Summary
Embryonic stem cell-derived mesenchymal stromal cells (MSCs; also known as mesenchymal stem cells) represent a promising source for bone regenerative medicine. Despite remarkable advances in stem cell biology, the molecular mechanism regulating differentiation of human embryonic stem cells (hESCs) into MSCs remains poorly understood. Here, we report that inhibition of IκB kinase (IKK)/nuclear factor kappa B (NF-κB) signaling enhances differentiation of hESCs into MSCs by expediting the loss of pluripotent markers and increasing the expression of MSC surface markers. In addition, a significantly higher quantity of MSCs was produced from hESCs with IKK/NF-κB suppression. These isolated MSCs displayed evident multipotency with capacity to terminally differentiate into osteoblasts, chondrocytes, and adipocytes in vitro and to form bone in vivo. Collectively, our data provide important insights into the role of NF-κB in mesenchymal lineage specification during hESC differentiation, suggesting that IKK inhibitors could be utilized as an adjuvant in generating MSCs for cell-mediated therapies.
Keywords: embryonic stem cells, MSCs, IKK, NF-κB, mesoderm
Graphical Abstract
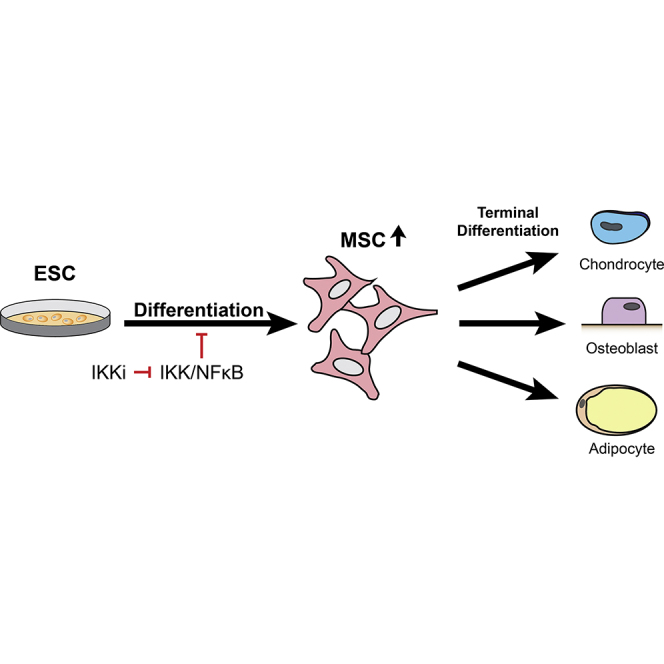
Highlights
-
•
NF-κB signaling inhibits differentiation of hESCs
-
•
Inhibition of NF-κB signaling enhances mesenchymal lineage specification of hESCs
-
•
Small-molecule IKK inhibitors help to enrich functional MSCs from hESCs
Wang, Hong, and colleagues show that inhibition of IκB kinase (IKK)/nuclear factor kappa B enhances differentiation of hESCs into MSCs by expediting the loss of pluripotent markers and enhancing mesenchymal differentiation. The small-molecule IKK inhibitor treatment significantly generates more MSCs from hESCs, which display multipotency with capacity to differentiate into osteoblasts, chondrocytes, and adipocytes in vitro and to form bone in vivo.
Introduction
Human mesenchymal stromal cells (MSCs; also known as mesenchymal stem cells) have gained considerable attention for their promising potential in cell-mediated therapy. MSCs are progenitor cells whose capacity to differentiate into osteoblasts and modulate the immune response makes them instrumental in bone regenerative medicine (Kode et al., 2009, Stappenbeck and Miyoshi, 2009, Alvarez et al., 2015, Deng et al., 2015). Clinical trials have already demonstrated that implantation of MSCs is an effective and safe treatment modality in defect repair (Bianco et al., 2013, Quarto et al., 2001, Wang et al., 2012). Although MSCs used in cell therapies are mostly isolated from adult bone marrow, proliferation and differentiation capacity of these MSCs from bone marrow (BMSCs) has been shown to decline as the donor patient ages (Kern et al., 2006, Quarto et al., 2001). Due to these shortcomings of BMSCs, human embryonic stem cells (hESCs), which have the potential to provide an unlimited supply of MSCs, are potential alternative sources for MSCs (Choo and Lim, 2011, Thomson, 1998). hESC-derived MSCs are similar to BMSCs biologically and functionally, but with higher osteogenic potential and proliferation rates as well as less immunogenicity (Li et al., 2013). Three methods have been developed to promote the differentiation of hESCs into MSCs: (1) the formation of three-dimensional embryonic bodies, (2) the culture of hESCs on stromal cells, and (3) the culture of hESCs as monolayers (Arpornmaeklong et al., 2009, Barberi et al., 2005, Villa-Diaz et al., 2012, Karp et al., 2006). However, the molecular mechanism by which hESCs commit to MSC fate remains elusive.
The nuclear factor kappa B (NF-κB) is known for its key role in the regulation of many cellular processes. The classical NF-κB is a heterodimer of p50 and p65/RelA proteins, in which the p65/RelA subunit has transactivation activity. The IκB kinase (IKK) complex mediates the degradation of IκBs to activate NF-κB (Krum et al., 2010). Recent studies have suggested that NF-κB may also play a role in stem cell self-renewal and differentiation (Armstrong et al., 2006, Dreesen and Brivanlou, 2007). The NF-κB subunits, including p65, p50, IκBα, and IκBβ, were found to be present throughout differentiation of hESCs (Yang et al., 2010). Although NF-κB signaling activity is low in hESCs, its inhibition leads to significant cell differentiation (Armstrong et al., 2006), suggesting that basal level of NF-κB activity is required for hESC identity. On the other hand, p65 overexpression caused loss of pluripotency and hESC differentiation (Luningschror et al., 2012). Moreover, it has been shown that Nanog maintains the pluripotency of mouse ESCs by binding to and suppressing the functions of NF-κB transcriptional activity (Torres and Watt, 2008). Given these seemingly contradictory reports, the precise regulatory functions of NF-κB in hESC differentiation require further investigation.
In this report, we investigated the effect of IKK/NF-κB signaling in regulating differentiation of hESCs into MSCs. Here, we demonstrated that inactivation of the IKK/NF-κB signaling pathway by the small-molecule IKK inhibitor (IKKi) and p65 knockdown promotes hESC differentiation into MSCs on monolayer culture. IKKi-treated hESCs generated a significantly increased MSC population and exhibited multipotency.
Results
Inhibition of NF-κB Signaling Promotes hESC Differentiation and Enhances MSC Marker Expression
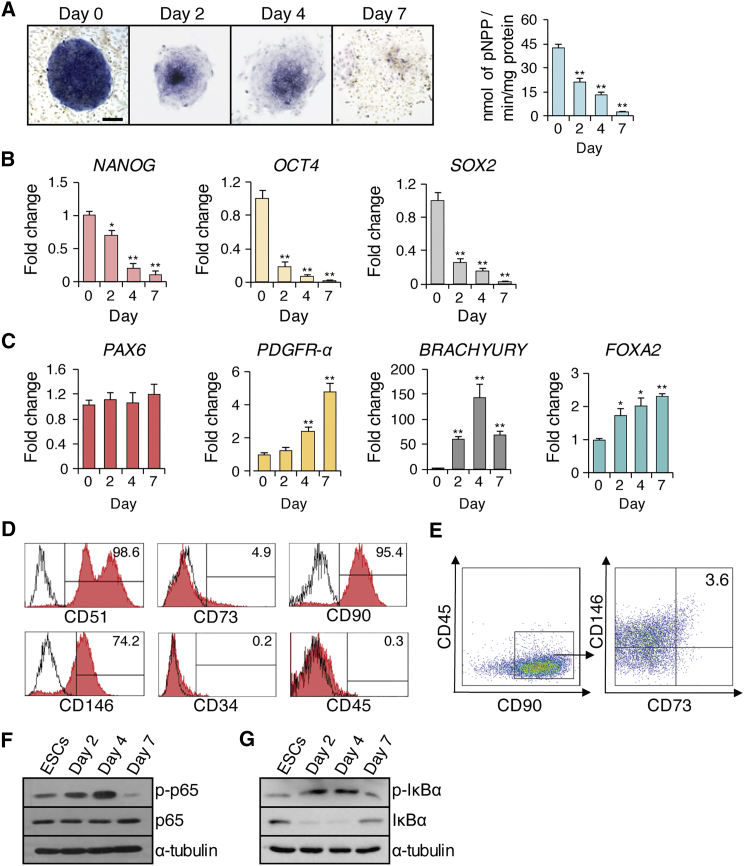
hESCs can be differentiated into MSCs when they are cultured in a monolayer without feeder cells (Karp et al., 2006). To induce this differentiation, we seeded the H1 hESC aggregates onto tissue culture dishes without feeder cells. We found that the alkaline phosphatase activity of hESCs decreased progressively and dramatically in the 7 days following culturing in a monolayer (Figure 1A). Real-time RT-PCR revealed that the expression of pluripotent markers, including NANOG, OCT4, and SOX2, gradually declined in parallel with alkaline phosphatase activity, indicating hESC differentiation (Figure 1B).
Figure 1.
Spontaneous Differentiation of hESCs without the Feeder Cell Layer
(A) Alkaline phosphatase staining and quantitative alkaline phosphatase activity assay at 0, 2, 4, and 7 days. Scale bar indicates 200 μm.
(B) qRT-PCR results of pluripotent markers (NANOG, OCT4, POU5F1) at 0, 2, 4, and 7 days.
(C) qRT-PCR results of an ectodermal gene (PAX6), mesodermal genes (PDGFR-α and BRACHYURY), and an endodermal gene (FOXA2) at 0, 2, 4, and 7 days.
(D) Flow cytometry analysis of cells expressing MSC surface markers.
(E) Four-color flow cytometry analysis for CD45, CD90, CD73, and CD146 expression was used to isolate CD73+CD90+CD146+CD45− MSCs.
(F) Western blot of p65 and phosphorylated p65 at 0, 2, 4, and 7 days of hESC differentiation.
(G) Western blot of IκBα and phosphorylated IκBα at 0, 2, 4, and 7 days of H1 hESC differentiation. Three independent experiments were performed.
∗p < 0.05, ∗∗p < 0.001.
We then examined the expression pattern of markers indicative of the three germ layers. While the ectodermal marker gene PAX6 was not induced, the endodermal marker gene FOXA2 and two mesodermal markers PDGFR-α and BRACHYURY were significantly elevated upon monolayer culture, indicating their preferential differentiation toward these two specific lineages (Figure 1C). Furthermore, when the MSC surface markers were assessed after 7 days of differentiation, more than 95% of cells were positive for CD51 and CD90, but negative for CD34 and CD45 (Figure 1D). However, CD146+ cells were 74.2% and CD73+ cells were relatively low at 4.9% (Figure 1D). Based on these results, we selected the combination markers of CD73+CD90+CD146+CD45− to isolate MSCs, excluding CD51 due to its high level of expression at 99% (Figure 1D). We were able to obtain 3.6% CD73+CD90+CD146+CD45− MSCs of the total differentiated cells from H1 hESCs (Figure 1E). Similarly, MSCs could also be generated from H9 hESCs (Figures S1A–S1E).
IKK directly phosphorylates the p65 transactivation domain on serine 536 (S536), a process that is correlated with IKK/NF-κB activity (Yang et al., 2003). To examine the status of the IKK/NF-κB signaling pathway, we screened for phosphorylated p65 during hESC differentiation. Consistent with previous studies (Foldes et al., 2010, Kang et al., 2007), phosphorylated status of p65 and IκBα was low, suggesting that NF-κB activity is present but low in H1 hESCs (Figures 1F and 1G). Interestingly, phosphorylation of p65 and IκBα increased progressively in the first 4 days of H1 or H9 hESC differentiation, but decreased to the basal level on day 7 (Figures 1F, 1G, and S1F). In contrast, the components of non-canonical NF-κB signaling, p100 and p52 (Bakkar et al., 2012), were minimally affected (Figure S1G).
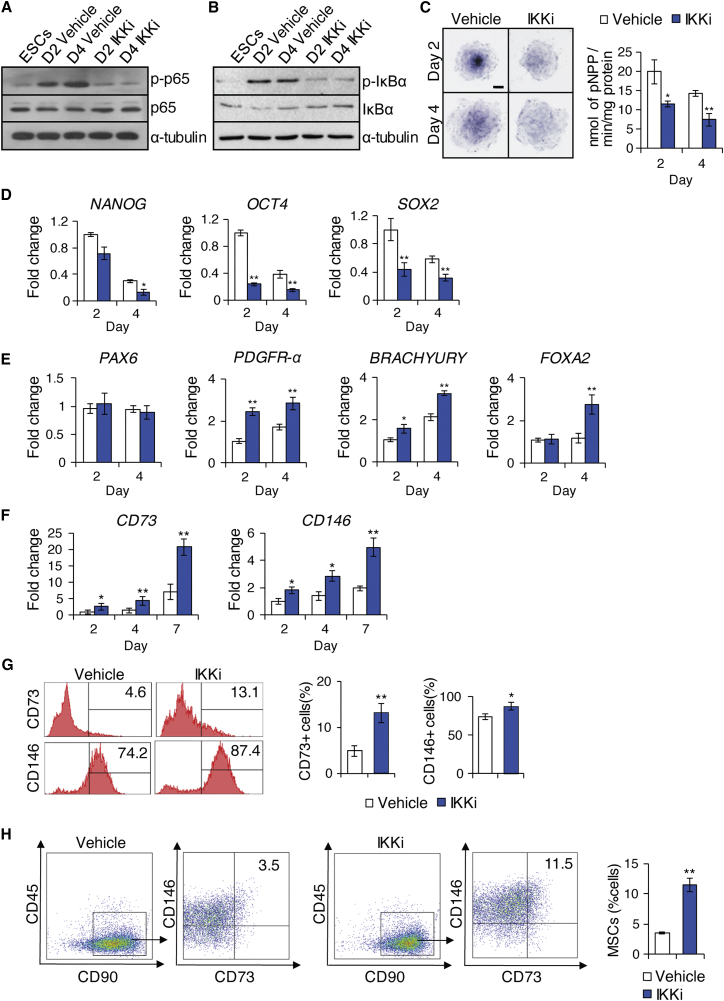
To determine whether IKK/NF-κB signaling plays a functional role in hESC differentiation into MSCs, we used IKKi to inhibit the IKKβ subunit of IKK complex, blocking IKK-mediated phosphorylation-induced proteasomal degradation of IκB, and thereby disabling activation of NF-κB (Baxter et al., 2004, Park et al., 2006). We treated the H1 hESC monolayer culture daily with IKKi (1 μM) from day 1 to day 4 during which IKK/NF-κB activity was found to be upregulated. This treatment resulted in significant inhibition of p65 and IκBα phosphorylation (Figures 2A and 2B), as well as reduction in alkaline phosphatase activity (Figure 2C) compared with vehicle control at days 2 and 4 of H1 hESC differentiation. Similarly, during the 7-day differentiation period, IKKi treatment consistently expedited the loss of pluripotency in H1 hESCs as determined by further suppressed mRNA expression levels of pluripotent markers, including NANOG, OCT4, and SOX2 (Figure 2D). Germ layer marker examination revealed that mesodermal markers PDGFR-α and BRACHYURY were found to be significantly upregulated as a result of treatment at days 2 and 4 of hESC differentiation (Figure 2E). The endodermal marker FOXA2 was also upregulated at day 4 (Figure 2E). In contrast, the ectodermal marker PAX6 gene expression remained unchanged (Figure 2E). MSC marker assessment showed that CD73 and CD146 were significantly upregulated following 4 days of IKKi treatment (Figure 2F); such upregulation was further confirmed by flow cytometry analysis (Figure 2G). IKKi treatment also generated a 3-fold increase in the proportion of CD73+CD90+CD146+CD45− MSCs in the total differentiated H1 hESC population, compared with vehicle control treatment (Figure 2H). Similarly, we found that IKKi treatment also significantly generated more MSCs from H9 hESCs (Figure S2).
Figure 2.
Effect of IKKi Treatment on Mesenchymal Lineage Specification of hESCs
(A) Western blot of p65 and phosphorylated p65 at 0, 2, and 4 days of hESC differentiation.
(B) Western blot of IκBα and phosphorylated IκBα at 0, 2, and 4 days of hESC differentiation.
(C) Alkaline phosphatase staining and quantitative alkaline phosphatase activity assay at 2 and 4 days. Scale bar indicates 200 μm.
(D) qRT-PCR results of NANOG, OCT4, and POU5F1 at 2 and 4 days.
(E) qRT-PCR results of PAX6, PDGFR-α, BRACHYURY, and FOXA2 at 2 and 4 days.
(F) qRT-PCR results of CD73 and CD146 at 2, 4, and 7 days.
(G) Flow cytometry analysis of cells with or without IKKi treatment examining MSC marker expression.
(H) Four-color flow cytometry analysis for CD45, CD90, CD73, and CD146 expression to isolate CD73+CD90+CD146+CD45− MSCs following 7 days of hESC differentiation with or without IKKi. Proportions of CD73+CD90+CD146+CD45− MSCs generated. Three independent experiments were performed.
∗p < 0.05, ∗∗p < 0.001.
IKKi-Treated Cells Exhibit Enhanced Osteogenic and Chondrogenic Potentials
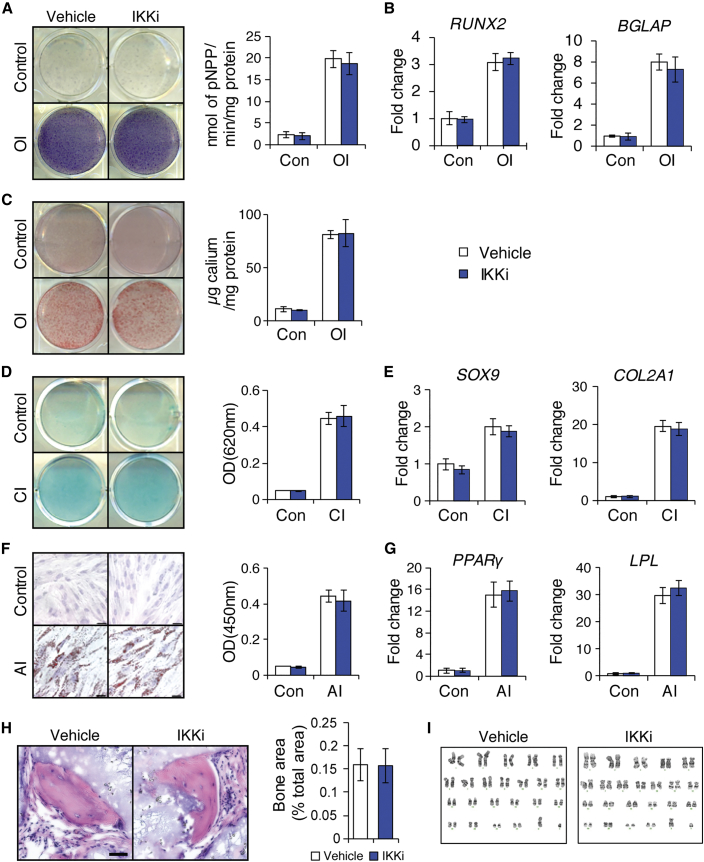
As IKKi treatment during hESC differentiation generated a larger CD73+CD90+CD146+CD45− MSC population, we examined whether inhibiting NF-κB signaling by IKKi enhances terminal differentiation capacity of differentiated hESCs. After H1 hESCs were grown in monolayer culture for 7 days with and without IKKi, these differentiated and unsorted hESCs were further induced to undergo osteogenic differentiation with osteogenic induction (OI) medium in the absence of IKKi for 7 days. As shown in Figure S3A, alkaline phosphatase activity was significantly elevated in IKKi-pretreated cells compared with control cells. In addition, real-time RT-PCR showed elevated expression levels of osteogenic markers, including RUNX2 and BGLAP (Figure S3B). Consistent with 7-day osteogenic induction results, IKKi-pretreated cells were able to form more mineralized nodules than control cells after prolonged treatment with OI medium for 14 days as demonstrated by Alizarin red staining (Figure S3C).
Furthermore, we evaluated and compared the chondrogenic capacity of differentiated H1 hESCs under chondrogenic conditions. IKKi-pretreated cells showed the presence of increased glycosaminoglycans as demonstrated by Alcian blue staining following prolonged treatment with chondrogenic induction medium for 21 days (Figure S3D). mRNA expression levels of chondrogenic marker genes including SOX9 and COL2A1 were also significantly upregulated (Figure S3E).
Inhibition of NF-κB Signaling by p65 Depletion Promotes hESC Differentiation and Enhances MSC Marker Expression
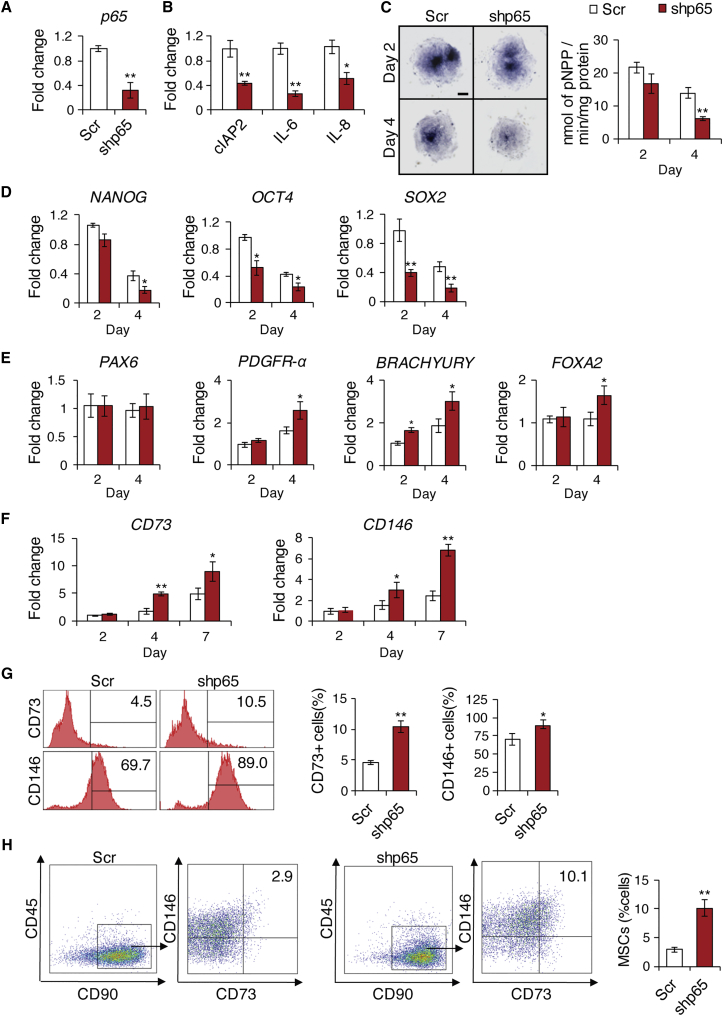
To further confirm that inhibition of NF-κB promotes hESC differentiation, we knocked down p65 in hESCs using lentiviruses expressing p65 small hairpin RNAs (shRNAs), targeting the 3′ UTR. The depletion of p65 in H1 hESCs was confirmed by real-time RT-PCR (Figure 3A) and by significantly suppressed expression of NF-κB target genes, including cIAP2, IL-6, and IL-8 (Figure 3B). Consistent with our IKKi treatment results, this p65 deficiency showed the decline of alkaline phosphatase activity at days 2 and 4 of the differentiation process (Figure 3C). Moreover, real-time RT-PCR revealed accelerated loss of the pluripotent markers NANOG, OCT4, and SOX2 during hESC differentiation (Figure 3D). As with IKKi treatment, the endodermal marker FOXA2 and two mesodermal markers PDGFR-α and BRACHYURY were found to be significantly elevated while the ectodermal marker PAX6 was unchanged (Figure 3E). The expression of CD73 and CD146 was also significantly upregulated in a time-dependent manner, with the most drastic differences at day 7 of hESC differentiation (Figure 3F). Flow cytometry analysis confirmed the increased expression of CD73 and CD146 (Figure 3G). Finally, similar to IKKi treatment, NF-κB inhibition by p65 depletion increased the proportion of CD73+CD90+CD146+CD45− MSCs by 3-fold (Figure 3H).
Figure 3.
Effect of p65 Knockdown on Differentiation and Mesenchymal Lineage Specification of hESCs
(A) qRT-PCR showing the deletion of p65 mRNA by shp65 in H1 hESCs.
(B) qRT-PCR results of NF-κB target genes (cIAP2, IL-6, IL-8) in Scr or shp65 transduced H1 hESCs at 4 days.
(C) Alkaline phosphatase staining and quantitative alkaline phosphatase activity assay for hESCs after transfection with Scr or shp65 lentivirus at 2 and 4 days. Scale bar indicates 200 μm.
(D) qRT-PCR results of NANOG, OCT4, and POU5F1 at 2 and 4 days.
(E) qRT-PCR results of PAX6, PDGFR-α, BRACHYURY, and FOXA2 at 2 and 4 days.
(F) qRT-PCR results of CD73 and CD146 at 2, 4, and 7 days.
(G) Flow cytometry analysis of cells expressing MSC markers.
(H) Four-color flow cytometry analysis for CD45, CD90, CD73, and CD146 to isolate CD73+CD90+CD146+CD45− MSCs following 7 days of H1 hESC differentiation. Three independent experiments were performed.
∗p < 0.05, ∗∗p < 0.001.
MSCs Derived from IKKi-Treated hESCs Maintain Multipotency In Vitro and Form Bone In Vivo
Since IKKi treatment generated significantly more MSCs from hESCs, it was important to determine whether these MSCs maintained multipotential properties and formed bone tissues in vivo. We sorted MSCs derived from IKKi-treated H1 hESCs and vehicle-treated hESCs and compared the terminal differentiation capacity to develop into osteoblasts, chondrocytes, and adipocytes in the absence of IKKi. Induced to undergo osteogenic differentiation, both groups displayed similar alkaline phosphatase activity and mineralization capacity (Figures 4A and 4C). Real-time RT-PCR also showed no significant differences in the expression of the osteogenic marker genes, RUNX2 and BGLAP (Figure 4B). Similarly, no significant changes were observed in chondrogenic potential, as determined by Alcian blue staining and expression of the chondrogenic marker genes, SOX9 and COL2A1 (Figures 4D and 4E). Induced to undergo adipogenic differentiation with adipogenic induction media for 21 days, oil red O staining revealed lipid deposit formation (Figure 4F) and the quantification and expression of adipogenic marker genes, PPAR-γ and LPL, showed no difference in adipogenic potential between the two groups (Figures 4F and 4G). Importantly, both populations were able to form bone in vivo (Figure 4H). To confirm ectopic bone regeneration, we examined the expression of human osteocalcin in these mineralized tissues. Osteocytes and lining osteoblasts were positively stained in both groups (Figure S4). To examine whether IKKi treatment might induce chromosomal abnormality at the genomic level, we performed G-banded chromosome studies of 20 mitoses. The results revealed that both groups exhibited a normal male karyotype with no gross abnormalities (Figure 4I).
Figure 4.
Characterization of Sorted MSCs Derived from IKKi-Treated hESCs
(A) Alkaline phosphatase staining and quantitative alkaline phosphatase activity assay after 14 days of osteogenic induction (OI).
(B) qRT-PCR results of osteogenic markers (RUNX2 and BGLAP) after 7 days of OI.
(C) ARS staining and quantification after 14 days of OI.
(D) Alcian blue staining and quantification after 21 days of chondrogenic induction.
(E) qRT-PCR results of chondrogenic markers (SOX9 and COL2a1) expression after 14 days of chondrogenic induction (CI).
(F) Oil red O staining and quantification after 21 days of adipogenic induction (AI). Scale bar indicates 200 μm.
(G) qRT-PCR results of adipogenic markers (PPAR-γ and LPL) expression after 14 days of AI.
(H) Bone formation in vivo by sorted MSCs derived from IKKi-treated H1 hESCs in six immunocompromised mice. Bar indicates 100 μm.
(I) Karyotype analysis of sorted MSCs derived from IKKi-treated H1 hESCs. For all in vitro experiments, three independent experiments were performed.
Discussion
For successful utilization of hESCs to generate MSCs for cell-mediated therapies, it is critical to develop a better understanding of the molecular mechanisms that govern their differentiation into specific progenitor cells. Here, we identified an important mechanism that facilitates differentiation of pluripotent hESCs into multipotent MSCs. By inhibiting the NF-κB signaling pathway, hESCs were differentiated more readily into MSCs, thereby enhancing lineage-specific terminal differentiation into osteoblasts and chondrocytes.
Recent studies have suggested that NF-κB signaling is involved in both maintenance and differentiation of ESCs (Dreesen and Brivanlou, 2007). While a low but detectable level of NF-κB activity was found in both mouse ESCs (mESCs) and hESCs (Torres and Watt, 2008, Yang et al., 2010), the functional role of NF-κB signaling was found to be contradictory. In mESCs, endogenous NF-κB activity and target gene expression was observed to have increased during the differentiation process. In addition, forced expression of the NF-κB subunit p65 caused mESC differentiation and loss of pluripotency (Luningschror et al., 2012). Conversely, NF-κB inhibition in mice increases expression of pluripotency markers (Dutta et al., 2011). miR-290, an ESC-specific microRNA cluster that targets p65, maintains pluripotency in mESCs by repressing NF-κB signaling (Luningschror et al., 2012). Similarly, Nanog’s binding to NF-κB inhibits the transcriptional activity of NF-κB and collaborates with Stat3 to promote self-renewal and impair differentiation (Torres and Watt, 2008). In contrast, it is reported that NF-κB inhibition in hESCs leads to hESC differentiation and loss of pluripotency (Armstrong et al., 2006). Using two different approaches for inhibiting NF-κB, our findings confirmed the notion that NF-κB inhibition promoted hESC differentiation. Importantly, we found that inhibition obtained significantly higher yields of MSCs. It was reported that there was a significant increase in expression of the NF-κB pathway components, p50 and phosphorylated form of p65, during embryonic body-mediated differentiation of hESCs grown in mouse embryonic feeder (MEF) layers (Yang et al., 2010). Similarly, here we demonstrated an increase in phosphorylated p65, indicative of heightened IKK/NF-κB activity, as hESCs differentiated in the monolayer culturing system and lost their pluripotency. It is noteworthy that such heightened activity was present only during the initial 4 days of differentiation and quickly returned to the basal level. When this transient upregulation of IKK/NF-κB signaling was inhibited, hESC differentiation was enhanced, suggesting that IKK/NF-κB activity may act as a compensatory mechanism to suppress its differentiation into progenitor cells during the early stages. Indeed, such a compensatory mechanism was also seen in previous studies. NF-κB activity is high during the development of bone but decreases in adult bone (Krum et al., 2010). Previously, we have found that the inhibition of NF-κB signaling promotes osteogenic differentiation of MSCs in vivo and in vitro (Chang et al., 2009, Chang et al., 2013). Altogether, our results suggest that NF-κB is a negative regulator of MSC differentiation from ESCs and bone formation.
It has previously been reported that differentiation of hESCs without the embryonic body stage led to an increase in the capacity of hESCs to differentiate into osteogenic progenitor cells (Karp et al., 2006). Consistent with these findings, our differentiation culture method resulted in cells with increased mesodermal and endodermal lineage marker gene expression, while the expression of the ectodermal lineage marker, PAX6, was unchanged. These results differ distinctly from embryonic bodies in which the inhibition of NF-κB promotes ectodermal lineage alone. Such discrepancies may stem from differences in culturing conditions including dosing. Yang et al. (2010) used a 20 μM dose of IKKi daily for 6 days, which is significantly higher than the concentration used in this study, 1 μM. This super-physiological concentration of IKKi may be responsible for the significant cell death and morphology changes that Yang et al. (2010) observed in hESCs. During our dosing optimization studies, we initially experienced massive cell death with higher doses, leading us to decide on a 1 μM dose. While IKKi at this low dose not only induces minimal cell death and proliferation impairment, it is sufficient to significantly suppress IKK activity, as demonstrated by a reduction in the S536 phosphorylated form of p65. As such, evaluating the role of NF-κB signaling by directly comparing different culturing conditions warrants closer examination.
In summary, we demonstrated that hESCs are a reliable resource to generate functional MSCs, making hESC-derived MSCs a promising alternative to BMSCs, which require invasive harvesting and show diminishing differentiation capacity with age. We also provide evidence that inhibition of NF-κB signaling promotes differentiation of hESCs into MSCs. By inhibiting IKK/NF-κB signaling, greater quantities of MSCs can be obtained more efficiently. Purified MSCs generated with IKKi treatment had the capacity for in vivo bone formation, offering tremendous benefits for bone tissue engineering. Therefore, the use of IKKi to inhibit NF-κB signaling to derive and enrich functional MSCs from hESCs may represent an efficient method that holds great promise for regenerative medicine in the future.
Experimental Procedures
Cell Culture
hESC-related work was approved by UCLA Embryonic Stem Cells Research Oversight Committee (IRB: 10-001711-CR-00001). H1 and H9 hESCs were obtained from UCLA Broad Stem Cell Research Center. hESCs (passages 35–45) were cultured on a mitotically inactivated MEF layer, as previously described (Thomson, 1998). To induce differentiation of hESCs, we detached hESC colonies using type IV collagenase (1 mg/ml), and hESC aggregates were plated into tissue culture dishes and grown in DMEM, containing 15% fetal bovine serum, 1% L-glutamine, 1% non-essential amino acid, and 1% penicillin-streptomycin for 1 day. Subsequently, IKKi (1 μM, Calbiochem) was added during ESC differentiation as indicated. After 7 days of differentiation, the derived cells were trypsinized to generate a single-cell suspension for further differentiation.
shRNA Knockdown
To knockdown p65 during differentiation of hESCs, we packaged and generated the lentiviruses expressing shRNA p65 or scramble shRNA (Scr) in 293T cells as described previously (Ramadoss et al., 2011). For viral infection, ESC aggregates were plated overnight and then infected with lentiviruses in the presence of polybrene (6 μg/ml) for 24 hr. After 3 days of infection, pools of colonies were selected with puromycin (Thermo Fisher Scientific) for 3 days at a concentration of 0.4 μg/ml. The target sequence for p65 shRNA was 5′-GTGACAAGGTGCAGAAAGA-3′.
All procedures were performed in accordance with the approved protocol by the University of California, Los Angeles (UCLA), and were oversight by UCLA Animal Research Committee (ARC). Detailed procedures are listed in Supplemental Experimental Procedures.
Author Contributions
D.P., C.Z., and R.A. performed the experiments and analyzed the data. C.H. and C.Y.W. designed experiments and wrote the manuscript.
Acknowledgments
This work was supported by the UCLA Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research Innovation Award and NIH/NIDCR grant R01DE016513. We thank Dr. Jinghua Tang for technical support on cell culture.
Published: March 10, 2016
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.02.006.
Contributor Information
Christine Hong, Email: chong@dentistry.ucla.edu.
Cun-Yu Wang, Email: cwang@dentistry.ucla.edu.
Supplemental Information
References
- Alvarez R., Lee H.L., Wang C.Y., Hong C. Characterization of the osteogenic potential of mesenchymal stem cells from human periodontal ligament based on cell surface markers. Int. J. Oral Sci. 2015;7:213–219. doi: 10.1038/ijos.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong L., Hughes O., Yung S., Hyslop L., Stewart R., Wappler I., Peters H., Walter T., Stojkovic P., Evans J. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- Arpornmaeklong P., Brown S.E., Wang Z., Krebsbach P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009;18:955–968. doi: 10.1089/scd.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N., Ladner K., Canan B.D., Liyanarachchi S., Bal N.C., Pant M., Periasamy M., Li Q., Janssen P.M., Guttridge D.C. IKKα and alternative NF-κB regulate PGC-1β to promote oxidative muscle metabolism. J. Cell Biol. 2012;196:497–511. doi: 10.1083/jcb.201108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi T., Willis L.M., Socci N.D., Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A., Brough S., Cooper A., Floettmann E., Foster S., Harding C., Kettle J., McInally T., Martin C., Mobbs M. Hit-to-lead studies: the discovery of potent, orally active, thiophene carboxamide IKK-2 inhibitors. Bioorg. Med. Chem. Lett. 2004;14:2817–2822. doi: 10.1016/j.bmcl.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Bianco P., Cao X., Frenett P.S., Mao J.J., Robey P.G., Simmons P.J., Wang C.Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Wang Z., Tang E., Fan Z., McCauley L., Franceschi R., Guan K., Krebsbach P.H., Wang C.Y. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Liu F., Lee M., Wu B., Ting K., Zara J.N., Soo C., Al Hezaimi K., Zou W., Chen X. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl. Acad. Sci. USA. 2013;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo A., Lim S.K. Derivation of mesenchymal stem cells from human embryonic stem cells. Methods Mol. Biol. 2011;690:175–182. doi: 10.1007/978-1-60761-962-8_12. [DOI] [PubMed] [Google Scholar]
- Deng P., Chen Q.M., Hong C., Wang C.Y. Histone methyltransferases and demethylases: regulators in balancing osteogenic and adipogenic differentiation of mesenchymal stem cells. Int. J. Oral Sci. 2015;7:197–204. doi: 10.1038/ijos.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen O., Brivanlou A.H. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- Dutta D., Ray S., Home P., Larson M., Wolfe M.W., Paul S. Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance. Stem Cells. 2011;4:618–628. doi: 10.1002/stem.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldes G., Liu A., Badiger R., Paul-Clark M., Moreno L., Lendvai Z., Wright J.S., Ali N.N., Harding S.E., Mitchell J.A. Innate immunity in human embryonic stem cells: comparison with adult human endothelial cells. PLoS One. 2010;5:e10501. doi: 10.1371/journal.pone.0010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.B., Kim Y.E., Kwon H.J., Sok D.E., Lee Y. Enhancement of NF-kappaB expression and activity upon differentiation of human embryonic stem cell line SNUhES3. Stem Cells Dev. 2007;16:615–623. doi: 10.1089/scd.2007.0014. [DOI] [PubMed] [Google Scholar]
- Karp J.M., Ferreira L.S., Khademhosseini A., Kwon A.H., Yeh J., Langer R.S. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kode J.A., Mukherjee S., Joglekar M.V., Hardikar A.A. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- Krum S.A., Chang J., Miranda-Carboni G., Wang C.-Y. Novel functions for NFκB: inhibition of bone formation. Nat. Rev. Rheumatol. 2010;6:607–611. doi: 10.1038/nrrheum.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li O., Tormin A., Sundberg B., Hyllner J., Le Blanc K., Scheding S. Human embryonic stem cell-derived mesenchymal stroma cells (hES-MSCs) engraft in vivo and support hematopoiesis without suppressing immune function: implications for off-the shelf ES-MSC therapies. PLoS One. 2013;8:e55319. doi: 10.1371/journal.pone.0055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luningschror P., Stocker B., Kaltschmidt B., Kaltschmidt C. miR-290 cluster modulates pluripotency by repressing canonical NF-kappaB signaling. Stem Cells. 2012;30:655–664. doi: 10.1002/stem.1033. [DOI] [PubMed] [Google Scholar]
- Park B.K., Zhang H., Zeng Q., Dai J., Keller E.T., Giordano T., Gu K., Shah V., Pei L., Zarbo R.J. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat. Med. 2006;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S.M., Mukhachev V., Lavroukov A., Kon E., Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- Ramadoss S., Li J., Ding X., Al Hezaimi K., Wang C.Y. Transducin beta-like protein 1 recruits nuclear factor kappaB to the target gene promoter for transcriptional activation. Mol. Cell. Biol. 2011;31:924–934. doi: 10.1128/MCB.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappenbeck T.S., Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- Thomson J.A. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Torres J., Watt F.M. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat. Cell Biol. 2008;10:194–201. doi: 10.1038/ncb1680. [DOI] [PubMed] [Google Scholar]
- Villa-Diaz L., Brown S., Liu Y., Ross A., Lahann J., Parent J., Krebsbach P. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Qu X., Zhao R.C. Clinical applications of mesenchymal stem cells. J Hematol. Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Tang E., Guan K., Wang C.-Y. IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- Yang C., Atkinson S.P., Vilella F., Lloret M., Armstrong L., Mann D.A., Lako M. Opposing putative roles for canonical and noncanonical NFkappaB signaling on the survival, proliferation, and differentiation potential of human embryonic stem cells. Stem Cells. 2010;28:1970–1980. doi: 10.1002/stem.528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.