Abstract
Objective(s):
The aim of this study was to evaluate, for the first time, whether the effects of low-dose adropin administration is effective in rats with hyperlipidemia.
Materials and Methods:
Twenty one Wistar albino female rats were randomly divided into 3 groups and fed with high-fat diet for 4 weeks to establish the hyperlipidemia model. Meanwhile, adropin was administrated intraperitonealy (2.1 μg/kg/day), once a day for continuous 10 days. Then, body weights and serum biochemical parameters, adropin, insulin and blood glucose levels were determined. Additionally, in liver tissue, inducible nitric oxide synthase (iNOS), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) mRNA gene expressions were evaluated by RT-PCR.
Results:
The results showed that intraperitoneal administration of adropin to hyperlipidemic rats for 10 days were extremely effective in decreasing the levels of serum triglycerides (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and gamma glutamil transferase (GGT) and increasing the levels of high density lipoprotein cholesterol (HDL-C). It could decrease mRNA expressions of pro-inflammatory cytokines TNF-α and IL-6 via regulating the expressions of iNOS. In addition, treatment with adropin showed a significant reduction in blood glucose, serum insulin levels, HbA1c (%), and HOMA-IR, and increase in serum adropin levels.
Conclusion:
Adropin may ameliorate lipid metabolism, reduce insulin resistance, and inhibit hepatocytes inflammation. Thus, adropin had significant therapeutic benefits and could be suggested as a potential candidate agent against hyperlipidemia.
Keywords: Adropin, Blood glucose, High-fat diet, Insulin, Lipid metabolism
Introduction
Hyperlipidemia causes abnormally elevated levels of lipids and lipoproteins in the blood including total cholesterol, triglycerides and is a lipid metabolism disorder (1). Hyperlipidemia can significantly enhance the risk of cardiovascular diseases such as coronary artery disease, atherosclerosis, cerebrovascular disease and peripheral vascular disease (2, 3). In addition, hyperlipidemia may result in solid organ injury, including damage to the liver and kidneys (4). High-fat diet induces the development of intra-abdominal fat deposits and hepatic steatosis (5, 6) and plays an important role in the improvement of insulin resistance and increasing glucose levels and causes hyperinsulinemia (7, 8).
Adropin is a new peptide hormone that regulates lipid metabolism. Adropin, a 76 amino acid secreted peptide is expressed in the brain, liver, and plasma and is encoded by the energy homeostasis associated (Enho) gene (9). Adropin plays an important role in the regulation of lipid and glucose homeostasis, which protects against the hepatosteatosis and hyperinsulinemia associated with obesity (10). Intraperitoneal injection of adropin has resulted in reduction of food intake and decrease in body weight in obese mice (9).
Adropin is an endocrine factor that plays important roles in the regulation of energy metabolism, insulin resistance, and endothelial functions. Although adropin effects on regulation of glucose and lipid homeostasis have been studied, the physiological effects of adropin on hyperlipidemia is not exactly known. Adropin has not been studied in hyperlipidemic rats using the high-fat diet model so far. Therefore, this study aimed to investigate the possible alterations in blood glucose and lipid metabolism in response to adropin in hyperlipidemic and healthy rats.
Materials and Methods
Animals
Female Wistar rats (10 weeks old) were obtained from Dumlupınar University Experimental Animal Laboratory, Kütahya, Turkey. They were kept under a controlled 12 hr light/12-hr dark cycle with a humidity of 55 ± 5% and room temperature of 23± 1°C. The rats had free access to food ad libitum. The experimental procedures were accepted by the Dumlupınar University Ethics Committee of Animal Care and Usage, Kütahya, Turkey.
Experimental design
The animals were randomly separated into three experimental groups as follows:
Group I: control (C, n=7) group received normal pellet diet.
Group II: hyperlipidemia (H, n=7) group received high-fat diet.
Group III: adropin treatment (A, n=7) group received high-fat diet and treated with adropin (2.1 μg/kg body weight (bw)/day).
Rats from groups H and A were fed with modified high-fat diet (10 ml/kg, orally) (11) for 4 weeks (12). Rats from group C were fed with normal pellet diet. Rats from group A intraperitonealy received adropin (Phoenix Pharmaceuticals, ABD) dissolved in distilled water for 10 days (13).
Tissue preparation and blood sampling
At the end of the experimental period, all animals were anesthetized with ketamine/xylazine HCl (75/10 mg/kg intraperitoneally). Blood samples were collected from the aorta without anticoagulant, left for 10 min and then, centrifuged for 15 min at 3,500 r/min to obtain serum which was stored at -20 °C until biochemical analysis for determination of serum insulin, adropin, TC, TG, HDL-C, LDL-C, AST, ALT, ALP, and GGT. Liver tissue samples were collected in liquid nitrogen for molecular studies.
Biochemical analyses
Determination of serum lipids
Serum concentrations of TC, TG, HDL-C, and LDL-C were measured by using a Beckman Coulter AU680 (Beckman Coulter, Miami, FL, USA) analyzer.
Determinationof liver function
Liver function was evaluated by assessing serum ALT, AST, ALP, and GGT levels using a Beckman Coulter AU680 (Beckman Coulter, Miami, FL, USA) analyzer.
Body weight and blood glucose measurement
Body weight and blood glucose levels were measured before starting the study and at the end of the experiment. Blood samples were collected from the tail of each animals after 12 hr fasting. The tail was cleaned with alcohol and about 1 mm of its end was cut and a drop of blood was used for the blood glucose test using a glucometer (eBsensor, Turkey).
Determination of serum ınsulin and adropin
According to the instruction of the manufacturer, serum concentrations of insulin and adropin were analyzed by an ELISA assay kit using the chemiluminescence method (Cusabio, Wuhan Huameı Bıotech Co, China) by an ELISA microplate reader (Spectrostar Nano, BMG, Labtech, Allmendgrün, Ortenberg, Germany).
Determination of HbA1c%
For Glycosylated hemoglobin A1c (HbA1c%) measurements, blood samples were collected into 2.0 ml dipotassium (K2) ethylene diamine tetraacetic acid (EDTA) vacuum tubes (BD Vacuteiner® BD-Plymouth, UK). After blood samples were collected, HbA1c% measurement was immediately performed without delay. Measurement of HbA1c% was performed using Tosoh G8 HPLC Analyzer (Tosoh Bioscience, Inc., San Francisco, CA).
Determination of HOMA-IR, HOMA-β
Homeostatic model assessment (HOMA-IR) score was calculated according to the formula: HOMA-IR [(Fasting serum insulin in U/l X fasting blood glucose in mmol/l)/22.5] using fasting serum insulin and fasting blood glucose concentrations measured at the end of the experimental period. β-cell function was determined with the HOMA-β, with the formula [serum insulin (U/l)×20/glucose (mmol/l)−3.5] (14).
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from liver tissue samples by GeneJET RNA Purification Kit (Thermo, Cat No: # K0732) according to the manufacturer's protocol. Total mRNA concentrations were measured at 260 nm using a Maestro Nano Micro-Volume spectrophotometer (Maestrogen Inc., Las Vegas, NV). Samples were stored at -80°C until further analysis. Complementary DNAs (cDNA) were quantified by EasyScript™ cDNA Synthesis Kit (Applied Biological Materials, Richmond, BC, Canada). cDNAs were stored at -20 °C until analysis by RT-PCR.
PCR was achieved in a total volume of 25 µl containing DNA template, 10X PCR-buffer, PCR grade H2O, 25 mmol/L MgCl2 and 10 mmol/l concentration of primers. RT-PCR was performed according to the manufacturer's instructions. The forward and reverse primers for TNF-α were 5’-CCA CCA CGC TCT TCT GTC TAC-3’ and 5’-GCT ACG GGC TTG TCA CTC G-3’, 148 bp, for IL-6 were 5’-CTT CCA GCC AGT TGC CTT CTT G-3’ and 5’-TGG TCT GTT GTG GGT GGT ATC C-3’, 109 bp, for iNOS were 5’-TTG GAG CGA GTT GTG GAT TGT TGT TC-3’ and 5’-GGT GAG GGC TTG CCT GAG TG AGC-3’, 126 bp. As a control for the presence of amplifiable RNA, β-actin primers were conceived. The forward and reverse primers for β-actin were 5’-CTA TCG GCA ATG AGC GGT TCC -3’ and 5’-TGT GTT GGC ATA GAG GTC TTT ACG -3’, 147 bp. The PCR products were electrophoresed on 2% agarose gel and added to ethidium bromide, making sure to include DNA markers of adequate size. The densitometry analysis of each PCR band was done using image J program. Optical density was calculated for gene products and results were expressed as the ratio of the density of TNF-α, IL-6, iNOS mRNA to β-actin mRNA.
Statistical analysis
Statistical analysis was done with SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) 16.0 pocket program. All results were given as mean ± standard error (SE). Comparisons among groups were made with Kruskal-Wallis test, and between two groups were made with Mann-Whitney U test. Values smaller than 0.05 were accepted as statistically significant.
Results
Effect of adropin on body weight and lipids
Table 1 depicts the levels of body weight, serum TC, TG, HDL-C and LDL-C levels of groups C, H and A.
Table 1.
Effect of adropin on body weight and lipids of control, hyperlipidemic, adropin treatment groups
| Groups | C (n=7) | H (n=7) | A (n=7) | P |
|---|---|---|---|---|
| Initial body weight (g) | 244.7 ±7.62 | 240.2 ±4.91 | 247.8 ±3.24 | 0.613 |
| Final body weight (g) | 256.5 ±9.13a | 311.0 ±12.3ab | 242.5 ±17.9b | 0.003 |
| TC (mg/dl) | 72.7 ±3.93a | 106.8 ±9.99a | 81.7 ± 6.43 | 0.014 |
| TG (mg/dl) | 99.5 ±14.3a | 148.1 ±16.8a | 80.8 ±18.5 | 0.081 |
| HDL-C (mg/dl) | 60.8 ±6.14a | 43.8 ±0.96ab | 57.5 ±5.57b | 0.031 |
| LDL-C (mg/dl) | 18.1 ±1.76a | 29.1 ±2.47ab | 18.4 ±2.64b | 0.011 |
C: control, H: hyperlipidemic, A: adropin treatment groups, TC: total cholesterol, TG: triglycerides, HDL-C: high-density lipoproteincholesterol, LDL-C: low-density lipoprotein-cholesterol.
p: Shows the differences between all groups (Kruskal-Wallis test).
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test).
On day 1 of the experiment, initial body weight in each group were not significantly different (P=0.613). At the end of the experiment, compared to group C, the final body weight of group H was significantly increased (P=0.002). Compared to group H, final body weight of group A was remarkably decreased (P=0.001) (Table 1).
There were statistically significant differences in the level of serum TC among the groups (P=0.014). The highest level of serum TC was found in group H as compared to group C (P= 0.004). No significant differences were observed in serum TG levels among the groups (P=0.081). But, the high level of TG was significant in group H compared to group C (P= 0.038). There were statistically significant differences in serum HDL-C and LDL-C levels among groups C, H and A (P=0.031 and P= 0.011, respectively). Significant decrease in serum HDL-C levels (P=0.026 and P=0.026) and a significant increase in LDL-C levels (P=0.004 and P=0.017) were observed in group H when compared to groups C and A (Table 1).
Effect of adropin on blood glucose, HbA1c (%), HOMA-IR, HOMA-β and serum insulin levels
Table 2 depicts the levels of blood glucose, HbA1c (%), HOMA-IR, HOMA-β and serum insulin in groups C, H and A.
Table 2.
Effect of adropin on blood glucose, HbA1c (%), HOMA-IR, HOMA-β and serum insulin levels of control, hyperlipidemic, adropin treatment groups of rats
| Groups | C (n=8) | H (n=8) | A (n=8) | P |
|---|---|---|---|---|
| Blood glucose (mg/dl) | 101.8 ±16.3ab | 235.2 ±29.1ac | 154.0 ±13.6bc | 0.010 |
| HbA1c (%) | 0.95 ±0.16a | 2.05 ±0.16ab | 1.00 ±0.02b | 0.001 |
| HOMA-IR | 20.6 ±3.76a | 73.5 ±12.2ab | 29.6 ±7.86b | 0.006 |
| HOMA-β | 5892.6 ±2730.0ab | 205.0 ±21.9a | 258.4 ±76.5b | 0.002 |
| Insulin (nIU/ml) | 103.4 ±13.2a | 127.2 ±10.0b | 61.8 ±8.76ab | 0.009 |
C: control, H: hyperlipidemic, A: adropin treatment groups; P: Shows the differences between all groups (Kruskal-Wallis test)
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test)
There were statistically significant differences in blood glucose, HbA1c (%), HOMA-IR, HOMA-β and serum insulin levels among groups C, H and A (P≤0.05). The hyperlipidemic rats showed a significant (P≤0.05) increase in the levels of glucose, HbA1c (%) and HOMA-IR compared to groups C and A and decrease in HOMA-β compared to group C. In addition, serum insulin level in group H was not significantly different from that of group C, but was significantly different from that of group A (Table 2).
Effect of adropin on liver enzyme markers
Table 3 depicts the levels of serum AST, ALT, ALP and GGT activities in groups C, H and A. There were statistically significant differences in the level of serum AST, ALT and GGT among the groups (P=0.012, P=0.007 and P=0.006, respectively), but no significant differences in the level of serum ALT (P=0.111) were seen. While the level of serum ALT and GGT was increased significantly (P≤0.05) in group H, increase in AST and ALP levels was not significant compared to group C. In addition, serum AST, ALT, ALP and GGT levels decreased in group A compared to group H (Table 3).
Table 3.
Effect of adropin on liver enzyme markers of control, hyperlipidemic, and adropin-treated groups of rat
| Groups | C (n=7) | H (n=7) | A (n=7) | P |
|---|---|---|---|---|
| AST (U/l) | 147.0 ±11.5a | 162.7 ± 10.3b | 82.3 ±5.65ab | 0.012 |
| ALT (U/l) | 72.5 ± 2.41a | 89.4 ± 4.48ab | 65.4 ± 3.90b | 0.007 |
| ALP (U/l) | 176.5 ± 25.9 | 229.7 ± 16.1a | 184.2 ± 18.9a | 0.111 |
| GGT (U/l) | 1.28 ± 0.18a | 2.42 ± 0.20ab | 1.42 ± 0.20b | 0.006 |
C: control, H: hyperlipidemic, A: adropin treatment groups, AST: aspartate transaminase, ALT: alanine transaminase, ALP: alkaline phosphatase, GGT: gama glutamil transferaz; P: Shows the differences between all groups (Kruskal-Wallis test)
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test)
Levels of serum adropin
Figure 1 shows the levels of adropin in serum in groups C, H and A. There were no significant differences in serum adropin levels among groups C (44.7±1.51), H (40.9 ± 1.38), A (50.6 ± 4.71), (P = 0.101). However, the level of serum adropin in group A was significantly higher than that in group H(P = 0.05) (Figure 1).
Figure 1.
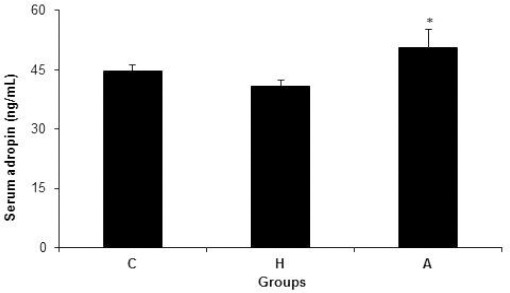
Serum adropin concentrations in control, hyperlipidemic, and adropin-treated groups of rats; * Shows significance between H and A groups (P≤0.05) (Mann Whitney U test); C: control, H: hyperlipidemic, and A: adropin-treated groups
Effect of adropin on TNF-α, IL-6 and iNOS mRNA expression in the liver tissue
Figure 2 shows mRNA expression of TNF-α in the liver tissue in groups C, H and A. The differences in the TNF-α mRNA expression levels in the liver tissue among groups C (0.97±0.08), H (1.28±0.15) and A (0.83±0.05) were significant (P=0.009). The expression of TNF-α was increased in group H when compared to groups C and A (P=0.05 and P=0.002, respectively) (Figure 2).
Figure 2.
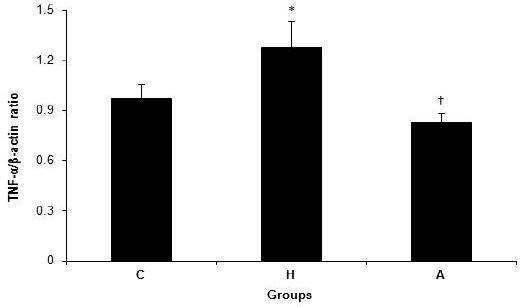
TNF-α/β-actin ratio in control, hyperlipidemic, and adropin-treated groups of rat; * Shows significance between groups C and H (P≤0.05) (Mann Whitney U test), † Shows significance between groups H and A (P≤0.05) (Mann Whitney U test); C: control, H: hyperlipidemic, A: adropin treatment groups, TNF-α: Transforming growth factor-alpha.
The changes in the level of mRNA expression of IL-6 in the liver tissue in groups C, H and A are shown in Figure 3. There were no significant differences in IL-6 mRNA expression levels in the liver tissue among groups C (1.17 ± 0.18), H (1.34± 0.11), and A (1.00± 0.02) (P=0.155). On the other hand, the level of of mRNA expression of IL-6 in the liver tissue was decreased significantly (P=0.035) in group A compared to group H (Figure 3).
Figure 3.
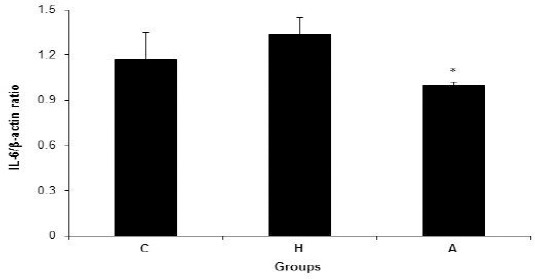
IL-6/β-actin ratio in control, hyperlipidemic, and adropin-treated groups of rats; * Shows significance between groups H and A (P≤0.05) (Mann Whitney U test); C: control, H: hyperlipidemic, A: adropin treatment groups, IL-6: Interleukin 6
Figure 4 shows mRNA expression of iNOS in the liver tissue in groups C, H and A. There were significant differences in iNOS mRNA expression levels in the liver tissue among groups C (1.02±0.16), H (1.72±0.25), A (0.89±0.04) (P=0.030). The expression of iNOS was increased in group H when compared to groups C and A (P=0.05 and P=0.011) (Figure 4).
Figure 4.
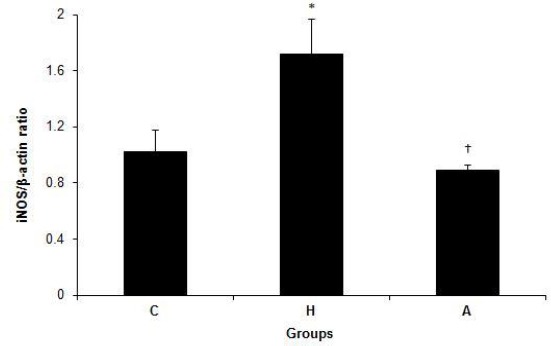
iNOS/β-actin ratio in control, hyperlipidemic, and adropin-treated groups of rats; * Shows significance between groups C and H (P≤0.05) (Mann Whitney U test); † Shows significance between groups H and A (P≤0.05) (Mann Whitney U test); C: control, H: hyperlipidemic, A: adropin treatment groups, iNOS: inducible nitric oxide synthase
Discussion
Hyperlipidemia is a state associated with elevation in the levels of TC, TG, and phospholipids, and also changes in lipoproteins (15) which leads to the development of various disorders including coronary artery disease, myocardial infarction (16) and diabetes mellitus (17) and has emerged as an important public health problem. This study was carried out in order to find the influence of daily intraperitoneal administration of adropin for 10 days on plasma glucose and lipid profile in hyperlipidemic rats.
In this study, a model of hyperlipidemia was successfully induced by high-fat diet in rats. Body weight and serum concentrations of TC, TG and LDL-C were significantly increased, while serum concentrations of HDL-C were significantly reduced. Miao et al showed that serum TC, TG and LDL-C were significantly increased, whereas HDL-C level was significantly decreased in diet-induced hyperlipidemic rats compared to the control group (18). These results seem compareble with our results and can be considered as an indicator of occurence of hyperlipidemia in rats. Adropin treatment resulted in reductions in both TC and TG levels, but was not statistically significant. In addition, intraperitoneal administration of adropin significantly decreased body weight and the levels of LDL-C and increased the levels of HDL-C in group A. Butler et al demonstrated that adropin levels are negatively correlated with TG in metabolic syndrome patients after gastric bypass surgery (19). Yildirim et al recently showed that serum adropin level was negatively correlated with serum lipid markers including TC, LDL-C, and TG in policystic ovarian syndrome (PCOS) patients (20). This finding suggested that adropin could decrease the elevated activity of lipids in hyperlipidemic rats and had effects on ameliorating lipid metabolism disease.
There were significant increases in serum adropin levels in group A compared to group H. Reduction in lipid activity in group A might be a result of an increase in the levels of serum adropin. Kumar et al reported that the amount of adropin in circulation is augmented commensurate to the increase in dietary fat content and adropin levels were decreased in mice fed with a high-carbohydrate-low-fat diet, while increased in mice fed with a low-carbohydrate-high-fat diet. (21). In a study by Kumar et al, it was observed that energy homeostasis associated (Enho) gene expression level was significantly lower in the liver tissues of rats with diet-induced obesity and adropin administration to rats with diet-induced obesity decreases hepatosteatosis (9). In this study and previous studies on this subject, differences in dose and type of administration of adropin led to the differences in the results.
In this study, blood glucose, HbA1c (%) and HOMA-IR scores increased and a decrease was found in HOMA-β levels in group H compared to group C. Eu et al demonstrated that mean blood glucose concentration and HOMA-IR index were increased in high-fat diet-induced obese rats for 28 days compared to the controls (22). In our study, intraperitoneal administration of adropin significantly decreased the levels of fasting blood glucose, HbA1c (%), HOMA-IR and insulin levels in group A. The reason for the reduction of these parameters in adropin-treated group might arise from increasing the levels of serum adropin. Adropin may lead to inhibiting the production of glucose metabolism directly. In studies performed on diet-induced obese mice with insulin resistance, it was reported that adropin treatment ameliorated glucose tolerance, insulin action and metabolic flexibility towards glucose utilization (23). Aydın et al reported that there was a negative correlation between HbA1c (%) and adropin concentrations (24). Yildirim et al recently showed that serum adropin level was negatively correlated with fasting serum insulin levels, HOMA-IR in PCOS patients (20). The study demonstrated that lower adropin levels were associated with insulin resistance in humans, as in mice (21). In the study performed by Kumar et al it was shown that increased amounts of adropin in circulation, decrease insulin resistance and glucose intolerance that occur in response to metabolic stress (9). Our present results demonstrated that adropin could decrease the elevated activity of glucose and insulin in hyperlipidemic rats.
In this study, an increase was observed in serum ALT, ALP and GGT levels in group H compared to group C, but the high level of serum AST was not significant. Abbas et al revealed that high-fat diet caused a significant increase in serum levels of ALT, AST, ALP, and GGT in comparison to control (25). After treatment with adropin, it was found that adropin could decrease the level of AST, ALT, ALP and GGT in group A. There is no study in the literature focusing on the relation between adropin and hepatic marker enzymes that can be compared with the results of this study. Our present results demonstrated that adropin could decrease the elevated liver enzymes activities and has potential hepatoprotective effects in hyperlipidemic rats.
In this study, TNF-α mRNA expression in the liver tissue were higher in group H than in the healthy rats (controls), indicating that hyperlipidemia induces the upregulation of pro-inflammatory cytokines and leads to liver tissue damage. In addition, in group H, IL-6 levels are elevated, but not statistically significant. Ding et al have demonstrated that renal expression of TNF-α and IL-6 were increased in the hyperlipidemic group when compared to the normal control group (26). In the present study, adropin was shown to reduce mRNA expression levels of TNF-α and IL-6 in hyperlipidemic rat model. There is no study in the literature considering the relation between adropin, TNF-α and IL-6 that can be compared with the results of this study. Lovren et al reported that adropin-treated endothelial cells showed proliferation, migration, and capillary-like tube formation as well as less permeability and TNF-α-induced apoptosis (13).
In this study, iNOS mRNA expression in the liver tissue was significantly increased in group H compared to groups C and A. A study by Wang et al (27) showed that the expression of iNOS mRNA expression increased in rats with hyperlipidemia. A significant decrease in the iNOS mRNA expression in group A is seen when compared to group O. Kuloglu and Aydin showed that the densities of adropin and iNOS immuno-reactivity rised with the severity of the diabetes. In addition, increased levels of adropin and iNOS in the kidney demonstrate that these substances are involved in the pathophysiology of diabetes; this creates a compensative mechanism against the damage inflicted by the disease (28). Increased expression of TNF-α, IL-6 and iNOS mRNA seen in hyperlipidemic rats was decreased in adropin-treated rats. Our present results demonstrated that adropin suppressed inflammation in hyperlipidemiarats.
Conclusion
In this research, exposure to a high-fat diet, even if it does not result in obesity, may be a risk factor for metabolic disorders. As a result, serum adropin levels are decreased in hyperlipidemic rats in which hyperlipidemia was induced by administering high fat diet for four weeks. Adropin treatment can prevent hyperlipidemia. These findings suggest that adropin could have a strong anti-hyperlipidemia activity and also ameliorate other lipid metabolism disorder-related complications, presumably by reducing inflammatory cytokines, developing glucose metabolism, insulin sensitivity and intervention improved the level of serum of lipid and liver enzyme markers in the hyperlipidemic rats. We assume that further comprehensive studies are required in order to have a better understanding of the relation between hyperlipidemia and adropin.
Acknowledgment
The authors are grateful to Dumlupınar Research Center (DPU-ILTEM) for using the laboratory and equipments, to Didem Ocak and Arif Soylu for their help in experimental research. There is no financial source for our work.
Conflict of interest
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors declared no conflict of interest.
References
- 1.Suk HY, Zhou C, Yang TT, Zhu H, Yu RY, Olabisi O, Yang X, Brancho D, Kim JY, Scherer PE, Frank PG, Lisanti MP, Calvert JW, Lefer DJ, Molkentin JD, Ghigo A, Hirsch E, Jin J, Chow CW. Ablation of calcineurin Aβreveals hyperlipidemia and signaling cross-talks with phosphodiesterases. J Biol Chem. 2013;288:3477–3488. doi: 10.1074/jbc.M112.419150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pöss J, Custodis F, Werner C, Weingärtner O, Böhm M, Laufs U. Cardiovascular disease and dyslipidemia: beyond LDL. Curr Pharm Des. 2011;17:861–870. doi: 10.2174/138161211795428858. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 2011;31:189–196. doi: 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- 4.Lu ZL, Xu ZM, Kou WR, Zhao SP. Advance in basic and clinical research of Xuezhikang capsule. Chin J Integr Med. 2006;12:85–93. doi: 10.1007/BF02857352. [DOI] [PubMed] [Google Scholar]
- 5.Priego T, Sa´nchez J, Pico´ C, Palou A. Sex-differential expression of metabolism- related genes in response to a high-fat diet. Obesity. 2008;16:819–826. doi: 10.1038/oby.2007.117. [DOI] [PubMed] [Google Scholar]
- 6.Taraschenko OD, Maisonneuve IM, Glick SD. Sex differences in high fat-induced obesity in rats: effects of 18-methoxycoronaridine. Physiol Behav. 2011;103:308–314. doi: 10.1016/j.physbeh.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez ML. The metabolic syndrome. Nutr Rev. 2007;65:S30–S34. doi: 10.1111/j.1753-4887.2007.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 8.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, Ferrante AW, Jr, Mynatt RL, Burris TP, Dong JZ, Halem HA, Culler MD, Heisler LK, Stephens JM, Butler AA. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydin S, Kuloglu T, Aydin S, Eren MN, Yilmaz M, Kalayci M, Sahin I, Kocaman N, Citil C, Kendir Y. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol Cell Biochem. 2013;380:73–81. doi: 10.1007/s11010-013-1660-4. [DOI] [PubMed] [Google Scholar]
- 11.Ai J, Wang N, Du J, Yang M, Liu P, Du Z. Establishment of type 2 diabetic animal model in in Wistar rats. Chin Pharmacol Bull. 2004;20:1309–1312. [Google Scholar]
- 12.Yang R, Guo P, Song X, Liu F, Gao N. Hyperlipidemic guinea pig model: mechanisms of triglyceride metabolism disorder and comparison to rat. Biol Pharm Bull. 2011;34:1046–1051. doi: 10.1248/bpb.34.1046. [DOI] [PubMed] [Google Scholar]
- 13.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, Al-Omran M, Teoh H, Verma S. Adropin is a novel regulator of endothelial function. Circulation. 2010;14:185–192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim MA, Islam MS. Anti-diabetic effects of the acetone fraction of Senna singueana stem bark in a type 2 diabetes rat model. J Ethnopharmacol. 2014;28(153):392–409. doi: 10.1016/j.jep.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Bagdade JD, Helve E, Taskinen MR. Effect of continuous insulin infusion therapy lipoprotein surface and core lipid composition in IDDM. Metabolism. 1991;40:445–449. doi: 10.1016/0026-0495(91)90222-i. [DOI] [PubMed] [Google Scholar]
- 16.Onody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrate formation in rat hearts. Cardiovasc Res. 2003;58:663–670. doi: 10.1016/s0008-6363(03)00330-4. [DOI] [PubMed] [Google Scholar]
- 17.Shew WH, Jeng CY, Lee WJ, Lin SY, Pei D, Chen YT. Simvastatin treatment in postprandial hypertri-glyceridemia in type 2 diabetes mellitus patients with combined hyperlipidemia. Metabolism. 2001;50:355–359. doi: 10.1053/meta.2001.21026. [DOI] [PubMed] [Google Scholar]
- 18.Miao H, Chen H, Pei S, Bai X, Vaziri ND, Zhao YY. Plasma lipidomics reveal profound perturbation of glycerophospholipids, fatty acids, and sphingolipids in diet-induced hyperlipidemia. Chem Biol Interact. 2015;25:79–87. doi: 10.1016/j.cbi.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O’Keeffe M, St-Onge MP, Ravussin E, Havel PJ. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab. 2012;97:3783–3791. doi: 10.1210/jc.2012-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yildirim B, Celik O, Aydin S. Adropin: a key component and potential gatekeeper of metabolic disturbances in policystic ovarian syndrome. Clin Exp Obstet Gynecol. 2014;41:310–312. [PubMed] [Google Scholar]
- 21.Kumar KG, Zhang J, Gao S, Rossi J, McGuinness OP, Halem HH, Culler MD, Mynatt RL, Butler AA. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 2012;20:1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eu CH, Lim WY, Ton bin Abdul SH, Kadir K. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010;29(9):81. doi: 10.1186/1476-511X-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S, McMillan RP, Zhu Q, Lopaschuk GD, Hulver MW, Butler AA. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol Metab. 2015;17(4):310–324. doi: 10.1016/j.molmet.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydin S. Three new players in energy regulation: preptin, adropin and irisin. Peptides. 2014;56:94–110. doi: 10.1016/j.peptides.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Abbas AM, Sakr HF. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J Physiol Biochem. 2013;69:737–750. doi: 10.1007/s13105-013-0250-y. [DOI] [PubMed] [Google Scholar]
- 26.Ding M, Si D, Zhang W, Feng Z, He M, Yang P. Red yeast rice repairs kidney damage and reduces inflammatory transcription factors in rat models of hyperlipidemia. Exp Ther Med. 2014;8:1737–1744. doi: 10.3892/etm.2014.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang WQ, Zhang HF, Gao GX, Baı QX, Lı R, Wang XM. Adiponectin Inhibits Hyperlipidemia-Induced Platelet Aggregation via Attenuating Oxidative/Nitrative Stress. Physiol Res. 2011;60:347–354. doi: 10.33549/physiolres.932044. [DOI] [PubMed] [Google Scholar]
- 28.Kuloglu T, Aydin S. Immunohistochemical expressions of adropin and inducible nitric oxide synthase in renal tissues of rats with streptozotocin-induced experimental diabetes. Biotech Histochem. 2014;89:104–110. doi: 10.3109/10520295.2013.821713. [DOI] [PubMed] [Google Scholar]


