Abstract
Background:
OnabotulinumtoxinA is widely used in treating neurogenic detrusor overactivity (NDO). We carried out a systematic review and meta-analysis to assess the efficacy and safety of the drug for treating NDO.
Methods:
We searched the following databases: Medline, EMBASE, and the Cochrane Controlled Trials Register. All published randomized double-blind, placebo-controlled trials of onabotulinumtoxinA for the treatment of NDO were identified in the analysis. The reference lists of the retrieved studies were also investigated.
Results:
Four publications involving a total of 807 patients were identified in the analysis, which compared onabotulinumtoxinA with placebo. The changes of the mean number of urinary incontinence per week (the standardized mean difference [SMD] = −10.91, 95% confidence intervals [CIs] = −14.18–−7.63, P < 0.0001); maximum cystometric capacity (SMD = 146.09, 95% CI = 126.19–165.99, P < 0.0001) and maximum detrusor pressure (SMD = −32.65, 95% CI = −37.83–−27.48, P < 0.0001) indicated that onabotulinumtoxinA was more effective than the placebo, despite the doses of onabotulinumtoxinA. Safety assessments primarily localized to the urinary tract indicated onabotulinumtoxinA were often associated with more complications. Urinary tract infections (relative risk [RR] =1.48, 95% CI = 1.20–1.81, P = 0.0002); hematuria (RR = 1.81, 95% CI = 1.00–3.24, P = 0.05) and urinary retention (RR = 5.87, 95% CI = 3.61–9.56, P < 0.0001).
Conclusions:
This meta-analysis indicates that onabotulinumtoxinA to be an effective treatment for NDO with side effects primarily localized to urinary tract.
Keywords: Meta-analysis, Neurogenic Detrusor Overactivity, OnabotulinumtoxinA, Randomized Controlled Trial
INTRODUCTION
Overactive bladder (OAB), defined by the International Continence Society as urgency with or without urinary incontinence (UI), usually associated with frequency and nocturia,[1] is a multifactorial and common health disorder associated with detrimental effects on quality-of-life and huge economic burden.[2,3] Neurogenic detrusor overactivity (NDO) is defined as a special kind of OAB when there is a relevant underlying neurological condition spinal cord injury or multiple sclerosis.[1] It is caused by spontaneous, involuntary contractions of the bladder wall during urinary filling that can be associated with reduced bladder wall compliance and elevated filling pressures.[4] Antimuscarinic agents are the current pharmacological mainstay for OAB.[5,6] However, many patients discontinue their use due to inadequate efficacy and/or intolerable side effects.[7,8]
OnabotulinumtoxinA, a specific formulation of botulinum toxin A, is a neuromodulator that inhibits vesicle-mediated neurotransmission and reduces muscle spasticity, which has emerged as an effective second line therapy in the management of NDO with a recent European consensus giving it a Grade A recommendation for use in this condition.[9] The efficacy of intradetrusor botulinum toxin type A injection in treating NDO was first reported in 1999.[10] In NDO, intradetrusor botulinum toxin type A has been shown to significantly decrease UI episodes and improve urodynamic parameters at doses of 200 U and 300 U in several randomized placebo-controlled trials.[11,12,13,14] However, modulation of neuromuscular transmission may also result in urinary retention and so on and for this reason botulinum toxin type A is still under debate.[15]
The goal of the present study was to perform a meta-analysis to evaluate the safety and efficacy of onabotulinumtoxinA in treating NDO, which may resolve some of the current controversies over use of the drug.
METHODS
Search strategy
Medline (1966 to November 2013), EMBASE (1974 to November 2013), and Cochrane Controlled Trials Register databases were searched to identify randomized controlled trials (RCTs) that referred to the impact of onabotulinumtoxinA in treating NDO; we also searched the reference lists of the retrieved studies. The following search terms were used: OnabotulinumtoxinA, NDO, RCT.
Inclusion criteria and trial selection
Randomized controlled trials that met the following criteria were included: (1) The study design included treatment with onabotulinumtoxinA; (2) the study provided accurate data that could be analyzed, including the total number of subjects and the values of each index; and (3) the full text of the study could be accessed. When the same study was published in various journals or different years, the most recent publication was used for the meta-analysis. If the same group of researchers studied a group of subjects with multiple experiments, then each study was included. A flow diagram of the study selection process is presented in Figure 1.
Figure 1.
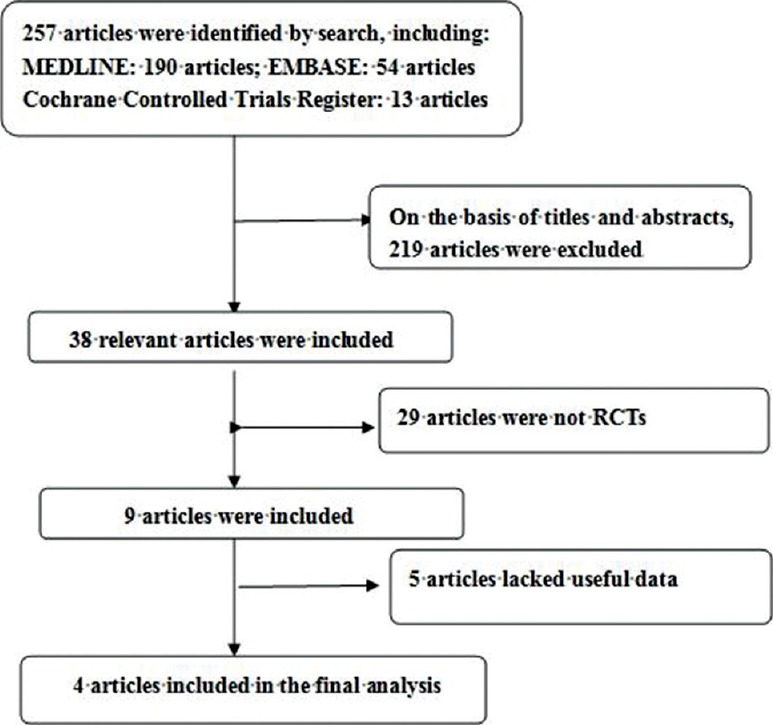
A flow diagram of the study selection process.
Quality assessment
The quality of the identified RCTs was assessed in terms of how patients were allocated to the arms of the study, the concealment of allocation procedures, blinding, and data loss due to attrition. The studies were then classified qualitatively according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v. 5.1.0.[16] Based on the quality assessment criteria, each study was rated and assigned to one of the three following quality categories: A – If all quality criteria were adequately met, the study was deemed to have a low risk of bias; B – If one or more of the quality criteria was only partially met or was unclear, the study was deemed to have a moderate risk of bias; or C – If one or more of the criteria was not met or not included, the study was deemed to have a high risk of bias. Differences were resolved by discussion among the authors.
Data extraction
The following information was collected for each study: (1) The name of the RCT; (2) the study design and sample size; (3) the therapy that the patients received; (4) the country in which the study was conducted; and (5) data including the mean number of UI per week, maximum cystometric capacity (MCC), maximum detrusor pressure (MDP) during first involuntary detrusor contraction, urinary tract infection (UTI), hematuria, and urinary retention.
Statistical analysis and meta-analysis
The meta-analysis of comparable data was carried out using RevMan v.5.1.0 (Cochrane Collaboration, Oxford, UK).[16] The data of changes in the mean number of UI per week, MCC and MDP during first involuntary detrusor contraction were continuous data, which were used for assessing the efficacy. And the data of changes in the mean number of UI per week were determined as the main parameters. The data of common adverse events [AEs] (e.g. UTI, hematuria and urinary retention) were discontinuous data. We estimated the relative risk (RR) for dichotomous outcomes and the standardized mean difference (SMD) for continuous outcomes pooled across studies by using the DerSimonian and Laird random-effects model.[17] A “fixed-effects” statistical model was used if there was no conspicuous heterogeneity, and a “random-effects” model was used if heterogeneity was detected. Tests for heterogeneity were performed using Chi-square tests with the significance level set at P < 0.1. Furthermore, for a more acute analysis, a subgroup analysis was performed for some conditions.
RESULTS
Characteristics of the individual studies
The database search found 257 articles that could have been included in our meta-analysis. Based on the inclusion and exclusion criteria, 219 articles were excluded after reading the titles and abstracts of the articles. Twenty-nine articles were not RCTs. Five articles lacked useful data. In all, four articles[11,12,13,14] reporting data from a total of four RCTs that compared onabotulinumtoxinA with placebo, were included in the analysis [Figure 1]. The baseline characteristics of the studies included in our meta-analysis are listed in Table 1. All the included patients were administered by intravesical injections in the detrustor and evaluated at weeks 6 after the treatment.
Table 1.
Study and patient characteristics
| Author | Therapy in experimental group | Therapy in control group | Country | Sample size | Dosage | Inclusion population | ||
|---|---|---|---|---|---|---|---|---|
| OnabotulinumtoxinA | Placebo | |||||||
| 200 U | 300 U | |||||||
| Schurch et al.[11] | OnabotulinumtoxinA | Placebo | Belgium, France and Switzerland | 19 | 19 | 21 | 200 U/300 U | Patients with NDO for at least 6 weeks, inadequately treated with anticholinergic therapy |
| Herschorn et al.[13] | OnabotulinumtoxinA | Placebo | Canada | - | 29 | 29 | 300 U | Patients with NDO for 1 month or greater, 1 or more UI episodes per day, inadequately treated with anticholinergic therapy |
| Cruz et al.[12] | OnabotulinumtoxinA | Placebo | Europe, North America, Latin America, South Africa, and Asia Pacific | 92 | 91 | 92 | 200 U/300 U | Patients with NDO for 3 months or greater, 14 or more UI episodes per week, inadequately treated with anticholinergic therapy |
| Ginsberg et al.[14] | OnabotulinumtoxinA | Placebo | US and Europe | 135 | 127 | 149 | 200 U/300 U | Patients with NDO for 3 months or greater, 14 or more UI episodes per week, inadequately treated with anticholinergic therapy |
UI: Urinary incontinence; NDO: Neurogenic detrusor overactivity.
Quality of the individual studies
All four RCTs were double blinded, and all described the randomization processes that they had used. All included a power calculation to determine the optimal sample size [Table 2]. The level of quality of each identified study was A [Table 2].
Table 2.
Quality assessment of individual study
| Author | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow-up (%) | Calculation of sample size | Intention-to- treat analysis | Level of quality |
|---|---|---|---|---|---|---|---|
| Schurch et al.[11] | A | A | A | 0 | Yes | Yes | A |
| Herschorn et al.[13] | A | A | A | 10 | Yes | Yes | A |
| Cruz et al.[12] | A | A | A | 1.4 | Yes | Yes | A |
| Ginsberg et al.[14] | A | A | A | 2.2 | Yes | Yes | A |
A: All quality criteria met (adequate): low risk of bias.
Efficacy
Mean number of urinary incontinence per week
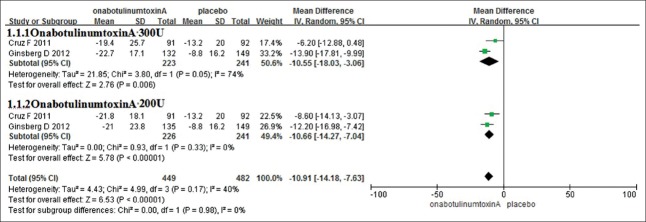
Two RCTs, representing 932 participants (450 in the onabotulinumtoxinA group and 482 in the control group), included the data of changes in the mean number of UI per week [Figure 2]. According to the doses of the onabotulinumtoxinA, the analyses were divided into two subgroups: Group onabotulinumtoxinA 300 U and group onabotulinumtoxinA 200 U. In the subgroup analyses, the changes of the mean number of UI per week were greatly reduced in both group onabotulinumtoxinA 300 U (SMD = −10.55, 95% confidence interval [CI] = −18.03–−3.06, P < 0.0001) and group onabotulinumtoxinA 200 U (SMD = −10.66, 95% CI = −14.27–−7.04, P < 0.0001). Combining the results, without the heterogeneity (I2 = 0), the changes of the mean number of UI per week decreased (SMD = −10.91, 95% CI = −14.18–−7.63, P < 0.0001). This result suggests that onabotulinumtoxinA of both 300 U and 200 U showed statistically significant reductions in the mean number of UI per week compared with placebo.
Figure 2.
The changes of mean number of urinary incontinence per week.
Maximum cystometric capacity and maximum detrusor pressure during first involuntary detrusor contraction
Two of the RCTs included the MCC improvement data representing a cohort of 932 participants (450 in the onabotulinumtoxinA group and 482 in the control group) [Table 3]. In the subgroup analyses, no heterogeneity was found between the RCTs in which treatment was given the onabotulinumtoxinA 300 U, the SMD was 151.54, and the 95% CI was 122.98–180.10, P < 0.0001. For the study that used onabotulinumtoxinA 200 U, the SMD was 140.94 (95% CI = 113.19–168.68, P < 0.0001). Combining the results, without the heterogeneity (I2 = 0), the MCC increases significantly. (SMD = 146.09, 95% CI = 126.19–165.99, P < 0.0001). This result suggests that regardless of the doses, onabotulinumtoxinA had significantly greater increases in the MCC. Two of the RCTs included the MDP during first involuntary detrusor contraction improvement data representing a cohort of 932 participants (450 in the onabotulinumtoxinA group and 482 in the control group) [Table 3]. In the subgroup analyses, the MDP during first involuntary detrusor contraction was greatly reduced in both group onabotulinumtoxinA 300 U (SMD = −31.94, 95% CI = −39.09–−24.79, P < 0.0001) and group onabotulinumtoxinA 200 U (SMD = −33.44, 95% CI = −40.94–−25.94, P < 0.0001). Combining the results, without the heterogeneity (I2 = 0), the MDP during first involuntary detrusor contraction decreases significantly. (SMD = −32.65, 95% CI = −37.83–−27.48, P < 0.0001). This result suggests that onabotulinumtoxinA of both 300 U and 200 U reduced the MDP during first involuntary detrusor contraction significantly.
Table 3.
Analysis outcomes of MCC and MDP
| Items | Number of studies | SMD | 95% CI | P |
|---|---|---|---|---|
| MCC | ||||
| OnabotulinumtoxinA 300 U | 2 | 151.54 | 122.98–180.10 | <0.0001 |
| OnabotulinumtoxinA 200 U | 2 | 140.94 | 113.19–168.68 | <0.0001 |
| Overall | 2 | 146.09 | 126.19–165.99 | <0.0001 |
| MDP | ||||
| OnabotulinumtoxinA 300 U | 2 | −31.94 | −39.09–24.79 | <0.0001 |
| OnabotulinumtoxinA 200 U | 2 | −33.44 | −40.94–25.94 | <0.0001 |
| Overall | 2 | −32.65 | −37.83–27.48 | <0.0001 |
MCC: Maximum cystometric capacity; SMD: The standardized mean difference; MDP: Maximum detrusor pressure; CI: Confidence interval.
Safety
Urinary tract infection hematuria and urinary retention
Four RCTs, representing 1049 participants (508 in the onabotulinumtoxinA group and 541 in the control group), included the UTI data. According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of RR was 1.48, and the 95% CI was 1.20–1.81, P = 0.0002. Four RCTs, representing 1049 participants (508 in the onabotulinumtoxinA group and 541 in the control group), included the hematuria data. The pooled estimate of RR was 1.81, and the 95% CI = 1.00–3.24, P = 0.05. And three of the RCTs included urinary retention data representing a cohort of 969 (470 in the onabotulinumtoxinA group and 499 in the control group), the pooled estimate of RR was 5.87, 95% CI = 3.61–9.56, P < 0.0001 [Table 4]. The result suggests that onabotulinumtoxinA was often associated with complications primarily localized to the urinary tract.
Table 4.
Analysis outcomes of adverse events
| Adverse events | Number of studies | RR | 95% CI | P |
|---|---|---|---|---|
| Urinary tract infection | 4 | 1.48 | 1.20–1.81 | 0.0002 |
| Hematuria | 4 | 1.81 | 1.00–1.34 | 0.0500 |
| Urinary retention | 4 | 5.87 | 3.61–9.56 | <0.0001 |
CI: Confidence interval; RR: Relative risk.
DISCUSSION
Neurogenic detrusor overactivity can be managed by various treatment modalities, including bladder and behavioral training, biofeedback, electrical stimulation, botulinum toxin, surgery or pharmacotherapy.[18] The current first-line pharmacotherapeutic treatment options indicated for NDO are muscarinic receptor antagonists, such as solifenacin, tolterodine, fesoterodine, and so on. However, NDO patients may have a suboptimal response or find that antimuscarinic therapy is limited by associated AEs.[19,20] For these patients, there has not been another class of oral therapeutic agents available; therefore, there is a need for a new treatment option for NDO that is effective and well-tolerated, with a distinct mechanism of action. Efficacy of onabotulinumtoxinA injection, a specific formulation of botulinum toxin type A, into the detrusor muscle has been demonstrated in both neurogenic and idiopathic patients with incontinence.[21,22] OnabotulinumtoxinA may be a useful treatment to augment existing NDO treatment options ranging from noninvasive therapies to implantable neuromodulators or other invasive surgical options.
Our study reveals that onabotulinumtoxinA is superior to placebo in improving the mean number of UI per week, MCC and the MDP during first involuntary detrusor contraction. The results demonstrate that treatment with onabotulinumtoxinA provides both statistically significant and more importantly, clinically relevant improvements in the NDO symptoms regardless of the doses. The significant clinical efficacy of onabotulinumtoxinA may be explained by its proposed dual mechanism of action in targeting both the afferent and efferent neuronal pathways of bladder control. OnabotulinumtoxinA blocks the release of acetylcholine and other neurotransmitters and may decrease the expression of sensory receptors.[23,24]
The adverse reaction such as UTI, hematuria, and urinary retention induced by onabotulinumtoxinA were higher than placebo. No clinically relevant changes were observed in other safety parameters, such as fatigue, dysuria, constipation, and so on. Overall, treatment with onabotulinumtoxinA was well-tolerated, with AEs primarily limited to localized urologic events.
As the doses of onabotulinumtoxinA were 200 U or 300 U, so we can conclude that onabotulinumtoxinA 200 U or 300 U is an effective treatment for NDO symptoms. OnabotulinumtoxinA was administered via cystoscopy evenly spaced but avoiding the trigone in all of the included RCTs. Kuo[25] and Manecksha et al.[26] conducted two RCTs and reported that bladder base/trigone injection is as safe and effective as bladder body injections with or without trigone involvement. Besides, efficacy and safety data were concluded from 6 weeks, the data of longer term safety and efficacy of onabotulinumtoxinA cannot be extrapolated from the included RCTs. Kennelly et al.[27] focused on the results of repeated treatment for up to five treatment cycles and reported significantly decreased UI episodes.
This meta-analysis includes studies which are all findings from randomized double-blind, placebo-controlled trials. According to the quality-assessment scale that we developed, the quality of the individual studies in the meta-analysis was conforming. The results of this analysis acquire great importance from the scientific standpoint but also in the everyday clinical practice. Several potential limitations should be considered in our meta-analysis. First, only four RCTs with a limited number of patients assessing the efficacy and safety of onabotulinumtoxinA were included, and these insufficient data may thus affect the final conclusion. Second, the smaller number of participants, lack of uniformity of patient cohorts, different doses of medications may also result in a bias. Third, the longer term safety, efficacy, and persistence of onabotulinumtoxinA cannot be extrapolated from this article. In addition, unpublished studies’ data were not included in the analysis. More high-quality trials with larger samples are proposed to learn more about the efficacy and safety of different doses of onabotulinumtoxinA on NDO. This meta-analysis indicates that onabotulinumtoxinA to be an effective treatment for NDO symptoms with side effects primarily localized to urinary tract.
Footnotes
Edited by: Li-Min Chen
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol. 2006;50:1306–14. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Sacco E, Tienforti D, D’Addessi A, Pinto F, Racioppi M, Totaro A, et al. Social, economic, and health utility considerations in the treatment of overactive bladder. Open Access J Urol. 2010;2:11–24. doi: 10.2147/rru.s4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getsios D, El-Hadi W, Caro I, Caro JJ. Pharmacological management of overactive bladder: A systematic and critical review of published economic evaluations. Pharmacoeconomics. 2005;23:995–1006. doi: 10.2165/00019053-200523100-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chapple CR. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55:33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- 6.Garely AD, Burrows LJ. Current pharmacotherapeutic strategies for overactive bladder. Expert Opin Pharmacother. 2002;3:827–33. doi: 10.1517/14656566.3.7.827. [DOI] [PubMed] [Google Scholar]
- 7.Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int J Clin Pract. 2011;65:567–85. doi: 10.1111/j.1742-1241.2010.02626.x. [DOI] [PubMed] [Google Scholar]
- 8.Brostrøm S, Hallas J. Persistence of antimuscarinic drug use. Eur J Clin Pharmacol. 2009;65:309–14. doi: 10.1007/s00228-008-0600-9. [DOI] [PubMed] [Google Scholar]
- 9.Apostolidis A, Dasgupta P, Denys P, Elneil S, Fowler CJ, Giannantoni A, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: A European consensus report. Eur Urol. 2009;55:100–19. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–7. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 11.Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: Results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200. doi: 10.1097/01.ju.0000162035.73977.1c. [DOI] [PubMed] [Google Scholar]
- 12.Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: A randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742–50. doi: 10.1016/j.eururo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Herschorn S, Gajewski J, Ethans K, Corcos J, Carlson K, Bailly G, et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: A randomized, double-blind trial. J Urol. 2011;185:2229–35. doi: 10.1016/j.juro.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187:2131–9. doi: 10.1016/j.juro.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 15.Knuepfer S, Juenemann KP. Experience with botulinum toxin type A in the treatment of neurogenic detrusor overactivity in clinical practice. Ther Adv Urol. 2014;6:34–42. doi: 10.1177/1756287213510962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, v.5.1. [Last updated on 2011 Mar 05]. Available from: http://www.cochrane.handbook-org/2011 .
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008;14:291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–82. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 21.Anger JT, Weinberg A, Suttorp MJ, Litwin MS, Shekelle PG. Outcomes of intravesical botulinum toxin for idiopathic overactive bladder symptoms: A systematic review of the literature. J Urol. 2010;183:2258–64. doi: 10.1016/j.juro.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dmochowski R, Sand PK. Botulinum toxin A in the overactive bladder: Current status and future directions. BJU Int. 2007;99:247–62. doi: 10.1111/j.1464-410X.2007.06575.x. [DOI] [PubMed] [Google Scholar]
- 23.Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644–50. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama T, Chancellor MB, Oguma K, Yamamoto Y, Suzuki T, Kumon H, et al. Botulinum toxin type A for the treatment of lower urinary tract disorders. Int J Urol. 2012;19:202–15. doi: 10.1111/j.1442-2042.2011.02946.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuo HC. Bladder base/trigone injection is safe and as effective as bladder body injection of onabotulinumtoxinA for idiopathic detrusor overactivity refractory to antimuscarinics. Neurourol Urodyn. 2011;30:1242–8. doi: 10.1002/nau.21054. [DOI] [PubMed] [Google Scholar]
- 26.Manecksha RP, Cullen IM, Ahmad S, McNeill G, Flynn R, McDermott TE, et al. Prospective randomised controlled trial comparing trigone-sparing versus trigone-including intradetrusor injection of abobotulinumtoxinA for refractory idiopathic detrusor overactivity. Eur Urol. 2012;61:928–35. doi: 10.1016/j.eururo.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Kennelly M, Dmochowski R, Ethans K, Karsenty G, Schulte-Baukloh H, Jenkins B, et al. Long-term efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: An interim analysis. Urology. 2013;81:491–7. doi: 10.1016/j.urology.2012.11.010. [DOI] [PubMed] [Google Scholar]