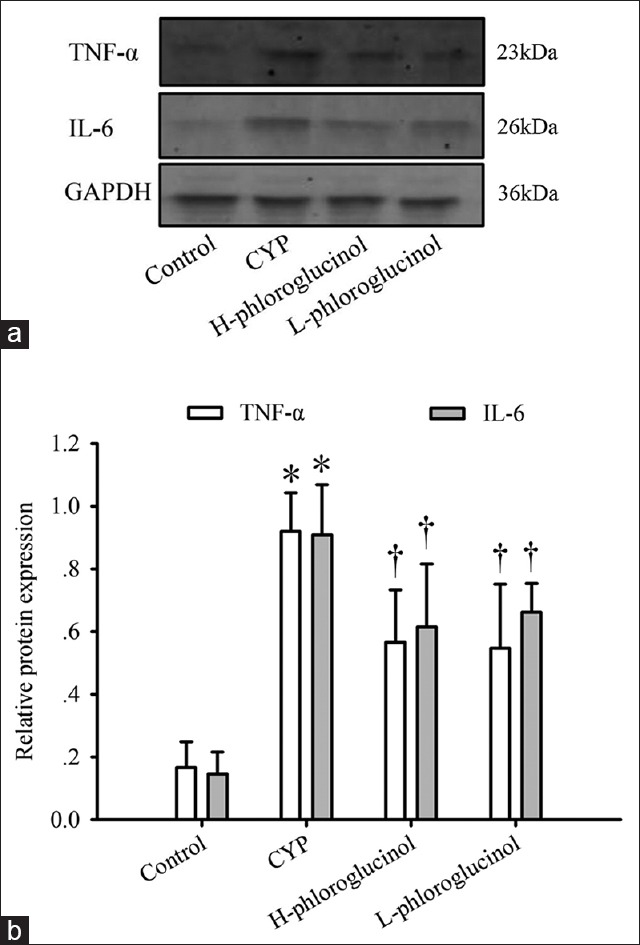
Effect of phloroglucinol on cyclophosphamide (CYP)-induced tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) protein expression. Representative Western blotting images (a) and statistical analysis (b) of TNF-α and IL-6 are shown. Values are expressed as the mean ± standard error, n = 8. *P < 0.01 (vs. the control group), †P < 0.01 (vs. the CYP-treated group).
DISCUSSION
Interstitial cystitis is a chronic disease characterized by chronic inflammation, mast cell activation, leaky epithelium, urinary frequency, and urinary urgency.[6] I.p. injection with CYP could reproduce most features of IC in humans such as increasing voiding frequency, decreasing urine volume per void, and increasing permeability of the bladder wall in rats.[18] CYP is an antineoplastic agent that is widely used for the treatment of malignancies such as lymphoma and leukemia. CYP administered in the peritoneum is converted to the toxic metabolite acrolein in the kidney, which accumulates in the bladder and injures the urinary tract. In the present study, CMG showed that repetitive CYP administration increased the voiding frequency and decreased the micturition pressure; the histological changes such as epithelial damage, hemorrhage, cellular infiltration and edema induced by CYP were the same as in previous reports;[18] we also observed that mast cell proliferation and activation were increased in rats with CYP-induced cystitis. These results are consistent with those of the previous study.[19]
Phloroglucinol is an antispasmodic agent that is used to treat acute pain related to functional disorders of the digestive tract and bile duct,[10] but its mechanism of action is still unknown. Further studies are needed to clarify this issue. This study showed that the application of phloroglucinol suppressed bladder overactivity and ameliorated histological damage in the CYP-induced cystitis rat model; rats treated with phloroglucinol had reduced mast cell proliferation. These data indicated that phloroglucinol might be effective at treating IC. To our knowledge, this is the first report demonstrating the efficacy of phloroglucinol for bladder inflammation. We speculate that the mechanism of the improvement in bladder overactivity and histological damage by phloroglucinol is due to the suppression of bladder oxidative stress and inflammation.
Reactive oxygen species include the hydroxyl radical, superoxide anion, hydrogen peroxide, and singlet oxygen. ROS play integral roles in intracellular signaling, physiological immunological responses, and gene expression under physiological conditions. Excessive ROS production results in cellular and tissue damage.[20,21] Oxidative stress reflects an imbalance between reactive species generation and the ability of antioxidant systems to buffer these radicals.
Inflammatory cells are the main source of ROS. In addition to directly releasing ROS, the activated inflammatory cells produce ROS via the MPO pathway, which catalyzes H2O2 and the chloride ion to form HOCl, an ROS with much stronger oxidative properties.[22] It is common knowledge that a certain level of MPO activity is important in the defense against microbial infection under normal conditions. However, the overactivation of MPO leads to a significant increase in MPO-generated oxidants (such as HOCl), which in turn results in tissue oxidative injury.[23] In addition to this role of being a substrate for MPO, H2O2 is a substrate for CAT. H2O2 is degraded to water and oxygen in the presence of CAT.[24] Therefore, the balance between MPO and CAT activity is critical for the redox status in the CYP-treated rats.
The toxic effects of CYP are partly due to the increased production of free radicals, which is increased in CYP-treated rats. The overproduction of ROS is one of the reasons for the urothelial injury.[5,25] In the present study, we found that CYP-treatment significantly increases inflammatory cell infiltration and MPO activity while decreasing CAT activity in the bladder tissue. This finding suggests that the oxidative status overwhelmed the reductive status and that the increased ROS cause direct oxidative damage to the bladder.
Previous research showed that phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells, a neuroblastoma cell line.[9] Our results showed that phloroglucinol exerts its protective effect on CYP-induced bladder injury through regulating the activities of MPO and CAT the bladder tissues.
It was proposed that proinflammatory cytokines are involved in sensitization of bladder afferent pathways under inflammatory conditions and that the sensitization of the bladder afferent pathways might be involved in the pathogenesis of IC.[4]
Tumor necrosis factor-α is a potent inducer of the inflammatory response and a regulator of immunity. Its biological function includes the modulation of growth differentiation and proliferation of a variety of cell types, release of pro-inflammatory mediators and apoptosis. Many different immune and nonimmune cell types can produce TNF-α, including macrophages, monocytes, neutrophils, mast cells, eosinophils, fibroblasts, epithelial cells, and smooth muscle cells. Potentially noxious stimuli (physical, chemical or immunological) can rapidly induce the production and release of TNF-α.[26] Interestingly, mast cells are known to release and respond to TNF-α, indicating a positive autocrine loop that leads to the augmentation of mast cell activation.[27]
Interleukin-6, a pro-inflammatory cytokine produced by various cell types (endothelial cells, macrophages, fibroblasts, and mast cells), was closely related to symptom severity in a rat cystitis model.[3] It was reported that IL-6 enhances detrusor smooth muscle contractility, which might result in increased voiding frequency.[28]
Previous study showed that phloroglucinol exhibited the inhibitory effects on the production of inflammatory mediators such as TNF-α and IL-6 in RAW264.7 cells (a murine macrophage-like cell line) stimulated by lipopolysaccharide.[29] Our research shows that the TNF-α and IL-6 expression levels are significantly increased in the bladder of CYP-treated rats compared with control rats and that the expression of these two pro-inflammatory cytokines decreased in the phloroglucinol-treated group, indicating that chronic inflammation can be repressed by phloroglucinol.
In conclusion, the present study suggests that CYP-induced cystitis is related to oxidative stress and ROS formation due to inflammation. At the tested concentrations, phloroglucinol ameliorated bladder symptoms and histological changes related to CYP-induced cystitis by inhibiting the activity of MPO while increasing the activity of CAT and suppressing TNF-α and IL-6 production. Given these results, phloroglucinol could be a candidate therapeutic agent for IC.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hanno P, Lin A, Nordling J, Nyberg L, van Ophoven A, Ueda T, et al. Bladder pain syndrome committee of the international consultation on incontinence. Neurourol Urodyn. 2010;29:191–8. doi: 10.1002/nau.20847. [DOI] [PubMed] [Google Scholar]
- 2.Davis NF, Brady CM, Creagh T. Interstitial cystitis/painful bladder syndrome: Epidemiology, pathophysiology and evidence-based treatment options. Eur J Obstet Gynecol Reprod Biol. 2014;175:30–7. doi: 10.1016/j.ejogrb.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Lv YS, Yao YS, Lin ME, Rong L, Deng BH, Huang J, et al. Interleukin-6 levels in female rats with protamine sulfate-induced chronic cystitis treated with hyaluronic acid. Int J Urol. 2013;20:1017–22. doi: 10.1111/iju.12090. [DOI] [PubMed] [Google Scholar]
- 4.Bae WJ, Ha US, Kim S, Kim SJ, Hong SH, Lee JY, et al. Reduction of oxidative stress may play a role in the anti-inflammatory effect of the novel herbal formulation in a rat model of hydrochloric acid-induced cystitis. Neurourol Urodyn. 2015;34:86–91. doi: 10.1002/nau.22507. [DOI] [PubMed] [Google Scholar]
- 5.Al-Malki AL. Synergestic effect of lycopene and melatonin against the genesis of oxidative stress induced by cyclophosphamide in rats. Toxicol Ind Health. 2012;30:570–75. doi: 10.1177/0748233712459916. [DOI] [PubMed] [Google Scholar]
- 6.Giannantoni A, Bini V, Dmochowski R, Hanno P, Nickel JC, Proietti S, et al. Contemporary management of the painful bladder: A systematic review. Eur Urol. 2012;61:29–53. doi: 10.1016/j.eururo.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 7.Chassany O, Bonaz B, Bruley DES Varannes S, Bueno L, Cargill G, Coffin B, et al. Acute exacerbation of pain in irritable bowel syndrome: Efficacy of phloroglucinol/trimethylphloroglucinol. A randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2007;25:1115–23. doi: 10.1111/j.1365-2036.2007.03296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang KA, Zhang R, Chae S, Lee SJ, Kim J, Kim J, et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem Biol Interact. 2010;185:215–26. doi: 10.1016/j.cbi.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Lee K, Kang KA, Lee NH, Hyun JW, Kim HS. Phloroglucinol exerts protective effects against oxidative stress – Induced cell damage in SH-SY5Y cells. J Pharmacol Sci. 2012;119:186–92. doi: 10.1254/jphs.12056fp. [DOI] [PubMed] [Google Scholar]
- 10.Li NS, Luo XJ, Zhang YS, He L, Liu YZ, Peng J. Phloroglucinol protects gastric mucosa against ethanol-induced injury through regulating myeloperoxidase and catalase activities. Fundam Clin Pharmacol. 2011;25:462–8. doi: 10.1111/j.1472-8206.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 11.Li TT, Zhang YS, He L, Li NS, Peng J, Li YJ. Protective effect of phloroglucinol against myocardial ischaemia-reperfusion injury is related to inhibition of myeloperoxidase activity and inflammatory cell infiltration. Clin Exp Pharmacol Physiol. 2011;38:27–33. doi: 10.1111/j.1440-1681.2010.05457.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QH, Zhou ZS, Lu GS, Song B, Guo JX. Melatonin improves bladder symptoms and may ameliorate bladder damage via increasing HO-1 in rats. Inflammation. 2013;36:651–7. doi: 10.1007/s10753-012-9588-5. [DOI] [PubMed] [Google Scholar]
- 13.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One. 2008;3:e2096. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang B, Liu X, Chen H, Liu D, Kuang Y, Xing B, et al. Ischemic postconditioning attenuates renal ischemic/reperfusion injury in mongrel dogs. Urology. 2010;76:1519.e–7. doi: 10.1016/j.urology.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R, Chen NH. Osthole improves chronic cerebral hypoperfusion induced cognitive deficits and neuronal damage in hippocampus. Eur J Pharmacol. 2010;636:96–101. doi: 10.1016/j.ejphar.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Cao Y, Li L, Liang Y, Tian X, Mo N, et al. Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun. 2014;449:74–80. doi: 10.1016/j.bbrc.2014.04.153. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Pak SC, Jeon S, Kim DI. Modified yukmijihwangtang suppresses the production of proinflammatory cytokines in the intravesical hydrochloric acid-induced cystitis rat model via the NF-κB pathway. Am J Chin Med. 2012;40:321–34. doi: 10.1142/S0192415X12500255. [DOI] [PubMed] [Google Scholar]
- 18.Joshi SK, Mikusa JP, Weaver B, Honore P. Morphine and ABT-594 (a nicotinic acetylcholine agonist) exert centrally mediated antinociception in the rat cyclophosphamide cystitis model of visceral pain. J Pain. 2008;9:146–56. doi: 10.1016/j.jpain.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Golubeva AV, Zhdanov AV, Mallel G, Dinan TG, Cryan JF. The mouse cyclophosphamide model of bladder pain syndrome: Tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol Rep. 2014;2:e00260. doi: 10.1002/phy2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Salam OM, Youness ER, Mohammed NA, Morsy SM, Omara EA, Sleem AA. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J Med Food. 2014;17:588–98. doi: 10.1089/jmf.2013.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–16. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 22.Rees MD, Bottle SE, Fairfull-Smith KE, Malle E, Whitelock JM, Davies MJ. Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides. Biochem J. 2009;421:79–86. doi: 10.1042/BJ20090309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y, Robich MP, et al. Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation. 2009;120:S22–30. doi: 10.1161/CIRCULATIONAHA.108.842724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wölkart G, Kaber G, Kojda G, Brunner F. Role of endogenous hydrogen peroxide in cardiovascular ischaemia/reperfusion function: Studies in mouse hearts with catalase-overexpression in the vascular endothelium. Pharmacol Res. 2006;54:50–6. doi: 10.1016/j.phrs.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Ayhanci A, Yaman S, Sahinturk V, Uyar R, Bayramoglu G, Senturk H, et al. Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats. Biol Trace Elem Res. 2010;134:98–108. doi: 10.1007/s12011-009-8458-y. [DOI] [PubMed] [Google Scholar]
- 26.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 27.Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-kappa B and TNF-alpha: A positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–93. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- 28.Weng TI, Wu HY, Lin PY, Liu SH. Uropathogenic Escherichia coli-induced inflammation alters mouse urinary bladder contraction via an interleukin-6-activated inducible nitric oxide synthase-related pathway. Infect Immun. 2009;77:3312–9. doi: 10.1128/IAI.00013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MM, Kim SK. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem Toxicol. 2010;48:2925–33. doi: 10.1016/j.fct.2010.07.029. [DOI] [PubMed] [Google Scholar]