Abstract
Obesity, insulin resistance and increased propensity for type 2 diabetes and cardiovascular disease result from an imbalance between energy intake and expenditure. The cloning of genes involved in energy homeostasis produced a simple feedback model for the homeostatic regulation of adipose mass. Serum leptin secreted from adipocytes signals nutrient sufficiency, curbing appetite and supporting energy expenditure. A rapid decline in leptin during nutrient scarcity instigates adaptive mechanisms, including increased appetite and reduced energy expenditure. Hypothalamic melanocortin neurons are important mediators of this response, integrating inputs of energy status from leptin with other peripheral signals. While this feedback response prolongs survival during fasting, other mechanisms allowing the prediction of nutrient availability also confer a selective advantage. This adaptation has been commonly studied in rodents using restricted feeding (RF) paradigms constraining food intake to limited periods at 24h intervals. RF rapidly elicits rhythmic bouts of activity and wakefulness anticipating food presentation. While the response exhibits features suggesting a clock-like mechanism, the neuromolecular mechanisms governing expression of food anticipatory behaviors are poorly understood. Here we discuss a model whereby melanocortin neurons regulating the homeostatic adaptation to variable caloric availability also regulate inputs into neural networks governing anticipatory rhythms in wakefulness, activity and metabolism.
Keywords: Melanocortins, Hypothalamus, Rhythms, Energy Homeostasis
Introduction
Energy homeostasis involves mechanisms that balance calorie intake with the energy requirements associated with growth, organ and tissue maintenance, and physical effort associated with nutrient acquisition 1. At the tissue level, it also involves regulating metabolite levels in key storage organs (e.g. triacylglycerol in adipose tissue, glycogen in liver) as well as in the blood (e.g. glycemia, lipidemia) 2, 3. A failure to rigorously maintain energy homeostasis with time can adversely affect health, leading to either to obesity 1 or cachexia 4. Of particular concern is the increased prevalence of the cluster of metabolic disorders commonly associated with obesity and insulin resistance, including type 2 diabetes and cardiovascular disease. Collectively, if unchecked the increased incidence of these disorders may negate the achievements in public health observed in the 20th century and reduce life expectancy in the 21st century 1, 5.
The increased occurrence of obesity and type 2 diabetes linked to abundant calories and reduced physical activity has motivated interest in determining the molecular mechanisms associated with energy homeostasis 1. That the hypothalamus has a critical role in maintaining energy homeostasis by controlling appetite and metabolism has been known for nearly a century 6. More recently, our understanding of the neuromolecular mechanisms involved in the hypothalamic regulation of energy homeostasis has been facilitated by studies describing the interactions between central nervous system (CNS) sites controlling behaviour and peripheral metabolism with signals received from peripheral organ systems 7–9. Neurons residing in the hypothalamus and brain stem receive a continuous flow of information about energy status from the gastrointestinal tract and peripheral organs, with the adipocytokine leptin having a predominant and non-redundant role as an indicator of energy status 10. In turn, the CNS rapidly responds to regulate ingestive behavior and the autonomic nervous system and neuroendocrine axes that exert a tight control on the metabolic activity of peripheral tissues to maintain energy homeostasis 11.
The ability to adapt to non-caloric environmental cues, including photoperiod (day length), temporal shifts in food availability and ambient temperature is also an important component of systems involved in energy homeostasis. Most life forms on Earth, including prokaryotes and plants, have evolved intrinsic clock mechanisms that synchronize their physiology with environment cues, allowing for adaptations anticipating changes in the environment 12, 13. Indeed, we are all subject to an internal clock mechanism that compartmentalizes the circadian day into periods dominated by sleep or motor activity that in the past was centered on foraging for food, or preparing food stores for periods of the annual cycle when food is less abundant. Many aspects of mammalian behavior and physiology including sleep/wake cycles, locomotor activity, blood pressure, body temperature, hormone secretions and metabolic pathways exhibit daily rhythmicity under the control of circadian clocks 14. Emerging evidence indicates that disturbances of circadian rhythms due to either mutations in clock genes 15 or lifestyle modifications 16 increases risk of developing metabolic disorders. Recent data also suggest a fundamental link between the cellular timekeeping mechanisms responsible for maintaining circadian rhythms with the regulation of essential metabolic processes including gluconeogenesis and lipogenesis 14, 17–22.
During periods of nutrient scarcity, the availability of food can become the dominant zeitgeber over riding the intrinsic tendency toward diurnal or nocturnal behavior 23. The location and mechanisms of this “food entrainable oscillator” (FEO) has been the subject of much debate and controversy 24, 25. At one level, the central nervous system must be involved, as the process involves the expression of complex behaviors including sleep cycles, the stimulation of appetite and motivation to seek food. The hypothalamus has been proposed to play an important function as a conduit for peripheral signals in this process 26, 27. On the other hand, every cell in the body exhibits the potential for autonomous regulation of clock activity by energy status, suggesting a distributed response that is independent of a “central” site of control 20, 21, 28.
Here we discuss our recent data suggesting that the melanocortin system is required for expression of rhythms that anticipate food presentation 29. Hypothalamic melanocortin neurons are devoted to nutrient sensing and the integration of many signals of energy homeostasis that, in turn, influences a distributed network in the CNS that affects satiety and peripheral metabolism 30. We posit that these neurons also coordinate feeding-related inputs into a distributed network that governs expression of rhythms in activity during period of restricted feeding.
Neuroanatomy and molecular composition of the mammalian biological clock
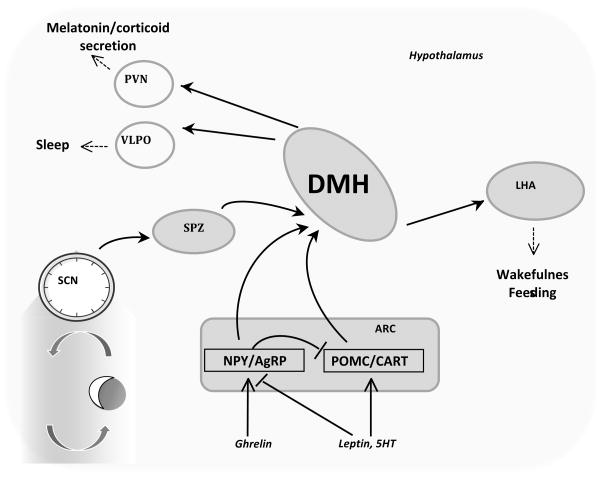
The intent here is not to provide an exhaustive review of the circadian literature; for this the reader is directed to recent reviews 14, 31, 32. Hypothalamic structures involved in maintaining circadian rhythms have been the subject of investigation for nearly 4 decades since the publication of Stephan and Zucker reporting the results from studies examining the effect of lesions in the suprachiasmatic area in rats 33. Overall, the hypothalamus acts as a relay center to process information not only from the environment but also from systemic inputs to affect the multiple systems controlling behavior and homeostasis (Fig. 1). The master clock responsible for maintaining a robust circadian rhythm in the absence of photic cues (i.e., constant dark) resides in the suprachiasmatic nucleus of the hypothalamus (SCN) 14. The SCN receives information concerning photoperiod from the retinohypothalamic tract, and sends projections to the dorsomedial hypothalamus (DMH) through the subparaventricular zone of the paraventricular nucleus (spPVNz) 26, 27. The DMH functions as a relay center, sending projections to areas involved in regulating neuroendocrine systems (the paraventricular nucleus), thermoregulation (medial preoptic area) and sleep cycles (the lateral hypothalamus, ventrolateral preoptic nucleus) 26, 27. The DMH may also be involved in the integration of photic cues with other signals, including signals of food intake (Fig. 1) 26, 34.
Figure 1. Neuroanatomic and neuroendocrine connections in the hypothalamus.
The SCN is the master clock, and sends information through the subparaventricular zone (SPZ) and dorsomedial nucleus of the hypothalamus (DMH). An unbiased survey of clock activity in rats subject to restricted suggests the induction of a rhythm in the DMH that persists in the absence of feeding79. Lesions studies also suggest that the DMH is important for expression of circadian rhythms 80,81. Projections from DMH extend to other regions of the hypothalamus such as the lateral hypothalamus (LHA), the ventrolateral preoptic nucleus (VLPO), and the paraventricular nucleus (PVN). These areas involved in sleep cycles (LHA, VLPO), neuroendocrine function (PVN) and feeding behavior (LHA). The arcuate nucleus (ARC) is a center involved in regulating feeding behavior. ARC neurons expressing the neuropeptides AgRP and neuropeptide Y (NPY) are orexigenic, while those expressing POMC (which is post translationally modified to produce α-MSH) and cocaine-and-amphetamine regulated transcript (CART) are anorectic. These neurons integrate many signals of energy status and food intake, regulating satiety and energy expenditure through Mc4r not shown in this diagram. NPY/AgRP and POMC/CART neurons may transmit signals of energy status into the DMH expressing Mc3r. Alternatively, neurons expressing Mc3r reside in other areas of the brain, such as the LHA and ventral tegmental area102, involved in complex behavioral processes. An involvement of these neurons in the expression of rhythms in arousal and food seeking behavior is also possible.
At a molecular level, a clock mechanism involves a core oscillator comprised of nuclear transcription factors and transcriptional repressors that establishes a rhythm of approximately 24h. Directly or indirectly, these transcription factors affect the activity of a large proportion of the transcriptome (~3000 genes) 35. Circadian oscillators are comprised of transcriptional activators and repressors assembled in an autoregulatory loop that generates rhythmic patterns of gene expression that varies by tissue type (Fig. 2). Brain and muscle ARNT-like protein 1 (Bmal1), circadian locomotor output cycles kaput (Clock) and neuronal PAS domain protein 2 (Npas2, a Clock homolog) are transcription factors that form a positive limb. Bmal1-Clock or Bmal1-Npas2 heterodimers bind to E-box enhancer elements within the promoters of the Period (Per) and Cryptochrome (Cry) genes to activate transcription. The reason for the existence of two heterodimers containing Bmal1 and either Clock or Npas2 is unclear at this time. However, the analysis of circadian rhythms in single and double Clock−/− Npas2−/− mutant mice suggests a partial redundancy of function 36. In the SCN, Npas2 can compensate for the absence of Clock to maintain a rhythm as assessed by measurement of wheel running activity, and measurement of activity of the Per2 promoter as a clock output 36, 37. However, Npas2 is unable to compensate for the loss of Clock in peripheral tissues, resulting in the loss of rhythm of Per2 reporter 37.
Figure 2. Molecular mechanisms of mammalian biological clock.
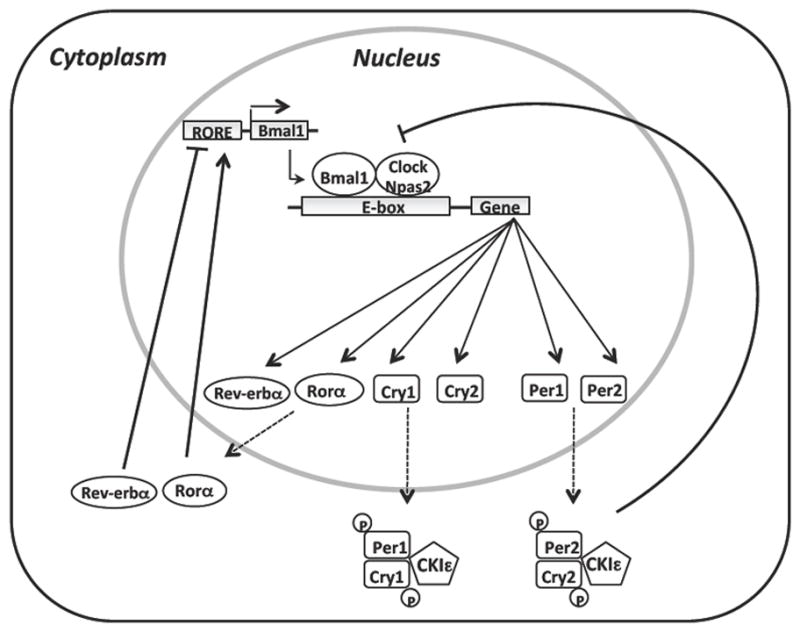
The mammalian circadian clock consists of a network of transcription-translation feedback loops that oscillates with a periodicity of 24h. This core oscillator includes different transcription factors such as Brain and muscle ARNT-like protein 1 (Bmal1), circadian locomoter output cycles kaput (Clock) and neuronal PAS domain protein 2 (Npas2). These transcription factors can heterodimerize and activate transcription of downstream targets such as Period genes (Per1, Per2) and Cryptochrome genes (Cry1, Cry2), Rorα and Rev-erbα, which contain an E-box enhancer elements within their promoters. Upon translation, Per and Cry proteins heterodimerize and translocate to the nucleus to inhibit the action of Bmal1-Npas2/Clock complex. Casein kinase ε (CKIε) protein regulates expression of Per and Cry proteins through phosphorylation. A secondary inhibitory feedback loop composed of Rev-erbα and Rorα drives the rhythmic transcription of Bmal1. Rev-erbα represses Bmal1 expression while Rorα activates its transcription.
The negative limb of the clock involves the Period (Per1, Per2) and Cryptochrome (Cry1, Cry2) proteins. As the levels of cytosolic Per and Cry proteins increase, they form heterodimers that translocate to the nucleus where they act to repress their own transcription through direct interaction with Bmal1-Clock or Bmal1-Npas2 heterodimers. In addition to regulation at the level of transcription, Per and Cry proteins are also regulated at the post-transcriptional level through phosphorylation by the casein kinase epsilon (CKIε). An additional feedback loop involves the nuclear receptors Rev-erbα and RORα 38. The transcription of Rev-erbα is activated by Bmal1-Clock or Bmal1-Npas2 heterodimers bind to E-box enhancer elements. Then Rev-erbα represses the transcription of Bmal1 through shared ROR binding elements (RORE) within the Bmal1 promoter. With the repression of Bmal1 transcription, the expression levels of Rev-erbα also collapses, thus removing an inhibitor of Bmal1 transcription and facilitating reinitiation of the cycle (Fig. 2).
Biological clocks and energy homeostasis
A role for the circadian clock in energy homeostasis has been suggested by several studies reporting that voluntary or genetic disruption of rhythms of behavior and physiology impacts health. Clinical studies have demonstrated that sleep restriction, shift work and night eating conditions increase risk for obesity, hypertension, cardiovascular disease and other components of the metabolic syndrome 39. Furthermore, some reports suggest that a loss of circadian rhythms of glucose metabolism may contribute to the development of type 2 diabetes 40, 41. Circadian rhythms in both insulin secretion and sensitivity are altered in patients with type 2 diabetes 42. Gene expression profiling studies have also identified a large number of transcripts (approximately 5–15% of the transcriptome) that exhibit rhythmic expression in different active metabolic organs such as liver, skeletal muscle, brown and white adipose tissue 43–45. Many of the identified genes are involved in different metabolic processes such as glucose and lipid metabolism, oxidative phosphorylation and detoxification pathways, suggesting again that clock system might have an essential role in the regulation of the major metabolic pathways.
Further evidence linking the circadian system with energy homeostasis is demonstrated from the analysis of mice with disrupted clock gene function 17, 46. Turek et al. reported that mice which are homozygous carriers of the ClockΔ19 mutation mice not only exhibited dampened diurnal rhythms in locomotor activity, but also an altered feeding rhythm with increased food intake during the day. These mice are hyperphagic, and exhibit a more severe diet-induced obese phenotype compared to wild type controls. The development of many characteristics of the metabolic syndrome, including hyperglycemia, hyperlipidemia, hyperleptinemia and hepatic steatosis is more severe in ClockΔ19 mutants 15. The involvement of the internal clock in regulating the rhythm of food intake was also demonstrated recently in a report examining Per2 knockout mice, which also exhibit abnormal circadian rhythms of food intake, particularly when fed a high fat diet 47. ClockΔ19 mutant mice also exhibit adipocyte hypertrophy and reduced expression of hypothalamic peptides associated with energy balance such as ghrelin, orexins and CART (cocaine and amphetamine regulated transcript).
Interestingly, an important metabolic role for peripheral clocks has also been reported in a context of normal feeding behavior and locomotor activity. Global Bmal1 knockout mice display abnormal feeding behavior and locomotor activity, altered daily oscillations in glucose and triglycerides plasma levels, reduced gluconeogenesis, reduced subcutaneous fat and decreased lifespan 48–50. The analysis of mice with specific hepatic disruption of the Bmal1 gene indicates that this transcription factor is crucial for circadian regulation of hepatic glucose regulatory genes and for systemic glucose homeostasis 50. Bmal1 also has an important role in adipocyte function, promoting adipocyte differentiation and lipogenesis in mature adipocyte 51, 52. There are other components of the core clock machinery that have been reported to have a crucial role in physiologic process. For example, Rev-erbα has been demonstrated as an important regulator of lipid and lipoprotein metabolism, adipogenesis, and vascular inflammation 38.
These studies have suggested a link between the systems that maintain circadian rhythms with energy homeostasis, and established a causal relationship between clock disorders and energy imbalance. However, the reciprocal relationship is also true, with mutations or challenges disrupting normal metabolic regulation affecting the activity of the circadian clock. For example, mice fed a high fat diet display altered circadian behavioral in feeding, locomotor activity, sleep/wake cycle as well as altered oscillations of clock gene expression in peripheral metabolic tissues 53, 54. Collectively, these studies suggest that clock genes regulate energy homeostasis either directly through the regulation of gene expression, or indirectly by influencing the timing of food intake (Fig. 3).
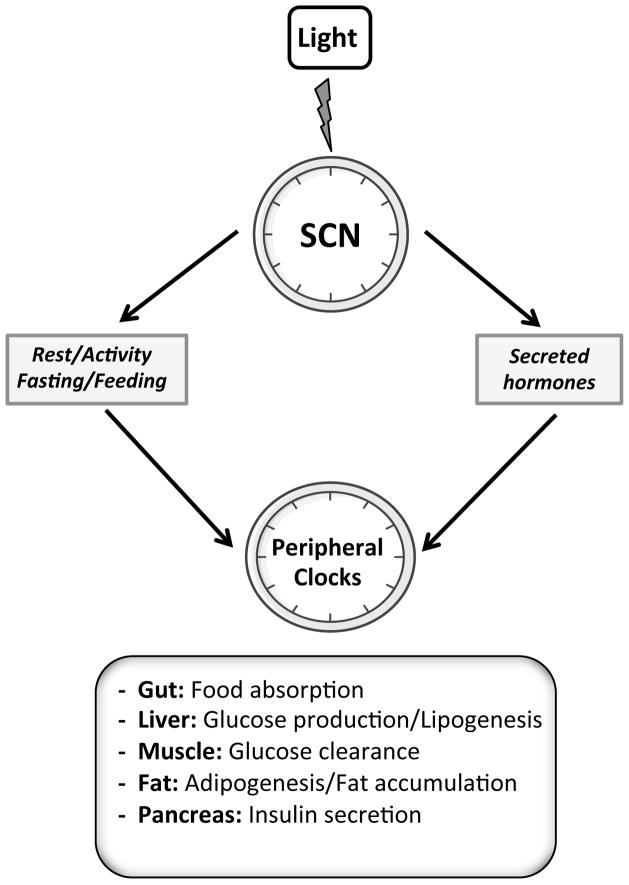
Figure 3. Entrainment of peripheral clocks by SCN.
The master pacemaker encoding the mammalian clocks is located in the suprachiasmatic nucleus of the hypothalamus (SCN). Clocks residing in the SCN are entrained by light signals transmitted through the retinohypothalamic tract. The synchronization of other oscillators throughout the body by the SCN is presumably indirect via behavioral rhythms (e.g., feeding, physical activity) or rhythmically secreted hormones.
How do clocks monitor calories?
The clock machinery is linked to energy metabolism pathways at multiple levels, and clock oscillators are regulated by factors that influence the supply of energy. In vitro studies have demonstrated that nutrient signals (glucose, fatty acids, and sterols) or circulating hormones (insulin, leptin, and glucocorticoids) are involved in the entrainment of clock gene expression 55–58. Moreover, the activity of clocks in tissues involved in energy metabolism, including the liver and adipose tissues, rapidly entrains to meal time independently of clocks in the SCN 59. These observations have led to efforts to understand how clocks in the periphery respond to caloric cues. The regulation of clock activity by calories may occur at two levels. The analysis of clock activity in cultured cells suggests autonomous regulation by altered energy status. At the level of the whole organism, hypothalamic structures residing outside the SCN may be involved in integrating inputs from signals of food intake and energy reserves with signals of photoperiod received from the retina.
Nuclear receptors that sense metabolites are a potential input by which energy metabolism can regulate clock activity autonomously 60. Nuclear receptors were initially perceived as acting as core transcriptional regulators that control major aspects of energy metabolism in response to activation by diverse intracellular metabolites including fatty acids, steroids or xenobiotic compounds 61. Interestingly, of the 45 known nuclear receptors 25 exhibit marked rhythms of expression 62. The dual role of nuclear receptors in coordinating energy metabolism and circadian clock has been illustrated by the studies on Rev-erbα and RORα. As previously described, Rev-erbα and RORα are components of the core clock circuitry that regulate Bmal1 expression. They are also important regulators of lipid metabolism, adipocyte differentiation and vascular inflammation 38, 63. Other nuclear receptors may also regulate clock activity. Recent studies have observed that transcription factors involved in regulating carbohydrate and lipid metabolism including Peroxisome proliferator activated receptor-alpha (Pparα), Pparγ coactivator 1 (Pgc1α) and Sirtuins regulate clock activity. Liu et al. reported that Pgc1α, a transcriptional coactivator that regulates adaptive energy metabolism, is essential not only for regulation of metabolic gene expression but also for normal clock function 19. Pgc1α null mice display both metabolic and circadian abnormalities including impaired diurnal rhythms of activity, body temperature and metabolic rate 19, 64. Pgc1α regulates transcription of Bmal1 and Rev-erbα through coactivation of the ROR receptors 19. Pgc1α is thus not only important for regulating metabolism and clock activity, but may also be a key component of the circadian oscillator that integrates the mammalian clock and energy metabolism 19.
Another nuclear receptor implicated in clock gene regulation is PPARα, which regulates lipid synthesis, storage and fatty acid oxidation 65, 66. PPARα has been reported to be involved in the adaptive response to feeding cues, and is required for adapting fatty acid metabolism during fasting 67. Rhythmic expression of PPARα has been observed in peripheral tissues such as liver, heart, kidney 43. PPARα has been shown to bind directly to the Bmal1 promoter by chromatin immunoprecipitation 68. Reciprocally, Bmal1/Clock heterodimers can regulate PPARα expression by binding to functional E-box element in PPARα promoter 68–70. Treatment of mice with fibrates, which are agonists of PPARα, increases expression of Bmal1 in the liver, an effect which is not observed in Pparα−/− mice 68.
Sirt1 is a NAD+ protein deacetylase linked to metabolism and aging 71, and has recently been also shown to interact directly with clock genes and to regulate their expression by inducing chromatin remodelling 18, 72. Sirt1 binds with Clock-Bmal1 heterodimers in a circadian manner and promotes the deacetylation and degradation of Per2 18. Genetic ablation of the Sirt1 gene or pharmacological inhibition of Sirt1 activity is associated with abnormal circadian rhythms of Bmal1 expression 72.
Clock oscillators can themselves act directly as sensors for feeding related signals and the cellular oxidative level. The transcriptional activity of Clock-Bmal1 heterodimers has been shown to be strongly influenced by the ratio of NAD+/NADH, which is closely tied to cellular energy metabolism 28. Indeed, Clock, Npas2, Bmal1 and Per2 oscillators all contain a PAS domain (PAS comes from the first letters of Per, Arnt and Sim), that can detect redox state, hypoglycaemia, oxygen balance, and xenobiotic metabolism. NADH enhances DNA binding of Clock-Bmal1 and Npas2-Bmal1 heterodimers, whereas NAD+ inhibits binding 28.
Several studies have reported that peripheral tissue oscillators can function independently, and even become uncoupled from the SCN in response to hormonal signals 55 or RF 59, 73. Restricting food intake to the day time results in a phase shift in the rhythmic expression of clock genes in the peripheral tissues of mice. Some studies indicate that the activity of the master clock in SCN remains locked to the light-dark cycle 59, 73, however RF when combined with calorie restriction does alter the circadian clock in the SCN 74. Interestingly, clock gene expression in the liver is rapidly entrained by RF within two days, prior to the expression of FAA 73. The ability of the RF to rapidly entrain peripheral oscillators indicates that food is a very potent synchronizer (or zeitgeber) for peripheral clocks. This nutritional regulation of clock genes in peripheral tissues may play a direct role in coordinating metabolic oscillations suggesting then that peripheral oscillators are directly responsive to cellular energy status 75.
Central nervous system and expression of anticipatory behavior
A growing body of literature has described in detail a mechanism for the regulation of clock activity by cellular energy status. However, it is also evident that the expressions of rhythms in activity involve areas of the central nervous system involved in complex behavior. At this time, it is not clear how the cellular response to altered energy status is translated to coordinate rhythms with nutrient availability. In addition, whether the adaptive response to restricted feeding is indeed a decentralized response, or is coordinated by a distinct group (or groups) or neurons in the CNS, is unclear. Certainly, the master clock required for maintaining rhythms resides within the hypothalamus. In mammals, lesioning and transplantation studies demonstrate that SCN is necessary for daily rhythms in locomotor activity and feeding 76–78. The SCN receives signals of light from the retina via the retinohypothalamic tract. Under conditions of continuous food availability, the SCN is thought to entrain clocks in peripheral tissues indirectly, either through behavioral rhythms (rest/activity or fasting/feeding cycles) 79 or through secreted hormones displaying a circadian rhythm 31 (Fig. 2). However, there is also evidence suggesting that areas of the hypothalamic outside the SCN are required for maintaining the expression of rhythms in behavior associated with restricted feeding. Moreover, the SCN is connected to regions of the hypothalamus thought to regulate feeding behavior and energy metabolism.
Destruction of specific hypothalamic areas using neurotrophic toxins has been instrumental in the identification of neuronal populations outside of the SCN critical for maintaining circadian rhythms of arousal, core temperature and locomotor activity under conditions of ad libitum feeding or during restricted feeding. The subparaventricular zone (SPZ) and dorsomedial nucleus of the hypothalamus (DMH) may be necessary for organizing circadian rhythms of body temperature, sleep/wake cycle, locomotor activity, feeding and corticoid production 34 (Fig. 1). Specifically, neurons within the dorsal and ventral SPZ are necessary for circadian rhythms of body temperature and sleep/waking, respectively 80. The DMH, a region involved in the regulation of food intake and satiety, has been also implicated in the ability of the organisms to express food anticipatory activity 81, 82. When food access is limited to defined periods outside of the normal feeding period, animals rapidly develop food seeking behavior and wakefulness that anticipate food presentation 83. It has been reported that this adaptive response is blocked in animals with cell-specific lesions in the DMH. Indeed, some reports have suggested that DMH lesions resulted in severe impairment of feeding, sleep/wake cycles, locomotor activity and corticoid secretion 84, 85. Projections from DMH to other regions of the brain include the ventrolateral preoptic nucleus, the paraventricular nucleus, and the lateral hypothalamus which regulate sleep, corticoid release, and wakefulness/feeding respectively (Fig. 1). The ventromedial hypothalamus (VMH) has also been hypothesized to be linked with the FEO 86, while VMH activation has been observed to be associated with increased arousal in anticipation of food presentation 87. As the DMH appears to be a key site for the expression of food-entrainable circadian rhythms, it has been suggested that this area may be a source of inputs in the food entrainable oscillator (FEO) and the expression of food anticipatory activity (FAA) 81.
However, controversy remains concerning the role of DMH. Landry et al. have reported that DMH-lesioned rats can still anticipate mealtime, and that this anticipation persists during total food deprivation 88. Two other reports have also suggested that the DMH is not required for the expression of food entrainment 89, 90. However, other studies have produced results suggesting that the DMH (and the VMH) are important for maintaining circadian rhythms and entrainment of food seeking behavior 82, 87. These hypothalamic nuclei are also crucial in the regulation of appetite control, energy expenditure, and metabolism, suggesting an interconnection between systems regulating metabolism and circadian rhythms 6. Due to the difficulty to identify a distinct anatomical location for the FEO, it has been suggested that it may be distributed or non-local system 24.
The melanocortin system integrates systemic signals of energy status to regulate energy homeostasis
While the hypothalamus has been proposed to contain sites where caloric cues are integrated with outputs from the SCN to regulate the expression of rhythms, the actual identity of these neurons is unknown. Neurons in the lateral hypothalamus expressing the neuropeptide orexin have an important role in regulating sleep state 26, and may also be involved in increasing wakefulness in anticipation of food presentation 91, 92. Hypothalamic melanocortin neurons are another well defined system involved in the regulation of energy homeostasis 9, 93. In the brain, the melanocortin system is composed of primary neurons expressing proopiomelanocortin (Pomc), agouti related peptide (AgRP), and secondary neurons expressing melanocortin receptors 9, 93. The activity of Pomc and AgRP is regulated by many peripheral signals of energy status, including leptin, insulin, glucose, cholecystokinin, PYY3-36, and ghrelin 30. Orexin may also regulate the activity of AgRP neurons, suggesting a connection with a system involved in regulating arousal. Recent studies using scheduled feeding protocols have also indicated that melanocortin neurons respond rapidly to meal ingestion 94, 95. Collectively, the published data support the hypothesis that Pomc neurons transmit an anorectic signal, while the output of AgRP neurons is primarily orexigenic, and that these neurons respond rapidly to signals of caloric intake and energy status.
Five melanocortin receptors have been cloned, and all are 7 transmembrane g-protein coupled receptors 9. Of these, the Mc3r and melanocortin-4 receptor (Mc4r) have been identified as important regulators of energy homeostasis. These receptors can be activated by α melanocyte-stimulating hormones (αMSH), a peptide derived from post-translational processing of POMC in the arcuate nucleus of the hypothalamus. Both Mc3r and Mc4r are expressed in brain areas known to be involved in regulating energy balance, including the hypothalamus. Disruption or blockade of Mc3r and Mc4r activity using central administered non-selective antagonists is associated with hyperphagia and weight gain 96–99, as well as altered lipid metabolism and nutrient partitioning in peripheral tissues 100, 101. The results from studies investigating the phenotype of Mc3r and Mc4r gene in knockout mouse models indicate that the acute regulation of satiety, energy expenditure and glucose disposal by melanocortins in mediated by the Mc4r, and not Mc3r 97–99, 102–105.
While the importance of the Mc4r in maintaining appropriate metabolic homeostasis has been intensively studied, the role of the Mc3r remains unclear. Mc3r knockout mice (Mc3r−/−) exhibit an obese phenotype characterized by modest increase in fat mass and reduced lean mass as well as increased metabolic efficiency and sensitivity to high fat diet 97, 98. The role of the Mc3r in the regulation of food intake is complex. Feeding studies with Mc3r−/− mice have reported varied results including no difference in food consumption, modest hypophagia, and even a modest hyperphagia during the lights-on phase 93, 98. On the B6 background, the measurement of food intake using an automated system suggests that Mc3r−/− mice may be mildly hyperphagic when first provided a purified high fat diet. This hyperphagia is primarily due to an increased food intake during the daytime, suggesting that Mc3r might function in the circadian regulation of ingestive behaviour 93. Further evidence of reduced amplitude of night-time (peak) versus day-time (nadir) feeding was also observed in a separate cohort of mice fed a purified low fat diet 29. Interestingly, dense expression of Mc3r mRNA has been observed in VMH and DMH 106. As previously mentioned, these are two hypothalamic sites are involved in regulating food intake and that may also function to maintain circadian rhythms 81, 82, 86.
MC3R are required for entrainment to restricted feeding
During our interrogation of the phenotype of Mc3r−/− mice, we developed an interest in how organisms adapt anticipatory rhythms to nutrient intake. The provision of a limited amount of food on a recurring basis rapidly elicits food anticipatory behavior, a phenomenon that displays features suggesting the involvement of a clock or hour glass mechanism 23. Based on observations from studies examining daily rhythms in rats with various hypothalamic lesions 82, 84, 86, we developed the hypothesis that the melanocortin system acts through the melanocortin-3 receptor (Mc3r) to link signals of energy status with system expressing rhythms of food anticipatory behavior 29. The original unpublished studies used Mc3r knockout mice (B6.129S4-Mc3rtm1Cone/J) on the original out bred C57BL/6J (B6) and 129/Sv backgrounds developed in Dr Roger Cone’s laboratory at the Vollum Institute in Portland, Oregon 98. More recently, studies have used mice back crossed >10 generations onto the B6 background 29.
Recent studies in our laboratory suggest that Mc3r may regulate behaviour via effects on circadian rhythms in the brain 29. Our findings indicate that Mc3r are involved in the expression of adaptive entrainment of behaviour in response to feeding cues 29. By using scheduled feeding protocols associated with a caloric restriction, we have demonstrated that the Mc3r gene is necessary for the coordinated development of anticipatory activity and increased wakefulness associated with restricted feeding. Moreover, analysis of cortical gene expression revealed severe abnormalities in rhythmic expression of clock genes such as Bmal1, Npas2 and Per2 in Mc3r −/− at both RF and ad libitum feeding. These observations suggest that Mc3r containing neurons may regulate inputs maintaining proper cyclic oscillation of clock genes in the forebrain. This alteration in oscillation in Bmal1, Npas2 and Per2 gene expression could play a role in the impaired entrainment of Mc3r−/− mice to RF.
Studies have reported altered arousal rhythms in mice with mutated Bmal1/Mop3 gene expression 107. Moreover, delayed expression or loss of FAA has been also reported in animals with Npas2 or Per2 disruption 108, 109. However, a recent study has raised questions about the interpretation of those results, reporting that clock mutant mice with total disruption of Bmal1 or Per1/Per2 still exhibit normal FAA both in a light dark cycle and in a constant darkness conditions 90. Involvement of the known clock genes in daily rhythms of FAA is controversial as reports are conflicting 85, 109. In a recent study, Storch et al. emphasized the importance of the proper evaluation of the FAA. In particular, they have demonstrated that reduced FAA observed in Bmal1 knockout mice during an abrupt shift from constant food availability to restricted food availability is secondary to a lethargic and moribund state 90. Studies examining the response of mutants to restricted feeding thus require a careful assessment of the health and metabolic status of the animal.
Decreased FAA in Mc3r−/− mice is likely not a consequence of any disability, as we have not observed increased mortality in Mc3r−/− mice subject to restricted feeding. Moreover, the assessment of spontaneous locomotory activity and energy expenditure in Mc3r−/− mice and wild type animals is not consistent with illness-like behaviour in the former 29. It is possible that Mc3r are involved in transmitting information regarding energy status into the DMH (Fig. 1). However, Mc3r mRNA is expressed in nearly 30 areas in the central nervous system that may also be involved in expression of complex behaviours 106. More precise studies that will clearly define the role of Mc3r in anticipatory and reward-seeking behaviour are warranted. This unique function of the Mc3r to signal calorie intake to clocks governing circadian rhythms in arousal, food seeking behaviour and metabolism also suggests a novel output of the Mc3r for regulating energy homeostasis. It will also be of interest to determine whether Mc3r in the central nervous system synchronize the activity of peripheral clocks, which have a critical role in maintaining energy homeostasis, with food intake.
Conclusions
Biological clocks promote the synchronization of behavioural and physiologic processes with environmental cues, and facilitate the expression of rhythms that anticipate changes in the environment. During the past decade, significant advances have been made in determining how the circadian clocks function, and have contributed to a better understanding how circadian rhythms are involved in energy homeostasis. Clocks integrate cues from environment and systemic signals of energy status to regulate diverse cellular and physiological function. The integration of the circadian clock and energy metabolism is controlled by biological signals at multiple levels. Our results imply that Mc3r could be important mediators in transmitting feeding related inputs into neurons governing the development of anticipatory activity especially during periods of nutrient scarcity.
It is also important to acknowledge the significant controversy associated with this field of research, and in the interpretation of data 24, 25. The results from recent studies suggesting a critical role for the known clocks in the expression of food entrainable rhythms 85, 108, 109 have been questioned by other experiments suggesting an alternative interpretation of food anticipatory activity 25, 90. Significant questions concerning the restricted feeding protocol have been raised, leading to a re-interpretation of published data. It also appears likely that an abrupt phase shift to limited nutrient availability is too severe for clock mutants to adapt too, and that the failure to express behavioral rhythms is due to metabolic distress. The known clock genes may thus not be required specifically for the expression of rhythms, but may have a critical role in metabolic adaptation. In this context, the Mc3r knockout mouse may provide a unique genetic model for investigating mechanisms responsible for the expression of rhythms in food anticipatory activity.
Acknowledgments
This work is been supported by a Grant from the National Institutes of Health (DK073189) to AAB. AAB and GMS also acknowledge financial support provided by the Pennington Biomedical Research Foundation and the American Diabetes Association.
References
- 1.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 2.Caspi L, Wang PY, Lam TK. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 2007;6:99–104. doi: 10.1016/j.cmet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher AL, Marks DL. Central mechanisms controlling appetite and food intake in a cancer setting: an update. Curr Opin Support Palliat Care. 2007;1:306–11. doi: 10.1097/SPC.0b013e3282f14c4e. [DOI] [PubMed] [Google Scholar]
- 5.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 6.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 8.Coppari R, Ramadori G, Elmquist JK. The role of transcriptional regulators in central control of appetite and body weight. Nat Clin Pract Endocrinol Metab. 2009;5:160–6. doi: 10.1038/ncpendmet1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–49. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 10.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–90S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 12.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–3. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 13.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–42. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–5. doi: 10.1038/nm0106-54. discussion 55. [DOI] [PubMed] [Google Scholar]
- 18.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–28. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–81. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–4. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–7. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41:81–6. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 24.Davidson AJ. Search for the feeding-entrainable circadian oscillator: a complex proposition. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1524–6. doi: 10.1152/ajpregu.00073.2006. [DOI] [PubMed] [Google Scholar]
- 25.Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, Pevet P, Shibata S. Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms. 2009;7:3. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–52. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- 27.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 28.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–4. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 29.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–55. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 31.Kornmann B, Schaad O, Reinke H, Saini C, Schibler U. Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb Symp Quant Biol. 2007;72:319–30. doi: 10.1101/sqb.2007.72.041. [DOI] [PubMed] [Google Scholar]
- 32.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 33.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–7. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–5. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007;17:R538–9. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 38.Duez H, Staels B. Rev-erb alpha gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 39.Ramsey KM, Bass J. Obeying the clock yields benefits for metabolism. Proc Natl Acad Sci U S A. 2009;106:4069–70. doi: 10.1073/pnas.0901304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 41.Prasai MJ, George JT, Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5:89–95. doi: 10.3132/dvdr.2008.015. [DOI] [PubMed] [Google Scholar]
- 42.Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–8. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 43.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 44.Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006;2:e16. doi: 10.1371/journal.pcbi.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatfield D, Schibler U. Circadian glucose homeostasis requires compensatory interference between brain and liver clocks. Proc Natl Acad Sci U S A. 2008;105:14753–4. doi: 10.1073/pnas.0807861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–60. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–6. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zvonic S, Floyd ZE, Mynatt RL, Gimble JM. Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis. Obesity (Silver Spring) 2007;15:539–43. doi: 10.1038/oby.2007.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav. 2006;87:255–62. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 56.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002;277:44244–51. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- 57.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–15. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 58.Froy O, Miskin R. The interrelations among feeding, circadian rhythms and ageing. Prog Neurobiol. 2007;82:142–50. doi: 10.1016/j.pneurobio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr. 2007;27:219–40. doi: 10.1146/annurev.nutr.27.061406.093546. [DOI] [PubMed] [Google Scholar]
- 61.McEwan IJ. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. doi: 10.1007/978-1-60327-575-0_1. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–10. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 63.Lin JD, Liu C, Li S. Integration of energy metabolism and the mammalian clock. Cell Cycle. 2008;7:453–7. doi: 10.4161/cc.7.4.5442. [DOI] [PubMed] [Google Scholar]
- 64.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 66.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–98. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–27. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- 69.Inoue I, Shinoda Y, Ikeda M, Hayashi K, Kanazawa K, Nomura M, Matsunaga T, Xu H, Kawai S, Awata T, Komoda T, Katayama S. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12:169–74. doi: 10.5551/jat.12.169. [DOI] [PubMed] [Google Scholar]
- 70.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J. 2005;386:575–81. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 74.Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem. 2003;384:711–9. doi: 10.1515/BC.2003.079. [DOI] [PubMed] [Google Scholar]
- 75.Bechtold DA. Energy-responsive timekeeping. J Genet. 2008;87:447–58. doi: 10.1007/s12041-008-0067-6. [DOI] [PubMed] [Google Scholar]
- 76.Welsh D, Richardson GS, Dement WC. Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol Behav. 1988;43:771–77. doi: 10.1016/0031-9384(88)90375-7. [DOI] [PubMed] [Google Scholar]
- 77.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 78.Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci. 2006;26:6406–12. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 80.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–74. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–5. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 83.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–95. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 84.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–7. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci. 1998;18:3843–52. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci U S A. 2007;104:20078–83. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–78. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- 89.Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29:1447–60. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- 90.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–13. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Shiromani PJ. Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain Res. 2008;1205:47–54. doi: 10.1016/j.brainres.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–90. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–21. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Singru PS, Sanchez E, Fekete C, Lechan RM. Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology. 2007;148:638–46. doi: 10.1210/en.2006-1233. [DOI] [PubMed] [Google Scholar]
- 96.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 97.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 98.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 99.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–11. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 100.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–88. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148:6186–94. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 102.Sutton GM, Josephine Babin M, Gu X, Hruby VJ, Butler AA. A derivative of the melanocortin receptor antagonist SHU9119 (PG932) increases food intake when administered peripherally. Peptides. 2008;29:104–11. doi: 10.1016/j.peptides.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–49. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 106.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 108.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–83. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 109.Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol. 2006;16:2016–22. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]