Abstract
Translesion synthesis by specialized DNA polymerases is an important strategy for mitigating DNA damage that cannot be otherwise repaired either due to the chemical nature of the lesion. Apurinic/Apyrimidinic (abasic, AP) sites represent a block to both transcription and replication, and are normally repaired by the base excision repair (BER) pathway. However, when the number of abasic sites exceeds BER capacity, mitochondrial DNA is targeted for degradation. Here, we used two uracil-N-glycosylase (UNG1) mutants, Y147A or N204D, to generate AP sites directly in the mtDNA of NIH3T3 cells in vivo at sites normally occupied by T or C residues, respectively, and to study repair of these lesions in their native context. We conclude that mitochondrial DNA polymerase γ (Pol γ) is capable of translesion synthesis across AP sites in mitochondria of the NIH3T3 cells, and obeys the A-rule. However, in our system, base excision repair (BER) and mtDNA degradation occur more frequently than translesion bypass of AP sites.
Keywords: Abasic sites, Base Excision Repair, DNA polymerase gamma, mitochondrial DNA damage, translesional synthesis
Introduction
In mammalian cells, genetic information is stored in two locations: in the nucleus and in mitochondria. Nuclear DNA (nDNA) is organized into chromosomes of which two sets are present per cell: one paternal and another maternal. In contrast, mitochondrial DNA (mtDNA) inheritance in animals is (with a few exceptions) exclusively maternal, and this DNA species is highly redundant, with typically a few hundred to a few thousand copies per cell. Human mtDNA is a 16 568 bp circular molecule crucial for proper mitochondrial function and cellular ATP production. It encodes 13 protein components of the mitochondrial oxidative phosphorylation (OXPHOS) system (Anderson et al., 1981). These polypeptides are encoded using a genetic code distinct from that used in the nucleus, and, therefore, they require a separate translational apparatus, some components of which (22 tRNAs and two rRNAs) also are encoded in mtDNA (Herrmann et al., 2012). In many (but not all, Noll et al., 1990) cell types, the bulk of ATP is produced by OXPHOS in mitochondria. Since mtDNA encodes components for four out of five mitochondrial respiratory complexes, it is not surprising that mutations in mtDNA have been associated with various human pathologies, such as mitochondrial diseases (Schapira, 2012; Ylikallio & Suomalainen, 2012; Zheng et al., 2012), diabetes (Bannwarth et al., 2011; Maassen et al., 2005; Supale et al., 2012), cancer (Wallace, 2012; Yu, 2012), neurodegenerative disorders (Milone, 2012), and many others. Therefore, understanding cellular mechanisms for the maintenance of mtDNA integrity is of utmost importance as it can provide targets for clinical interventions aimed at the prevention and treatment of human diseases.
Genomic DNA is under continuous assault by endogenous and environmental insults that may result in base loss and formation of non-instructional apurinic/apyrimidinic (AP) sites. AP sites can be generated through spontaneous or enzymatic hydrolysis of the N-glycosyl bond between the deoxyribose and base. The rate of spontaneous depurination has been estimated to be ~10 000/cell/day (Lindahl & Nyberg, 1972; Nakamura et al., 1998). In addition, AP sites can be generated as intermediates during the base excision repair of oxidized and alkylated bases. Unrepaired AP sites represent non-instructional lesions that are cytotoxic and mutagenic, and can block DNA synthesis (Gentil et al., 1990; Guillet & Boiteux, 2002; Loeb & Preston, 1986; Yu et al., 2003). When Pol encounters an AP site in vitro, it can bypass this lesion by inserting a nucleotide. The identity of the base added and, therefore, mutagenic specificity can depend on the polymerase and on auxiliary proteins (Strauss, 2002). Thus, it was established that Escherichia coli Pol I and V preferentially insert A opposite the AP sites in vitro (Boiteux & Laval, 1982; Kunkel et al., 1983; Tang et al., 1999) and in vivo (Schaaper et al., 1983). Similarly, purified calf thymus Pol α (Shibutani et al., 1997) and human Pol β in the presence of Pol ε (Villani et al., 2011) also insert an A opposite the AP site. In contrast, under similar conditions, Pol λ induces single or double deletions (Villani et al., 2011), whereas Pol ι has a slight preference for inserting G (Nair et al., 2009). In vivo, yeast cells repair an AP site in plasmid DNA by inserting a C (Gibbs & Lawrence, 1995), whereas mammalian cells incorporate nucleotides in the order of preference G>A>T>C (Neto et al., 1992). Overall, however, most DNA polymerases of both pro- and eukaryotic origin most commonly insert an A opposite the AP site. This preferential insertion has become known as the A-rule (Strauss, 2002).
While in the mammalian nucleus as many as 12 (Friedberg et al., 2000) different DNA polymerases may participate in the repair of AP sites, mitochondria contain only one replicative DNA polymerase, Pol γ, which is responsible for both DNA replication and repair (Copeland & Longley, 2003). Recently, a second DNA polymerase, PRIMPOL, has been described in mitochondria (Garcia-Gomez et al., 2013). This polymerase is capable of synthesizing primers using either nucleoside triphosphates (NTPs) or deoxynucleoside triphosphates (dNTPs), conferring the ability to re-prime and restart replication downstream of DNA lesions (Guilliam et al., 2015). However, in vitro PRIMPOL appears to skip an AP site thus generating a deletion (Garcia-Gomez et al., 2013). In vitro, Xenopus laevis and human mitochondrial Pol γ obey the A-rule (Liu et al., 2008; Pinz et al., 1995). However, neither the efficiency of translesion synthesis across AP sites by Pol γ, nor the identity of the inserted base has been determined in vivo. Here, we report an implementation of a system for inducible generation of AP sites in mtDNA in vivo and describe its application for studying the efficiency of translesion synthesis through AP sites by Pol γ.
Methods
Cells and DNA constructs
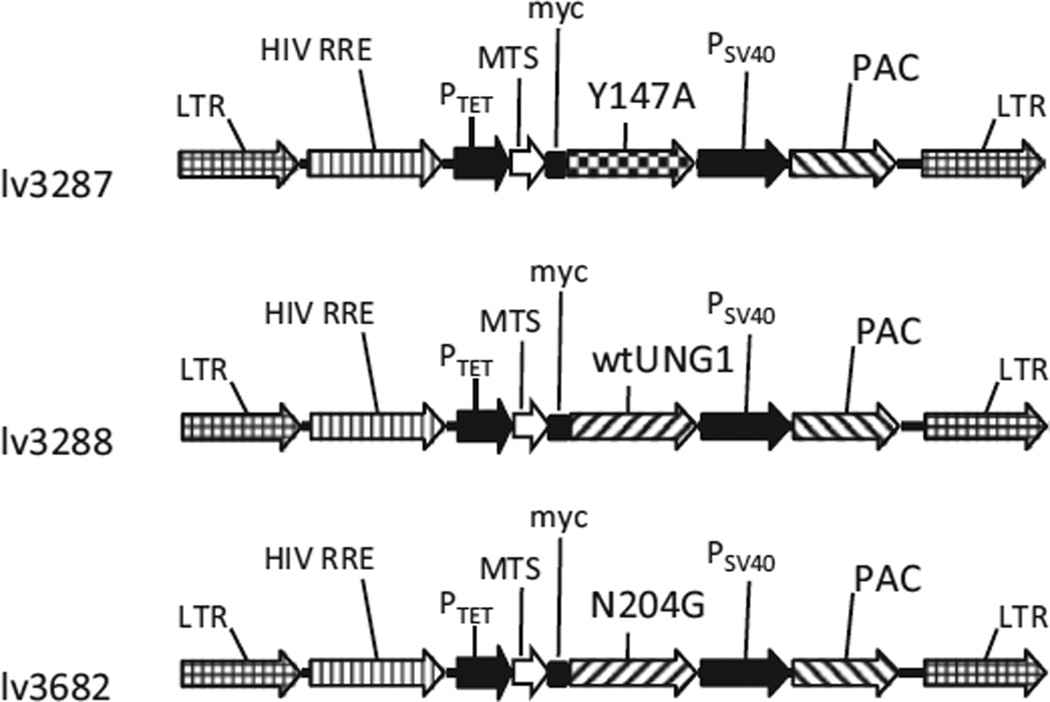
3T3 cells and their derivatives were propagated in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum, 50 µg/ml gentamycin, 50 µg/ml uridine, and 1 mM sodium pyruvate in a humidified atmosphere containing 5% CO2 at 37 °C. For inducible expression, 3T3 cells were modified by introducing a Tet-On advanced transactivator with retrovirus rv2641. The constructs for inducible expression of the wild type (WT) and the Y147A mutant UNG1 were described previously (lv3288 and lv3277, Addgene plasmids # 46885 and #46883, respectively) (Shokolenko et al., 2013). The N204D mutation (Kavli et al., 1996) was introduced into UNG1 by overlap extension PCR (Ho et al., 1989) using primers UNG1N204Df (GGTGTTCTCCTTC TCGACGCTGTCCTCACG) and UNG1N204Dr (CGTGAGGAC AGCGTCGAGAAGGAGAACACC). The N204D mutant was modified as follows: the native matrix targeting sequence (MTS) of UNG1 was removed and replaced with a combination of MTS of human ornithine transcarbamylase (OTC) and a myc-tag. For inducible lentiviral expression this construct was inserted into pMA2780 (Addgene plasmid #25438) thus creating pMA3682 (Figure 1).
Figure 1.
Vector maps. HIV RRE, human immunodeficiency virus rev response element; LTR, lentiviral long terminal repeat; MTS, mitochondrial matrix targeting sequence of human ornithine transcarbamylase; the Y147A, mutant UNG1 gene; N204D, mutant UNG1 gene; wtUNG1, wild type UNG1 gene; myc, myc tag epitope; PAC, puromycin resistance gene; PSV40, SV40 promoter; PTet, doxycycline-regulated promoter; wtUNG1, wild type human UNG1 gene; wPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
Production of lentiviral supernatants and infection of target cells
Lentivirus-containing supernatants were produced by CaPO4-mediated transfection of the HEK293FT cell line, using established protocols (Zufferey et al., 1997). Gag, Pol, and Env functions for lentiviral constructs were provided in trans by cotransfecting the vector plasmid with two helper plasmids, psPAX2 and pMD2.G (Addgene). Target cells were infected with lentiviruses in 35-mm dishes at 30% confluence by incubating them overnight with corresponding supernatant in the presence of 10 µg/mL polybrene (Sigma-Aldrich Corp., St. Louis, MO). The next day, the supernatant was removed and cells were allowed to recover for 24 h in DMEM, after which cells were trypsinized, and serial dilutions were transferred into 145-mm dishes. Transduced cells were selected with puromycin (2 µg/mL) for 6 d. Individual colonies were picked and analyzed for inducible protein expression by western blotting and for inducible loss of mtDNA by qPCR.
Determination of mtDNA copy number
Precise determination of mtDNA copy number was achieved with the help of the duplex TaqMan qPCR with the following primers and probes. Mouse mtDNA: rtF-mtDNA (ACTTCTAACTAA AAGAATTACAGC), rtR-mtDNA (TAGACGAGTTGATT CATAAAATTG), mtDNA-probe (6-FAM/CCCGAAACC/ZEN/AAACGAGCTACCT/IAbFQ). Mouse nDNA: rtF-mTert (CCT CAAGCATTCACCTCTTCTTTG), rtR-mTert (CCAAGGACCT GCTCGATGAC), mTret-probe (TEX613-Y/ACCACCCTCTCTG ACCTCCAGCCA/IAbRQ). To generate a standard curve, a calibrator plasmid (pMA2789), which contains cloned nuclear and mitochondrial targets in 1:1 ratio, was used.
Western blotting
Protein extracts from treated and control cells were prepared using lysis solution containing 10 mM Tris-HCl, 1% SDS, 1× EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentrations were measured using the BCA assay (Pierce, Rockford, IL). Proteins were separated by PAGE and transferred to PVDF membranes, blocked, and incubated with primary and secondary antibodies using standard techniques (Sambrook & Russel, 2001). Blots were developed with SuperSignal West Pico and exposed to CL-Xposure film (both Pierce, Rockford, IL). Primary antibodies were α-myc tag (Cell Signaling), α-HSP60 (mitochondrial, BD Biosciences, San Jose, CA), α-cytochrome oxidase subunit 1 (AbCam, Cambridge, UK).
mtDNA mutagenesis
mtDNA mutation loads were determined by a PCR-cloning-sequencing approach as described earlier (Shokolenko et al., 2009). Total DNA was extracted with the help of a Blood and Tissue kit (Qiagen, Valencia, CA) from uninduced cells and from cells that were induced for various periods of time and then allowed to grow without induction for up to 168 h to restore mtDNA levels. To ensure representativeness of the results, three different ~1 kb regions of mtDNA were amplified with the following primers: 1f (AAA GCA TCT GGC CTA CAC CCA GAA), 1r (ACC CTC GTT TAG CCG TTC ATG CTA), 2f (AAA GCC CAC TTC GCC ATC ATA TTC), 2r (TAC TGT TGC TTG ATT TAG TCG GCC), 3f (AGC CCA TGT TGA AGC TCC AAT TGC), and 3r (TGT GGT GGT GTA CAG TGG GAA GTT).
Statistical analyses
Multiple comparisons were performed using a one-way ANOVA.
Results
Experimental system
In this study, we took advantage of the regulated expression of two distinct mitochondrially targeted mutants of human UNG1, Y147A, and N204D (Kavli et al., 1996). The Y147A mutant has expanded substrate specificity and can hydrolyze a glycosidic bond of the thymidine in DNA, thus creating AP sites at the locations of T residues. Similarly, an expanded substrate range allows the N204D mutant to generate AP sites at the locations of C residues (Kavli et al., 1996). The fate of these AP sites can go one of the three ways: (1) they can be faithfully repaired by BER machinery (Krokan & Bjoras, 2013), (2) they can be bypassed through translesion synthesis by Pol γ (Liu et al., 2008; Pinz et al., 1995) or PRIMPOL (Garcia-Gomez et al., 2013), or (3) the mtDNA molecule containing the lesion can be destroyed (Shokolenko et al., 2013). Replicative bypass of AP sites generated by the Y147A mutant with incorporation of any base but A opposite the lesion will result in a mutation of A to T, C, or G on the opposite DNA strand. Similarly, when AP sites generated by N204D are bypassed by Pol γ in vivo, incorporation of any base but G opposite the lesion will manifest itself as a mutation of G to C, T, or A on the complementary DNA strand. Lesion bypass by PRIMPOL is expected to result in single-nucleotide deletions of A (T) or G (C) (Garcia-Gomez et al., 2013). Therefore, translesion synthesis across abasic sites in mtDNA can be tracked by sequencing mtDNA after exposing it to UNG1 mutants in vivo. Importantly, excessive generation of AP sites in mtDNA as a result of overexposure to UNG1 mutant proteins will result in mtDNA degradation, which may prevent detection of mutations. Therefore, mtDNA should be exposed to a pulse of mutant UNG1 that results in a submaximal mtDNA loss. This would ensure that mtDNA is both damaged (as reported by degradation), and that some damaged mtDNA molecules are salvaged by either BER or by a translesion bypass (as reported by submaximal level of degradation). The latter process will be detectable as mutagenesis of either A or G residues (depending on whether the Y147A or the N204D mutant is used). Importantly, methylation studies suggest that, with the exception of a few binding sites for regulatory factors, mtDNA is fairly uniformly accessible in vivo to mitochondrially targeted methylase (Rebelo et al., 2009). Therefore, it is reasonable to expect similar accessibility of mtDNA to UNG1 mutants used in this study.
Optimization of the experimental system
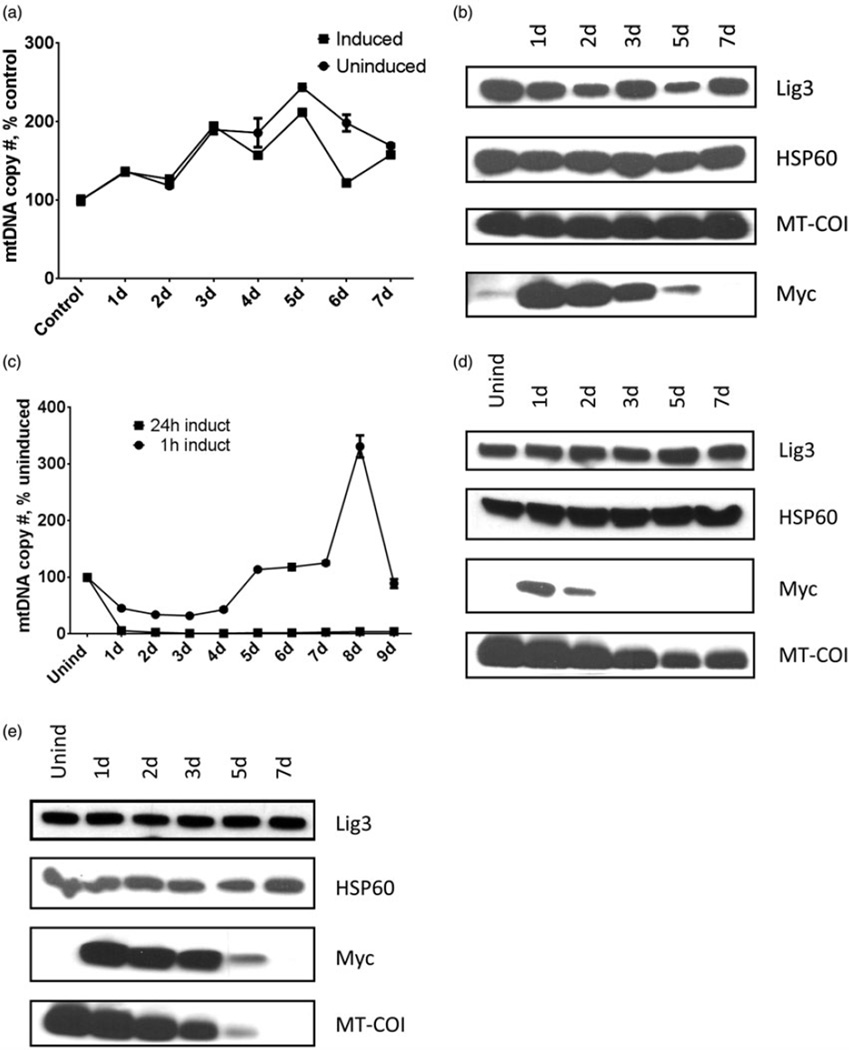
To ensure uniformity of induction of the UNG1 variants 3T3 Tet-On cells transduced with lentiviruses lv3287, lv3288, and lv3682 were clonally selected, and clones were individually tested for inducible expression of the transgene and for the loss of mtDNA in response to induction. Induction of the cells transduced with lv3288 encoding WT UNG1 for 24 h did not result in changes in mtDNA copy number beyond those normally observed in the growing culture (Figure 2a). Accordingly, cellular levels of subunit I of the cytochrome oxidase encoded by mtDNA (MT-COI) remained stable, while the removal of doxycycline after induction resulted in the gradual loss of UNG1 expression as reported by a myc tag (Figure 2b).
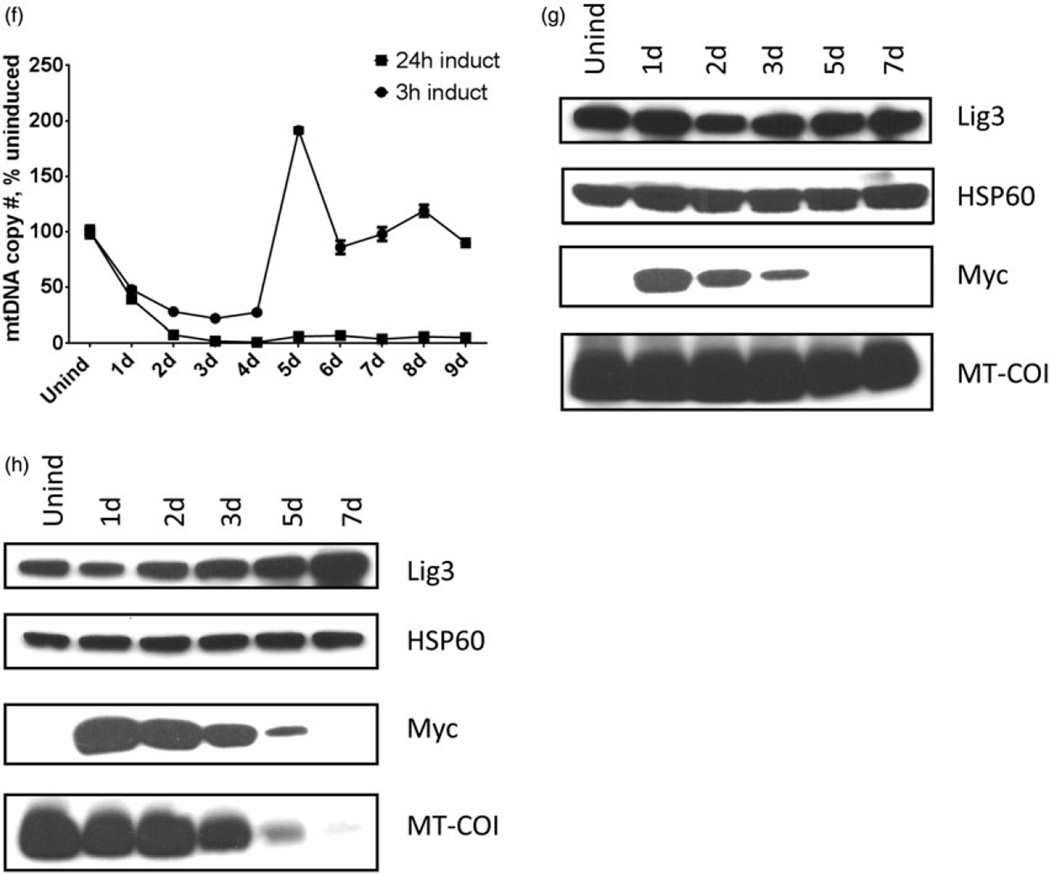
Figure 2.
Correlation between the expression of UNG1 variants, mtDNA content, and expression of the mtDNA. Cells expressing wtUNG1 or either the Y147A or the N204D mutant were induced with empirically chosen regimens (see text), and mtDNA content (a, c, and f) as well as expression of the myc-tagged UNG1 variants and mtDNA-encoded MT-COI subunit were monitored by western blotting. DNA ligase III (Lig3) and HSP60, loading controls. (a and b) Cells were induced to express wtUNG1 for 24 h with 2 µg/ml of doxycycline. (c, d, and e) Cells were induced to express the Y147A mutant with 0.5 µg/ml of doxycycline for either 1 h (D) or 24 h. (e, f, g, and h) Cells were induced to express the N204D mutant with 2 µg/ml of doxycycline for either 3 h (g) or 24 h. (h) Plotted values are means of triplicate measurements ± SD.
To optimize induction of the Y147A and N204D mutants, both the concentration of doxycycline and the induction time were varied. Ultimately, the Y147A mutant was induced for 1 h with 0.5 µg/ml of doxycycline. Under this regimen, mtDNA was initially depleted down to 32% of the control value (at 3 d after induction), and subsequently recovered to original levels (full recovery at 5 d after induction). In contrast, when induction time was increased to 24 h while keeping doxycycline concentration the same, mtDNA loss was greater in magnitude (down to 1–4% of the control), and recovery of mtDNA copy number was not observed during 9 d of washout (Figure 2c). In agreement with the duration of induction, cellular levels of the Y147A mutant were lower in cells induced for 1 h versus those induced for 24 h, and induced protein in these cells persisted for a shorter period of time. In cells induced for 1 h, levels of the mitochondrially encoded MT-COI generally followed changes in mtDNA copy number in that MT-COI levels initially decline with decline of mtDNA copy number and then showing partial recovery between days 5 and 7 (Figure 2d). Since mtDNA levels failed to recover in cells induced for 24 h, MT-COI levels in these cells eventually dropped below the level of detection (Figure 2e).
To achieve similar levels of mtDNA damage, the N204D mutant required a higher concentration and a longer period of induction. This may reflect its fourfold lower specific activity (Kavli et al., 1996). Induction of the N204D mutant with 2 µg/ml of doxycycline for 3 h resulted in an mtDNA depletion profile similar to that observed during induction of the Y147A mutant with 0.5 µg/ml of doxycycline for 1 h (Figure 2f). As with Y147A mutant, the minimal mtDNA copy number (22% of the control value) was achieved at 3 days after induction, and mtDNA copy number fully recovered 5 d after induction (Figure 2f). The profiles of expression for MT-COI and myc-tagged N204D mutant were also remarkably similar to those observed with the Y147A, except myc-tagged N204D mutant persisted longer during the washout (Figure 2g and h). The latter is most likely due to the higher concentration of doxycycline and longer period of induction used for the N204D mutant. Thus, the induction regimens utilized in this study provide enough mutant UNG1 variants to ensure extensive yet submaximal mtDNA damage (as reported by the partial loss of mtDNA).
Translesion bypass of AP sites in mitochondria is less efficient than either BER or mtDNA degradation
As expected, induction of the WT UNG1 did not reduce mtDNA copy number or expression of the mtDNA-encoded MT-COI subunit (Figure 2a and b). In contrast, in cells expressing either Y147A or N204D mutant, mtDNA levels remain reduced between 1 and 5 d after induction (Figure 2c and f). This is presumably because of the negative balance between mtDNA replication and repair on one hand, and the persistence of the mutant UNG1 variants (Figure 2d and g), which continue to inflict mtDNA damage and, therefore, channel mtDNA to a degradation pathway (Shokolenko et al., 2013). From the point of induction up to day 3 post-induction, this balance favors mtDNA degradation. Thereafter, mtDNA levels begin to recover, presumably due to continued reduction in the intracellular levels of the mutant UNG variants. Since intracellular mtDNA levels remain reduced for at least 72 h after induction, it is safe to conclude that mtDNA is exposed to damage in the form of abasic sites for at least this long. At day 3 after induction, cells expressing Y147A or N204D mutant have lost 68% or 78% of mtDNA, respectively, presumably due to the damage that overwhelmed both mtDNA replication and repair of AP sites through both BER and translesion synthesis across AP sites by Pol γ. This suggests a high probability that residual mtDNA received and repaired damage, and even underwent multiple cycles of damage and repair at different positions. If translesion bypass of AP sites is a major pathway mitigating this type of mtDNA damage, surviving mtDNA should contain multiple bypassed lesions, which should manifest as mutations in at least one of the systems used. As indicated above, the choice of two UNG1 mutants with different specificities ensures that translesion bypass of AP sites is mutagenic in at least one of the systems.
Analysis of mtDNA mutations in experimental samples revealed the lack of statistically significant increase in A>G (T>C) transitions (Figure 3a) or A>T (T>A) transversions (Figure 3c). Statistically, only G>A (C>T) mutations were increased and only in cells induced to express the N204D mutant. In cells expressing N240D variant of UNG1, increase in G>A (C>T) mutations in mtDNA is anticipated if the A-rule (insertion of an A residue opposite AP sites) holds in vivo as it does in vitro (Liu et al., 2008; Pinz et al., 1995). Accordingly, no mutagenesis is observed in cells induced to express the Y147A mutant. Indeed, this mutant generates AP sites by removing T residues, and insertion of an A opposite such AP sites is non-mutagenic. The frequency of G>A (C>T) mutations was very low, suggesting that in vivo AP sites are mitigated predominantly by BER and mtDNA turnover, i.e., pathways that are not associated with significant mutagenesis.
Figure 3.
mtDNA mutagenesis as a consequence of AP site bypass in vivo. Cells were induced to express either WT or one of the mutants as described in the text, and were allowed to recover for 7 d, or were left uninduced. Total cellular DNA collected at 7 d time point was subjected to PCR-cloning-sequencing using high-fidelity Phusion polymerase, and the combined equivalent of at least 10 mitochondrial genomes was sequenced per treatment condition (three samples per condition). Mutation frequency A>G + T>C (a), G>A + C>T (b), and A>T + T>A (c) was expressed per mitochondrial genome equivalent (16 300 bp). *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Elucidation of the in vivo processing and mutagenic consequences of specific DNA lesions remains challenging in both pro- and eukaryotic systems. Despite significant progress achieved in understanding the nuclear processing of DNA damage, our understanding of similar processes in mitochondria remains limited. This is largely due to unavailability of methods for efficient genetic transformation of mitochondria in vivo. Here, we used targeted enzymatic damage to specific nucleotides to generate AP sites in mtDNA in vivo and to study repair of these lesions in mitochondria. One important advantage of our experimental system is that, in contrast to some other techniques that rely on transfection of host cells with extrachromosomal elements which contain defined lesions but lack native (e.g., nucleosomal) organization characteristic of the endogenous genome, we introduce AP sites directly into the mitochondrial genome, and therefore study repair of these lesions in their natural context.
Our results indicate that while Pol γ is capable of translesion synthesis across AP sites in vivo and obeys the A-rule during this synthesis, BER and mtDNA degradation are likely to be the main pathways for the processing of AP sites in vivo. The system employed allows for the easy discrimination of contribution of the translesion synthesis versus combined BER and mtDNA degradation by analyzing mtDNA mutagenesis. Indeed, translesion synthesis in all systems studied so far is heavily biased towards insertion of a specific base across the lesion (which may vary from system to system and organism to organism). On the other hand, BER and mtDNA degradation are error-free processes. One potential complication to using a single specific UNG1 mutant is that a bias of the translesion bypass system towards a cognate base may mask mutagenesis. For example, if mitochondrial translesion bypass system is biased towards insertion of an A residue (the A-rule), then little or no mutagenesis will be observed with Y147A mutant, which cleaves T residues off mtDNA. However, a parallel experiment with N204D mutant allows us to remedy this potential problem. Indeed, this mutant cleaves C residues off mtDNA, and, therefore, adherence of the mitochondrial translesion bypass system to the A-rule will result in an increased number of G>A transitions with N204D mutant. Similarly, if mitochondrial translesion bypass system follows the C-rule, one would expect an increased number of A>C and G>C transversions with Y147A and N204D mutants, respectively. Finally, a T-rule would result in increased number of A>T and G>T transversions, while G-rule would result in increased number of A>G transitions and no mutagenesis in Y147A and N204D mutants, respectively. Our results clearly indicate that mitochondria follow the A-rule.
The fact that mtDNA copy number remained suppressed for over 72 h due to persistent generation of AP sites in mtDNA and channeling of the extensively damaged mtDNA molecules to degradation suggests that surviving mtDNA has undergone multiple rounds of AP site introduction/repair. The net result is an average of about one AP site bypass per mtDNA molecule during this period, as evidenced by the load of G>A (C>T) mutations in cells induced to express N204D mutant of UNG1 (Figure 3b).
Our findings are consistent with observations made in vitro with E. coli Pol I, which barely replicated across abasic sites under physiological conditions. However, Mn2+ greatly stimulates bypass of AP sites by this enzyme (Boiteux & Laval, 1982). In yeast, it has also been reported that C is only infrequently inserted opposite AP sites and that inactivation of REV1 does not greatly affect mutagenesis of AP sites (Nair et al., 2009).
The overall balance between observed extent of mtDNA degradation (78%) and a requirement for multiple AP lesions per molecule for channeling mtDNA to degradation on one hand, and a modest overall total load of G>A mutations (about 1 per mtDNA molecule) observed with N204D mutant suggests that Pol γ only inefficiently bypasses abasic sites in vivo, and that BER and mtDNA degradation are responsible for the processing of the majority of AP sites in mtDNA in vivo. Also, the lack of statistically significant increase in the frequency of mtDNA deletions as well as their spectrum in cells induced to express Y147A and N204D mutants suggests minimal contribution of PRIMPOL to translesion synthesis across AP sites in vivo (results not shown).
Conclusion
Pol γ is capable of translesion synthesis across AP sites in mitochondria of NIH3T3 cells and obeys the A-rule during this synthesis. However, BER and mtDNA degradation are likely to be the main pathways for the processing of overwhelming amounts AP sites in this cell line.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Anderson S, Bankier AT, Barrell BG, De Bruijn MH, Coulson AR, Drouin J, Eperon IC, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bannwarth S, Abbassi M, Valero R, Fragaki K, Dubois N, Vialettes B, Paquis-Flucklinger V. A novel unstable mutation in mitochondrial DNA responsible for maternally inherited diabetes and deafness. Diabetes Care. 2011;34:2591–2593. doi: 10.2337/dc11-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S, Laval J. Coding properties of poly(deoxycytidylic acid) templates containing uracil or apyrimidinic sites: In vitro modulation of mutagenesis by deoxyribonucleic acid repair enzymes. Biochemistry. 1982;21:6746–6751. doi: 10.1021/bi00269a020. [DOI] [PubMed] [Google Scholar]
- Copeland WC, Longley MJ. DNA polymerase gamma in mitochondrial DNA replication and repair. Sci World J. 2003;3:34–44. doi: 10.1100/tsw.2003.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Feaver WJ, Gerlach VL. The many faces of DNA polymerases: Strategies for mutagenesis and for mutational avoidance. Proc Natl Acad Sci USA. 2000;97:5681–5683. doi: 10.1073/pnas.120152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ggarcia-Gomez S, Reyes A, Martinez-Jimenez MI, Chocron ES, Mouron S, Terrados G, Powell C, et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol Cell. 2013;52:541–553. doi: 10.1016/j.molcel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil A, Renault G, Madzak C, Margot A, Cabral-Neto JB, Vasseur JJ, Rayner B, et al. Mutagenic properties of a unique abasic site in mammalian cells. Biochem Biophys Res Commun. 1990;173:704–710. doi: 10.1016/s0006-291x(05)80092-0. [DOI] [PubMed] [Google Scholar]
- Gibbs PE, Lawrence CW. Novel mutagenic properties of abasic sites in Saccharomyces cerevisiae. J Mol Biol. 1995;251:229–236. doi: 10.1006/jmbi.1995.0430. [DOI] [PubMed] [Google Scholar]
- Guillet M, Boiteux S. Endogenous DNA abasic sites cause cell death in the absence of Apn1, Apn2 and Rad1/Rad10 in Saccharomyces cerevisiae. EMBO J. 2002;21:2833–2841. doi: 10.1093/emboj/21.11.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliam TA, Jozwiakowski SK, Ehlinger A, Barnes RP, Rudd SG, Bailey LJ, Skehel JM, et al. Human PrimPol is a highly error-prone polymerase regulated by single-stranded DNA binding proteins. Nucleic Acids Res. 2015;43:1056–1068. doi: 10.1093/nar/gku1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JM, Longen S, Weckbecker D, Depuydt M. Biogenesis of mitochondrial proteins. Adv Exp Med Biol. 2012;748:41–64. doi: 10.1007/978-1-4614-3573-0_3. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kavli B, Slupphaug G, Mol CD, Arvai AS, Peterson SB, Tainer JA, Krokan HE. Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMJO J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]
- Krokan HE, Bjoras M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5:a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Schaaper RM, Loeb LA. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983;22:2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Liu P, Qian L, Sung JS, de Souza-Pinto NC, Zheng L, Bogenhagen DF, Bohr VA, et al. Removal of oxidative DNA damage via FEN1-dependent long-patch base excision repair in human cell mitochondria. Mol Cell Biol. 2008;28:4975–4987. doi: 10.1128/MCB.00457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Maassen JA, Janssen GM, ‘t Hart LM. Molecular mechanisms of mitochondrial diabetes (MIDD) Ann Med. 2005;37:213–221. doi: 10.1080/07853890510007188. [DOI] [PubMed] [Google Scholar]
- Milone M. Mitochondria, diabetes, and Alzheimer’s disease. Diabetes. 2012;61:991–992. doi: 10.2337/db12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. DNA synthesis across an abasic lesion by human DNA polymerase iota. Structure. 2009;17:530–537. doi: 10.1016/j.str.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J, Walker VE, Upton PB, Chiang SY, Kow YW, Swenberg JA. Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer Res. 1998;58:222–225. [PubMed] [Google Scholar]
- Neto JB, Gentil A, Cabral RE, Sarasin A. Mutation spectrum of heat-induced abasic sites on a single-stranded shuttle vector replicated in mammalian cells. J Biol Chem. 1992;267:19718–19723. [PubMed] [Google Scholar]
- Noll T, Wissemann P, Mertens S, Krutzfeldt A, Spahr R, Piper HM. Hypoxia tolerance of coronary endothelial cells. Adv Exp Med Biol. 1990;277:467–476. doi: 10.1007/978-1-4684-8181-5_52. [DOI] [PubMed] [Google Scholar]
- Pinz KG, Shibutani S, Bogenhagen DF. Action of mitochondrial DNA polymerase gamma at sites of base loss or oxidative damage. J Biol Chem. 1995;270:9202–9206. doi: 10.1074/jbc.270.16.9202. [DOI] [PubMed] [Google Scholar]
- Rebelo AP, Williams SL, Moraes CT. In vivo methylation of mtDNA reveals the dynamics of protein–mtDNA interactions. Nucleic Acids Res. 2009;37:6701–6715. doi: 10.1093/nar/gkp727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning. [Google Scholar]
- Schaaper RM, Kunkel TA, Loeb LA. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci USA. 1983;80:487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial diseases. Lancet. 2012;379:1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- Shibutani S, Takeshita M, Grollman AP. Translesional synthesis on DNA templates containing a single abasic site. A mechanistic study of the “A rule”. J Biol Chem. 1997;272:13916–13922. doi: 10.1074/jbc.272.21.13916. [DOI] [PubMed] [Google Scholar]
- Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37:2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokolenko IN, Wilson GL, Alexeyev MF. Persistent damage induces mitochondrial DNA degradation. DNA Repair (Amst) 2013;12:488–499. doi: 10.1016/j.dnarep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss BS. The “specificity“ rule revisited: Polymerases as determinants of mutational specificity. DNA Repair (Amst) 2002;1:125–135. doi: 10.1016/s1568-7864(01)00014-3. [DOI] [PubMed] [Google Scholar]
- Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic b cells. Trends Endocrinol Metab. 2012;23:477–487. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Tang M, Shen X, Frank EG, O’Donnell M, Woodgate R, Goodman MF. UmuD’(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G, Hubscher U, Gironis N, Parkkinen S, Pospiech H, Shevelev I, Dicicco G, et al. In vitro gap-directed translesion DNA synthesis of an abasic site involving human DNA polymerases epsilon, lambda, and beta. J Biol Chem. 2011;286:32094–32104. doi: 10.1074/jbc.M111.246611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikallio E, Suomalainen A. Mechanisms of mitochondrial diseases. Ann Med. 2012;44:41–59. doi: 10.3109/07853890.2011.598547. [DOI] [PubMed] [Google Scholar]
- Yu M. Somatic mitochondrial DNA mutations in human cancers. Adv Clin Chem. 2012;57:99–138. doi: 10.1016/b978-0-12-394384-2.00004-8. [DOI] [PubMed] [Google Scholar]
- Yu SL, Lee SK, Johnson RE, Prakash L, Prakash S. The stalling of transcription at abasic sites is highly mutagenic. Mol Cell Biol. 2003;23:382–388. doi: 10.1128/MCB.23.1.382-388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Ji Y, Guan MX. Mitochondrial tRNA mutations associated with deafness. Mitochondrion. 2012;12:406–413. doi: 10.1016/j.mito.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]