Abstract
Objective(s):
Analgesic effects of oxytocin and it's the other physiological effects were well-known. The aim of present study was determination of nitric oxide role on analgesic effects of oxytocin in mice.
Materials and Methods:
216 male Albino mice were divided randomly into two experimental groups, tail flick and formalin test. Each experimental group consists of three main groups including: saline, L-arginine (50 mg/kg) and L-NAME (10 mg/kg) intraperitoneal (IP) injection. 15 min after injection in each of the following groups, the animals in each groups divided to the three subgroups including: saline (n=12), oxytocin (1 mg/kg) (n=12) and oxytocin (1 mg/kg) + atosiban (1 mg/kg) (n=12) was injected IP and then after 30 min of use the formalin test and tail flick were to evaluate the response to pain.
Results:
Area under the curve (AUC) in the late phase of the formalin test, in sub-groups oxytocin + saline and L-NAME were significantly decreased compared with saline + saline group (P<0.05 to P< 0.001), and AUC in L-arginine + saline and atosiban + saline + oxytocin were significantly increased compared with oxytocin + saline group (P<0.05). Tail flick tests as well as a significant reduction in the AUC in oxytocin + L-arginine and atosiban + saline + oxytocin groups were compared with Oxytocin + Saline group (P<0.001).
Conclusion:
Oxytocin has analgesic effects in the acute and late phase of pain in the formalin test. Moreover, exogenous increasing of nitric oxide reduced the analgesic effect of oxytocin.
Keywords: Analgesia, Mice, Nitric Oxide, Oxytocin
Introduction
Pain is a noxious and a harmful felling of a living organism that arising from inflamed or injured tissues. Otherwise, responses to noxious stimuli may be enhanced (hyperalgesia) or normally innocuous stimuli. More studies confirm that in normal conditions, Aβ fibers are mainly relevant with non-noxious input from particular encapsulated receptors. In difference small Aδ fibers and C fibers respond to noxious stimuli (1). Noxious stimuli are mediated by glutamate (excitatory amino acid) which acting on α-amino-3-hydroxy-5-methylisoxazole (AMPA) receptors (2).
Oxytocin is a mammalian neurohypophysial hormone that synthesized in the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus. Oxytocinergic neurons display widespread projections through the central nervous system (CNS). Oxytocin receptors also widely distribute in the CNS, including hypothalamus, thalamus olfactory system, cortex, and dorsal horn in the spinal cord (3).
Nitric Oxide (NO) is a significant mediator of nociception in acute and chronic pain conditions (4, 5) at the peripheral and central levels (6, 7). The experimental and clinical studies demonstrated that NO has analgesic potential and could induce analgesia (8). The L-arginine-NO pathway has a critical role in the N-methyl-D-aspartate (NMDA)-mediated and glutamate nociceptive responses. The results of considering the role of aspartame on formalin test in mice approved the activation of NMDA receptors could modulate pain-related behavior via NO-cyclic guanosine monophosphate (NO/cGMP)-glutamate release cascade (9). NO/cGMP signaling pathway has play an essential role in developing endurance to opioid analgesia (10).
Different biological functions of Oxytocin done by it receptor that coupled to GTP binding proteins (G q/11) which stimulate together with Gβγ the activity of phospholipase C-β isoforms (3).
Intraperitoneal or intracisternal injection of oxytocin was showed the anti-nociceptive effect in rat or mice (11, 12). Moreover, when oxytocin injected into periaqueductal gray matter and raphe Magnus nucleus remarkable reduced nociceptive response in rats (13, 14). Direct application or electrical stimulation of the paraventricular nucleus of hypothalamus which led to endogenous released of oxytocin on the spinal cord produced a clear analgesic effect (15). Intrathecally injection of naloxone before the stimulation of the paraventricular nucleus and oxytocin administration reduced significantly analgesic effect of oxytocin (6). Oxytocin inhibits sensory glutamatergic transmission between afferent fibers and dorsal horn neurons in the spinal cord (16, 17).
According to the above mentioned studies we aimed to determine the rolls of NO on anti- nociceptive effects of oxytocin in mice.
Materials and Methods
Experimental Animals
In this study 216 male albino mice (20-25 g) were used. Animals were prepared from animal house of Medical School of Iran University that housed in 12 hr/12 hr light/dark cycle at 22±2°C. Food and water was freely available. The experimental protocols were approved by the Animal Experimentation Ethics Committee (AEEC) of Medical School of Iran University.
Drugs
In this experiment, these chemicals were used: oxytocin, atosiban, L-NAME powder and L-arginine powder (Sigma Co., USA).
Protocol
The animals were divided randomly into two experimental groups, tail flick and formalin test. Each experimental group consists of three main groups including: saline, L-arginine (50 mg/kg) and L-NAME (10 mg/kg) IP injection. 15 min after injection in each of the following groups, the animals in each groups divided to the three subgroups including: saline (n=12), oxytocin (1 mg/kg) (n=12) and oxytocin (1 mg/kg) + atosiban (1 mg/kg) (n=12) was injected IP and then after 30 min of use the formalin test and tail flick were to evaluate the response to pain.
Tail flick test
Tail flick test is an acute model of pain. In this study it was used to assess the anti-nociceptive effect of the drugs by measuring the latency of response (18). In this method, animal tail is exposed to a powerful light beam and the response latency period for flicking the tail off the beam is recorded (19). We apply radiant heat to the tail at 5-8 cm from the tip by using a tail flick apparatus. Tail flick latency time was estimated as the time from the onset of the heat exposure to the withdrawal of the tail. They adjusted the intensity of radiant heat to yield the baseline latencies of 2-4 sec. In order to avoid tissue damages the heat stimulus was discontinued after 7 sec (Cut off point =7 sec). In this study, tail flicking done 30, 45 and 60 min after injection (20).
Experimental design
216 male Albino mice were divided into 18 groups: The animals were divided randomly into two experimental groups’ tail flick and Formalin test. Each experimental group consists of three main groups including: saline, L-arginine (50 mg/kg) and L-NAME (10 mg/kg) IP injection. 15 min after injection in each of the following groups, the animals in each groups divided to the three subgroups including: saline (n=12), oxytocin (1 mg/kg) (n=12) and oxytocin (1 mg/kg)+atosiban (1 mg/kg) (n=12) was injected IP and then after 30 minutes of use the formalin test and tail flick were to evaluate the response to pain (Table 1). This interventional study was conducted using 216 male Albino mice.
Table 1.
Experimental design
| Tail flick test | Saline (IP) | Saline (n=12) Oxytocin (1 mg/kg) (n=12) Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) |
| L-arginine (50 mg/kg) (IP) | Saline (n=12) Oxytocin (1 mg/kg) (n=12) Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) |
|
| L-NAME (10 mg/kg) (IP) | Saline (n=12) Oxytocin (1 mg/kg) (n=12) Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) |
|
| Formalin test | Saline (IP) | Saline (n=12) Oxytocin (1 mg/kg) (n=12) Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) |
| L-arginine (50 mg/kg) (IP) | Saline (n=12) Oxytocin (1 mg/kg) (n=12) |
|
| L-NAME (10 mg/kg) (IP) | Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) Saline (n=12) Oxytocin (1mg/kg) (n=12) Oxytocin (1 mg/kg) + Atosiban (1 mg/kg) (n=12) |
|
Formalin test
After intrathecal administration of the last drug the formalin test was performed 10 min. Animals were placed in acrylic testing chambers individually (30 × 30 × 30 cm) and they were allowed to acclimate for at least 60 min. we placed a mirror at a 45° angle below the floor of the chamber to allow an unobstructed view of the mouse paws. Then, 20 μl of formalin 2.5% was injected SC. into the plantar surface of the left hind paw. The behaviors were observed for the next 60 min and they were quantified as described by Dubuisson and Dennis (1977) as:
0 = normal weight bearing on the injected paw.
1 = limping during locomotion or resting the paw lightly on the floor.
2 = elevation of the injected paw so that at most the nails touch the floor.
3 = licking, biting or shaking the injected paw.
A trained observer scored subjects behaviors continuously every 15 sec intervals. The trained observer kept “blind” to each subject's treatment condition.
A biphasic response of nociceptive behavior in mouse was the result of subcutaneous formalin injection. The early phase (phase 1) starts immediately after formalin application, followed by a lull and a more prolonged but delayed second phase (phase 2). Average of scores was considered as the phase 1 in the first 5 min and the area under curve (AUC) of pain scores during 10–60 min after formalin injection was considered as phase 2 (21).
Maximum possible effect (%MPE)
Time course of anti-nociceptive response was formed by plotting the mean of latency times as a function of time for of individual drug. Anti-nociception was quantified as either tail flick latency time or %MPE or AUC which includes both maximum effects and duration of action (18). The %MPE was calculated as [(T1 - T0) / (T2 - T0)] × 100. T0 and T1 were the tail flick latencies before and after drug administration. T2 was the cut off time.
The AUC was calculated considering Tail Flick Latency time (TFL) from 15 to 90 post-injection based on Trapezoid rules (18) as follows: AUC = 15 × TFL[(MIN 15) + (MIN 30) + (MIN 45) + (MIN 60) + (MIN 90) / 2].
Statistical analysis
All data were presented as mean ± SEM. SPSS 16 was applied for the analysis and the results were statistically analyzed by one-way analysis of variance (ANOVA) followed by Tukey test and P<0.05 were considered to significant.
Results
The result showed that area under the curve (AUC) in the acute phase of the formalin test, in sub-groups oxytocin + saline and L-NAME + saline were significantly decreased compared with saline + saline group (P<0.05, P<0.001), and also AUC in oxytocin+
Saline compared to L-NAME + Saline and L-arginine + Saline were significantly decreased (P<0.05 and P<0.01 respectively) (Figure 1).
Figure 1.
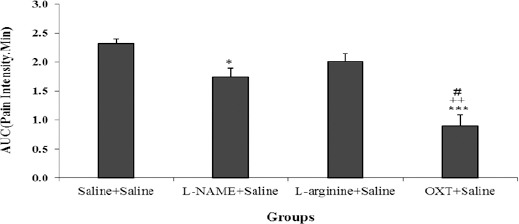
Comparison of AUC (area under the curve) between different groups in the acute phase of the formalin test. Values are means±SEM (n=12). *P<0.05, ***P<0.001 compared to saline + saline group; ++P<0.01 compared between OXT + saline and L-arginine + saline group; #P<0.05 compared between OXT + saline and L-NAME + saline group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
In the late phase of the formalin test also AUC in sub-groups ooxytocin + saline and L-NAME + saline were significantly decreased compared with saline + saline group (P<0.01, P<0.001), and also AUC in oxytocin + saline compared to L-NAME + saline and L-arginine + saline were significantly decreased (P<0.05, P<0.001 respectively) (Figure 2).
Figure 2.
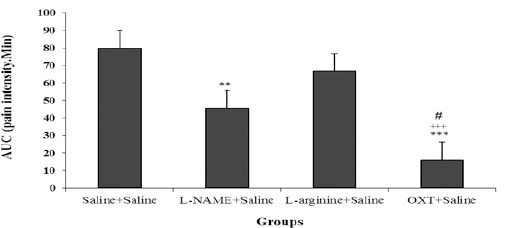
Comparison AUC (area under the curve) between different groups in the chronic phase of the formalin test. Values are means±SEM (n=12). **P<0.01 and ***P<0.001 compared to saline + saline group; ++P<0.01 compared between OXT + saline and L-arginine + saline group; #P<0.05 compared between OXT + saline and L-NAME + saline group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
AUC in the acute phase of the formalin test, in sub-groups oxytocin + L-arginine, oxytocin+ L-NAME and atosiban + oxytocin + saline group (Figure 3).
Figure 3.
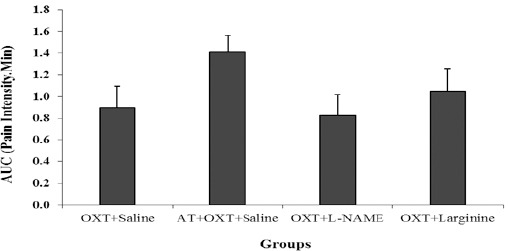
Comparison AUC (area under the curve) in Atosiban (AT)+ OXT + saline, OXT + L-NAME and OXT + L-arginine groups with OXT+ saline group in the acute phase of the formalin test. Values are means±SEM (n = 12). Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
But in the late phase of the formalin test AUC in sub-groups Oxytocin + L-arginine and atosiban + saline + oxytocin were significantly increased compared with Oxytocin + saline group (P<0.05) (Figure 4).
Figure 4.
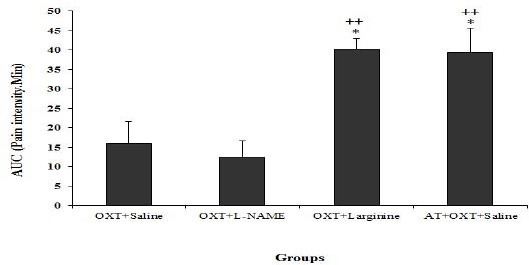
Comparison AUC (area under the curve) in OXT+ L-NAME, OXT+L-arginine and Atosiban (AT) + OXT + saline groups with OXT + saline group in the chronic phase of the formalin test. Values are means±SEM (n=12). ++P<0.01 compared to OXT+L arginine group; *P<0.05 compared to OXT+ saline group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
The tail flick test results show that the MPE (Figure 5A) and AUC (Figure 5B), in oxytocin + saline increased significantly compared with saline + saline, L-NAME+ saline and L-arginine + saline groups (P<0.001), but the MPE and AUC between L-NAME+ saline and L-arginine + saline groups with saline + saline group were not significant. Furthermore, the MPE (Figure 6A) and AUC (Figure 6B), in oxytocin + L-NAME increased significantly compared with oxytocin + L-arginine and oxytocin + atosiban + saline groups (P<0.001), but not significant compared to the oxytocin +L-NAME group.
Figure 5.
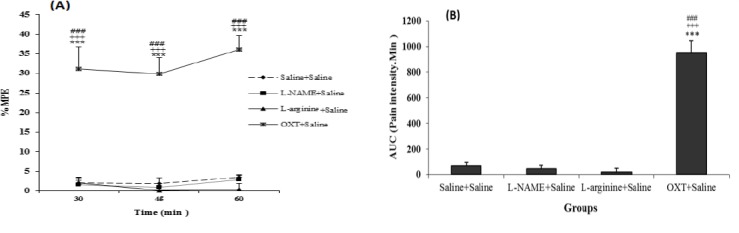
Comparison between L-NAME + saline, L-arginine + saline and OXT + Saline groups with saline + saline group in the acute phase of the tail flick test. (A): MPE (Maximum possible effect) (B): AUC (area under the curve). Values are means±SEM (n=12). ***P<0.001 compared to saline + Saline group; +++P<0.01 compared between OXT + saline and L-arginine + Saline; ###P<0.001 compared between OXT + saline and L-NAME + saline group. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
Figure 6.
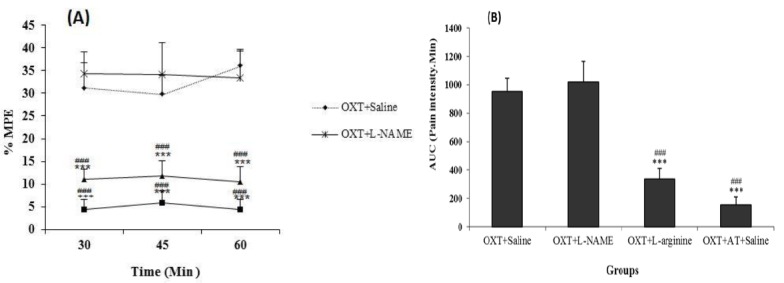
Comparison between OXT + L-NAME, OXT + L-arginine and OXT + AT + saline groups with OXT + saline group in the chronic phase of the tail flick test. MPE (maximum possible effect) (A) and AUC (area under the curve) (B). Values are means±SEM (n=12). ***P<0.001 compared to OXT + saline group; ###P<0.001 compared to OXT+L-NAME. Statistical analyses were made using the one-way ANOVA followed by the Tukey's test Post hoc
Discussion
In different studies, several tests were used to assess the pain in order to evaluate the analgesic effect of oxytocin, but all of them were associated with acute pain in form of thermal or mechanical. Here, tail flick test related to acute pain, and formalin test was used in order to evaluate the analgesic effects of oxytocin. In this study, mice were received normal saline and oxytocin showed decreased responses in both phases of the formalin test and also decreased responses in the tail flick test. Our results showed that oxytocin not only in acute pain, but also played a role in chronic pain which leading to decrease the pain responses in both phases. Results of other studies have also shown that oxytocin has analgesic effect (22) and part of analgesic effect of that is related to inhibition of spinal glutamatergic transmission (23, 24). One of the proposed mechanisms for the analgesic effect of oxytocin in normal animals is the action of oxytocin on its G protein receptor which leads to metabolisation of intracellular calcium and subsequently a nonspecific cation channel open and potassium channel close (15, 25). It was demonstrated that oxytocin can block glutamate activation and causing decreased nociceptive responses in spinal cord cells (17). On the other hand glutamate → NMDA → NO pathway is part of a metabolic chain in pain process in spinal cord. Oxytocin is also affected on the recipient of the Mu (µ) and Kappa (κ) Opioid and causing the analgesia (26).
Paraventricular nucleus stimulation through oxytocinergic descending path leads to analgesic effect (27). The accumulation of the effects of oxytocin, are on accumbency nuclei, limitans thalamic nucleus, Motor trigeminal, sustantia gelatinosa cores, trigeminal core of the spinal cord and spinal cord (28). In another study it was showed that the injection of oxytocin into posterior vagus motor nuclei stimulates gall bladder via NMDA receptor – NO – cGMP pathway that indicates another evidence of oxytocin involvement in this pathway (29). It was also reported that in acute and chronic recurrent pain a significant change is occurred in content of oxytocin in human cerebrospinal fluid (CSF) and plasma (30). A mechanism for peripheral analgesic effect of oxytocin is inhibition of membrane depolarization of sensory neurons sensitive to Capsaicin and the increase in intracellular calcium (31). Some studies show that injection of formalin into the metatarsus increased glutamate release only during the acute phase while in the chronic phase such change does not occur (32) and subcutaneous administration of oxytocin, reduce the inflammation which induced by carrageenan injection (33). Injection of formalin intraplantar causing increase in glutamate release in acute phase (32) and increase nitrite and nitrate in spinal cord dorsal horn (34). Nitrite and nitrate release induced by formalin injection may be caused by increasing glutamate levels. Because oxytocin has an inhibitory effect on glutamate activity then, reduced responses in acute formalin test phase could related to decreased production of nitrite and nitrate induced by glutamate.
Also, in this study it was showed that anti-nociceptive effect of oxytocin in acute phase of formalin test was not antagonized by atosiban probably could due to indirect effect of oxytocin in suppress of glutamate release. It was also reported that intrathecal injection of atosiban has a hyperalgesic effect in rats with inflammation. On the other hand, an intrathecal injection of atosiban alone has no effect on the mechanical and thermal stimulation in normal rats this means that endogenous oxytocin does not interfere in spinal analgesic action in normal rats (30). But in current study atosiban antagonized anti-nociceptive effect of oxytocin in chronic phase of formalin test and this indicate Oxytocin via its receptors in spinal cord dorsal horn causing anti-nociceptive effect. In a study it was reported that oxytocin reduces the pain by effect on its receptor in dorsal horn of the spinal cord (35). The results showed that in tail flick test oxytocin caused increase in latency and atosiban antagonized this effect that was expectable because these effects were shown in previous study (36).
Another finding of this study was that the L-NAME at a dose of 10 mg/kg has analgesic effect on both phases of the formalin test, which this effect was more pronounced in chronic phase. That it was predictable because, as stated before, the main effects of nitric oxide is in the chronic phase of the formalin test. Other studies indicate that NOS inhibitors reduce the pain that induced by formalin injection and change the electrophysiological activity of dorsal horn neurons (37, 38). L-NAME significantly reduced the release of glutamate and finally formalin induced nitrite and nitrate during the acute phase of the formalin test (39). This finding may have confirmed the findings of current study showed that the L-NAME effect on the acute phase of the formalin test.
In the chronic phase of the formalin test and tail flick test groups which received oxytocin and L-arginine showed significant differences compared with group which received oxytocin and normal saline. So that L-arginine injected with oxytocin may lead to reduced analgesic effect of oxytocin while L-arginine alone has no effect on the pain response in mentioned tests. It has been reported that chronic administration of L-arginine reduces the analgesic effect of morphine (39). Another study suggested that the analgesic effects of oxytocin, at least in part of its effects induced by the δ and κ opioid receptors (15). It was proposed that reduction of anti-nociceptive effect of morphine through administration of L-arginine is accompanied to reduced morphine concentration in different region of brain and spinal cord (40). Some of studies showed that anti-nociceptive effect of oxytocin at least partly caused by κ and δ opioid receptors (24). These receptors are in periaqueductal grey matter (PAG) and oxytocin in this region via mentioned receptors caused its anti-nociceptive effect partly and effect of L-arginine to reduce anti-nociceptive effect of oxytocin in this manner can described. The injection of L-NAME with oxytocin was no effect on oxytocin-induced analgesic responses. The effect of NO in pain transmission is dual and contradictory. Increase nitric oxide has an analgesic effect whereas L-NAME induces analgesic effect in some models of acute and chronic pain (38). Since the L-NAME has a decreasing effect on the production of nitrite and nitrate produced in the spinal cord followed by formalin injection and L-arginine administration as a NO donor in formalin injection can increase the levels of nitrite and nitrate surface of the spinal cord and debilitating the analgesic effect of oxytocin.
Conclusion
Our data suggested oxytocin has significant roles in the acute and chronic pain. Administration of L-arginine in the spinal cord can increase the levels of NO in the spinal cord which reduced the analgesic effect of oxytocin but L-NAME had no role in anti-nociceptive effect of oxytocin.
Acknowledgment
This work was supported by grants from Research Affairs of Iran University of Medical Sciences, the Specialized Research Fund (No. 89299). The authors would like to thank Dr Mahmoud Hosseini for his guidance and advice throughout preparation of this article.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Priebe HJ. Textbook of Anaesthesia. In: Aitkenhead AR, Rowbotham DJ, Smith G, editors. 4th Edn. Edinburgh: Churchill Livingstone; p. 806. indexed;illustrated. Price£54.95. British J Anaesthesia 2001. [Google Scholar]
- 2.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuro-pharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 3.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 4.Toriyabe M, Omote K, Kawamata T, Namiki A. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesio-logy. 2004;101:983–990. doi: 10.1097/00000542-200410000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. 2010;6:13. doi: 10.1186/1744-8069-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freire MA, Guimaraes JS, Leal WG, Pereira A. Pain modulation by nitric oxide in the spinal cord. Front Neurosci. 2009;3:175–181. doi: 10.3389/neuro.01.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung E, Burke B, Bieber AJ, Doss JC, Ohgami Y, Quock RM. Dynorphin-mediated antinociceptive effects of L-arginine and SIN-1 (an NO donor) in mice. Brain Res Bull. 2006;70:245–250. doi: 10.1016/j.brainresbull.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Abdollahi M, Nikfar S, Abdoli N. Potentiation by nitric oxide synthase inhibitor and calcium channel blocker of aspartame-induced antinociception in the mouse formalin test. Fundam Clin Pharmacol. 2001;15:117–123. doi: 10.1046/j.1472-8206.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir E, Bagcivan I, Durmus N, Altun A, Gursoy S. The nitric oxide-cGMP signaling pathway plays a significant role in tolerance to the analgesic effect of morphine. Can J Physiol Pharmacol. 2011;89:89–95. doi: 10.1139/y10-109. [DOI] [PubMed] [Google Scholar]
- 11.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on nociception and morphine antinociception. Neuropeptides. 1993;24:125–129. doi: 10.1016/0143-4179(93)90075-l. [DOI] [PubMed] [Google Scholar]
- 12.Zubrzycka M, Fichna J, Janecka A. Inhibition of trigemino-hypoglossal reflex in rats by oxytocin is mediated by mu and kappa opioid receptors. Brain Res. 2005;1035:67–72. doi: 10.1016/j.brainres.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Ge Y, Lundeberg T, Yu LC. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Res. 2002;927:204–207. doi: 10.1016/s0006-8993(01)03346-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang JW, Lundeberg T, Yu LC. Antinociceptive role of oxytocin in the nucleus raphe magnus of rats, an involvement of mu-opioid receptor. Regul Pept. 2003;115:153–159. doi: 10.1016/s0167-0115(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 15.Condés-Lara M, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J, López Hidalgo M, Freund-Mercier MJ. Paraventricular hypothalamic influences on spinal nociceptive processing. Brain Res. 2006;1081:126–137. doi: 10.1016/j.brainres.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, et al. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condés-Lara M, González NM, Martínez-Lorenzana G, Delgado OL, Freund-Mercier MJ. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res. 2003;976:75–81. doi: 10.1016/s0006-8993(03)02690-8. [DOI] [PubMed] [Google Scholar]
- 18.Keyhanfar F, Meymandi MS, Sepehri G, Rastegaryanzadeh R, Heravi G. Evaluation of antinociceptive effect of pregabalin in mice and its combination with tramadol using tail flick test. Iran J Pharm Res. 2013;12:483–493. [PMC free article] [PubMed] [Google Scholar]
- 19.Leandri M, Leandri S, Ghignotti M, Cilli M, Lunardi G. The ITFR, impulsive tail flick reflex by short duration nociceptive stimuli. J Neurosci Methods. 2011;199:69–77. doi: 10.1016/j.jneumeth.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Lundeberg T, Uvnäs-Moberg K, Agren G, Bruzelius G. Anti-nociceptive effects of oxytocin in rats and mice. Neuroscience Letters. 1994;170:153–157. doi: 10.1016/0304-3940(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 21.Naderi N, Shafaghi B, Khodayar MJ, Zarindast MR. Interaction between gamma-aminobutyric acid GABA B and cannabinoid CB 1 receptors in spinal pain pathways in rat. Eur J Pharm. 2005;514:159–164. doi: 10.1016/j.ejphar.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28:1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Condés-Lara M, González NM, Martínez-Lorenzana G, Delgado OL, Freund-Mercier MJ. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res. 2003;976:75–81. doi: 10.1016/s0006-8993(03)02690-8. [DOI] [PubMed] [Google Scholar]
- 24.Condes-Lara M, Maie IA, Dickenson AH. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Res. 2005;1045:124–133. doi: 10.1016/j.brainres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupré A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas-Piloni G, López-Hidalgo M, et al. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Yu LC. Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats. Regul Pept. 2004;120:53–58. doi: 10.1016/j.regpep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Liu CY, Xie DP, Liu KJ, Zhou YQ, Liu JZ. Oxytocin microinjected into dorsal motor nucleus of the vagus excites gallbladder motility via NMDA receptor-NO-cGMP pathway. Brain Res. 2005;1032:116–122. doi: 10.1016/j.brainres.2004.10.057. [DOI] [PubMed] [Google Scholar]
- 30.Yu S-Q, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983:13–22. doi: 10.1016/s0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]
- 31.Hobo S, Hayashida K, Eisenach JC. Oxytocin inhibits the membrane depolarization-induced increase in intracellular calcium in capsaicin sensitive sensory neurons: a peripheral mechanism of analgesic action. Anesth Analg. 2012;114:442–449. doi: 10.1213/ANE.0b013e31823b1bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda K, Sakurada C, Takahashi M, Yamada T, Sakurada T. Characterization of nociceptive responses and spinal releases of nitric oxide metabolites and glutamate evoked by different concentrations of formalin in rats. Pain. 2001;92:107–115. doi: 10.1016/s0304-3959(00)00476-0. [DOI] [PubMed] [Google Scholar]
- 33.Petersson M, Wiberg U, Lundeberg T, Uvnäs-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479–1484. doi: 10.1016/s0196-9781(01)00469-7. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe C, Okuda K, Sakurada C, Ando R, Sakurada T, Sakurada S. Evidence that nitric oxide-glutamate cascade modulates spinal antinociceptive effect of morphine: a behavioural and microdialysis study in rats. Brain Res. 2003;990:77–86. doi: 10.1016/s0006-8993(03)03440-1. [DOI] [PubMed] [Google Scholar]
- 35.Wrobel L, Schorscher-Petcu A, Dupré A, Yoshida M, Nishimori K, Tribollet E. Distribution and identity of neurons expressing the oxytocin receptor in the mouse spinal cord. Neurosci Lett. 2011;495:49–54. doi: 10.1016/j.neulet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 36.Uvnäs-Moberg K, Bruzelius G, Alster P, Bileviciute I, Lundeberg T. Oxytocin increases and a specific oxytocin antagonist decreases pain threshold in male rats. Acta Physiol Scand. 1992;144:487–488. doi: 10.1111/j.1748-1716.1992.tb09327.x. [DOI] [PubMed] [Google Scholar]
- 37.Homayoun H, Babaie A, Gharib B, Etminani A, Khavandgar S, ManiDehp A, et al. The involvement of nitric oxide in the antinociception induced by cyclosporin A in mice. Pharmacol Biochem Behav. 2002;72:267–272. doi: 10.1016/s0091-3057(01)00774-2. [DOI] [PubMed] [Google Scholar]
- 38.Bulutcu F, Dogrul A, Güç MO. The involvement of nitric oxide in the analgesic effects of ketamine. Life Sci. 2002;71:841–853. doi: 10.1016/s0024-3205(02)01765-4. [DOI] [PubMed] [Google Scholar]
- 39.Sakurada C, Sugiyama A, Nakayama M, Yonezawa A, Sakurada S, Tan-No K, et al. Antinociceptive effect of spinally injected L-NAME on the acute nociceptive response induced by low concentrations of formalin. Neurochem Int. 2001;38:417–423. doi: 10.1016/s0197-0186(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 40.Bhargava HN, Bian J-T, Kumar S. Mechanism of attenuation of morphine antinociception by chronic treatment with L-arginine. J Pharmacol Exp Therap. 1997;281:707–712. [PubMed] [Google Scholar]


