Abstract
Purpose
Retinopathy of prematurity (ROP) is a disorder of developing retinal blood vessels in preterm infants. The purpose of this nested study was to investigate the effects of higher (91–95%) and lower (85–89%) oxygen saturation (SpO2) targeting on retinal blood vessel growth in preterm infants.
Methods
Retinal blood vessel growth in the higher (91–95%) and lower (85–89%) oxygen saturation (SpO2) targeting groups was compared. Suitable RetCam (Clarity, Pleasanton, CA, USA) images collected in the BOOST-II UK trial were used. The distances between the centre of the optic disc and the ROP ridge in the temporal and nasal retina were measured in pixel units.
Results
Images from 38 infants were studied, 20 from the higher SpO2 target group and 18 from the lower SpO2 target group. On average, temporal blood vessels extended further from the optic disc than nasal blood vessels, mean (standard deviation (SD)) 463.39 (55.05) pixels compared with 360.13 (44.47) pixels, respectively, P<0.0001. Temporal blood vessels extended less far from the optic disc in the higher SpO2 target group than in the lower SpO2 target group: mean (SD) 449.83 (56.16) pixels compared with 480.02 (49.94), respectively, P=0.055. Nasal retinal blood vessel measurements were broadly similar in the higher and lower SpO2 target groups; mean (SD) 353.96 (41.95) compared with 370.00 (48.82) pixels, respectively, P=0.38.
Conclusions
Relatively high oxygen saturation targeting (91–95%) was associated with a trend (P=0.055) towards reduced retinal blood vessel growth in this study of preterm infants.
Introduction
Retinopathy of prematurity (ROP) is a disorder of developing retinal blood vessels in preterm infants. Normal vascular development starts at the optic nerve and radiates peripherally,1 by vasculogenesis and vascular endothelial growth factor (VEGF)-mediated angiogenesis processes.2
Clinical and animal studies have suggested that ROP occurs in two phases.3 Phase 1 is associated with the higher blood and tissue oxygen levels in the preterm infant than occur in utero. This follows preterm birth into environmental air, exacerbated by supplemental oxygen. Increased oxygen levels are thought to result in downregulation of VEGF, with delayed vascularisation. However, in vivo human studies have not yet been conducted. Phase 2 consists of a vasoproliferative response following reduced blood vessel development in phase 1. The attenuated vasculature can no longer supply sufficient oxygen and nutrients to the growing avascular peripheral retina, and this results in greatly increased expression of VEGF. In addition to oxygen-mediated effects, phase 1 is associated with low levels of circulating IGF1 and phase 2 with more normal levels of IGF1.4, 5 IGF1 is required for VEGF-mediated angiogenesis.6, 7
Risk factors for ROP may be divided into prenatal and postnatal factors. Prenatal factors affect the degree of retinal blood vessel development present at the time of preterm birth. The incidence of ROP is higher in infants of lower gestational age and birth weight, and in infants small for gestational age.8 Postnatal factors reflect divergence from in utero conditions.9 Supplemental oxygen represents the best understood postnatal risk factor for ROP. Randomised controlled trials have shown that restricted oxygen therapy results in a lower incidence of severe ROP, but mortality is increased.3, 10, 11, 12
The aim of this nested study was to investigate the effects of higher (91–95%) and lower (85–89%) oxygen saturation (SpO2) targeting on retinal blood vessel growth in preterm infants. Data from infants included in the BOOST-II UK (Benefits of Oxygen Saturation Targeting) randomised controlled trial11 were analysed. Our hypothesis was that exposure to higher SpO2 targeting inhibits blood vessel growth compared with lower SpO2 targeting.
Subjects and methods
Analyses were based on eye examination data obtained as part of the BOOST-II UK trial.11 973 infants aged less than 28 weeks' gestation at birth were enrolled. In 12 of the study centres, retinal images were obtained by a consultant paediatric ophthalmologist using a contact widefield digital fundus camera (RetCam, Clarity systems, Pleasanton, CA, USA) at the time of ROP screening examinations. The images were uploaded onto a secure study server for reading and analysis.
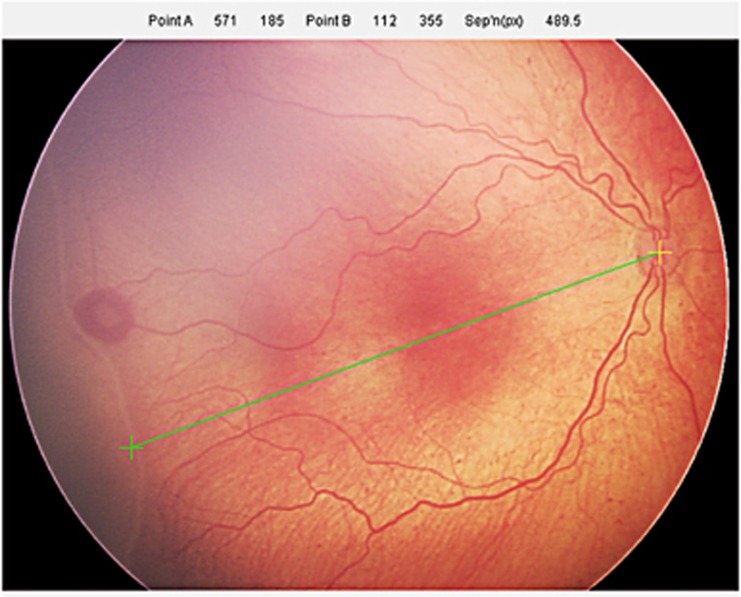
Good quality images that included adequate views of the optic disc, the temporal retinal blood vessel arcades, and a temporal or nasal ROP demarcation line or ridge were selected. Our analysis was restricted to images obtained from screening examinations on infants less than 36 weeks (252 days) postmenstrual age (PMA) at the time of examination, as the trial intervention finished at this age. All measurements were performed by observers who were masked to the trial group allocation. Measurements were made using bespoke digital imaging software (http://www.VisionResearch.org) that allowed accurate linear measurements to be made (Figure 1). The angle of measurement from the optic disc was that judged to fall on a line midway between the temporal vascular arcades. This applied to both temporal and nasal measurements. Measurement was then made between the centre of the optic disc and the point at the outer margin of the ROP ridge. Measurements were expressed in camera pixel units. Each measurement was made independently on three occasions by two observers (RBRM and BWF) and the six results were averaged.
Figure 1.
Measurement of the distance from the centre of the optic disc to the outer edge of the ROP ridge, along an axis bisecting the temporal blood vessel arcades.
The set of images from each infant examination was incomplete in many cases. Images of adequate quality for measurement from both the temporal and nasal retina of both eyes were only available for two examinations. For each infant examination, a temporal retinal measurement from one eye and a nasal retinal measurement from one eye were analysed, where available. When a temporal or a nasal measurement was available from both eyes, one eye was randomly selected.
Intra-observer measurement variation was assessed by obtaining the difference between the highest and lowest measurement of the three measurements performed by each observer, and inter-observer measurement variation was assessed by obtaining the difference between the averaged measurements obtained by each observer, for each image.
Statistical analysis
Demographic and clinical data were summarised with counts for categorical variables, means (standard deviations) for normally distributed continuous variables and medians (with simple ranges) for other continuous variables. Comparative statistical analysis entailed calculating the mean difference (plus 95% CI) for normally distributed continuous outcomes. Agreement between the two observers was assessed using Bland–Altman plots.13 Analysis was first performed using all available examinations. However, as there were multiple examinations for some individuals, and these were technically statistically not independent, a further analysis was performed, restricted to one examination for each infant. All analyses were performed using GraphPad Prism 6 software (GraphPad, La Jolla, CA, USA).
Ethics approval statement
This study was a nested study within the BOOST-II UK trial, approved by the Trent Research Ethics Committee, reference 06/MRE04/91.
Results
At the close of the BOOST-II UK study data collection, the retinal image database contained 2,022 images taken during 432 eye examinations, from 134 infants. Ninety-nine images taken during 57 screening examinations from 38 infants were of sufficient quality to allow retinal blood vessel measurements to be obtained. Sixty-five were images of the temporal retina and thirty-four of the nasal retina. The median gestational age of the 38 infants studied was 25 weeks+0 days (range 22 weeks+6 days to 27 weeks+56 days), and the median birth weight was 740 g (range 450–1,200 g). Twenty-one infants were female. Maternal ethnicity was white in all cases. Eighteen infants were from the low oxygen saturation targeting group, and twenty were from the high oxygen saturation targeting group.
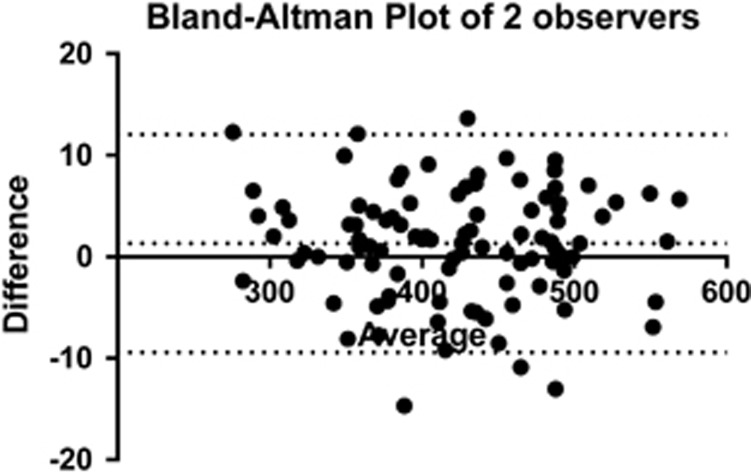
Agreement between the two observers is shown in Figure 2. The 95% confidence intervals were −9.43 to +12.04 pixels and the measurement bias between the two observers was a mean (SD) of 1.31 (5.48) pixels. The repeatability coefficient for measurements was 7.63 pixels for BWF and 9.31 pixels for RBRM.
Figure 2.
Bland–Altman plot of pixel measurements made by the two observers. Dashed lines show 95% confidence intervals.
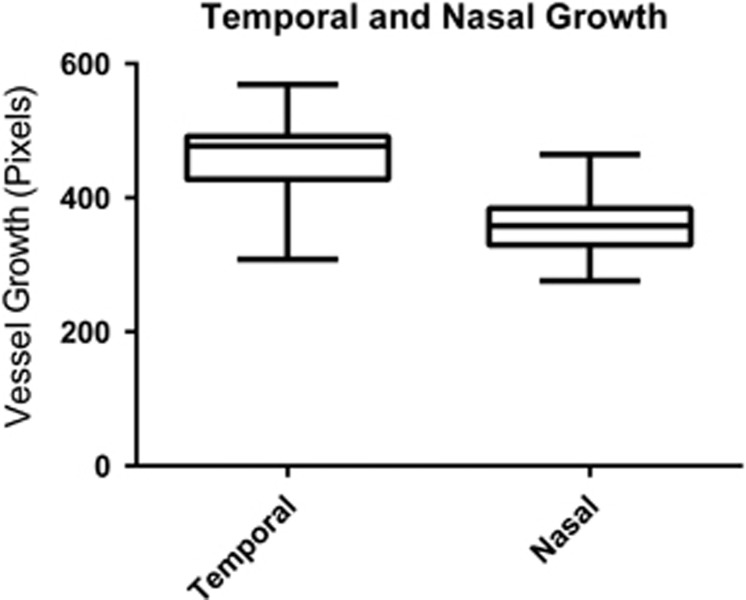
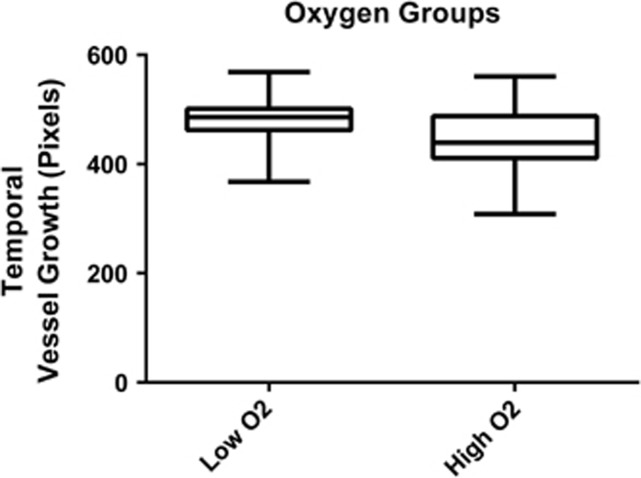
On average, temporal blood vessels extended further from the optic disc than nasal blood vessels, mean (SD) 463.39 (55.05) pixels compared with 360.13 (44.47) pixels, P<0.0001 (Figure 3). The timing of these examinations was 242.33 (7.51) days PMA for the temporal group and 239.35 (9.23) days PMA for the nasal group (P=0.20). Temporal vessels extended less far from the optic disc in the higher SpO2 target group than the lower SpO2 target group, mean (SD) 449.83 (56.16) pixels compared with 480.02 (49.94) pixels, respectively, P=0.055, (Figure 4). There was no evidence of a difference between the two groups for nasal retinal blood vessel growth, mean (SD) 353.96 (41.95) pixels, compared with 370.00 (48.82) pixels, respectively, P=0.38.
Figure 3.
Growth of retinal blood vessels of the temporal and nasal retina expressed as camera pixels from the centre of the optic disc to the intersection with the ROP ridge. Box and whiskers plot.
Figure 4.
Growth of retinal blood vessels of the temporal retina in the low and high SpO2 targeting groups expressed as camera pixels from the centre of the optic disc to the intersection with the ROP ridge. Box and whiskers plot.
In 26 infants, measurements were available from a single examination. For eight infants, measurements were available from two examinations, in two infants from three examinations, in one infant from four examinations and in one infant from five examinations. Further analysis was performed by selecting one examination from each infant, to produce ‘per patient' analysis rather than ‘per examination' analysis. The median PMA at the time of examination for all study examinations was 34 weeks+1 day. In order to analyse a single set of measurements from each infant, the examination closest to this time was selected for each infant. Measurement of one temporal and one nasal image per infant showed that temporal blood vessels grew further from the optic disc than nasal blood vessels; mean (SD) 461.08 (59.55) pixels compared with 355.47 (42.83) pixels, P<0.0001. Temporal vessels extended less far in the higher SpO2 target group than in the lower SpO2 target group, mean (SD) 453.73 (64.72) pixels compared with 469.91 (53.54) pixels, however, there was no statistical evidence of a difference (P=0.45). Nasal vessels extended mean (SD) 351.25 (45.98) pixels in the higher SpO2 target group, compared with 361.66 (39.81) pixels in the lower SpO2 target group, P=0.61.
Discussion
In this study, we found that retinal blood vessels extended further in the temporal retina than the nasal retina. This is consistent with a previous study.14 ROP frequently develops first in the nasal retina in the most immature infants.15 The mean PMA at which nasal measurements were made was 3 days earlier than for temporal measurements. Images were selected, based on the presence of an ROP ridge. It is conceivable that development of an ROP ridge causes reduced forward growth of retinal blood vessels. As this occurs earlier in the nasal retina than in the temporal retina, the effect may contribute to the nasal/temporal asymmetry observed. However, our data were of insufficient quality or quantity to analyse these possible effects, which therefore remain speculative.
Temporal retinal vessels extended less distance from the optic disc in the higher SpO2 (91–95%) target group than in the lower SpO2 (85–89%) target group. Although the BOOST-II UK trial measured the effects of SpO2 targeting, the actual oxygen saturations achieved were confirmed to be different in the two groups.11 Relative hyperoxia during the early postnatal period in preterm infants is thought to reduce normal ‘physiological hypoxia' control of retinal blood vessel angiogenesis. We found a trend towards an association between oxygen saturation targeting and retinal blood vessel growth. However, with a P value of 0.55, the association was not robust. We have not demonstrated causation. Models of ROP have shown impaired blood vessel growth in animals raised in a hyperoxic environment when compared with those raised in room air.16, 17, 18, 19 A number of clinical studies have shown abnormal retinal blood vessel growth in infants treated with supplemental oxygen, but have not quantified the effect.3
Our measurements benefited from the use of two observers with a high level of concordance. However, it is likely that error occurred due to limitations in the extent to which each observer was able to accurately and consistently position the screen cursor at reference points determined using a subjectively judged angle. Measurement of the extent of temporal vascularisation is made more difficult by the presence of a temporal ‘notch' in some eyes. We chose an anatomically consistent reference point in relation to the temporal vessel arcades in order to reduce variation based on asymmetry of the extent of temporal retina vascularisation. An alternative approach would have been to make measurements at multiple points along the ROP ridge, as was done in the study by Gallagher et al.14
Comparisons of measurements were based on the assumption that optical magnification was identical in all eyes, which although likely,20 may not have been the case. A further source of error may have resulted from measurement of two-dimensional images of three-dimensional curved structures. Linear magnification of radially orientated lines is greatest in the centre of each image and much smaller near their edge. Future work should address this issue through mathematical modelling of the coordinates of each reference point relative to the centre of the image.
Despite the methodological limitations of this study, the results lend some support to the hypothesis that higher oxygen levels inhibit blood vessel growth in the developing retina, compared with lower oxygen levels. The limited number of observations available to us restricted the statistical confidence of this result. Although we found a trend, the association remains unproven. We have not demonstrated causation. Our data are unique, and no further oxygen trials in preterm infants are planned. Should further clinical oxygen trials in preterm infants be conducted, an attempt should be made to replicate and extend our findings. In addition, further work is required to better define how retinal vessel development sensitivity to oxygen saturation varies with PMA.
Acknowledgments
This work was supported by the MRC, grant number G0400415, and by the Royal College of Surgeons Edinburgh.
Footnotes
Professor Fielder reports personal fees from Clarity Medical Systems (makers of RetCam), outside the submitted work. The remaining authors declare no conflict of interest.
References
- Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res 1997; 65(4): 555–568. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that 'physiological hypoxia' is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci 1995; 36(7): 1201–1214. [PubMed] [Google Scholar]
- Fleck BW, Stenson BJ. Retinopathy of prematurity and the oxygen conundrum: lessons learned from recent randomized trials. Clin Perinatol 2013; 40(2): 229–240. [DOI] [PubMed] [Google Scholar]
- Smith LE, Hard AL, Hellstrom A. The biology of retinopathy of prematurity: how knowledge of pathogenesis guides treatment. Clin Perinatol 2013; 40(2): 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, Niklasson A et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003; 112(5): 1016–1020. [DOI] [PubMed] [Google Scholar]
- Hellström A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, de Lacerda L et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab 2002; 87(7): 3413–3416. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA 2001; 98(10): 5804–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal CA, Fleck BW, Wright E, Graham C, McIntosh N. Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed 2009; 94(3): F193–F195. [DOI] [PubMed] [Google Scholar]
- Wikstrand MH, Hard AL, Niklasson A, Smith L, Löfqvist C, Hellström A. Maternal and neonatal factors associated with poor early weight gain and later retinopathy of prematurity. Acta Paediatr 2011; 100(12): 1528–1533. [DOI] [PubMed] [Google Scholar]
- Chen ML, Guo L, Smith LE, Dammann CE, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics 2010; 125(6): e1483–e1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow-Mordi WO, Darlow BA. Oxygen saturation and outcomes in preterm infants. N Engl J Med 2013; 368(22): 2094–2104. [DOI] [PubMed] [Google Scholar]
- Cross KW. Cost of preventing retrolental fibroplasia? Lancet 1973; 2(7835): 954–956. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1(8476): 307–310. [PubMed] [Google Scholar]
- Gallagher K, Moseley MJ, Tandon A, Watson MP, Cocker KD, Fielder AR. Nasotemporal asymmetry of retinopathy of prematurity. Arch Ophthalmol 2003; 121(11): 1563–1568. [DOI] [PubMed] [Google Scholar]
- Fielder AR, Shaw DE, Robinson J, Ng YK. Natural history of retinopathy of prematurity: a prospective study. Eye 1992; 6: 233–242. [DOI] [PubMed] [Google Scholar]
- Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol 1954; 38(7): 397–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res 1994; 36(6): 724–731. [DOI] [PubMed] [Google Scholar]
- Dhaliwal CA, Wade J, Gillespie T, Aspinall P, McIntosh N, Fleck BW. Early retinal blood vessel growth in normal and growth restricted rat pups raised in oxygen and room air. Br J Ophthalmol 2011; 95(11): 1592–1596. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994; 35(1): 101–111. [PubMed] [Google Scholar]
- McLoone E, O'Keefe M, Donoghue V, McLoone S, Horgan N, Lanigan B. RetCam image analysis of optic disc morphology in premature infants and its relation to ischaemic brain inury. Br J Ophthalmol 2006; 90: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]