Abstract
Pain and tobacco smoking are highly prevalent and comorbid conditions that impose considerable burdens on individuals and health care systems. A recently proposed reciprocal model suggests that these conditions interact in a bidirectional manner, resulting in greater pain and the maintenance of tobacco addiction. Anxiety and depression are common among smokers in pain and have been identified as central mechanisms of interest. There is emerging evidence that smokers with anxiety/depression may experience more severe pain and functional impairment, greater pain-induced motivation to smoke, and increased sensitivity to pain during periods of smoking abstinence. Based on empirical findings, we hypothesize that these experiences may engender expectations that abstaining from smoking will exacerbate both pain and negative affect, thus eroding self-efficacy for smoking cessation and increasing perceived barriers to quitting. The goal of this narrative review is to examine the role of anxiety/depression in complex pain–smoking relations so as to advance evolving theoretical perspectives and inform the development of tailored interventions.
Keywords: pain, smoking, negative affect, anxiety, depression, comorbidity
The high prevalence and societal impact of both pain and tobacco smoking are substantial and warrant continued research into their etiology and treatment. Moreover, evidence of significant comorbidity between these conditions offers an opportunity to examine mechanisms and modifiable risk factors that can inform the development of novel interventions. One rapidly emerging, yet still understudied area of clinical and empirical interest in complex pain–smoking relations, is the role of co-occurring anxiety and depression (Ditre, Brandon, Zale, & Meagher, 2011). For example, a growing body of evidence suggests that smokers with elevated symptoms of anxiety and depression may experience greater pain and functional impairment, increased motivation to smoke in response to pain, and greater sensitivity to pain when attempting to abstain from smoking. Furthermore, anxiety or depression may amplify the extent to which pain serves as a barrier to smoking cessation. We utilized a narrative approach to review an emerging literature examining the role of anxiety, depression, and related symptoms/sequelae in bidirectional associations between pain and tobacco smoking. It is our hope that this work will inform evolving theoretical perspectives, future research, and the development of tailored smoking treatments for smokers with co-occurring pain and anxious/depressive symptoms.
Overview of Tobacco Smoking and Pain
Tobacco smoking is a significant public health problem that is responsible for greater than 40% of all premature deaths and disability in the United States (Centers for Disease Control and Prevention [CDC], 2010), with an estimated annual cost of greater than US$180 billion in direct medical expenses and lost productivity (CDC, 2008). Despite known health risks, nearly 19% of the U.S. adult population continues to smoke tobacco cigarettes (CDC, 2014). Like smoking, chronic pain is a critical national health concern that affects 22% to 43% of American adults (Gureje et al., 2008; Institute of Medicine [IOM], 2011). Pain is an inherently subjective and aversive experience that is comprised of sensory-physiological, cognitive-evaluative, and motivational-affective components (International Association for the Study of Pain [IASP], 1994; Turk & Melzack, 2011). Chronic pain is typically distinguished from acute pain by applying various pain duration cutoffs (e.g., 3-12 months; Turk & Okifuji, 2001). Pain-related complaints motivate approximately 50% of all physician visits each year, and pain is responsible for greater than US$600 billion in annual health care costs and lost productivity (IOM, 2011; Mayo Clinic, 2001; Turk & Melzack, 2011).
Complex interrelations between pain and smoking have previously been reviewed and are beyond scope of this article (Ditre et al., 2011; Parkerson, Zvolensky, & Asmundson, 2013; Shi, Weingarten, Mantilla, Hooten, & Warner, 2010). Briefly, prevalence estimates indicate that individuals with chronic pain are about twice as likely to be current smokers relative to individuals without chronic pain (e.g., Strine & Hootman, 2007; Zvolensky, McMillan, Gonzalez, & Asmundson, 2010). Rates of smoking appear to be even higher among clinical pain samples, with several reports indicating that 49% to 68% of treatment-seeking pain patients currently smoke tobacco cigarettes (Hooten, Shi, Gazelka, & Warner, 2011; Jamison, Stetson, & Parris, 1991; Michna et al., 2004). Consistent with the perspective that some individuals may be “burdened” by characteristics that make smoking cessation more difficult (Hughes & Brandon, 2003; Irvin & Brandon, 2000), researchers have suggested that smokers with comorbid pain may constitute a recalcitrant subgroup that faces unique barriers to quitting (e.g., Ditre et al., 2011; Ditre, Langdon, Kosiba, Zale, & Zvolensky, 2015; Zale, Ditre, Dorfman, Heckman, & Brandon, 2014).
According to a recently proposed reciprocal model of pain and smoking (Ditre et al., 2011), interrelations between these conditions may be conceptualized as bidirectional in nature. Based on evidence derived from clinical, epidemiological, and laboratory-based research, this model posits that pain and smoking behavior interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of tobacco dependence. Research in this emerging domain can be usefully broken down into two directions of empirical inquiry: the effects of smoking on pain (e.g., tobacco smoking as a risk factor in the onset of painful conditions) and the effects of pain on smoking (e.g., pain as a proximal antecedent of smoking behavior). After examining each of these pathways in turn, we explicate the role of anxiety, depression, and related factors (e.g., negative affect, pain-related anxiety, anhedonia) in complex pain–smoking relations, and discuss implications for smoking cessation and the treatment of tobacco addiction.
Bidirectional Relations Between Pain and Tobacco Smoking
With regard to the effects of smoking on pain, there is accumulating evidence that tobacco smoking may worsen pain over time, and that pain may be amplified during the early stages of nicotine withdrawal. Smoking has been identified as a unique causal agent in the development of chronic low back pain (Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010) and rheumatoid arthritis (United States Department of Health and Human Services [USDHHS], 2014). Smoking has also been associated with the onset and exacerbation of numerous other painful conditions, including fibromyalgia, chronic headache, and osteoarthritis (Aamodt, Stovner, Hagen, Brathen, & Zwart, 2006; Amin et al., 2007; Goesling et al., 2015; Lee et al., 2010). Relative to nonsmokers, current smokers tend to report greater pain intensity and disability (e.g., pain-related interference with daily activities, work, relationships, physical functioning), and there is some evidence of covariation between pain/disability and severity of tobacco dependence (e.g., Hooten, Shi, et al., 2011; Hooten et al., 2009; Weingarten et al., 2008; Weingarten et al., 2009). Smokers are also more likely to experience deleterious pain treatment outcomes, including greater unemployment (Fishbain et al., 2008), more severe health role limitations (Hooten et al., 2009), and decreased efficacy of pharmacologic/surgical interventions (Glassman et al., 2007; Harty & Veale, 2010; Hooten, Shi, et al., 2011). Finally, smokers may experience greater sensitivity to pain (i.e., hyperalgesia) during periods of smoking abstinence. For example, rodent models have consistently demonstrated increased pain in the context of acute nicotine deprivation (e.g., Grabus, Martin, & Damaj, 2005; Jackson, McIntosh, Brunzell, Sanjakdar, & Damaj, 2009), and initial findings with human participants suggest a positive association between nicotine/tobacco withdrawal and self-reported pain intensity (Allen, Hatsukami, Christianson, & Brown, 2000; Cosgrove et al., 2010).
With regard to the effects of pain on smoking, researchers have long recognized that the desire to avoid or mitigate pain may serve as a potent reinforcer in the maintenance of tobacco dependence (e.g., Ditre et al., 2011; Fertig, Pomerleau, & Sanders, 1986; Fishbain et al., 2007; Silverstein, 1982; Zvolensky, McMillan, Gonzalez, & Asmundson, 2009). For example, smokers with chronic pain reliably endorse smoking cigarettes in response to painful episodes (Hooten, Vickers, et al., 2011; Jamison et al., 1991; Patterson et al., 2012). Data derived from ecological momentary assessment and experimental research paradigms further indicate that the experience of pain can increase urge to smoke and function as a proximal antecedent of smoking behavior (Dhingra et al., 2014; Ditre & Brandon, 2008), especially when individuals hold expectations for nicotine/tobacco-related pain reduction (Ditre, Heckman, Butts, & Brandon, 2010). Indeed, new meta-analytic findings show that nicotine delivered via tobacco smoke and other means (e.g., nicotine patch) can produce acute analgesic effects that may be characterized as small to moderate in magnitude (Ditre, Heckman, Zale, Kosiba, & Maisto, under review). Finally, consistent with evidence that pain can motivate smoking, there are some data to suggest that pain may impede smoking cessation. For example, smokers in pain (relative to no pain) tend to endorse greater difficulty when attempting to quit and less confidence in their ability to remain abstinent during future quit attempts (Ditre, Kosiba, Zale, Zvolensky, & Maisto, under review; Zale et al., 2014). In addition, both acute pain reactivity (Nakajima & al’Absi, 2011) and positive chronic pain status (Waldie, McGee, Reeder, & Poulton, 2008) have been linked with smoking relapse trajectories.
By integrating these directions of empirical inquiry, the reciprocal model of pain and smoking posits that smoking contributes to the onset and exacerbation of pain, which in turn motivates continued smoking, thus resulting in greater pain and the maintenance of tobacco addiction (Ditre et al., 2011). Within this model, negative affect (including the presence of co-occurring anxiety and depression) is hypothesized to play a key mechanistic role, which is consistent with the identification of negative affect as a principal component in theoretical conceptualizations of pain processing (Wade, Dougherty, Hart, Rafii, & Price, 1992) and addiction motivation (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Indeed, a growing body of evidence suggests that negative affect mediates pain–smoking relations and exacerbates the deleterious effects of pain on smoking cessation.
Anxiety and Depression in Bidirectional Pain–Smoking Relations
Anxiety and Depression
Anxiety and depression have been related to the onset and maintenance of both chronic pain and tobacco dependence. Whereas anxiety represents the anticipation of future threat, depression represents the presence of sad, empty, or irritable mood (Diagnostic and Statistical Manual of Mental Disorders [DSM-5]; American Psychiatric Association [APA], 2013). Symptoms of anxiety and depression can be experienced at varying levels of intensity and duration, with anxious/depressive disorders diagnosed according to established criteria. Thus, individuals may experience symptoms of anxiety or depression without meeting diagnostic criteria for a clinical disorder. Given that either manifestation is likely relevant to complex pain–smoking associations, in the following sections, we refer to anxiety/depression when discussing symptoms or disorders, with more granular distinctions made as relevant.
Respective associations between anxiety/depression and both tobacco smoking (e.g., Ameringer & Leventhal, 2010; Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007; Zvolensky et al., 2008) and pain (e.g., Asmundson, Abrams, & Collimore, 2008; Dersh, Polatin, & Gatchel, 2002; Goesling, Clauw, & Hassett, 2013) have been reviewed extensively. In brief, anxiety/depression tends to be more common and more severe among smokers, relative to nonsmokers (Breslau, 1995; Goodwin et al., 2014; Lasser et al., 2000; McCabe et al., 2004; Williams & Ziedonis, 2004), and has been associated with poorer cessation outcomes (Pratt & Brody, 2010). Similarly, persons with chronic pain (vs. no pain) are more likely to experience anxiety/depression (Currie & Wang, 2004, 2005; de Leeuw, Eisenlohr-Moul, & Bertrand, 2013; Ohayon & Schatzberg, 2003; Torelli, Lambru, & Manzoni, 2006), and those with elevated anxious/depressive symptoms or disorders (i.e., major depressive disorder, panic disorder, and generalized anxiety disorder) tend to experience poorer pain outcomes (e.g., greater pain intensity and disability; Bair, Robinson, Katon, & Kroenke, 2003; Dersh et al., 2002).
Although several studies of pain and smoking have included anxiety symptoms and disorders (i.e., panic disorder, generalized anxiety disorder, social phobia, agoraphobia, specific phobia, posttraumatic stress disorder) as covariates in statistical analyses (Zale & Ditre, 2013; Zvolensky et al., 2009, 2010), few have explicitly tested the mechanistic role of anxiety in pain–smoking interrelations. Research in this area has primarily focused on directional effects of pain on smoking through examination of anxiety states that are more proximal to the pain experience (e.g., pain-related anxiety). According to the fear-avoidance model of chronic pain, pain-related fear and anxiety (i.e., fearful or anxious responding to actual or anticipated pain) are important mechanisms that maintain pain and facilitate the transition from acute to chronic pain (Vlaeyen & Linton, 2000; Zale & Ditre, 2015). There is also emerging evidence that pain-related anxiety may contribute to the maintenance of tobacco dependence. Specifically, among smokers with chronic pain, greater levels of pain-related anxiety have been associated with smoking dependence motives (Ditre, Zale, Kosiba, & Zvolensky, 2013) and expectancies for negative affect reduction via tobacco smoking (Gonzalez, Hogan, McLeish, & Zvolensky, 2010). Smokers who report greater pain-related anxiety have also been shown to endorse greater levels of tobacco dependence and to perceive more barriers to smoking cessation (Ditre et al., 2015). Moreover, researchers have suggested that pain-related anxiety may contribute to the effects of pain on smoking by serving as a situational motivator of smoking, and that smokers who endorse greater levels of pain-related anxiety may favor the use of nicotine/tobacco over other more adaptive strategies for pain coping (Ditre et al., 2015; Ditre et al., 2013).
Depression is also highly prevalent among smokers with chronic pain (e.g., Hooten, Shi, et al., 2011; Hooten et al., 2009) and is emerging as a key mediator in directional effects of smoking on pain. For example, Goesling and colleagues (2015) recently found that, among treatment-seeking chronic pain patients, current smokers endorsed more severe depressive symptoms, which in turn was associated with greater levels of pain and functional interference. Depression was also identified as a mediator of associations between current smoking and both pain severity and pain-related interference among persons evaluated for admission to an outpatient pain treatment program (Goesling, Brummett, & Hassett, 2012). Similar findings derived from a study of patients receiving multidisciplinary pain treatment indicated that greater levels of depressive symptoms partially accounted for associations between current smoking status and pain severity (Hooten, Shi, et al., 2011). Results derived from a population-based study also support the notion that the presence of a major depressive disorder partially accounts for associations between current tobacco smoking and greater pain intensity (van Hecke et al., 2014). Finally, epidemiological data obtained from a nationally representative sample indicate that smoking may contribute to the onset or exacerbation of depressive symptoms, which subsequently may increase the risk of developing chronic pain (Shi, Hooten, Roberts, & Warner, 2010).
In addition to pain onset and severity, we are aware of one study that examined associations among smoking, pain, depression, and opioid analgesic use. Among patients receiving multidisciplinary pain treatment, both depressive symptoms and smoking status were individually associated with greater morphine dosages, but only depressive symptoms were no longer significant when entered simultaneously in a model that included smoking and pain (Hooten, Shi, et al., 2011). These findings suggest that although smoking, pain, depression, and opioid use appear to be interrelated, greater levels of depression among smokers may not be sufficient to account for why they needed higher opioid dosages. It is possible that smoking and opioid use demonstrate unique associations (e.g., above-and-beyond the influence of depression) due to complex nicotine-opioid interactions that can result in cross-tolerance and/or sensitization to the rewarding effects of opioid medications (Shi, Weingarten, et al., 2010; Vihavainen, Piltonen, Tuominen, Korpi, & Ahtee, 2008).
Negative Affect and Other Transdiagnostic Factors
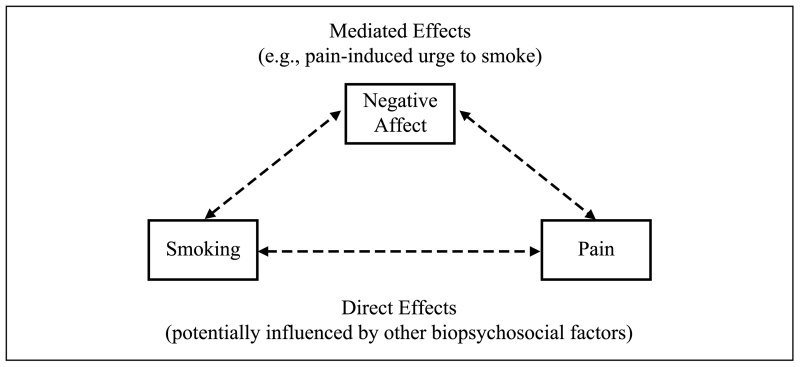
Although assessment of anxiety/depression offers considerable clinical and empirical utility, such broad classifications may obscure shared symptomatology or heterogeneity within disorders. Thus, researchers have also examined factors common across these disorders. First, negative affect represents a broad construct encompassing nonspecific states of subjective distress (Watson, Clark, & Tellegen, 1988), and has been implicated as a mediator in bidirectional pain–smoking relations (see Figure 1). Laboratory research has consistently demonstrated that negative affect induction (via the presentation of affectively-valenced picture cues) increases both pain sensitivity (e.g., de Wied & Verbaten, 2001; Kenntner-Mabiala, Weyers, & Pauli, 2007; Meagher, Arnau, & Rhudy, 2001; Rhudy, Bartley, & Williams, 2010) and smoking behavior (Kassel, Stroud, & Paronis, 2003), and negative affect is readily elicited through both pain- and smoking-related processes. Indeed, pain-related negative affect is a primary consequence of the pain experience (Wade, Dougherty, Archer, & Price, 1996), and a review of associations between smoking and negative affect concluded that negative affect can be exacerbated by chronic cigarette consumption and smoking withdrawal (Kassel et al., 2003). There is also experimental evidence that pain can be a potent motivator of urge to smoke, with directional effects partially mediated by pain-induced negative affect (Ditre & Brandon, 2008). Findings of partial mediation are consistent with the perspective that complex pain–smoking relations are likely driven by a variety of biopsychosocial mechanisms, including, but not limited to, acute negative affective states (Ditre et al., 2011).
Figure 1.
Negative affect as mediator of bidirectional pain–smoking relations.
In addition to negative affect, transdiagnostic factors (which represent characteristic responses to affective stimuli and states) are of increasing empirical interest. Three such factors were identified in a recent review, which concluded that anhedonia (i.e., diminished pleasure and response to reward), anxiety sensitivity (i.e., fear of anxiety-related sensations), and distress tolerance (i.e., ability to tolerate distressing states) likely serve as common risk factors for both smoking and anxiety/depression, and contribute to the maintenance of tobacco dependence (Leventhal & Zvolensky, 2015). Our review of the literature also indicated that these exemplary factors are highly relevant to chronic pain conditions and may serve as a model for understanding complex associations among pain, smoking, and additional transdiagnostic factors that could be identified in future research. Indeed, chronic pain patients have reported greater levels of anhedonia and decreased responsiveness to rewards, relative to healthy controls (Elvemo, Landrø, Borchgrevink, & Håberg, 2015), and distress tolerance has been implicated in emotion regulation and coping among persons with chronic pain (Hamilton, Karoly, & Kitzman, 2004). Moreover, decades of research have consistently demonstrated that greater levels of anxiety sensitivity predict a range of negative pain-related outcomes, including greater use of analgesic medications, greater cognitive bias toward pain experiences, and greater activation of fear-avoidance mechanisms (e.g., pain-related fear) that contribute to the development and maintenance of pain-related disability (e.g., Asmundson & Norton, 1995; Keogh & Cochrane, 2002; Wong et al., 2014). Thus, we suggest that transdiagnostic factors relevant to anxiety/depression should also be considered when addressing complex interrelations among affect, pain, and tobacco smoking.
Implications for Smoking Cessation
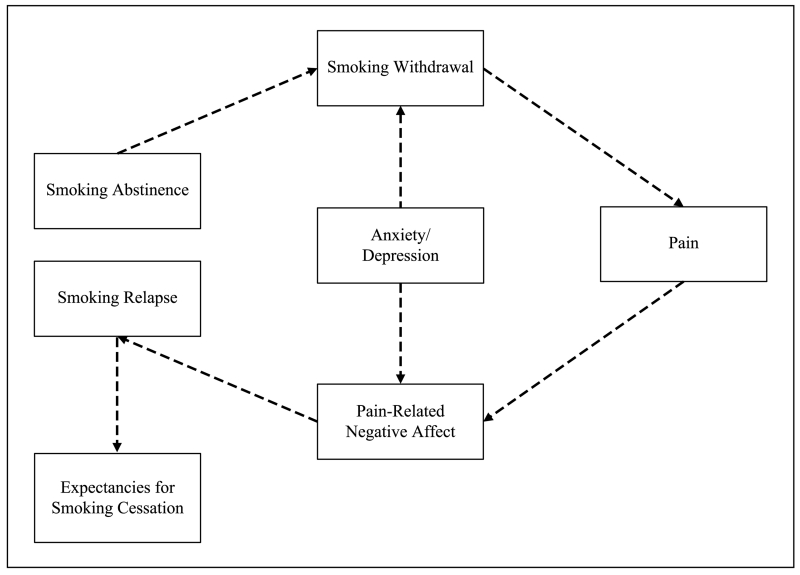
Within the framework of reciprocal pain–smoking relations, there are several reasons to suspect that anxiety/depression may undermine smoking cessation among persons with chronic pain. As depicted in Figure 2, we hypothesize that anxiety/depression exacerbates tobacco withdrawal severity, which in turn heightens pain sensitivity, resulting in greater negative affect (also potentially amplified by anxiety/depression), and subsequently precipitating relapse to smoking. First, smokers with anxious/depressive symptoms and disorders (e.g., major depressive disorder, panic disorder, generalized anxiety disorder) tend to experience greater levels of nicotine/tobacco withdrawal (i.e., an aversive state characterized by cognitive, affective, behavioral, and physiological symptoms; Hendricks, Ditre, Drobes, & Brandon, 2006; Hughes, 1991) when attempting to abstain from smoking (Leventhal, Ameringer, Osborn, Zvolensky, & Langdon, 2013; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008; Morissette et al., 2007; Weinberger, Desai, & McKee, 2010). Second, withdrawal severity is a hypothesized mechanism by which smokers may experience greater pain during periods of abstinence (e.g., Allen et al., 2000). Third, researchers have suggested that smokers who experience greater pain and pain-related negative affect during a quit attempt may return to smoking, in part, to alleviate increased pain sensitivity (Ditre et al., 2011; Shi, Weingarten, et al., 2010).
Figure 2.
Hypothesized role of anxiety and depression in relapse among smokers with chronic pain.
Note. Anxiety and depression are hypothesized to exacerbate smoking withdrawal, which increases pain sensitivity, results in greater negative affect, and precipitates relapse to smoking. Repeated cycles are hypothesized to engender negative expectations that undermine future cessation efforts.
We further hypothesize that repeated experiences of increasingly severe withdrawal and hyperalgesia (i.e., increased sensitivity to pain) when attempting to quit smoking may engender cognitive expectations that abstaining from tobacco will exacerbate both pain and negative affect, thus eroding self-efficacy for smoking cessation and increasing perceived barriers to quitting. Indeed, smokers with chronic pain who endorse greater withdrawal severity during past quit attempts have been shown to anticipate more severe withdrawal/negative affect during future quit attempts (Ditre, Kosiba, et al., under review). Consistent with evidence that past cessation failures may erode confidence for future success (Carey & Carey, 1993; Kirchner, Shiffman, & Wileyto, 2012), smokers in pain have also been shown to report lower levels of self-efficacy and greater perceived barriers to quitting, in part due to having experienced more severe withdrawal and greater difficulty during previous quit attempts (Ditre, Kosiba, et al., under review; Zale et al., 2014). Thus, smokers with comorbid anxiety, depression, and pain may be more likely to encounter difficulty maintaining abstinence, which may engender expectations that ultimately serve to undermine future quit attempts.
Tailored Approaches to Tobacco Dependence Treatment
Evidence reviewed herein suggests that smokers with comorbid pain and anxiety/depression are likely to experience unique challenges when attempting to quit smoking, and it follows that they may also require specialized treatment. Researchers have suggested that smokers in pain would benefit from tailored interventions that address smoking in the context of pain (e.g., Ditre et al., 2011; Zale et al., 2014), and we further recommend that clinicians also consider the role of anxiety/depression in pain–smoking relations. An integrated cognitive-behavioral approach that encourages consideration of comorbid conditions could provide an optimal model for intervention in this population (Mueser, Noordsy, Drake, & Fox, 2003).
Cognitive-behavioral therapy (CBT) provides the foundation for an efficacious smoking treatment that typically includes psychoeducation, cognitive restructuring, self-monitoring, coping skills training, and self-efficacy enhancement (Brown, 2011; Perkins, Conklin, & Levine, 2008; Reus & Smith, 2008). An integrated CBT-based cessation protocol for smokers with comorbid pain and anxiety/depression could incorporate functional analysis to aid in the identification of pain- and affect/mood-relevant proximal antecedents and consequences of smoking, psychoeducation to prepare these smokers for more severe withdrawal and increased sensitivity to pain during the early stages of a quit attempt, and cognitive restructuring that addresses the perceived utility of smoking for pain coping and affect regulation. Smokers in pain may also benefit from pre-cessation interventions that seek to enhance self-efficacy for quitting by teaching alternative and more adaptive (i.e., non-smoking-related) strategies for coping with both pain and anxiety/depression (e.g., Ditre, Kosiba, et al., under review; Zale et al., 2014).
Recommendations for the treatment of smokers with comorbid psychiatric conditions include the use of combination pharmacotherapy (Fagerstrom & Aubin, 2009), and there is reason to believe that smokers with pain and anxiety/depression may also benefit from tailored combinations of pharmacologic intervention. For example, researchers have suggested that high dose nicotine replacement therapy might confer acute analgesic benefits that help to mitigate pain during the early stages of quitting (Ditre, Heckman, et al., under review). In addition, bupropion is an efficacious smoking cessation aid that is also approved by the Food and Drug Administration (FDA) for treatment of depression (Fiore et al., 2008), and there is some evidence that smokers are less likely to relapse during the early stages of a quit attempt when bupropion and nicotine replacement therapy are combined (Fiore et al., 2008; Piper et al., 2007). Given evidence that smokers in pain (relative to no pain) are more likely to utilize pharmacotherapy for smoking cessation (Zale & Ditre, 2013), they may be especially amenable to dual treatment (e.g., bupropion plus combination/high dose nicotine replacement therapy).
Conclusion and Future Research Directions
Our review of the literature indicates that chronic pain and anxiety/depression each make smoking cessation more difficult, and that their co-occurrence may impede quitting to a greater extent than either in isolation. Specifically, we observed evidence that pain, smoking, and anxiety/depression are all highly prevalent and comorbid, and that negative affect is a likely mediator of reciprocal pain–smoking relations. We also integrated parallel lines of research to suggest that anxiety/depression may exacerbate the deleterious effects of pain on smoking cessation via processes such as greater pain-induced motivation to smoke, more severe withdrawal, and increased sensitivity to pain. Limitations of the currently reviewed literature include variability in the assessment of anxiety/depression, recent adjustments to the criteria used to classify and diagnose anxious/depressive disorders (DSM-5, APA, 2013), and relatively few studies that directly tested interrelations among pain, smoking and anxiety/depression. Additional research is needed to better understand the complex interplay of pain and anxiety/depression during the course of a smoking cessation attempt, and several promising lines of research warrant discussion.
First, ecological momentary assessment methods have recently been used to track near real-time covariation of pain intensity and smoking behavior (Dhingra et al., 2014), and future studies should apply these techniques to monitor temporal changes in pain intensity, anxiety/depression, negative affect, and withdrawal severity during the early stages of smoking abstinence. Future research would also benefit from assessing covariation between smoking-related outcome expectancies (Pain and Smoking Inventory; Ditre, Zale, Heckman, & Hendricks, under review) and transdiagnostic factors (Leventhal & Zvolensky, 2015) that are relevant to anxiety, depression, negative affect pain, and smoking prior to and over the course of a smoking cessation attempt. Although our review identified exemplary transdiagnostic factors that have been studied extensively for their role in associations between smoking and anxiety/depression, future work should seek to identify additional factors (e.g., pain catastrophizing) that may play an important role in bidirectional pain–smoking relations.
Future research would also benefit from examining overlap in neurobiological substrates associated with anxiety/depression, smoking behavior, and pain perception (Parkerson et al., 2013). For example, corticotropin-releasing factor (CRF) has been identified as a potential mechanism in the effects of pain on smoking (Ditre et al., 2011), and rodent models of nicotine self-administration implicate CRF1 receptors in the manifestation of anxiety-like behavior and increased pain during nicotine deprivation (Cohen et al., 2015). There is also an emerging consensus that activation of nicotinic acetylcholine receptors (nAChRs) plays a prominent role in pain–smoking processes. Greater nAChR availability has been associated with increased pain reactivity during smoking abstinence among humans (Cosgrove et al., 2010), and recent animal studies indicate that nAChR subunits that modulate nicotine analgesia are central in expression of anxiety- and depression-like behaviors (Semenova, Contet, Roberts, & Markou, 2012). Additional work is needed to better understand how overlapping neural mechanisms may contribute to the maintenance of tobacco dependence among smokers with comorbid chronic pain and anxiety/depression, as this work may also inform novel pharmacologic intervention approaches.
Finally, it is necessary to conduct randomized-controlled trials to test the utility of existing and novel smoking cessation treatments for smokers with comorbid pain and anxiety/depression. For example, it is not yet known whether treatment of anxiety/depression prior to a quit attempt (i.e., sequential treatment) may either aid in the management/mitigation of pain during smoking abstinence or decrease the extent to which pain precipitates relapse. Future research is also needed to better understand potentially complex inter-relations between pain, smoking, anxiety/depression and opioid use. For example, researchers should examine whether concurrent opioid use influences the treatment of tobacco dependence and anxiety/depression among smokers in pain. Pilot data suggest that varenicline (a prescription medication for smoking cessation) may be efficacious for the treatment of opioid tapering (Hooten & Warner, 2015), and additional research is needed to determine whether smokers who seek to taper their opioid medications should do so in conjunction with a quit attempt. Although we have suggested that some evidence-based treatments could be adapted to benefit smokers with chronic pain and anxiety/depression, such interventions have yet to be developed or tested. Future research may consider the utility of sequential and/or integrated treatments for pain and smoking cessation among individuals with comorbid anxiety and depression.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by Grant Nos. R21DA034285 and R21DA038204 awarded to Joseph W. Ditre by the National Institute on Drug Abuse, Grant No. F31DA039628 awarded to Emily L. Zale by the National Institute on Drug Abuse, and Grant No. K05AA16928 awarded to Stephen A. Maisto by the National Institute on Alcohol Abuse and Alcoholism.
Biographies
Author Biographies
Emily L. Zale is a doctoral candidate in the Deparmtent of Psychology at Syracuse University. Her primary research interests include relationships between substance use and chronic medical conditions, with a focus on tobacco smoking, alcohol, and prescription opioid use among persons in pain, and the development of novel interventions for co-ocurring substance use and medical conditions.
Stephen A. Maisto is a Professor of Psychology at Syracuse University. His research programs span several health behavior-related areas and include the assessment and modification of alcohol and other drug use, the integration of behavioral healthcare in medical settings, in particular primary care, HIV prevention and intervention, with focus on alcohol and other drug use as determining factors, and human laboratory research on the determinants and consequences of alcohol consumption.
Joseph W. Ditre is an Assistant Professor of Psychology at Syracuse University. His research cuts across basic and applied work in the areas of health psychology and behavioral medicine with a focus on testing bi-directional relations between the experience of acute/chronic pain and the use/misuse of addictive substances (e.g., nicotine/tobacco, alcohol, cannabis) and developing novel treatments for individuals with co-occurring medical and substance use disorders.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aamodt AH, Stovner LJ, Hagen K, Brathen G, Zwart J. Headache prevalence related to smoking and alcohol use. The Head-HUNT Study. European Journal of Neurology. 2006;13:1233–1238. doi: 10.1111/j.1468-1331.2006.01492.x. doi:10.1111/j.1468-1331.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine & Tobacco Research. 2000;2:231–241. doi: 10.1080/14622200050147493. doi:10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: An integrative review. Nicotine & Tobacco Research. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. doi:10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Niu J, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, Felson DT. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Annals of the Rheumatic Diseases. 2007;66:18–22. doi: 10.1136/ard.2006.056697. doi:10.1136/ard.2006.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJG, Abrams MP, Collimore KC. Pain and anxiety disorders. In: Zvolensky MJ, Smits JA, editors. Anxiety in health behaviors and physical illness. Springer; New York, NY: 2008. pp. 3–28. [Google Scholar]
- Asmundson GJG, Norton GR. Anxiety sensitivity in patients with physically unexplained chronic back pain: A preliminary report. Behaviour Research and Therapy. 1995;33:771–777. doi: 10.1016/0005-7967(95)00012-m. doi:10.1016/0005-7967(95)00012-M. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. doi:10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. doi:10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Brown RA. Intensive behavioral treatment. In: Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook: A guide to best practices. The Guilford Press; New York, NY: 2011. pp. 118–177. [Google Scholar]
- Carey KB, Carey MP. Changes in self-efficacy resulting from unaided attempts to quit smoking. Psychology of Addictive Behaviors. 1993;7:219–224. doi:10.1037/0893-164x.7.4.219. [Google Scholar]
- Centers for Disease Control and Prevention Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Vital signs: Current cigarette smoking among adults aged ≥ 18 years—United States, 2009. Morbidity and Mortality Weekly Report. 2010;59:1135–1140. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Current cigarette smoking among adults—United States 2005-2012. Morbidity and Mortality Weekly Report. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addiction Biology. 2015;20:56–68. doi: 10.1111/adb.12077. doi:10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee S, Bois F, Alagille D, Tamagnan GD, Staley JK. Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine & Tobacco Research. 2010;12:535–539. doi: 10.1093/ntr/ntq040. doi:10.1093/ntr/ntq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107:54–60. doi: 10.1016/j.pain.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Currie SR, Wang JL. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychological Medicine. 2005;35:1275–1282. doi: 10.1017/S0033291705004952. doi:10.1017/S0022181705004952. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Eisenlohr-Moul T, Bertrand P. The association of smoking status with sleep disturbance, psychological functioning, and pain severity in patients with temporomandibular disorders. Journal of Orofacial Pain. 2013;27:32–41. doi: 10.11607/jop.1040. doi:10.11607/jop.1040. [DOI] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosomatic Medicine. 2002;64:773–786. doi: 10.1097/01.psy.0000024232.11538.54. doi:10.1097/01.PSY.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- de Wied M, Verbaten MN. Affective pictures processing, attention, and pain tolerance. Pain. 2001;90:163–172. doi: 10.1016/s0304-3959(00)00400-0. doi:10.1016/S0304-3959(00)00400-0. [DOI] [PubMed] [Google Scholar]
- Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, Portenoy R. Ecological momentary assessment of smoking behavior in persistent pain patients. Clinical Journal of Pain. 2014;30:205–213. doi: 10.1097/AJP.0b013e31829821c7. doi:10.1097/AJP.0b013e31829821c7. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology. 2008;117:467–472. doi: 10.1037/0021-843X.117.2.467. doi:10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin. 2011;137:1065–1093. doi: 10.1037/a0025544. doi:10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman B, Zale EL, Kosiba JD, Maisto SA. Acute analgesic effects of nicotine and tobacco smoking in humans: A meta-analysis. Pain. doi: 10.1097/j.pain.0000000000000572. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology. 2010;119:524–533. doi: 10.1037/a0019568. doi:10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, Maisto SA. Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation. Annals of Behavioral Medicine. doi: 10.1007/s12160-016-9769-9. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Langdon KJ, Kosiba JD, Zale EL, Zvolensky MJ. Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addictive Behaviors. 2015;42:130–135. doi: 10.1016/j.addbeh.2014.11.032. doi:10.1016/j.addbeh.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, Heckman BW, Hendricks PS. A measure of pain and smoking expectancies: Development and initial validation of the Pain and Smoking Inventory. Nicotine & Tobacco Research. under review. [Google Scholar]
- Ditre JW, Zale EL, Kosiba JD, Zvolensky MJ. A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Experimental and Clinical Psychopharmacology. 2013;21:443–449. doi: 10.1037/a0034174. doi:10.1037/a0034174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvemo NA, Landrø NI, Borchgrevink PC, Håberg AK. Reward responsiveness in patients with chronic pain. European Journal of Pain. 2015 doi: 10.1002/ejp.687. Advance online publication. doi:10.1002/ejp.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K, Aubin HJ. Management of smoking cessation in patients with psychiatric disorders. Current Medical Research and Opinion. 2009;25:511–518. doi: 10.1185/03007990802707568. doi:10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addictive Behaviors. 1986;11:239–248. doi: 10.1016/0306-4603(86)90052-3. doi:10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Fishbain DA, Lewis JE, Cole B, Cutler RB, Rosomoff HL, Rosomoff RS. Variables associated with current smoking status in chronic pain patients. Pain Medicine. 2007;8:301–311. doi: 10.1111/j.1526-4637.2007.00317.x. doi:10.1111/j.1526-4637.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Lewis JE, Cutler R, Cole B, Steele Rosomoff R, Rosomoff HL. Does smoking status affect multidisciplinary pain facility treatment outcome? Pain Medicine. 2008;9:1081–1090. doi: 10.1111/j.1526-4637.2007.00306.x. doi:10.1111/j.1526-4637.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Glassman SD, Dimar JRI, Burkus K, Hardacker JW, Pryor PW, Boden SD, Carreon LY. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007;32:1693–1698. doi: 10.1097/BRS.0b013e318074c366. doi:10.1097/BRS.0b013e318074c366. [DOI] [PubMed] [Google Scholar]
- Goesling J, Brummett CM, Hassett AL. Cigarette smoking and pain: Depressive symptoms mediate smoking-related pain symptoms. Pain. 2012;153:1749–1754. doi: 10.1016/j.pain.2012.05.014. doi:10.1016/j.pain.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Goesling J, Brummett CM, Meraj TS, Moser SE, Hassett AL, Ditre JW. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Medicine. 2015;16:1433–1442. doi: 10.1111/pme.12747. doi:10.1111/pme.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goesling J, Clauw D, Hassett A. Pain and depression: An integrative review of neurobiological and psychological factors. Current Psychiatry Reports. 2013;15:1–8. doi: 10.1007/s11920-013-0421-0. doi:10.1007/s11920-013-0421-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Hogan J, McLeish AC, Zvolensky MJ. An evaluation of pain-related anxiety among daily cigarette smokers in terms of negative and positive reinforcement smoking outcome expectancies. Addictive Behaviors. 2010;35:553–557. doi: 10.1016/j.addbeh.2010.01.005. doi:10.1016/j.addbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Wall MM, Choo T, Galea S, Horowitz J, Nomura Y, Hasin DS. Changes in the prevalence of mood and anxiety disorders among male and female current smokers in the United States: 1990-2001. Annals of Epidemiology. 2014;24:493–497. doi: 10.1016/j.annepidem.2014.01.014. doi:10.1016/j.annepidem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Damaj MI. Nicotine physical dependence in the mouse: Involvement of the alpha7 nicotinic receptor subtype. European Journal of Pharmacology. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. doi:10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Alonso J. The relation between multiple pains and mental disorders: Results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. doi:10.1016/j. pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Karoly P, Kitzman H. Self-regulation and chronic pain: The role of emotion. Cognitive Therapy and Research. 2004;28:559–576. doi:10.1023/B:COTR.0000045565.88145.76. [Google Scholar]
- Harty LC, Veale DJ. Irish smokers with rheumatoid arthritis suffer more than their nonsmoking counterparts. Journal of Rheumatology. 2010;37:1062. doi: 10.3899/jrheum.091403. author reply 1063. doi:10.3899/jrheum.091403. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. doi:10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152:223–229. doi: 10.1016/j.pain.2010.10.045. doi:10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Bruce BK, Schmidt JE, Kerkvliet JL, Patten CA, Warner DO. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Medicine. 2009;10:347–355. doi: 10.1111/j.1526-4637.2008.00494.x. doi:10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, Warner DO. Smoking cessation and chronic pain: Patient and pain medicine physician attitudes. Pain Practice. 2011;11:552–563. doi: 10.1111/j.1533-2500.2011.00462.x. doi:10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Warner DO. Varenicline for opioid withdrawal in patients with chronic pain: A randomized, single-blinded, placebo controlled pilot trial. Addictive Behaviors. 2015;42:69–72. doi: 10.1016/j.addbeh.2014.11.007. doi:10.1016/j.addbeh.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology. 1991;104:409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Brandon TH. A softer view of hardening. Nicotine & Tobacco Research. 2003;5:961–962. doi: 10.1080/14622200310001615330. doi:10.1080/14622200310001615330. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Relieving pain in America: A blueprint for transforming prevention, care, education, and research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- International Association for the Study of Pain . Part III: Pain terms: A current list with definitions and notes on usage. In: Merskey H, Bogduk N, editors. Classification of chronic pain. 2nd ed. IASP Press; Seattle, WA: 1994. pp. 209–214. [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine & Tobacco Research. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2009;331:547–554. doi: 10.1124/jpet.109.155457. doi:10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors. 1991;16:103–110. doi: 10.1016/0306-4603(91)90002-y. doi:10.1016/0306-4603(91)90002-Y. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. doi:10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Weyers P, Pauli P. Independent effects of emotion and attention on sensory and affective pain perception. Cognition and Emotion. 2007;21:1615–1629. doi:10.1080/02699930701252249. [Google Scholar]
- Keogh E, Cochrane M. Anxiety sensitivity, cognitive biases, and the experience of pain. The Journal of Pain. 2002;3:320–329. doi: 10.1054/jpai.2002.125182. doi:10.1054/jpai.2002.125182. [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: Recurrent abstinence violation effects and lapse-relapse progression. Journal of Abnormal Psychology. 2012;121:187–197. doi: 10.1037/a0024451. doi:10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. doi:10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kim SH, Nah SS, Lee JH, Lee YA, Hong SJ, Kim SK. Smoking habits influence pain and functional and psychiatric features in fibromyalgia. Joint, Bone, Spine: Revue du Rhumatisme. 2010;78:259–265. doi: 10.1016/j.jbspin.2010.07.018. doi:10.1016/j.jbspin.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence. 2013;133:324–329. doi: 10.1016/j.drugalcdep.2013.06.015. doi:10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & Tobacco Research. 2008;10:507–517. doi: 10.1080/14622200801901971. doi:10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141:176–212. doi: 10.1037/bul0000003. doi:10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic . Managing pain: Attitude, medication and therapy are keys to control. Mayo Clinic Health Letter; 2001. Available from http://www.mayoclinic.com. [Google Scholar]
- McCabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zolvensky MJ. Smoking behaviors across anxiety disorders. Journal of Anxiety Disorders. 2004;18:7–18. doi: 10.1016/j.janxdis.2003.07.003. doi:10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: Effects of affective picture modulation. Psychosomatic Medicine. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. doi:10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: Importance of abuse history. Journal of Pain and Symptom Management. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. doi:10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychological Bulletin. 2007;133:245–272. doi: 10.1037/0033-2909.133.2.245. doi:10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Mueser K, Noordsy DL, Drake RE, Fox L. Integrated treatment for dual disorders: A guide to effective practice. The Guilford Press; New York, NY: 2003. [Google Scholar]
- Nakajima M, al’Absi M. Enhanced pain perception prior to smoking cessation is associated with early relapse. Biological Psychology. 2011;88:141–146. doi: 10.1016/j.biopsycho.2011.07.006. doi:10.1016/j.biopsycho.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Archives of General Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. doi:10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- Parkerson HA, Zvolensky MJ, Asmundson GJ. Understanding the relationship between smoking and pain. Expert Review of Neurotherapeutics. 2013;13:1407–1414. doi: 10.1586/14737175.2013.859524. doi:10.1586/14737175.2013.859524. [DOI] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. Journal of Pain. 2012;13:285–292. doi: 10.1016/j.jpain.2011.11.008. doi:10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-behavioral therapy for smoking cessation: A practical guidebook to the most effective treatments. Routledge; New York, NY: 2008. [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research. 2007;9:947–954. doi: 10.1080/14622200701540820. doi:10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005-2008. NCHS Data Brief. 2010;34:1–8. [PubMed] [Google Scholar]
- Reus VI, Smith BJ. Multimodal techniques for smoking cessation: A review of their efficacy and utilisation and clinical practice guidelines. International Journal of Clinical Practice. 2008;62:1753–1768. doi: 10.1111/j.1742-1241.2008.01885.x. doi:10.1111/j.1742-1241.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Bartley EJ, Williams AE. Habituation, sensitization, and emotional valence modulation of pain responses. Pain. 2010;148:320–327. doi: 10.1016/j.pain.2009.11.018. doi:10.1016/j.pain.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Semenova S, Contet C, Roberts AJ, Markou A. Mice lacking the beta4 subunit of the nicotinic acetylcholine receptor show memory deficits, altered anxiety- and depression-like behavior, and diminished nicotine-induced analgesia. Nicotine & Tobacco Research. 2012;14:1346–1355. doi: 10.1093/ntr/nts107. doi:10.1093/ntr/nts107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Hooten WM, Roberts RO, Warner DO. Modifiable risk factors for incidence of pain in older adults. Pain. 2010;151:366–371. doi: 10.1016/j.pain.2010.07.021. doi:10.1016/j. pain.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: Pathophysiology and clinical implications. Anesthesiology. 2010;113:977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. doi:10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: A meta-analysis. American Journal of Medicine. 2010;123:e7–e35. doi: 10.1016/j.amjmed.2009.05.028. doi:10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. Journal of Personality and Social Psychology. 1982;42:946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis & Rheumatism. 2007;57:656–665. doi: 10.1002/art.22684. doi:10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- Torelli P, Lambru G, Manzoni GC. Psychiatric comorbidity and headache: Clinical and therapeutical aspects. Neurological sciences: Official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27(Suppl. 2):S73–S76. doi: 10.1007/s10072-006-0574-2. doi:10.1007/s10072-006-0574-2. [DOI] [PubMed] [Google Scholar]
- Turk DC, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 3rd ed. The Guilford Press; New York, NY: 2011. pp. 3–18. [Google Scholar]
- Turk DC, Okifuji A. Pain terms and taxonomies of pain. In: Loeser JD, Chapman CR, Turk DC, Butler SH, editors. Bonica’s management of pain. 3rd ed. Lippincott Williams & Wilkins; Hagerstown, MD: 2001. pp. 17–25. [Google Scholar]
- United States Department of Health and Human Services . The health consequences of smoking—50 years of progress: A report of the surgeon general. U.S. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- van Hecke O, Torrance N, Cochrane L, Cavanagh J, Donnan PT, Padmanabhan S, Smith BH. Does a history of depression actually mediate smoking-related pain? Findings from a cross-sectional general population-based study. European Journal of Pain. 2014;18:1223–1230. doi: 10.1002/j.1532-2149.2014.00470.x. doi:10.1002/j.1532-2149.2014.00470.x. [DOI] [PubMed] [Google Scholar]
- Vihavainen T, Piltonen M, Tuominen RK, Korpi ER, Ahtee L. Morphine-nicotine interaction in conditioned place preference in mice after chronic nicotine exposure. European Journal of Pharmacology. 2008;587:169–174. doi: 10.1016/j.ejphar.2008.03.028. doi:10.1016/j.ejphar.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. doi:10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: A multivariate analytical approach. Pain. 1996;68:157–167. doi: 10.1016/S0304-3959(96)03162-4. doi:10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Hart RP, Rafii A, Price DD. A canonical correlation analysis of the influence of neuroticism and extraversion on chronic pain, suffering, and pain behavior. Pain. 1992;51:67–73. doi: 10.1016/0304-3959(92)90010-9. doi:10.1016/0304-3959(92)90010-9. [DOI] [PubMed] [Google Scholar]
- Waldie KE, McGee R, Reeder AI, Poulton R. Associations between frequent headaches, persistent smoking, and attempts to quit. Headache. 2008;48:545–552. doi: 10.1111/j.1526-4610.2007.01037.x. doi:10.1111/j.1526-4610.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi:10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug and Alcohol Dependence. 2010;108:7–12. doi: 10.1016/j.drugalcdep.2009.11.004. doi:10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11:643–653. [PubMed] [Google Scholar]
- Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, Oh TH. Impact of tobacco use in patients presenting to a multidisciplinary outpatient treatment program for fibromyalgia. Clinical Journal of Pain. 2009;25:39–43. doi: 10.1097/AJP.0b013e31817d105e. doi:10.1097/AJP.0b013e31817d105e. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addictive Behaviors. 2004;29:1067–1083. doi: 10.1016/j.addbeh.2004.03.009. doi:10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wong WS, Lam HMJ, Chow YF, Chen PP, Lim HS, Wong S, Fielding R. The effects of anxiety sensitivity, pain hypervigilance, and pain catastrophizing on quality of life outcomes of patients with chronic pain: A preliminary, cross-sectional analysis. Quality of Life Research. 2014;23:2333–2341. doi: 10.1007/s11136-014-0683-y. doi:10.1007/s11136-014-0683-y. [DOI] [PubMed] [Google Scholar]
- Zale EL, Ditre JW. Associations between chronic pain status, attempts to quit smoking, and use of pharmacotherapy for smoking cessation. Psychology of Addictive Behaviors. 2013;28:294–299. doi: 10.1037/a0032515. doi:10.1037/a0032515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Ditre JW. Pain-related fear, disability, and the fear-avoidance model of chronic pain. Current Opinion in Psychology. 2015;5:24–30. doi: 10.1016/j.copsyc.2015.03.014. doi:10.1016/j. copsyc.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Ditre JW, Dorfman ML, Heckman BW, Brandon TH. Smokers in pain report lower confidence and greater difficulty quitting. Nicotine & Tobacco Research. 2014;16:1272–1276. doi: 10.1093/ntr/ntu077. doi:10.1093/ntr/ntu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Leyro T, Bernstein A, Feldner MT, Yartz AR, Babson KA, Bonn-Miller MO. Tobacco use and panic psychopathology: Current status and future directions. In: Zvolensky MJ, Smits JA, editors. Anxiety in health behaviors and physical illness. Springer; New York, NY: 2008. pp. 3–28. [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research. 2009;11:1407–1414. doi: 10.1093/ntr/ntp153. doi:10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan KA, Gonzalez A, Asmundson GJG. Chronic musculoskeletal pain and cigarette smoking among a representative sample of Canadian adolescents and adults. Addictive Behaviors. 2010;35:1008–1012. doi: 10.1016/j.addbeh.2010.06.019. doi:10.1016/j.addbeh.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]