Abstract
Background:
Pseudomonas aeruginosa is among the most problematic hospital and community-acquired pathogens. Toxin-antitoxin (TA) systems are maintenance regulatory systems in bacteria and have recently been considered new targets for antimicrobial therapy. The prevalence and transcription of these systems in clinical isolates are still unknown.
Objectives:
The aim of this study was to characterize three types of TA systems (parDE, relBE, and higBA) among P. aeruginosa clinical isolates.
Materials and Methods:
We typed our clinical isolates by ERIC-PCR (enterobacterial repetitive intergenic consensus sequence-based polymerase chain reaction) and BOX-PCR. We then investigated 174 P. aeruginosa clinical isolates from three hospitals in Ahvaz, Iran, for the presence of TA system genes, and determined whether these systems were encoded on chromosomes or plasmids by amplification of the flanking regions.
Results:
Our results showed that in the 174 P. aeruginosa isolates, relBE and higBA were universal, but parDE was less prevalent. Both of the flanking regions of the parDE genes in all positive isolates were amplified. The flanking regions of nearly all relBE genes were amplified. Amplification was observed for the downstream sequence of every higBA locus, as well as for the region upstream of higBA, except in 14 strains.
Conclusions:
Based on the presence of TA systems in the majority of P. aeruginosa isolates, these could be used as a novel target for antimicrobial therapy.
Keywords: Gene Regulatory Networks, Resistance, Genotyping, Pseudomonas aeruginosa
1. Background
When toxin-antitoxin (TA) systems were first discovered, they were considered to be a plasmid maintenance mechanism in the post-segregational killing (PSK) phenomenon (1, 2). Toxin-antitoxin systems act through regulation mechanisms consisting of two components: a stable toxin and a labile antitoxin located on the same plasmid or the same loci of the chromosome. When the plasmid is present, it encodes the antitoxin, which attaches to the toxin and inactivates it, ensuring that only the daughter cells that inherit the plasmid survive after cell division. If the plasmid is absent in a daughter cell, the unstable antitoxin is degraded, and the stable toxin kills the daughter cell during PSK (3). Toxin is always a protein, but antitoxin can be a protein or RNA (4).
RelBE is one of the most-studied TA systems that mediate the stringent response when amino acid concentrations are very low in an environment of Escherichia coli. This response eventually causes the bacteriostasis to save the energy, improving the survival of the bacterium (5). ParD is a plasmid antitoxin that forms a dimer in a ribbon-helix-helix DNA-binding structure. It stabilizes plasmids by inhibiting ParE toxin in cells that express ParD and ParE, and regulates its own promoter (parDE). Activation of ParE toxin leads to inhibited cell division, but not to cell growth (6, 7). The higBA locus was first identified in a temperature-sensitive plasmid (Rts1) of Proteus vulgaris. It differs from other characterized TA loci because the toxin-encoding gene (higB) lies upstream of the antitoxin-encoding gene (higA); however, like other TA loci, the antitoxin represses transcription of the operon. There is a weak sequence similarity between higB, relE, and parE, which provides conflicting evidence about the cellular role of higB (8).
Pseudomonas aeruginosa is one of the most commonly considered Gram-negative aerobic bacilli in the differential diagnosis of a number of Gram-negative infections. Consideration of this organism is important because it causes severe hospital-acquired infections, especially in immune-compromised hosts, is often antibiotic-resistant, which complicates the choice of therapy, and is associated with a high mortality rate (9). TA system genes have been identified in many prokaryotic genomes; however, their role in cell physiology is unclear (10). A bioinformatics evaluation of 126 bacterial genomes indicated the presence of TA loci genes (11). The bioinformatics data introduced three TA loci on P. aeruginosa PAO1, including relBE, parDE, and higBA (11, 12).
Repetitive element-based PCR (rep-PCR) is a method for the molecular typing of bacterial genomes, examining strain-specific patterns obtained from PCR amplification of the repetitive DNA elements present within bacterial genomes. Three classes of repetitive elements are used for typing purposes: the repetitive extragenic palindromic (REP) sequence, the enterobacterial repetitive intergenic consensus (ERIC) sequence, and the BOX elements (13).
2. Objectives
The aim of this study was to characterize three types of TA systems, parDE, relBE, and higBA, among our P. aeruginosa clinical isolates.
3. Materials and Methods
3.1. Bacterial Isolates
One hundred seventy-four clinical isolates were collected from three medical centers in Ahvaz, southwest Iran: the Golestan and Emam general hospitals (n = 130) and the special burn unit at Taleghani Hospital (n = 44). These clinical isolates were collected from a variety of clinical specimens, such as urine, blood, feces, bronchial washings, sputum, wound swabs, throat swabs, and ulceration swabs. The isolates were identified as P. aeruginosa according to their morphology, Gram staining, oxidase test, OF test, pyocianin and pyoverdin production, and growth on cetrimide agar (Merck, Germany) plates. The control strain PAO1 was also examined. Stock cultures were stored in TSB (Merck, Germany) containing 20% glycerol (Merck, Germany) at −80°C.
3.2. DNA Extraction
Isolates were grown in TSB (Merck, Germany) at 37°C overnight, then DNA was extracted using the genomic DNA extraction kit (Bioneer, South Korea).
3.3. ERIC-PCR and BOX-PCR
Two typing methods (ERIC-PCR and BOX-PCR) were used to genotype the P. aeruginosa clinical isolates, in order to study the bacterial genetic diversity within this complex group. The ERIC and BOX primer sequences (Macrogen, South Korea) were used in PCR to detect differences in the number and distribution of these repetitive sequences in the bacterial genome (Table 1) (14, 15).
Table 1. Primers Used for Typing Pseudomonas aeruginosa Clinical Isolates.
PCR amplification was performed in a final volume of 25 μL containing 3 μL of purified total DNA, 8 μL of PCR master mix (containing Taq polymerase, MgC12, dNTPs, and PCR buffer; Amplicon, Denmark), and 2.5 pM of each primer (Macrogen, South Korea). PCR was carried out in a thermal cycler apparatus (PeqStar; PeqLab, Germany) with an initial denaturation step at 95°C for 3 minutes, followed by 35 cycles including denaturation at 94°C for 1 minute, annealing (at 52°C for ERIC-PCR and at 48°C for BOX-PCR) for 1 minute and extension at 72°C for 2 minutes, with a final extension step at 72°C for 5 minutes.
3.4. Detection of TA System Genes and Analysis of Flanking Region Amplification
Gene-specific internal primers (Macrogen, South Korea) were used to amplify the relBE, parDE, and higBA TA genes, and the intergenic primers (Macrogen, South Korea) were used to amplify the upstream and downstream of the flanking regions. The sequences of the primers used for this purpose are listed in Table 2 (17).
Table 2. Primers Used for Detection of TA System Genes and Analysis of Flanking Regions in Pseudomonas aeruginosa Clinical Isolates.
| Primer name | 5'-3' Sequence | Expected Fragment, bp | Application | Reference |
|---|---|---|---|---|
| parDE | 556 | PCR | (15) | |
| F | GCGGCTGACCTGGATTTATC | |||
| R | CCAAGCAGTAGCGGATCAATTG | |||
| relBE | 505 | PCR | (15) | |
| F | CAGGGGGTAATTTCGACTCTG | |||
| R | ATGAGCACCGTAGTCTCGTTC | |||
| higBA | 469 | PCR | (15) | |
| F | CTCATGTTCGATCTGCTTGC | |||
| R | CAATGCTTCATGCGGCTAC | |||
| parD-UP | 679 | PCR, flanking | (15) | |
| (+)CGGTGATCTTTGCCAACACTAAG | ||||
| (−)CTTCCGCTCAGCATATGACTC | ||||
| parE-down | 676 | PCR, flanking | (15) | |
| (+)TGAGTCTTCTGGGGGTGCTG | ||||
| (−)GGAATTCCACACCATCCGC | ||||
| relE-up | 548 | PCR, flanking | (15) | |
| (+)CCGGAAAAAGCGCGAGAAAGC | ||||
| (−)GGGGGCTGCAATGAGCCTG | ||||
| relB-down | 646 | PCR, flanking | (15) | |
| (+)GTGCTCATTTTCTGATCAACTTCG | ||||
| (−)GTGACGCTCTCCGACAGCTTC | ||||
| higA-UP | 823 | PCR, flanking | (15) | |
| (+)GATCCGACCCCTTCCGTCTAAACG | ||||
| (−)GTAGCCGCATGAAGCATTG | ||||
| higB-down | 712 | PCR, flanking | (15) | |
| (+)CAGGTGGAGAGCGCAGGTC | ||||
| (−)CAATTGTCCCAACGCCTCCTTCG | ||||
| higA-up-PA7 | 803 | PCR, flanking | (15) | |
| (+)GTTTGCCACGTTTGCATGCAG | ||||
| (−)CGCTCAGTTCTGGATGAATCTCC | ||||
| higB-down-PA7 | 655 | PCR, flanking | (15) | |
| (+)GCATCGCCGATTCCAAGTG | ||||
| (−)GCAACGTGTGTTCGTCACC |
PCR amplification was performed in a final volume of 25 μL containing 3 μL of purified total DNA, 8 μL of PCR master mix (containing Taq polymerase, MgC12, dNTPs, and PCR buffer; Amplicon, Denmark), and 2.5 pM of each primer (Macrogen, South Korea). PCR was carried out in a thermal cycler apparatus (PeqStar; PeqLab, Germany) with an initial denaturation step at 94°C for 5 minutes, followed by 30 cycles including denaturation at 94°C for 1 minute, annealing at 52°C for 1 minute, and extension at 72°C for 1 minute, with a final extension step at 72°C for 5 minutes.
3.5. Electrophoresis
The PCR products were separated on 1% agarose gels (SinaClon, Iran) in 1X tris/borate/EDTA buffer (SinaClon, Iran). Bands were visualized under UV gel documentation and photographed. ethidium bromide (Merck, Germany) as a stain was added to the agarose gel (SinaClon, Iran) during preparation to give a concentration of 0.2 μl/mL.
4. Results
The similarities in the band patterns were analyzed by Dice and clustered by UPGMA methods. If any two isolates had 80% similarity in band patterns, they were considered one type (Figures 1 - 3). The results of BOX typing are not shown.
Figure 1. Gel Electrophoresis for ERIC Types of Pseudomonas aeruginosa Clinical Isolates From Taleghani Hospital.
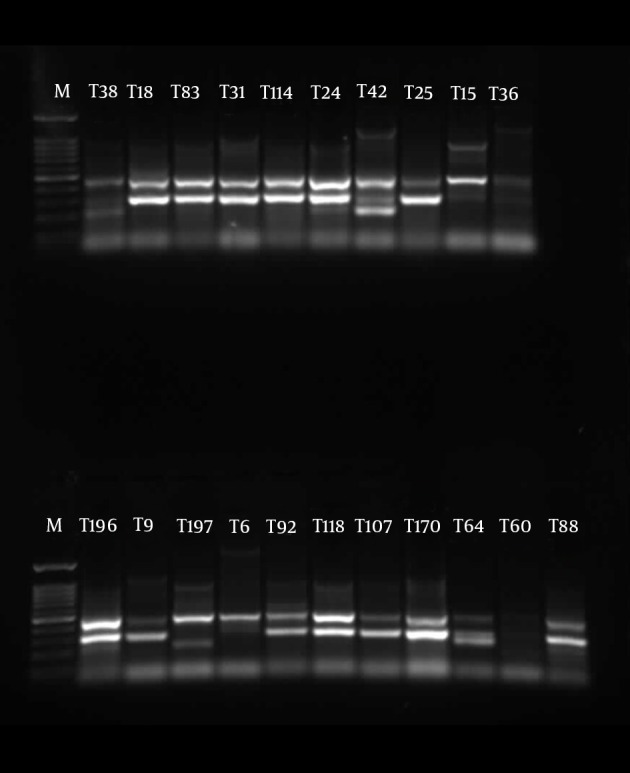
M, 100 bp DNA ladder plus (SinaClon, Iran); T lines, isolates from Taleghani Hospital.
Figure 3. Gel Electrophoresis for ERIC Types of Pseudomonas aeruginosa Clinical Isolates From Golestan Hospital.
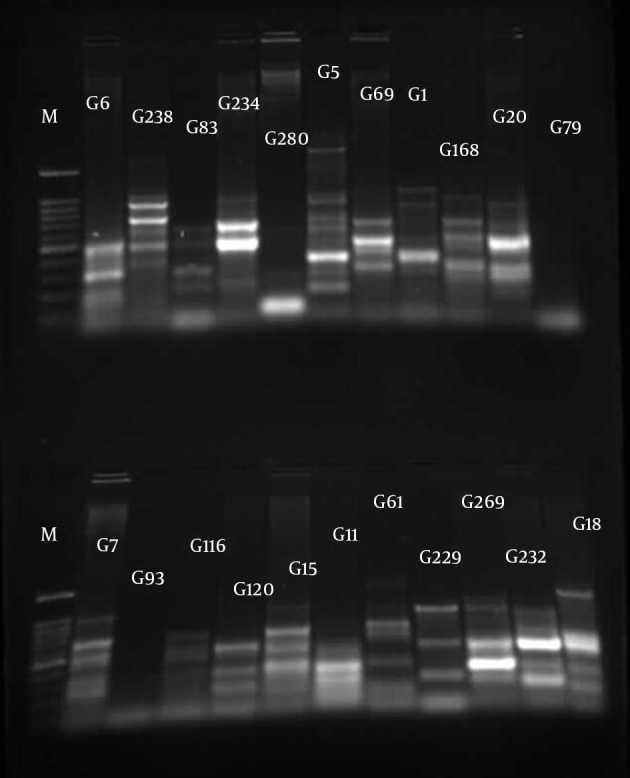
M, 100 bp DNA ladder plus (SinaClon, Iran); G lines, Pseudomonas aeruginosa isolates from Golestan Hospital.
Figure 2. Gel Electrophoresis for ERIC Types of Pseudomonas aeruginosa Clinical Isolates From Emam Hospital.

1, 100 bp DNA ladder (Fermentas, Latvia); E lines, Pseudomonas aeruginosa isolates from Emam Khomeini Hospital.
Detection of TA-associated genes carried out by primers was designed according to the homolog genes found in P. aeruginosa PAO1. For the 174 P. aeruginosa isolates, relBE (174/174, 100%) and higBA (174/174, 100%) were universal, whereas parDE (53/174, 30%) was less prevalent (Figures 4 - 6).
Figure 4. Gel Electrophoresis for Detection of higBA Loci in Pseudomonas aeruginosa Clinical Isolates.
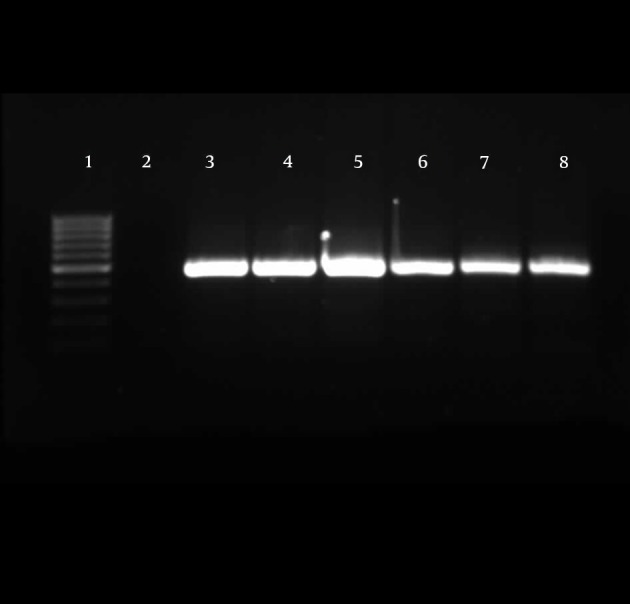
The expected size was 469 bp. All isolates were positive for these loci. Line 1, 100 bp DNA ladder (Fermentas, Latvia); line 2, negative control; lines 3 -8, positive loci.
Figure 6. Gel Electrophoresis for Detection of parDE Loci in Pseudomonas aeruginosa Clinical Isolates.
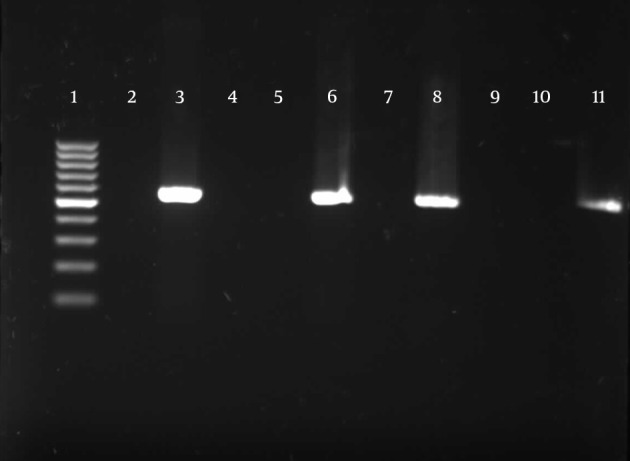
The expected size was 556 bp. Only 30% of the isolates were positive for these loci. Line 1, 100 bp DNA ladder (Fermentas, Latvia); line 2, negative control; lines 3, 6, 8, and 11, positive loci; lines 4, 5, 7, 9, and 10, negative loci.
Figure 5. Gel Electrophoresis for Detection of relBE Loci in Pseudomonas aeruginosa Clinical Isolates.

The expected size was 505 bp. All isolates were positive for these loci. Line 1, 100 bp DNA ladder (Fermentas, Latvia); line 2, negative control; lines 3 - 8, positive loci.
Comparison of the ERIC types of P. aeruginosa strains that carried parDE, relBE, and higBA showed that these strains have different band patterns. This indicates that there is no correlation between genome relatedness and carriage of TA systems. For analysis of flanking regions in TA genes, primers were designed based on the conserved sequence in P. aeruginosa strains PAO1 and PA7. We then investigated whether the TA genes were located on a plasmid or the chromosome of the P. aeruginosa isolates. For this purpose, the presence of a PCR product would suggest a chromosomal location for TA systems. In our P. aeruginosa clinical isolates, both flanking regions of the parDE genes in all isolates (174/174, 100%), and the flanking regions of nearly all relBE genes (170/174, 97%), were amplified. Amplification was observed for the downstream sequence of higBA loci (174/174, 100%), as well as for the region upstream of higBA, except for 14 strains (160/174, 76%). For these 14 strains, PCR was performed with primers that were designed based on the PA7 reference sequence, but no product was amplified.
5. Discussion
Multidrug-resistant P. aeruginosa (MRPA) is currently one of the major concerns in health care systems, as it causes nosocomial infections in hospitals throughout the world, including in Iran. Japoni et al. have reported that 22% of metallo-β-lactamase-producing P. aeruginosa isolates were completely resistant to imipenem, meropenem, piperacillin/tazobactam, ampicillin, and aztreonam (16), while Moniri and Tavajjohi detected a 30% rate of multidrug-resistant isolates in their study (17). Therefore, new strategies are needed to solve the problem of MRPA. Recently, some strategies have been based on designing a conjugate vaccine (18) or combining antibiotics with herbal extracts or other antimicrobials (19).
One of these strategies could be designing antimicrobials that can target TA systems. Generally, toxin molecules act as negative regulators in cell survival, and antitoxin molecules act as positive regulators. Under stress conditions, interactions between the expression levels of toxin-antitoxin molecules are vital for the life of bacteria; therefore, they are considered potential targets for the development of new antimicrobial agents (20). Few studies have been done to investigate clinical bacterial strains for the presence and functionality of TA systems. In this study, we used PCR to show that higBA and relBE are universal in a collection of P. aeruginosa clinical isolates, whereas parDE was less commonly observed. Our findings concur with and confirm the report by Williams et al. (15).
Previously, Moritz et al. showed that TA systems are universal in vancomycin-resistant enterococci (21), and we confirmed this data for P. aeruginosa. Hemati et al. reported a 100% prevalence of relBE in P. aeruginosa clinical isolates, which is the same as our results, but they did not study the parDE or higBA TA systems (22). Karimi et al. reported an 85% prevalence of relBE in E.coli clinical isolates (2). These results show that TA systems are more prevalent in nosocomial pathogens than was previously believed. However, more studies are needed to confirm, characterize, and explain the prevalence of disparate classes of TA systems in clinical isolates.
Our analysis of the TA flanking regions showed that a chromosomal location was conserved in all strains harboring the parDE gene, in almost all strains harboring relBE, and in the majority of strains harboring higBA. We could not amplify the upstream sequence of the higBA gene in 14 strains; however, the downstream sequence of the higBA locus was amplified. Therefore, we can conclude that the higBA gene is in a chromosomal locus. These results concur with those of Williams et al. (15). Their conservative genetic structure and universal presence in clinical isolates make TA systems some of the newest and most interesting antimicrobial targets for designing new drugs for use against bacteria such as MRPA. To reach this goal, it is imperative to evaluate the functionality and expression of TA systems in the life cycle of bacteria. This is the first report of the presence of three TA systems (parDE, relBE, and higBA) in P. aeruginosa clinical isolates in Iran.
Acknowledgments
The authors would like to thank the microbiology lab personnel at Golestan, Emam, and Taleghani hospitals for their assistance in collecting the bacterial clinical isolates.
Footnotes
Authors’ Contribution:Mohammad Savari performed practical experiments, wrote the manuscript, abstracted data, analyzed data, and performed statistical analysis and revision. Abbas Bahador developed the original idea and the protocol, set up the tests, wrote the manuscript, and performed interpretation of the results and revisions. Soodabeh Rostami performed practical experiments, revision, and sample collection. Alireza Ekrami performed practical experiments and sample collection.
Funding/Support:This study was supported by a grant from the research council at Tehran University of Medical Sciences.
References
- 1.Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science. 2003;301(5639):1496–9. doi: 10.1126/science.1088157. [DOI] [PubMed] [Google Scholar]
- 2.Karimi S, Ghafourian S, Taheri Kalani M, Azizi Jalilian F, Hemati S, Sadeghifard N. Association between toxin-antitoxin systems and biofilm formation. Jundishapur J Microbiol. 2015;8(1):e26627. doi: 10.5812/jjm.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nariya H, Inouye M. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell. 2008;132(1):55–66. doi: 10.1016/j.cell.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Van Melderen L. Toxin-antitoxin systems: why so many, what for? Curr Opin Microbiol. 2010;13(6):781–5. doi: 10.1016/j.mib.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Christensen SK, Mikkelsen M, Pedersen K, Gerdes K. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc Natl Acad Sci U S A. 2001;98(25):14328–33. doi: 10.1073/pnas.251327898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberer M, Zangger K, Prytulla S, Keller W. The anti-toxin ParD of plasmid RK2 consists of two structurally distinct moieties and belongs to the ribbon-helix-helix family of DNA-binding proteins. Biochem J. 2002;361(Pt 1):41–7. doi: 10.1042/0264-6021:3610041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiebig A, Castro Rojas CM, Siegal-Gaskins D, Crosson S. Interaction specificity, toxicity and regulation of a paralogous set of ParE/RelE-family toxin-antitoxin systems. Mol Microbiol. 2010;77(1):236–51. doi: 10.1111/j.1365-2958.2010.07207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian QB, Hayashi T, Murata T, Terawaki Y. Gene product identification and promoter analysis of hig locus of plasmid Rts1. Biochem Biophys Res Commun. 1996;225(2):679–84. doi: 10.1006/bbrc.1996.1229. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Zhao CJ, Wang H, Yu YS, Zhu ZH, Chu YZ, et al. [Antimicrobial resistance of Gram-negative bacilli isolated from 13 teaching hospitals across China]. Zhonghua Yi Xue Za Zhi. 2013;93(18):1388–96. [PubMed] [Google Scholar]
- 10.Wang X, Wood TK. Toxin-antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl Environ Microbiol. 2011;77(16):5577–83. doi: 10.1128/AEM.05068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33(3):966–76. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto C, Pellicer T, Balsa D, Christensen SK, Gerdes K, Espinosa M. The chromosomal relBE2 toxin-antitoxin locus of Streptococcus pneumoniae: characterization and use of a bioluminescence resonance energy transfer assay to detect toxin-antitoxin interaction. Mol Microbiol. 2006;59(4):1280–96. doi: 10.1111/j.1365-2958.2006.05027.x. [DOI] [PubMed] [Google Scholar]
- 13.Olive DM, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37(6):1661–9. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolska K, Szweda P. A comparative evaluation of PCR ribotyping and ERIC PCR for determining the diversity of clinical Pseudomonas aeruginosa isolates. Pol J Microbiol. 2008;57(2):157–63. [PubMed] [Google Scholar]
- 15.Williams JJ, Halvorsen EM, Dwyer EM, DiFazio RM, Hergenrother PJ. Toxin-antitoxin (TA) systems are prevalent and transcribed in clinical isolates of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett. 2011;322(1):41–50. doi: 10.1111/j.1574-6968.2011.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japoni A, Anvarinejad M, Farshad S, Giammanco GM, Rafaatpour N, Alipour E. Antibiotic Susceptibility Patterns and Molecular Epidemiology of Metallo-beta-Lactamase Producing Pseudomonas aeruginosa Strains Isolated From Burn Patients. Iran Red Crescent Med J. 2014;16(5):e26627. doi: 10.5812/ircmj.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moniri R, Tavajjohi Z. Detection of ESBLs and MDR in Pseudomonas aeruginosa in a tertiary-care teaching hospital. Arch Clin Infect Dis. 2011;6(1):18–23. [Google Scholar]
- 18.Najafzadeh F, Shapouri R, Rahnema M, Rokhsartalab Azar S, Kianmehr A. Pseudomonas aeruginosa PAO-1 Lipopolysaccharide-Diphtheria Toxoid Conjugate Vaccine: Preparation, Characterization and Immunogenicity. Jundishapur J Microbiol. 2015;8(6):e26627. doi: 10.5812/jjm.8(5)2015.17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafiei M, Abdi Ali A, Shahcheraghi F, Saboora A, Akbari Noghabi K. Eradication of Pseudomonas aeruginosa Biofilms Using the Combination of n-butanolic Cyclamen coum Extract and Ciprofloxacin. Jundishapur J Microbiol. 2014;7(2):e26627. doi: 10.5812/jjm.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SJ, Son WS, Lee BJ. Structural overview of toxin-antitoxin systems in infectious bacteria: a target for developing antimicrobial agents. Biochim Biophys Acta. 2013;1834(6):1155–67. doi: 10.1016/j.bbapap.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Moritz EM, Hergenrother PJ. Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci. Proc Natl Acad Sci U S A. 2007;104(1):311–6. doi: 10.1073/pnas.0601168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemati S, Azizi-Jalilian F, Pakzad I, Taherikalani M, Maleki A, Karimi S, et al. The correlation between the presence of quorum sensing, toxin-antitoxin system genes and MIC values with ability of biofilm formation in clinical isolates of Pseudomonas aeruginosa. Iran J Microbiol. 2014;6(3):133–9. [PMC free article] [PubMed] [Google Scholar]


